-
PDF
- Split View
-
Views
-
Cite
Cite
G. Lorimer Moseley, Michael K. Nicholas, Paul W. Hodges, Does anticipation of back pain predispose to back trouble?, Brain, Volume 127, Issue 10, October 2004, Pages 2339–2347, https://doi.org/10.1093/brain/awh248
Close - Share Icon Share
Abstract
Limb movement imparts a perturbation to the body. The impact of that perturbation is limited via anticipatory postural adjustments. The strategy by which the CNS controls anticipatory postural adjustments of the trunk muscles during limb movement is altered during acute back pain and in people with recurrent back pain, even when they are pain free. The altered postural strategy probably serves to protect the spine in the short term, but it is associated with a cost and is thought to predispose spinal structures to injury in the long term. It is not known why this protective strategy might occur even when people are pain free, but one possibility is that it is caused by the anticipation of back pain. In eight healthy subjects, recordings of intramuscular EMG were made from the trunk muscles during single and repetitive arm movements. Anticipation of experimental back pain and anticipation of experimental elbow pain were elicited by the threat of painful cutaneous stimulation. There was no effect of anticipated experimental elbow pain on postural adjustments. During anticipated experimental back pain, for single arm movements there was delayed activation of the deep trunk muscles and augmentation of at least one superficial trunk muscle. For repetitive arm movements, there was decreased activity and a shift from biphasic to monophasic activation of the deep trunk muscles and increased activity of superficial trunk muscles during anticipation of back pain. In both instances, the changes were consistent with adoption of an altered strategy for postural control and were similar to those observed in patients with recurrent back pain. We conclude that anticipation of experimental back pain evokes a protective postural strategy that stiffens the spine. This protective strategy is associated with compressive cost and is thought to predispose to spinal injury if maintained long term.
Introduction
Back pain is common, and is costly to the individual and the community. Up to 85% of people will experience an acute episode of back pain at some point in their life (Walker, 2000). Of this group, ∼5% will develop chronic unremittent back pain, as many as 70% will have recurrent back trouble (Garofalo and Polatin, 1999), and together they cost the UK >£500 million each year (Great Britain. Clinical Standards Advisory Group, 1994). Expected pain and injury are thought to be potent predictors of chronic unremittent back pain (Burton et al., 1995), but it is not known why some people develop chronic recurrent trouble. One possibility is that the strategy by which the spine is controlled during perturbation to posture is altered and this places spinal structures at risk.
Prior to limb movement, the brain prepares the trunk for the impending perturbation by preparatory activation of the trunk muscles, so-called anticipatory postural adjustments (Belen'kii et al., 1967). The normal strategy by which postural adjustments are controlled during limb movements involves differential activation of the deep and superficial trunk muscles (Hodges and Richardson, 1997, 1999; Moseley et al., 2002a,b). Anticipatory postural activation of the superficial trunk muscles involves evaluation of the direction and magnitude of the perturbation caused by movement. In contrast, anticipatory postural activation of the deep trunk muscles does not require evaluation of the spatial characteristics of the perturbation. This latter component of spinal control requires limited cortical resources and is not affected by competitive demands on attention (Moseley et al., 2004), or by altering the predictability of the required movement (Hodges and Richardson, 1999).
It has been shown that pain can alter the amplitude of muscle activity during movement (Arendt-Nielsen et al., 1996; Madeleine et al., 1999; Zedka et al., 1999). However, our group has also shown that pain alters the strategy by which the brain prepares for movement: experimental back pain elicited by intramuscular (i.m.) injection of hypertonic saline selectively delays anticipatory postural activation of the deep muscles (Hodges et al., 2003b). The same effect has been observed in people with chronic back trouble, even though they were pain free at the time of testing (Hodges and Richardson, 1996). In that patient group, the effect has been shown to reflect adoption of an alternative strategy by which anticipatory postural adjustments control the perturbation to the spine (Hodges, 2001). Extensive theoretical (Macintosh and Bogduk, 1986; Solomonow et al., 1998; Wilke et al., 1995), in vitro (Kaigle et al., 1995) and in vivo (Hodges et al., 2001, 2003a; Richardson et al., 2002) findings suggest that the new postural strategy serves to stiffen the spine and is probably protective. However, those findings also suggest that two aspects of the protective strategy are problematic if maintained long term. First, the primary role of the deep trunk muscles lies in fine-tuning of spinal control, which means the new postural strategy is probably associated with a reduction in spinal control. Secondly, the superficial trunk muscles impart stiffness to the spine via increased compression, and a sustained increase in loading of the spine is thought to stimulate nociceptors in spinal structures and predispose to injury (Gardner-Morse and Stokes, 1998; van Dieen et al., 2003).
