-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas J. Bartsch, György Homola, Armin Biller, Stephen M. Smith, Heinz-Gerd Weijers, Gerhard A. Wiesbeck, Mark Jenkinson, Nicola De Stefano, László Solymosi, Martin Bendszus, Manifestations of early brain recovery associated with abstinence from alcoholism, Brain, Volume 130, Issue 1, January 2007, Pages 36–47, https://doi.org/10.1093/brain/awl303
Close - Share Icon Share
Abstract
Chronic alcohol abuse results in morphological, metabolic, and functional brain damage which may, to some extent, be reversible with early effects upon abstinence. Although morphometric, spectroscopic, and neuropsychological indicators of cerebral regeneration have been described previously, the overall amount and spatial preference of early brain recovery attained by abstinence and its associations with other indicators of regeneration are not well established. We investigated global and local brain volume changes in a longitudinal two-timepoint study with T1-weighted MRI at admission and after short-term (6–7 weeks) sobriety follow-up in 15 uncomplicated, recently detoxified alcoholics. Volumetric brain gain was related to metabolic and neuropsychological recovery. On admission and after short-term abstinence, structural image evaluation using normalization of atrophy (SIENA), its voxelwise statistical extension to multiple subjects, proton MR spectroscopy (1H-MRS), and neuropsychological tests were applied. Upon short-term sobriety, 1H-MRS levels of cerebellar choline and frontomesial N-acetylaspartate (NAA) were significantly augmented. Automatically detected global brain volume gain amounted to nearly two per cent on average and was spatially significant around the superior vermis, perimesencephalic, periventricular and frontal brain edges. It correlated positively with the percentages of cerebellar and frontomesial choline increase, as detected by 1H-MRS. Moreover, frontomesial NAA gains were associated with improved performance on the d2-test of attention. In 10 age- and gender-matched healthy control subjects, no significant brain volume or metabolite changes were observed. Although cerebral osmotic regulations may occur initially upon sobriety, significant increases of cerebellar choline and frontomesial NAA levels detected at stable brain water integrals and creatine concentrations, serum electrolytes and red blood cell indices in our patient sample suggest that early brain recovery through abstinence does not simply reflect rehydration. Instead, even the adult human brain and particularly its white matter seems to possess genuine capabilities for regrowth. Our findings emphasize metabolic as well as regionally distinct morphological capacities for partial brain recovery from toxic insults of chronic alcoholism and substantiate early measurable benefits of therapeutic sobriety. Further understanding of the precise mechanisms of this recovery may become a valuable model of brain regeneration with relevance for other disorders.
Abbreviations
- 1H-MRS
proton MR spectroscopy
- NAA
N-acetylaspartate
- PBVC
percentage brain volume change
- SIENA
structural image evaluation using normalization of atrophy
Introduction
Chronic alcoholism is a major source for the morbidity and mortality of a large variety of diseases worldwide and can cause, among others, substantial cerebral damage. The existence of irreversible cerebral atrophy and dementia purely related to the toxic effects of alcohol and not to secondary disorders (such as nutritional Wernicke's encephalopathy and Korsakoff's amnestic syndrome) has been disputed (Victor et al., 1985). Nevertheless, it is generally agreed that chronic alcoholism results in grey and white matter brain injury which is neuropathologically detectable and accompanied by brain shrinkage, reductions of neuronal and glial markers as well as neuropsychological impairments (Kril et al., 1997; Harper et al., 2003).
Previous comparisons of alcoholic versus healthy subjects or non-heavily drinking controls by proton magnetic resonance spectroscopy (1H-MRS) have revealed reduced levels of brain N-acetylaspartate (NAA) in most (Fein et al., 1994; Jagannathan et al., 1996; Seitz et al., 1999; Bendszus et al., 2001; Schweinsburg et al., 2001; 2003; Bloomer et al., 2004; Durazzo et al., 2004; Meyerhoff et al., 2004; Viola et al., 2004) but not all investigations and brain regions studied (Schweinsburg et al., 2000; Parks et al., 2002; Ende et al., 2005; Mason et al., 2006). Similarly, lowered levels of choline have been detected (Jagannathan et al., 1996; Seitz et al., 1999; Bloomer et al., 2004; Viola et al., 2004; Ende et al., 2005; Mason et al., 2006), although this finding seems even more ambiguous (Schweinsburg et al., 2000, 2001, 2003) and dependent on localization (Bendszus et al., 2001; Parks et al., 2002). Shifts between different choline containing compounds may contribute to these heterogeneous results (Lee et al., 2003). Spectroscopic elevations of inositol compounds (Schweinsburg et al., 2000, 2001; Viola et al., 2004) due to alcohol toxicity and deviations of other metabolites such as γ-aminobutyric acid (Behar et al., 1999), glutamate, and glutamine have been reported but as yet these are still conflicting (Seitz et al., 1999; Braunova et al., 2000; Parks et al., 2002; Meyerhoff et al., 2004), related to comorbidities such as nicotine abuse (Mason et al., 2006), medical complications such as hepatic failure (Mesisca and Ross, quoted in Mason et al., 2005) or just the timepoint studied (Mason et al., 2003) and mostly difficult to detect reliably at conventional field strength and with appropriate signal-to-noise (S/N) ratios. Obviously, cross-sectional differences may depend on the tissue compartments and brain region studied as well as on gender (Schweinsburg et al., 2003), age, and concomitant complications such as clinical encephalopathy and metabolic alterations. In general, they are neither specific nor sufficient to reliably discriminate alcoholics from controls, and to separate potential pure alcoholic brain damage from secondary medical complications, the necessity to discriminate between cases of uncomplicated primary alcohol dependence and those suffering from secondary or concomitant diseases has been strongly emphasized (Victor et al., 1985; Kril et al., 1997; Harper et al., 2003).
Notably, cerebral volume loss, metabolic and neuropsychological impairments associated with chronic alcoholism may be in part reversible, even (and possibly primarily) during early stages of abstinence (Meyerhoff, quoted in Mason et al., 2005; Gazdzinski et al., 2005a). Upon longitudinal studies with sobriety, evidence for recovering NAA (Bendszus et al., 2001; Parks et al., 2002; Durazzo et al., 2006) as well as choline (Bendszus et al., 2001; Ende et al., 2005; Durazzo et al., 2006) levels has emerged, albeit somewhat equivocally with respect to other data (Martin et al., 1995; Schweinsburg et al., 2000; O'Neill et al., 2001; Ende et al., 2005). Nicotine abuse is strongly suspected to exacerbate brain NAA and choline loss in alcoholics (Durazzo et al., 2004) and to constrain their recovery upon abstinence (Durazzo et al., 2006). Regression of ventricular enlargement and sulcal widening upon abstinence has been reported more than two and a half decades ago (Carlen et al., 1978; Lishman, 1981) and substantiated thereafter (Schroth et al., 1988; Zipursky et al., 1989; Bendszus et al., 2001). This brain volume regain upon abstinence indicates that cerebral tissue shrinking is at least not entirely due to irreversible brain atrophy. Although there is some evidence that morphological restitution should not only be attributed to rehydration (Mander et al., 1989; Trabert et al., 1995; Schroth et al., 1998), the overall brain volume gain attained early upon abstinence, its localization and association with metabolic indicators for recovery from alcoholic brain damage as well as factors presumably limiting regeneration such as duration of dependence, are not satisfactorily established. The objective of the investigation reported here was the prospective, and longitudinal evaluation of morphometric, spectroscopic and neuropsychological effects of short-term sobriety in uncomplicated, not dehydrated or heavily smoking alcoholics without clinical encephalopathy.
Material and methods
The investigation presented was designed as a prospective longitudinal study collecting MR morphometry, 1H-MRS, and neuropsychological data as well as blood samples from alcohol-dependent patients and, in part, healthy controls at two timepoints, i.e. at enrolment and upon a 6–7 weeks sobriety follow-up.
Subjects
24 patients (age 41 ± 8; 29 to 58 years; 16 male, 8 female) afflicted with primary alcohol dependence according to DSM-IV and ICD-10 criteria and admitted to a specialized inpatient treatment unit were enrolled into the study. Diagnosis was established by a trained interviewer (G.A.W.) using the Semi-Structured Assessment for the Genetics of Alcoholism (Buchholz et al., 1994). Thereby, personal history of alcohol and drug abuse, occurrence of major alcohol-related life problems, and demographic information were recorded. Age for inclusion was set between 18 and 60 years, and active alcohol dependence (12 ± 7; 3 to 25 years duration) had to have lasted for the past 3 years or longer without previous full or partial remissions according to DSM-IV criteria. Alcohol consumption was estimated by (i) the number of days spent with active drinking per month (i.e. frequency of drinking), (ii) the average number of standard drinks containing 10 g of ethanol per drinking day (quantity of drinking), (iii) the maximum number of standard drinks consumed per drinking day, and (iv) the total number of standard drinks during the last 3 months before admission. Based upon multiaxial assessment, exclusion criteria were the following: a lifetime diagnostic history of any other psychiatric axis I or II disorder except alcohol dependence, previous alcohol withdrawal, nicotine abuse above an equivalent of 10 cigarettes per day, and presence of any other general medical condition or axis III illness as well as therapeutic need for psychoactive medication. Therefore, patients suffering from intoxication delirium upon admission, withdrawal delirium during actual detoxification, substance-induced persisting amnestic disorder (Korsakoff's syndrome), overt Wernicke's encephalopathy, central pontine or extrapontine myelinolysis (Machiafava-Bignami's disease), hepatic encephalopathy, or other alcohol-induced disorders were not eligible for the study. Initial MR morphometric, spectroscopic, and neuropsychological exams were carried out within the first four (2 ± 1; 1 to 4) days upon admission and within the first week after the last drink reported (3 ± 1; 2 to 7 days) when there was no evidence of persisting withdrawal and no blood alcohol was detected. Severity of withdrawal was monitored by brief clinical examination and heart rate as well as blood pressure measurements every 8 h for the first week upon admission. Abstinence was monitored carefully on a daily basis, and no psychoactive medication was administered. Upon short-term abstinence (i.e. 38 ± 3; 35 to 42 days after the initial exam), nine patients were non-compliant with their abstinence treatment (n = 8) or study enrolment (n = 1) leaving a total of 15 patients (aged 42 ± 8; 31 to 58 years; 10 male, 5 female) in the follow-up sample. Up to this timepoint, these patients had successfully maintained sobriety without evidence for prolonged or late withdrawal upon detoxification. There was no difference in gender, age or duration of dependence between compliant and non-compliant patients (Pcorrected ≥ 0.46, np = 3; Fisher's exact test, U-test). Furthermore, males were not significantly overrepresented in our final patient follow-up sample (n = 15), and these remaining men and women did not differ with regard to age (males: 39 ± 8; 31 to 58, females: 47 ± 6; 36 to 53 years) or duration of dependence (males: 12 ± 7; 3 to 23, females: 12 ± 7; 6 to 25 years; Pcorrected ≥ 0.21, np = 3; test of equal or given proportions test, U-test).
For control purposes, 10 healthy volunteers (47 ± 7; 30 to 55 years; 6 male, 4 female) were recruited via local newspaper advertisements. Gender and age distribution of the volunteers were matched to the patients studied, and the interval between their initial and follow-up exam (42 ± 7; 34 to 60 days) was not significantly different from the patients (Pcorrected ≥ 0.24, np = 3; Fisher's exact test, U-test). Detailed screening excluded any present or past alcohol- or other substance-related disorder as well as any relevant general medical condition. This was supported by normal CAGE questionnaire ratings (Mayfield et al., 1974) and blood samples drawn which were analysed for red blood cell (RBC) indices, haematocrit, carbohydrate-deficient transferrin (CDT), and γ-glutamyl-transferase (GGT) values.
All subjects provided written informed consent, and the study was approved by the local ethics committee.
MR morphometry (SIENA and its voxelwise extension across subjects)
All MR measurements were performed on a 1.5 tesla (63.7 MHz) Magnetom Vision scanner (Siemens Medical, Erlangen, Germany) running on Numaris3 (VA33D) with a circularly polarized transceiver head coil. T2-weighted TRUe Fast Imaging with steady-state precession spin echo (SE) localizers [TR/TE = 6.08/3.04 ms, flip angle (FA) = 70°] of the whole brain were acquired in axial, coronal, and sagittal 5 mm planes. Additionally, proton density (PD)- and T2-weighted SE sequences [TR/TE = 2100/20 and 80 ms, FA = 12°, phase-encoding (PhE) = left-to-right, field of view (FoV) = 256 × 256 mm2, 19 slices, slice thickness = 6 mm, 20% gap] centred and angulated parallel to the anterior commissure–posterior commissure line were used for neuroradiological screening.