Why then, does an alternative postural strategy associated with pain persist in some people even when they are pain free? One possibility is that the anticipation of pain alone is sufficient to cause the change in strategy. The anticipation of pain has been shown to disrupt cortical processing (Crombez et al., 1998), alter motor output associated with voluntary movements (Keefe et al., 1990) and activate cortical motor networks (Ploghaus et al., 1999). Further, the anticipation of pain has been implicated in the development of chronic unremittent pain syndromes (Lethem et al., 1983; Crombez et al., 1996), and when the anticipation of pain is associated with fear, it is thought to be more disabling than pain itself (Crombez et al., 1999).
We aimed to determine if the anticipation of experimental back pain was sufficient to alter the strategy by which the brain controls the spine during limb movement. It was hypothesized that anticipation of experimental back pain would selectively delay postural activation of the deep trunk muscles during single rapid arm movements and would reduce activation of the deep trunk muscles, and increase the activation of superficial trunk muscles, during repetitive arm movements.
Methods
Subjects
Eight subjects (five male) with a mean ± SD age, body mass and height of 32 ± 7 years, 63 ± 13 kg and 170 ± 11 cm, respectively, participated in this cross-sectional study. All subjects had also participated in a previous study (Moseley et al., 2004). Subjects were excluded if they had any diagnosed respiratory, neurological or psychiatric condition, or if they had experienced back pain in the previous 2 years. Subjects completed a general activity questionnaire and were screened using the Spielberger State–Trait Anxiety Inventory (STAI) (Spielberger et al., 1970) prior to data collection. Written informed consent was obtained from all subjects. All procedures were approved by the University of New South Wales Human Research Ethics Committee and conformed to the Declaration of Helsinki.
EMG
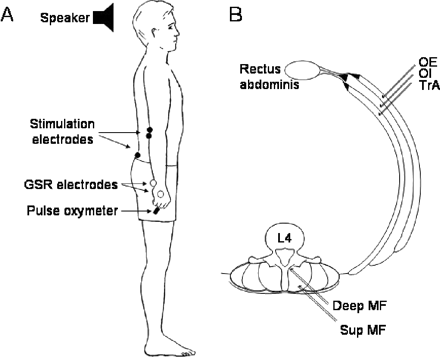
EMG of the deep trunk muscles [deep multifidus (MF) and transversus abdominus (TrA)] and superficial trunk muscles [superficial multifidus (Sup MF), obliquus externus (OE) and obliquus internus (OI)] was recorded with selective bipolar intramuscular electrodes (Teflon-coated stainless steel wire, 75 µm diameter with 1 mm Teflon removed and tips bent back) that were threaded into a hypodermic needle and inserted under ultrasound guidance (Fig. 1).
(A) Experimental set-up showing the site of electrodes for delivering the painful stimulation, recording the galvanic skin response (GSR) and recording EMG in anterior deltoid. Heart rate was recorded using a pulse oxymeter. (B) Intramuscular EMG electrodes inserted in the deep (Deep MF) and superficial (Sup MF) multifidus at the level of the lamina of the fourth lumbar vertebra. Intramuscular EMG electrodes also inserted through the anterolateral abdominal wall, ∼3 cm caudal to the inferior border of the ribs in obliquus externus (OE) and obliquus internus (OI) and transversus abdominis (TrA).
A pair of surface EMG electrodes (Ag/AgCl discs, 10 mm diameter, 20 mm inter-electrode distance) were placed over the left anterior deltoid muscle and a ground electrode over the right iliac crest. EMG data were amplified differentially with a gain of 10 000–50 000, band-pass filtered between 53 Hz and 1 kHz, and sampled at 2 kHz, using a Power1401 data acquisition system with Spike2 software (Cambridge Electronic Design, Cambridge, UK). Data were exported for analysis with Matlab 5.1 (Mathworks, Natic, MA).