For MR morphometry, sagittally oriented T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) (i.e. turbo fast low angle shot) pulse sequences (TR/TE = 9.70/4.0 ms, FA = 12°, PhE = posterior-to-anterior, FoV = 256 × 256 mm2, slab thickness = 186 mm) were recorded covering the entire head at an isotropic voxel size of 1 mm3. Axial PD-/T2-weighted and coronal T1-weighted reconstructions assured absence of gross parenchymal signal alterations, poliohaemorrhagic lesions, and overt mammillary body atrophy.
Structural image processing and data analysis was performed using FSL (FMRIB Software Library release 3.3, Author Webpage; Smith et al., 2004) by a single experienced neuroradiology resident (A.J.B.) blinded to patient and control data at the interactive stages. Images were loaded into FSLView, and myelencephalon and medulla spinalis were edited out, by hand, below the level of the pons at the medullopontine sulcus (seeFig. 1). Then, images were submitted to structural image evaluation using normalization of atrophy (SIENA version 2.4, part of FSL). To achieve optimal brain/non-brain separation for the individuals examined, thresholds for brain extraction were adjusted from 0.4 to 0.6, and optional standard space brain masking was used. To avoid the introduction of any systematic longitudinal bias, however, both options were fixed for any given subject. External skull border estimates were obtained. Brain-extracted images were then reinspected, and residual non-brain tissue (such as nasal or dural sinuses, skull and clivus, in particular) was segmented out by hand in FSLView. These cleaned brain images (with non-brain tissue, medulla spinalis and oblongata removed) were then fed back into SIENA (Smith et al., 2001, 2002) to estimate the final two-timepoint percentage brain volume change (PBVC). SIENA starts by extracting brain and skull images from the two-timepoint whole-head input data (Smith, 2002). The two brain images are then aligned to each other (Jenkinson and Smith, 2001, Jenkinson et al., 2002) using the skull images to constrain the registration scaling; both brain images are resampled into the space halfway between the two. Next, tissue-type segmentation is carried out (Zhang et al., 2001) combining grey and white matter estimates into a single class in order to find brain/non-brain edge points, and then perpendicular edge displacement (between the two timepoints) is estimated at these edge points. Finally, the mean edge displacement is converted into a (global) estimate of PBVC between the two timepoints.
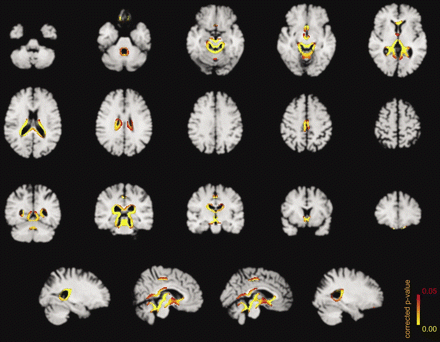
Significant morphometric edge flow indicating early brain regeneration through abstinence from alcoholism (voxelwise non-parametric SIENA statistics at Pcorrected ≤ 0.05; n = 15; overlay onto custom template space on skull-stripped single-patient MP-RAGE background for preservation of sulci and gyration; display in ‘radiological convention’ with left projected on right image side).
To allow for a voxelwise statistical extension to multiple subjects, standardized cerebral edge flow images were obtained (De Stefano et al., 2003). The subject-specific edge flow images (which encode amount and direction of perpendicular local brain edge motion between successive scans) were spatially dilated, affine transformed to standard space by FLIRT (FMRIB's Linear Image Registration Tool version 5.4.2), masked with a standard space brain edge image, smoothed by an isotropic Gaussian kernel (σ = 5 mm), and remasked. This produced cerebral edge flow images in standard space which were submitted to voxelwise statistical analysis. For investigation of the patients, standard space was defined by a skull-stripped custom template (seeFig. 1 for a patient background image in standard space). It was constructed from the sum of the skull-stripped initial MP-RAGE scans of all 24 patients studied which were co-registered by full affine transformations to the MNI152 template using FLIRT and smoothed by an isotropic Gaussian kernel (σ = 3 mm). To optimize the edge mask used, a group-specific brain edge image was generated for the patients (according to FSL's make_edges script).
MR spectroscopy (1H-MRS)
Localized 1H-MRS consisted of single voxel spectroscopy (SVS) measurements by a point-resolved spectroscopy (PRESS) SE sequence at TE = 135 ms (TR = 1500 ms, vector size 1024). For every voxel, two spectra with and without CHEmical-Shift Selective water suppression were obtained (128 versus 16 acquisitions). Fixed scanner calibration was confirmed by external in vitro standards. Isotropic voxels (20 × 20 × 20 mm3) were placed infratentorially in the left cerebellar hemisphere, whereas voxels of 30 × 40 × 20 mm3 were used on the supratentorial level for the mesial frontal lobes. Reproducible positioning was achieved as described previously by setting the cerebellar voxel with its medial edge in the left cerebellar hemisphere, close to the fourth ventricle, and with its superior edge below the tentorium, and by placing the frontal voxel with its inferior edge at the callosomarginal sulci and with its posterior edge at the central sulci (Bendszus et al., 2001).
For spectral analysis, the user-independent frequency domain-fitting routine by LCModel (version 6.1–4/LCMgui version 2.1–4; Author Webpage) was used. Eddy current correction was performed in the time domain by the voxels unsuppressed water reference Free Induction Decay dividing the water-suppressed signal by the phase factor of the water signal for each data point (Klose, 1990). Water integrals were obtained by extracting areas of unsuppressed water peaks from LCModel's detailed print output. S/N values were estimated by the ratio of the maximum in the spectrum-minus-baseline over the default analysis window to twice the root-mean-square residuals. Metabolite quantifications of NAA, choline, creatine, myo-inositol, and ethanol were based upon calibration measurements. Other metabolites (i.e. N-acetylaspartyl-glutamate, scyllo-inositol. glutamate, glutamine, γ-aminobutyric acid, macromolecules, phosphocholine, and glycerophosphocholine) were not considered in the analyses for these are either hard to resolve at 1.5 T with the particular PRESS sequence employed and/or because Cramér-Rao lower bounds were not consistently <50%. All metabolite quantifications were transformed into relative percentages of change adjusting for mean local brain volume change (based on individual flow images masked with jointly co-registered spectroscopy voxels) at short-term abstinence of the 15 patients studied twice and at follow-up of the 10 volunteers re-examined. The correction for within spectroscopy voxel brain volume change was made to exclude confounding effects by partial voluming, i.e. that a local brain volume difference itself propagates into metabolite changes.