Fear and pain
To ensure that arousal was similar between the two experimental conditions, i.e. painful stimulation of the low back and painful stimulation of the elbow, the galvanic skin response (GSR) and heart rate (HR) were recorded and reviewed in real time. GSR and HR data were sampled at 2 kHz with the EMG data. After each condition, two 11-point numerical rating scales (NRS) were conducted. In the first NRS, subjects were asked ‘How fearful of the impending pain were you during that trial?’ Zero represented ‘not at all’ and 10 represented ‘completely’. In the second NRS, subjects were asked ‘How would you rate on average the pain associated with each stimulation during that trial?’ Zero represented ‘no pain’ and 10 ‘worst possible pain’. The independence of these measures has been established previously (Moseley et al., 2003).
Experimental protocol
Postural activity of the trunk muscles during upper limb movements
Control data were recorded during single and repetitive movements of the arm, with the arm attached to a lightweight bar that had a potentiometer at the axis of shoulder rotation (Fig. 1). For the single movement trials, standing subjects were required to move their arm as quickly as possible in response to an auditory stimulus elicited from a speaker ∼1.5 m in front of the subject (Hodges and Richardson, 1997). Subjects were instructed to relax between trials. For the repetitive movement trials, subjects first stood relaxed for 10 s, and then moved their arm ‘as fast as possible’, between 15° flexion and 15° extension, for 10 s, while holding their breath at normal end expiratory volume (Hodges and Gandevia, 2000). Verbal feedback was provided about the time elapsed.
Painful stimulation of the back and elbow
Cutaneous electric painful stimulation to the back and elbow was used to elicit the anticipation of experimental pain. Cutaneous painful shock has been used extensively for experimental induction of pain (e.g. Gasser, 1929). This method is non-invasive and permits delivery of a stimulus of known intensity and duration (Handwerker and Kobal, 1993). Two pairs of surface electrodes (interelectrode distance 10 mm) were placed over (i) the posterior elbow of the right arm; and (ii) the right posterior upper pelvis (posterior superior iliac spine). Electrodes were placed over bone to minimize muscle contraction. In one subject, stimulation evoked muscle activity and the electrodes were moved. In two separate processes, stimuli (60 Hz, 100 ms train, 1 ms pulse duration) were presented at either the back or elbow with increasing intensity until the subject indicated that it was ‘moderately painful’. This was designated the pain stimulus. Determination of the pain stimulus and experimental trials for elbow and back stimulation were performed in random order.
For both experimental conditions, the subject was advised that, at random and without warning, they would receive a painful stimulus that was 80–120% of the previously determined intensity. To avoid the confounding effect of having to move in response to the auditory signal and not in response to the pain stimulus (thus creating a choice reaction task), the pain stimulus output elicited an auditory output via the same speaker and subjects were advised to respond to both stimuli. Approximately 10 stimuli were delivered during each condition. Movements performed while participants anticipated pain but were pain free were analysed and movements in response to the painful stimuli were not. Reported fear, GSR and HR returned to baseline levels between experimental conditions.
Data analysis
For single movements, spatial and temporal parameters of the EMG data were analysed. To remove bias, EMG traces were displayed individually without reference to muscle or trial. For each EMG trace, the onset of EMG was identified visually from the raw data as the point at which EMG increased above the baseline level. This technique is reliable and is preferred to computer-based methods (Hodges and Bui, 1996). The latency of the trunk muscle EMG response relative to the onset of deltoid EMG (‘onset latency’) was analysed. Trials were included if the onset of trunk muscle EMG occurred <200 ms before or after that of deltoid. The mean rectified EMG was determined for baseline during the period from 500 to 100 ms prior to deltoid EMG onset and for each of four 50 ms epochs from 100 ms before to 100 ms after deltoid EMG onset. The mean baseline EMG amplitude was subtracted from the mean magnitude for each epoch, and then normalized to the control condition for each subject.
For the repetitive movements, EMG data were high-pass filtered at 30 Hz, full-wave rectified, low-pass filtered at 100 Hz and time-normalized to 1000 samples per arm movement. Rectified EMG averages were triggered from the onset of forward movement of the arm, determined from the potentiometer data. Averages were displayed individually and without reference to the muscle being measured, and the time and amplitude of peaks and troughs were identified. A peak was recorded if the amplitude of the closest trough on either side was less than the peak in question by at least 15% of the maximum amplitude. Because the deep trunk muscles tend to demonstrate a biphasic pattern of activity during repetitive movements while the superficial trunk muscles usually demonstrate monophasic activity (Moseley et al., 2002a), we recorded the number of peaks in EMG activity for each muscle, for each subject and during each condition.