Neuropsychological assessment
Neuropsychological performance of the patients was assessed in the areas of attention and concentration (d2-test), long-term memory (Auditory Verbal Learning Test), and non-verbal (Standard Progressive Matrices) as well as verbal cognitive abilities (Vocabulary Test) by a single experienced psychologist (H.-G.W.). Due to its favourable time and compliance requirements, the d2-test of attention and concentration was applied twice to the patients, i.e. upon initial examination and after sobriety had been maintained by short-term abstinence. It is described in more detail below.
d2-Test of attention. This is a paper and pencil test asking for prompt detection of three subtly different target stimuli. Subjects are instructed to mark targets as fast and with as few errors as possible. Thus, speed and rule compliance are tested under a task-load condition. By subtracting omission and commission error count (i.e. the number of missing and false responses) from the total number of stimuli processed, an overall performance estimate is obtained. This reflects individual attention span, concentration ability and coordination. By norm tables of the test manual, it can be transformed into a standardized performance value (Brickenkamp, 1994). After a brief instruction, the test was administered individually within <5 min testing time. The difference of standardized performance values upon short-term abstinence was used to indicate d2-test performance changes. The internal test–retest reliability of the d2-test of attention has been proven to be extraordinary high and in the range of 0.95 to 0.98 for all parameters. Its criterion, construct, and predictive validity has been documented, and test values have been shown to be stable over an extended period of up to 23 months after initial testing.
The other three tests were only administered to the patients after sobriety had been maintained by short-term abstinence.
Blood alcohol content, red blood cell indices, serum electrolytes and enzymes
Blood samples (5 ml blood tubes, 5 ml EDTA blood tubes) were drawn from the patients' antecubital vein at their admission and upon short-term abstinence. Healthy volunteers were subjected to blood test only once at their initial exam. Blood alcohol content was established in serum samples by headspace gas chromatography with flame ionization detection yielding values ∼15% above whole blood alcohol levels. RBC indices, i.e. mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, and mean corpuscular volume, were determined along with haematocrit values by an automated counter employing the electrical impedance method (Counter S-Plus IV; Beckmann Coulter, Fullerton, USA). Serum electrolytes, i.e. sodium, potassium, and chloride, were measured by a potentiometric autoanalyser (Ektachem 700XRC; Eastman Kodak, Rochester, MN, USA). CDT and GGT were determined by a commercial double antibody radioimmunoassay kit (CDTect™; Axis-Shield PLC, Oslo, Norway) and enzymatically by a kinetic method (Oy Medix Biochemica AB; Kauniainen, Finland).
Statistical analysis
Standardized cerebral edge flow images were statistically analysed by permutation-based, voxelwise non-parametric testing (as implemented in the randomise tool, part of FSL). The positive effect of patients' short-term abstinence and upon follow-up of controls was modelled in a single condition/single image per subject, i.e. one sample t-test, type of design. Statistical inference was based on voxel-level thresholding at corrected P-values of 0.05 (corresponding to family-wise error rates of 5% for exhaustive inversion permutation distributions) adjusted for multiple comparisons across the number of voxels (i.e. number of comparisons np) within the tailored edge mask in custom template space by using null distributions of maximum {Pseudo-t}-test statistics (Nichols and Holmes, 2001). {Pseudo-t}-statistics were computed with variance regularization by smoothing with an isotropic Gaussian kernel (σ = 5 mm).
For other statistical analysis, the R environment for statistical computing (R version 2.3.1; R Development Core Team, 2006, Author Webpage) was used. Gaussian distribution of data variables was assessed by Shapiro-Wilk's test for normality. Based upon maintaining or rejecting the normality assumption, parametric or non-parametric tests for establishing differences or associations were applied as indicated. For Student's two sample t-test, variances were estimated separately for both groups, and the Welch modification to the degrees of freedom was used. For Mann–Whitney's U-test, continuity correction was performed for P-value approximation. Based upon directional assumptions, one-sided alternative hypotheses were specified for testing final PBVC, percentage of NAA and choline increase as well as improved d2-test performance upon short-term abstinence of the patients and their positive associations. Furthermore, one-tailed tests were applied for assessing presumably negative correlations of these parameters with age, duration of alcohol dependence according to DSM-IV criteria, and the four estimators of alcohol consumption. Linear regression fitting was plotted with realistic prediction hyperbolas at a coverage probability of 0.95 (using linesHyperb.lm of the sfsmics utilities package version 0.95–6 within R). To correlate d2-test performance changes with final PBVC or percentage of NAA and choline increase, Kendall's tau (τ) correlation coefficient was used for considerations of universality and interpretability. For corrected P-values outside statistical image analysis, multiple comparison correction was performed according to the lowest slope method of Benjamini and Hochberg (1995) by controlling the false discovery rate (using p.adjust within R), which is less stringent but more sensitive than family-wise error rates. The number of comparisons corrected for are indicated by np (based on the particular omnibus null-hypothesis specified appropriately). Significances (P ≤ 0.05) and trends (P ≤ 0.10) detected only at uncorrected levels are conditionally reported.
Results
MR morphometry (SIENA and its voxelwise extension across subjects)
For the 15 patients studied upon short-term abstinence, final PBVC automatically determined by SIENA was 1.85 ± 1.32% (−0.19 to 4.32%). Thus, a significant global volumetric brain gain was associated with abstinence (Pcorrected = 0.00, np = 6, t =5.42, df = 14, t-test; Table 1). No gender difference was observed for the patients' PBVC (males: 1.98 ± 1.52; −0.19 to 4.32, females: 1.60 ± 0.92; 0.71 to 2.96%; Pcorrected = 0.74, np = 6, t = 0.51, df = 12.25, t-test). Only the patient with the longest history of alcohol dependence (25 years), generated a negative final PBVC, −0.19%. Note that this value is within the magnitude of SIENA's established accuracy of ∼0.20 PBVC error (Smith et al., 2001).