Analysis in the frequency domain investigated the relationship between the frequency of EMG bursts in the trunk muscles and that of arm movement. Power spectra were generated by fast Fourier transform analysis. To evaluate similarity in frequency content, we calculated coherence, which is a measure of the presence of a constant temporal and spatial relationship between the phasic changes in two signals (Bendat and Piersol, 1966). Power and coherence were measured at the frequency of arm movement and have been used previously (Hodges and Gandevia, 2000; Hodges et al., 2003b; Zedka and Prochazka, 1997).
Statistical analysis
Student's t tests for dependent samples were used to compare arm acceleration, GSR, HR, reported expected pain and reported pain between conditions.
Single movement trials
For each muscle, the onset latency and the mean EMG amplitude for each epoch were averaged for all trials across the sample. As the data were distributed normally, a multivariate analysis of covariance (MANCOVA), with shoulder acceleration entered simultaneously as a covariate, was used to compare the onset latency between conditions for each muscle. A separate MANCOVA compared the mean rectified EMG amplitude for each epoch, for each muscle, between conditions. Post hoc testing of onset latency and the epoch data between conditions was undertaken with Duncan's multiple range tests.
Repetitive movement trials
Student t tests were used to compare the amplitude and frequency of arm movements between conditions. A multivariate analysis of variance (MANOVA) was used to compare the mean, maximum and minimum amplitude of time-locked EMG averages for each muscle between conditions. EMG data were assessed in the frequency domain by evaluating (i) the power of the EMG signal at the frequency of arm movement; and (ii) the coherence between the movement and EMG data at the frequency of arm movement. Although multiple measures elevate the probability of a type I error, a Bonferroni correction would elevate the probability of a type II error and reduce significance to P < 0.008, which we considered to be too conservative. In light of criticism in the literature of Bonferroni and other corrections (e.g. Perneger, 1998), we maintained significance at P = 0.05.
Results
Fear and pain measures
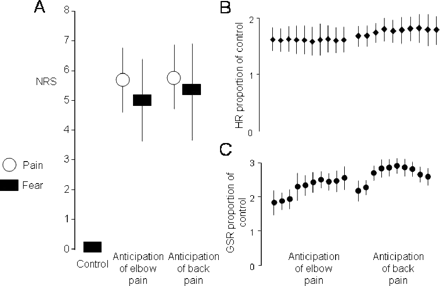
Reported fear, GSR and HR were greater during anticipated experimental elbow pain and during anticipated experimental back pain than during the control condition (P < 0.001 for all), but were not different between the elbow and back conditions (P = 0.1, 0.9 and 0.9 for reported fear, GSR and HR, respectively) (Fig. 2). For both conditions, GSR and HR both showed signs of habituation during the data collection period. The mean pain ratings were 4.3 for the elbow stimulation and 4.7 for the back stimulation, respectively, which were not different (P = 0.1). Taken together, these data suggest that the effects of expected shock and the intensity of expected pain were similar for the anatomically distinct conditions and that some habituation of the arousal effect occurred in each condition.
Reported pain (circles) and fear (boxes) (A), heart rate (HR) as a proportion of the mean heart rate during the control condition (B) and galvanic skin response (GSR) as a proportion of the mean galvanic skin response during the control condition (C). Note a similar response during both experimental conditions.
Single rapid arm movements
There was no effect of condition on the acceleration or amplitude of arm movement (P > 0.21 for all), which suggests that the arm was moved in a similar manner in all conditions.
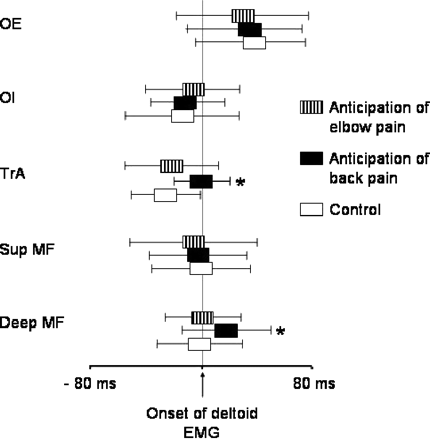
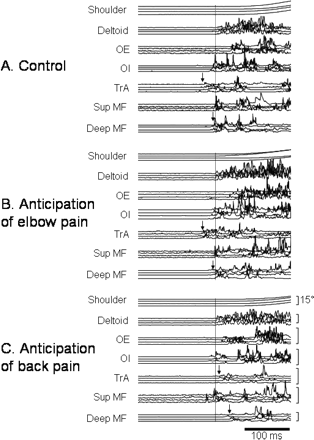
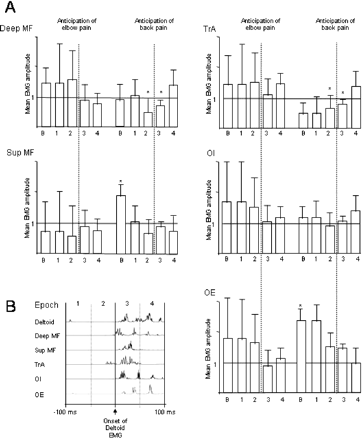
Relative to onset of deltoid EMG, the onset of EMG in the deep trunk muscles occurred later during anticipated experimental back pain than during the other conditions (P < 0.01). There was no effect of anticipated experimental elbow pain on the deep muscles (P > 0.1). For the superficial trunk muscles, there was an increase in the baseline EMG amplitude for OE and for Sup MF during anticipated experimental back pain (mean increase in EMG ∼120% for OE and ∼100% for Sup MF, P < 0.05 for both) (Fig. 3). In addition, for each subject, at least one superficial trunk muscle was active earlier during anticipated experimental back pain than during the other conditions, although there was not a consistent effect across the group for any muscle (P > 0.29 for all). EMG data from a single subject (Fig. 4) and averaged group data (Fig. 5) are shown. The onset latency data were corroborated by the epoch data, which showed a decrease in the mean EMG amplitude during epochs 2 and 3 (from 50 ms before to 50 ms after deltoid EMG onset) for the deep but not superficial muscles (Fig. 5). Each of the effects observed was maintained throughout the experimental condition and did not show signs of habituation with repeated trials.
(A) Group data showing the mean rectified EMG amplitude as a proportion of the amplitude for that epoch during the control trials, for baseline and for 50 ms epochs for 100 ms either side of the onset of deltoid EMG (shown with vertical dashed line). The asterisk and filled columns indicate the difference from the control condition (P < 0.05). Note the reduction in EMG for 50 ms either side of deltoid onset for the deep trunk muscles (TrA and deep MF) and the increase in baseline EMG for two of the superficial trunk muscles (OE and Sup MF) during expected back pain. (B) Calculation of the epochs according to the onset of deltoid EMG, for the 100 ms either side of deltoid onset.
Multiple trials from a representative subject during each condition. Intramuscular and surface (deltoid only) EMG data have been rectified and smoothed (t = 0.005). The vertical dotted line represents onset of deltoid EMG during forward arm movement. Vertical errors mark the approximate onset of transversus abdominis (TrA) and deep multifidus (Deep MF) during each condition. Square brackets mark 1 µV for EMG traces and 15° shoulder movement (shoulder). (A) Control data (no anticipation). (B) Anticipation of elbow pain. (C) Anticipation of back pain.
Group data showing mean (SEM) onset of EMG in obliquus externus (OE) and internus (OI), transversus abdominis (TrA), superficial (Sup MF) and deep (Deep MF) multifidus during single movements of the arm in standing. The vertical line indicates the onset of deltoid. The asterisk indicates the difference from the control condition (P < 0.05). Note a delay in the onset of EMG in the deep trunk muscles (TrA and deep MF) only during expected back pain.
Rapid repetitive arm movements
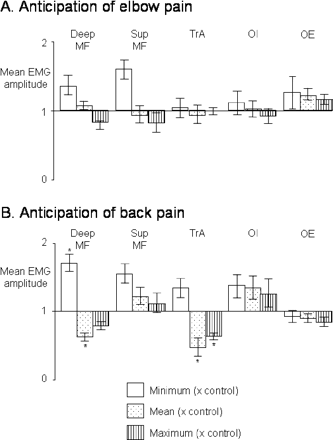
There was no effect of condition on the frequency or amplitude of arm movements (P > 0.34). The deep trunk muscles had a smaller maximum EMG amplitude, and a smaller mean EMG amplitude during anticipated experimental back pain than in the control trials (P < 0.04 for both) (Fig. 6). There were no differences between control and anticipated experimental elbow pain for any muscles, although a decrease in maximum EMG in deep MF approached significance (P > 0.07). Mean maximum, minimum and mean EMG of OE were greater during anticipated experimental elbow pain than during expected back pain (P = 0.03).