Morphometric brain volume (SIENA), spectroscopic (PRESS) and neuropsychological changes of short-term abstinent alcoholics and control subjects
| Method . | Parameter . | Alcohol-dependent patients upon short-term abstinence (n = 15) . | Non-alcoholic control subjects upon short-term follow-up (n = 10) . |
|---|---|---|---|
| SIENA | Percentage brain volume change (%) | ***1.85 ± 1.32†,†† | 0.03 ± 0.41 |
| 1H-MRS PRESS | cerebellar | **19 ± 20† | −1 ± 15 |
| Percentage choline change (%) | |||
| frontomesial | 9 ± 22†† | 3 ± 9 | |
| cerebellar | 10 ± 24 | 0 ± 12 | |
| Percentage NAA change (%) | |||
| frontomesial | *11 ± 15♠ | −1 ± 8 | |
| Neuropsychology | d2-test performance change (standardized units) | ×11 ± 26♠ | N.T. |
| Method . | Parameter . | Alcohol-dependent patients upon short-term abstinence (n = 15) . | Non-alcoholic control subjects upon short-term follow-up (n = 10) . |
|---|---|---|---|
| SIENA | Percentage brain volume change (%) | ***1.85 ± 1.32†,†† | 0.03 ± 0.41 |
| 1H-MRS PRESS | cerebellar | **19 ± 20† | −1 ± 15 |
| Percentage choline change (%) | |||
| frontomesial | 9 ± 22†† | 3 ± 9 | |
| cerebellar | 10 ± 24 | 0 ± 12 | |
| Percentage NAA change (%) | |||
| frontomesial | *11 ± 15♠ | −1 ± 8 | |
| Neuropsychology | d2-test performance change (standardized units) | ×11 ± 26♠ | N.T. |
Parameter changes = */**/*** Pcorrected < 0.05/0.01/0.001 (t-tests); ×Pcorrected < 0.05 (U-test); values: mean ± SD; N.T. = not tested; Positive correlations: †/††Pcorrected < 0.01 (Pearson's), ♠Pcorrected = 0.05 (Kendall's, single outlier of d2-performance change excluded).
Morphometric brain volume (SIENA), spectroscopic (PRESS) and neuropsychological changes of short-term abstinent alcoholics and control subjects
| Method . | Parameter . | Alcohol-dependent patients upon short-term abstinence (n = 15) . | Non-alcoholic control subjects upon short-term follow-up (n = 10) . |
|---|---|---|---|
| SIENA | Percentage brain volume change (%) | ***1.85 ± 1.32†,†† | 0.03 ± 0.41 |
| 1H-MRS PRESS | cerebellar | **19 ± 20† | −1 ± 15 |
| Percentage choline change (%) | |||
| frontomesial | 9 ± 22†† | 3 ± 9 | |
| cerebellar | 10 ± 24 | 0 ± 12 | |
| Percentage NAA change (%) | |||
| frontomesial | *11 ± 15♠ | −1 ± 8 | |
| Neuropsychology | d2-test performance change (standardized units) | ×11 ± 26♠ | N.T. |
| Method . | Parameter . | Alcohol-dependent patients upon short-term abstinence (n = 15) . | Non-alcoholic control subjects upon short-term follow-up (n = 10) . |
|---|---|---|---|
| SIENA | Percentage brain volume change (%) | ***1.85 ± 1.32†,†† | 0.03 ± 0.41 |
| 1H-MRS PRESS | cerebellar | **19 ± 20† | −1 ± 15 |
| Percentage choline change (%) | |||
| frontomesial | 9 ± 22†† | 3 ± 9 | |
| cerebellar | 10 ± 24 | 0 ± 12 | |
| Percentage NAA change (%) | |||
| frontomesial | *11 ± 15♠ | −1 ± 8 | |
| Neuropsychology | d2-test performance change (standardized units) | ×11 ± 26♠ | N.T. |
Parameter changes = */**/*** Pcorrected < 0.05/0.01/0.001 (t-tests); ×Pcorrected < 0.05 (U-test); values: mean ± SD; N.T. = not tested; Positive correlations: †/††Pcorrected < 0.01 (Pearson's), ♠Pcorrected = 0.05 (Kendall's, single outlier of d2-performance change excluded).
For the 10 control subjects examined twice, PBVC was 0.03 ± 0.41% (−0.43 to 0.60%). Thus, the healthy subjects did not show any evidence for a short-term brain volume change according to SIENA (Pcorrected = 0.91, np = 26, t = 0.16, df = 9, t-test; Table 1). Additionally, no significant mean group effect of spatial edge movement was detected for healthy volunteers at Pcorrected ≤ 0.05 (np = 40551, df = 9, 1024 permutations).
Upon short-term abstinence of the patient group, significant morphometric brain recovery was revealed at the superior vermis, perimesencephalic, infra- as well as supratentorial periventricular borders, and, to a lesser extent, on frontomesial and -orbital edges (Pcorrected ≤ 0.05, np = 41205, df = 14, 32 768 permutations; Fig. 1).
MR spectroscopy (1H-MRS)
LCModel's analysis delivered appropriate phasing and frequency assignments. Its results were examined for non-random residuals as well as baseline fitting. Estimated S/N values were 8 ± 3 (5 to 12) for infra- and 14 ± 2 (10 to 19; n = 50) for supratentorial spectra. Cerebellar spectra were, as expected, of somewhat poorer quality consistently exhibiting less S/N (seeFig. 2) and higher standard deviations even of the percentages of metabolite change (Table 1). Cramér-Rao lower bounds of the major metabolites NAA, choline, and creatine were <15%. Those of myo-inositol were <40% of the estimated frontomesial concentrations whereas cerebellar myo-inositol was in general not very reliably detected exhibiting Cramér-Rao lower bounds >50%. Cerebral ethanol was not detected in any of the spectra of patients or controls. For control subjects, insignificant metabolite changes upon follow-up, standard deviations of percentage metabolite change as well as Cramér-Rao lower bounds were compatible with previous reproducibility reports.