Group mean (SEM) for the peak (vertical pattern), mean (dotted pattern) and trough (open column) EMG amplitude normalized to the control condition for the expected elbow pain (A) and expected back pain (B). The asterisk indicates a significant difference from the control condition (P < 0.05).
Analysis in the frequency domain revealed less power of the EMG signal at movement frequency for Sup MF in both the experimental conditions than in control (P < 0.02), and less coherence between the movement and deep MF EMG data during anticipated experimental back pain than during expected elbow pain and control (P < 0.02). A similar pattern for TrA approached significance (P = 0.07). Each of the effects observed was maintained throughout performance of the task.
Discussion
We hypothesized that anticipation of experimental back pain would selectively delay postural activation of the deep trunk muscles during single rapid arm movements and would reduce activation of the deep trunk muscles, and increase the activation of superficial trunk muscles, during repetitive arm movements. The results support both hypotheses and suggest that when people anticipate back pain, there is a change in the strategy by which the CNS attempts to control the spine during the perturbation caused by movement. This change in strategy is not evoked when people anticipate elbow pain, even though the physiological and subjective measures suggested that fear of impending shock, and intensity of expected pain were similar between experimental conditions. Taken together, the results exclude the possibility that the impact on control strategy was caused by the attention requirements of the task or by a generic fear of impending shock.
There are several aspects of the current results that suggest that the strategy for spinal control was altered during anticipation of experimental back pain, even though the arm movements, and therefore the direction and magnitude of the perturbation caused by arm movement, remained constant. In single arm movements, postural activation of the deep trunk muscles was delayed, postural activation of at least one of the superficial trunk muscles was augmented and there was an increase in baseline activity for Sup MF and OE across the group. In repetitive arm movements, there was a decrease in the maximum and mean EMG amplitude of the deep trunk muscles and a reduction in the coherence of deep trunk muscle EMG with arm movement.
In both single and repetitive arm movements, the nature of the change in postural strategy is consistent with a protective response to stiffen the spine by co-contraction of the superficial trunk muscles. Although general spinal splinting may be beneficial in the short term, there is an associated cost. For instance, splinting reduces spinal flexibility, which is important for normal function and dampening of reactive forces (Hodges et al., 1999) and, according to biomechanics, must impart an increased compressive load on spinal structures (Cholewicki and McGill, 1994). Such an increase in loading has been shown to accelerate tissue degeneration (Ching et al., 2003), and sustained increased spinal load is thought to lead to stimulation of nociceptors in spinal structures and predispose to spinal injury (Gardner-Morse and Stokes, 1998). That said, although any increase in superficial trunk muscle co-contraction will impart some compressive load, the minimum change in EMG and the minimum duration of that change in EMG required to injure spinal tissue is yet to be determined. A second aspect of the results suggests increased risk associated with long-term alteration in spinal control. The deep trunk muscles are well suited to control shear (Hodges et al., 2003a) and are important in fine-tuning of spinal control (e.g. Kaigle et al., 1995). Therefore, delayed or reduced activity of the deep trunk muscles is likely to be associated with reduction of fine control, particularly between segments, which further places spinal structures at risk of nociceptive stimulation and injury. Based on the likely consequences of maintaining such a protective postural strategy long term, the current results raise the possibility that anticipation of back pain predisposes to back trouble. Although consistent with previous links between chronic pain-related disability and protective movement patterns (Lethem et al., 1983; Slade et al., 1983; Keefe et al., 1990; Vlaeyen and Linton, 2000, 1995), the current findings make the first link between anticipation of pain and subsequent nociception or injury, or both.
Elbow pain did not impart an alteration in postural strategy. Although anatomical specificity of the effect of anticipated experimental pain on postural responses is a new finding, it corroborates data obtained from patients. For example, when exposed to personally relevant stressors, patients with chronic jaw pain, but not those with chronic back pain, demonstrated increased jaw muscle activity (Flor et al., 1991, 1992). Similar specificity has been shown for frontalis muscle activity in patients with chronic headache (Flor and Turk, 1989), and paraspinal muscle activity in patients with chronic back pain (Flor et al., 1992). Together, those data suggest that the effect on motor output (i.e. increased muscle activity) is anatomically specific to the area of pain, or the anatomical context of expected pain and/or (re)injury. In the current experiment, the effect on motor output (i.e. altered postural response of the deep trunk muscles) only occurred when the impending pain was anatomically specific (i.e. back pain), which is not surprising in light of the potential benefit of the alternative postural strategy, i.e. to stiffen the spine and limit perturbation of the affected part.