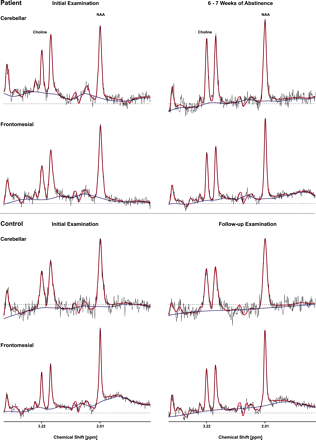
Representative cerebellar and frontomesial MR 1H-spectra (TR = 1500 ms/TE = 135 ms, PRESS) of a patient selected from our sample (n = 15) of uncomplicated alcoholics upon short-term abstinence and of a healthy control subject upon follow-up (red: LCModel fit, blue: baseline; displayed range: 1.00–4.00 p.p.m.).
PRESS water integrals did not change significantly in patients upon short-term abstinence (percentage cerebellar change: 1 ± 8; −22 to 11%, Pcorrected = 0.77, np = 26, t = 0.51; percentage frontomesial change: −2 ± 6; −11 to 15%, Pcorrected = 0.62, np = 26, t = 1.35; df = 14) or controls upon follow-up (cerebellar: 3 ± 8; −8 to 22%, Pcorrected = 0.62, np = 26, t = −1.32; frontomesial: −1 ± 7; −12 to 8%, Pcorrected = 0.77, np = 26, t = 0.48; df = 9; t-tests). In addition, levels of infra- and supratentorial creatine and myo-inositol remained constant in patients upon short-term abstinence as well as in controls upon follow-up (Pcorrected ≥ 0.62, np = 26; df = 14 and 9, respectively; t-tests). In the patients, percentage creatine change amounted to 2 ± 12% (−18 to 28%) on the infratentorial level and to 2 ± 9% (−12 to 25%) on the supratentorial level. In control subjects, percentage creatine change amounted to −1 ± 7% (−11 to 13%) on the infratentorial level and to 1 ± 6% (−6 to 12%) on the supratentorial level. For myo-inositol, percentages of cerebellar and frontomesial change were 2 ± 60% (−94 to 99%) and 10 ± 39% (−66 to 95%) in the patients, versus −2 ± 53% (−93 to 81%) and 6 ± 25% (−52 to 45%) in controls. Furthermore, controls did not exhibit any significant changes of infra- or supratentorial NAA or choline quantifications (cerebellar NAA change: 0 ± 12/−18 to 23%, frontomesial NAA change: −1 ± 8/−13 to 15%, cerebellar choline change: −1 ± 15/−32 to 13%, frontomesial choline change: 3 ± 9/−13 to 18%; Pcorrected ≥ 0.73, np = 26; df = 9, t-tests; Table 1). Notably, there were no significant group differences of infra- or supratentorial NAA, choline, creatine and myo-inositol quantifications, or water integrals between patients and controls in their initial exam and upon follow-up (Pcorrected ≥ 0.94, np = 20; t-tests; data not shown).
Upon short-term abstinence of the patients, cerebellar choline and frontomesial NAA levels were significantly augmented by 19 ± 20% (−7 to 58%, Pcorrected = 0.00, np = 6, t = 3.74) and 11 ± 15% (−17 to 36%, Pcorrected = 0.01, np = 6, t = 2.93), respectively (Table 1). There were strong trends for supratentorial choline (9 ± 22/−15 to 61%, Pcorrected = 0.06, np = 6, t = 1.64) and infratentorial NAA levels (10 ± 24/−43 to 61%, Pcorrected = 0.06, np = 6, t = 1.68; df = 14, t-tests) to increase as well. No gender differences were established for the patients' percentages of choline or NAA change upon short-term abstinence (Pcorrected ≥ 0.20, np = 6; df ≥ 5.93, t-tests).
Representative infra- and supratentorial spectra of a patient selected from our sample of uncomplicated alcoholics upon short-term abstinence and of a healthy control subject upon follow-up are shown in Fig. 2. The corresponding percentages of relative metabolite change (also adjusted for local brain volume differences) were the following: for the selected patient—cerebellar: NAA 12%/choline 50%, frontomesial: NAA 10%/choline 41%; for the selected control subject—cerebellar: NAA 3%/choline 13%, frontomesial: NAA 2%/choline 18%.
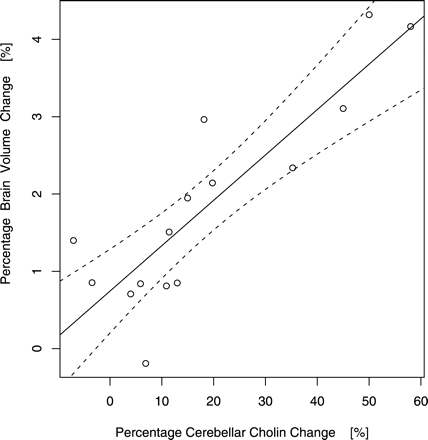
Most notably, final PBVC upon the patients' short-term abstinence was strongly and positively correlated with percentages of choline gain (cerebellar: Pcorrected = 0.00, np = 4, t = 6.23, r = 0.87, Fig. 3; frontomesial: Pcorrected = 0.00, np = 4, t = 3.86, r = 0.73) but not with percentages of cerebellar or frontomesial NAA change (Pcorrected ≥ 0.33; df = 13, Pearson's product moment correlations; Table 1).
Percentages of morphometric brain volume and spectroscopic cerebellar choline change (adjusted for local brain volume differences) upon short-term abstinence from alcoholism (linear regression/95% realistic prediction band, Pcorrected = 0.00; n = 15).
Attenuated frontomesial NAA (Puncorrected = 0.01, t = −2.53, r = −0.57) and cerebellar choline increases (Puncorrected = 0.01, t = −2.45, r = −0.56) as well as final PBVC values (Puncorrected = 0.05, t = −1.78, r = −0.44, Pearson's) of patients upon their short-term abstinence were associated with longer durations of illness at uncorrected significance levels but these correlations did not survive the multiple comparison correction (Pcorrected ≥ 0.15, np = 30) and are only conditionally reported here. There was no tendency, however, for cerebellar NAA and frontomesial choline changes at uncorrected or corrected levels to be negatively correlated with illness duration (Pcorrected ≥ 0.36, np = 30, Pearson's). Age and the four estimators of alcohol consumption did not reveal any significant associations with PBVC or the percentages of NAA and choline change on the infra- or supratentorial level (Pcorrected ≥ 0.29, np = 30, Pearson's).
Neuropsychological assessment (d2-test of attention, in particular)
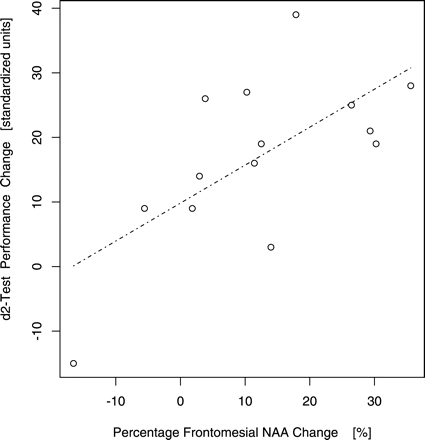
Standardized d2-test performance values of the patients increased by 11 ± 26 units (−68 to 39 units, Pcorrected = 0.02, np = 6, V = 99, U-test) upon short-term abstinence. No gender difference was detected for the patients' neuropsychological test improvement (males: 10 ± 30; −68 to 39, females: 13 ± 17; −15 to 28 units; Pcorrected = 0.98, np = 6, W = 25.5, U-test). Increase of standardized d2-performance was positively associated with the percentage of frontomesial NAA gain (Pcorrected = 0.05, np = 5, z = 2.25, τ = 0.46, odds ratio of concordant versus discordant change observations πc/πd = 2.70; Fig. 4; Table 1). At uncorrected levels, there was also a trend of standardized d2-performance gain to be correlated with the percentage of cerebellar NAA increase (Puncorrected = 0.08, z = 1.37, τ = 0.28, πc/πd = 1.78) and an association with the final PBVC (Puncorrected = 0.04, z = 1.87, τ = 0.38, πc/πd = 2.23) but these did not survive the multiple comparison correction (Pcorrected ≥ 0.10, np = 5) and are again only conditionally reported. Cerebellar or frontomesial choline gain were not correlated with standardized d2-performance increase at uncorrected or corrected significance levels (Pcorrected ≥ 0.12, np = 5, Kendall's rank correlations, single outlier of d2-performance change = −68 standardized units excluded).
Standardized d2-test performance change and spectroscopic frontomesial NAA change (adjusted for local brain volume differences) upon short-term abstinence from alcoholism (odds ratio of concordant versus discordant change observations πc/πd = 2.70, Pcorrected = 0.05; n = 14, one outlier of d2-performance change = −68 standardized units excluded).
Scores of the other three instruments (Auditory Verbal Learning Test, Standard Progressive Matrices, Vocabulary Test) were not associated with percentages of infra- or supratentorial NAA and choline increase and the final PBVC (Pcorrected ≥ 0.62, np = 5), or with age, duration of alcohol dependence and the four estimators of alcohol consumption (Pcorrected ≥ 0.66, np = 6, Kendall's).
Blood alcohol, red blood cell indices, haematocrit, serum electrolytes and enzymes
Blood samples contained no alcohol in any of the probes taken from the patients or controls. RBC indices, haematocrit, serum electrolytes and CDT as well as GGT values were within laboratory reference limits for patients as well as controls and exhibited no significant changes between study entry and upon the patients' follow-up (Pcorrected ≥ 0.62, np = 26; t-tests; data not shown).
Discussion
To our knowledge, this is the first study presenting integrated data on automatically detected global and regional brain volume regain, spectroscopic, and neuropsychological recovery in uncomplicated alcoholics upon short-term sobriety. Strict exclusion criteria limited the investigation to not heavily nicotine abusing patients without previous alcohol withdrawal and requiring no pharmacological support to achieve their abstinence which is certainly not typical for alcoholism but avoided interactions with secondary medical illnesses, alcohol withdrawal, administration of psychoactive medication etc. In fact, differences between our findings and previously published reports may arise from variations of the severity of alcoholism, toxicity and accompanying disorders or abuse between the actual samples studied. However, our results support the notion that remarkable morphological, metabolic and functional brain regeneration is not just feigned by rehydration and can be attained rapidly by abstinence (Mason et al., 2005).
For morphometry, we used normalized longitudinal brain change analysis by SIENA which is carried out in individual subject space and does not rely on grey versus white matter segmentation. Therefore, it avoids misregistrations into standard space possibly limiting other voxel-based morphometry approaches (Davatzikos, 2004) and is less contrast-to-noise dependent. Contrary to previous data on global brain volume regain upon short-term abstinence, which were derived by a somewhat similar but more MR contrast dependent method (Gazdzinski et al., 2005a) or computed tomography (Mann et al., 2005) but that did not attempt to control for hydration status, the patients' mean PBVC in our study exceeds the brain volume change of ∼1% detected by SIENA upon de- and rehydration (Duning et al., 2005; which is, in fact, in the magnitude of the positive intercept of the linear regression between global brain volume gain and rising cerebellar choline upon sobriety; seeFig. 3). Because we included no heavily nicotine abusing patients, this may also indicate less brain injury and better regeneration capacity for this patient population (Gazdzinski et al., 2005b; Durazzo et al., 2006). Global brain volume change of control subjects was, on the other hand, insignificant (Table 1) exhibiting standard deviations of about twice the measurement error reported for SIENA by a three-timepoint validation (Smith et al., 2002) and upon repositioning (Duning et al., 2005). Notably, 93% of our patients (14 out of 15 cases) achieved pronounced global brain regain beyond the maximum change (i.e. assuming null effect, the error spread) of controls, highlighting SIENA's discriminative power for the assessment of change. Novel localization of brain volume changes by SIENA's regional extension relies on transformation of individual edge flow images to standard space which was customized for the study presented. Because standardized flow images are limited to an outer edge contour and can not retain the entire subject-specific sulcation pattern, it is conceivable that regression of sulcal widening without outer brain edge shifts has led to a conservative or underestimation of regional (but not global) brain gain at hemispheric convexities upon recovery from alcoholism (Fig. 1). However, the spatial analysis of longitudinal brain volume change employed can be presumed to be particularly sensitive to ventricular edge motion. Given that a recent automated, cross-sectional and segmentation-based study failed to find ventricular enlargement due to chronic alcoholism (Gazdzinski et al., 2005b), this is noteworthy and further emphasizes the advantage of longitudinal morphometry. Our results suggest that both ventricular volume as well as brainstem atrophy associated with chronic heavy drinking and cross-sectionally confirmed by manual morphometry (Bloomer et al., 2004) can in fact regress upon short-term sobriety of uncomplicated alcoholics. Furthermore, longitudinal brain edge shifts upon recovery at corrected significance levels (tested non-parametrically to avoid violations of Gaussian random field assumptions by standardized flow images) included superior vermical, frontomesial as well as orbitofrontal edges and were spatially more extensive than the tissue contractions detected cross-sectionally at uncorrected significance levels (arguably derived by parametric inference) in chronic active heavy compared to light drinkers by deformation-based morphometry (Cardenas et al., 2005).