The possibility that the changes in postural adjustments observed here could be mediated at a motoneuron or spinal level cannot be excluded. Most data implicating spinal mechanisms in the changes in muscle activity during pain suggest that those mechanisms lead to reduced activity of agonists and increased activity of antagonists (e.g. Graven-Nielsen et al., 1997). That theory would predict, for example, a delay in activation of both superficial and deep extensor muscles and augmentation of superficial and deep abdominal muscles, which was not observed. Rather, we observed a delay in deep trunk muscles and augmentation of superficial trunk muscles. Notably, both Sup MF, an extensor, and OE, a flexor, demonstrated increased baseline activity during single arm movements. As such, the changes observed here are more consistent with an alteration of strategy. Moreover, similar changes have been shown in people with recurrent back trouble, in which they have been shown to reflect changes in postural strategy (Hodges, 2001).
A consideration for the generalizability of the current results is that painful cutaneous stimulation does not accurately simulate non-experimental back pain. We previously have demonstrated a similar change in postural strategy when normal subjects were given experimentally induced low back pain via i.m. injection of hypertonic saline, which more closely simulates non-experimental pain (Hodges et al., 2003b). We have also replicated those findings in a separate study that involved the same subjects as those used here (Moseley et al., 2004). Taken together, this series of studies demonstrates that asymptomatic controls who are anticipating back pain evoked by painful cutaneous stimulation, those who are experiencing back pain induced by i.m. injection of hypertonic saline and patients who suffer chronic recurrent episodes of back pain but who are pain free all demonstrate a similar postural strategy during arm movements. That strategy is different from pain-free controls under normal conditions. Thus, despite the limitations of painful cutaneous shock, it is sufficient to identify that relationship. That said, spinal and cortical projections from cutaneous input differ from those from muscle input, and painful cutaneous stimulation and painful muscle stimulation have different effects on short and long latency motor responses (Zedka et al., 1999), which suggests that experienced and anticipated cutaneous pain and muscle pain may involve distinct mechanisms and impart other distinct effects. It would therefore be valuable to replicate the current findings using a deeper painful stimulus such as i.m. injection of saline. This was not possible in our study because in order to evoke an authentic and sustained anticipation of pain, we considered it important to use a painful stimulus of known intensity and short duration, which permits task performance during expected pain but in the absence of pain. I.m. injection of algesic chemicals does not offer this advantage. We also chose not to use i.m. electrical stimulation because this would evoke a muscle response and it would be impossible to determine whether any effect was consequent to the anticipation of pain or the anticipation of postural perturbation imparted by a muscle response. Nonetheless, differences between experimentally induced pain and non-experimental pain should be remembered.
In summary, the anticipation of experimental back pain changes the strategy by which the CNS controls the spine during perturbation caused by limb movement. The alternative strategy serves to protect the trunk by stiffening the spine. The effect is unlikely to be caused by attention demand due to pain or other generalized effects of stress or fear because it is not present during anticipation of experimental elbow pain. Because this protective strategy is associated with a reduction in fine control and increased loading of spinal structures, it is thought to predispose spinal structures to nociception and injury if maintained long term. The main implications of the current work then, are that the alteration of postural strategy observed in recurrent back pain patients may be caused by the anticipation of back pain, and that anticipation of back pain predisposes the individual to back trouble.
G.L.M. is supported by a Clinical Research Fellowship Grant ID 210348 and P.W.H. is supported by a Senior Research Fellowship Grant ID 157203, both from the National Health & Medical Research Council of Australia.
References
Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study.
Belen'kii V, Gurfinkel VS, Pal'tsev Y. Control elements of voluntary movements. [Russian].
Burton AK, Tillotson KM, Main CJ, Hollis S. Psychosocial predictors of outcome in acute and subchronic low back trouble.
Ching CT, Chao DH, Yao FY, Holmes AD. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model.
Cholewicki J, McGill SM. EMG assisted optimization: a hybrid approach for estimating muscle forces in an indeterminate biomechanical model.
Crombez G, Vervaet L, Baeyens F, Lysens R, Eelen P. Do pain expectancies cause pain in chronic low back patients? A clinical investigation.