For spectroscopy data, relative percentages of metabolite change were derived and adjusted for within voxel brain volume changes (based on individual flow images). Because spectra were acquired at long TE and short TR, and relaxation time changes can not be entirely ruled out, these adjusted percentages of metabolite change remain somewhat semi-quantitative in nature. However, significant augmentation of cerebellar choline and frontomesial NAA levels upon short-term sobriety found in this study is in good accordance with previous longitudinal data (Bendszus et al., 2001; Parks et al., 2001; Ende et al., 2005; Durazzo et al., 2006) and substantiates evidence for neuronal and glial regeneration. Reversal of brain shrinkage could, in theory, be simulated by increased perfusion and intracranial blood volume (Trabert et al., 1995) but such an explanation would not be consistent with these spectroscopic data. Less pronounced and more variable increases of frontomesial choline and cerebellar NAA upon short-term abstinence may in part be due to partial voluming with more white than grey matter covered by cerebellar SVS and the reverse for frontomesial spectroscopy voxels. This notion is supported by consistently higher cerebellar choline levels compared with frontomesial SVS whereas NAA levels were comparable in the infra- and supratentorial voxels (data not shown), possibly also due to high neuronal density in the cerebellum. On average, the percentage of supratentorial choline change was lower than in the cerebellum whereas cerebellar NAA showed a similar percentage change as in frontomesial location but was just contaminated by higher variance (Table 1). Thus, it may be argued that qualitative changes of choline and NAA upon short-term abstinence from alcoholism seem quite consistent on the infra- and supratentorial level rendering these metabolites rather general markers of recovery.
The lack of significant group differences between patients and controls may be due to the sample size, less severe metabolic brain damage of uncomplicated, not heavily nicotine abusing alcoholics, or both. In any case, it emphasizes the superior power of longitudinal as opposed to cross-sectional comparisons. With regards to presumably increased vulnerability of females to alcoholic brain damage (Agartz et al., quoted in Mann et al., 2001, 2005), our data did not reveal significant gender differences for metabolic or morphological brain recovery upon short-term abstinence from alcoholism. The latter is in accordance to recent data (Mann et al., 2005) even though our sample size was rather small with very few women included and no significant gender difference for the duration of dependence. Thus, if there is telescoping brain injury in females it may not be easily reversible.
Longer duration of dependence may possibly, in some cases, lead to less frontomesial NAA, cerebellar choline, and global brain volume gains. Therefore, prolonged alcoholism at least potentially constrains metabolic as well as morphological brain recovery. Schweinsburg et al. (2004) have reported that longer history of active drinking is associated with more profound NAA reduction. This finding could correspond to prolonged alcoholism duration eventually limiting NAA restitution upon sobriety.
Global brain volume gain upon sobriety was significantly related to rising cerebellar and frontomesial choline but not NAA levels when corrected for maximal conceivable local brain volume changes within joint co-registered spectroscopy voxels. Thereby, our pilot evidence proposes that early brain volume regain upon sobriety is primarily driven by a cerebral choline increase which is supposed to indicate recovery from white matter damage potentially consistent with astrocytic regrowth and remyelination (Meyerhoff/quoted in Mason et al., 2005) and which could, in some instances, decrease the longer the illness. Since, in our morphometric assessment, a spatial preference for early brain regain has been delineated especially to periventricular edges (Fig. 1), white matter restoration may in fact prevail upon short-term abstinence of uncomplicated alcoholics.
NAA gains by abstinence were, on the other hand, below cerebellar choline increase but, at least on the supratentorial level, accompanied by cognitive improvement in the d2-test of attention. Even though attention, concentration, and performance speed changes upon abstinence may be linked to learning effects (Mann et al., 1999), neuropsychological improvement in this domain was, at an uncorrected significance level, also related to morphological brain regeneration. Nevertheless, the neuropsychological findings of our study remain yet quite exploratory in nature as only the d2-test of attention has been assessed longitudinally.
Slight NAA gains have been reported within the first week upon correction of severe dehydration (Lee et al., 1994). For our 6–7 week patient follow-up data, however, rehydration is rather unlikely to be the primary mechanism involved. Instead, our findings of unchanged cerebral water integrals, creatine and myo-inositol concentrations as well as serum electrolytes and RBC indices and of insignificant metabolite differences between our patients and controls are in line with the notion that early brain regain associated with abstinence from alcoholism cannot be sufficiently explained by simple rehydration. Furthermore, we did not observe a pathological shift or reversal of the patients' NAA/myo-inositol ratio associated with severe dehydration (Lee et al., 1994). Because myo-inositol presumably acts as an osmoregulator, this is further evidence for an osmotic steady-state in our patient (as well as control) sample. Thus, a reversal of more complex metabolic dysfunctions and, for example, axonal volume changes in cerebral white matter ought to contribute, at least in part, to the choline and NAA changes observed (De Stefano et al., 1995; Bjartmar et al., 2002). Notably, rising NAA levels may not necessarily reflect axonal or dendritic sprouting but also be related to (re-)myelination (Burri et al., 1991; Urenjak et al., 1992), and even adult human white matter harbours multipotential progenitor cells capable of neuro- as well as gliogenesis in vitro (Nunes et al., 2003).
In summary, sobriety has marked and early, globally averaged and regionally specific morphological, metabolic as well as functional benefits for convalescent alcoholics. Whether this is due to glial or neuronal viability or both remains somewhat unclear but prolonged dependence might possibly limit rapid recovery from white matter brain injury. Elucidating the precise nature and cellular mechanisms of this recovery further may serve as a valuable model of brain regeneration and, in the future, become relevant for other disorders with toxic insults of white matter, in particular.
We are extremely grateful to Stephen Provencher, PhD, for advice on calibration measurements, acquisition of model spectra, extraction of water integrals from unsuppressed spectra and improved baseline fitting by an optimized basis set.