Crombez G, Eccleston C, Baeyens F, Eelen P. Attentional disruption is enhanced by the threat of pain.
Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability.
Flor H, Turk DC. Psychophysiology of chronic pain: do chronic pain patients exhibit symptom-specific psychophysiological responses?
Flor H, Birbaumer N, Schulte W, Roos R. Stress-related electromyographic responses in patients with chronic temporomandibular pain.
Flor H, Birbaumer N, Schugens MM, Lutzenberger W. Symptom-specific psychophysiological responses in chronic pain patients.
Gardner-Morse MG, Stokes IA. The effects of abdominal muscle coactivation on lumbar spine stability.
Garofalo J, Polatin P. Low back pain: an epidemic in industrialised countries. In: Gatchel RJ, Turk DC, editors. Psychosocial factors in pain. Critical perspectives. New York: Guilford Press;
Gasser H, Erlanger J. The role of fiber size in the establishment of a nerve block by pressure of cocaine.
Graven-Nielsen T, Svensson P, Arendt-Nielsen L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function.
Great Britain. Clinical Standards Advisory Group. Epidemiology review: the epidemiology and cost of back pain. London: HMSO;
Handwerker HO, Kobal G. Psychophysiology of experimentally induced pain.
Hodges PW. Changes in motor planning of feedforward postural responses of the trunk muscles in low back pain.
Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography.
Hodges PW, Gandevia SC. Activation of the human diaphragm during a repetitive postural task.
Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis.
Hodges PW, Richardson CA. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement.
Hodges PW, Richardson CA. Transversus abdominis and the superficial abdominal muscles are controlled independently in a postural task.
Hodges PW, Cresswell A, Thorstensson A. Preparatory trunk motion accompanies rapid upper limb movement.
Hodges PW, Cresswell AG, Daggfeldt K, Thorstensson A. In vivo measurement of the effect of intra-abdominal pressure on the human spine.
Hodges PW, Kaigle Holm A, Holm S, Ekstrom L, Cresswell AG, Hansson T, et al. Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies.
Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC. Experimental muscle pain changes feedforward postural responses of the trunk muscles.
Kaigle AM, Holm SH, Hansson TH. Experimental instability in the lumbar spine.
Keefe FJ, Bradley LA, Crisson JE. Behavioral assessment of low back pain: identification of pain behavior subgroups.
Lethem J, Slade PD, Troup JD, Bentley G. Outline of a fear-avoidance model of exaggerated pain perception—I.
Macintosh JE, Bogduk N. The biomechanics of the lumbar multifidus.
Madeleine P, Lundager B, Voigt M, Arendt-Nielsen L. Shoulder muscle co-ordination during chronic and acute experimental neck-shoulder pain. An occupational pain study.
Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements.
Moseley GL, Hodges PW, Gandevia SC. External perturbation of the trunk in standing humans differentiallly activates components of the medial back muscles.
Moseley G, Brhyn L, Ilowiecki M, Solstad K, Hodges PW. The threat of predictable and unpredictable pain: differential effects on central nervous system processing?
Moseley GL, Nicholas MK, Hodges PW. Pain differs from non-painful attention-demanding or stressful tasks in its effect on postural control patterns of trunk muscles.
Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, et al. Dissociating pain from its anticipation in the human brain.
Richardson CA, Snijders CJ, Hides JA, Damen L, Pas MS, Storm J. The relation between the transversus abdominis muscles, sacroiliac joint mechanics, and low back pain.
Slade PD, Troup JD, Lethem J, Bentley G. The fear-avoidance model of exaggerated pain perception—II.
Solomonow M, Zhou BH, Harris M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine.
Spielberger CD, Gorsuch RL, Lushene RE. STAI manual for the State–Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press;
van Dieen JH, Selen LPJ, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature.
Vlaeyen JWS, Kole-Snijders AMJ, Boerne RGB, van Eelch. The role of fear of movement/(re)injury in pain disability.
Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998.
Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study.
Zedka M, Prochazka A. Phasic activity in the human erector spinae during repetitive hand movements.
Author notes
1Prince of Wales Medical Research Institute, Randwick, Sydney, 2Department of Physiotherapy, The University of Queensland, 3School of Physiotherapy, The University of Sydney and 4Pain Management Research Institute, Royal North Shore Hospital, Sydney, Australia