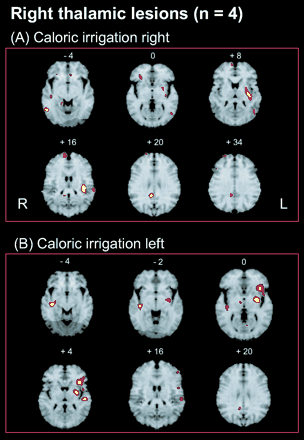
-
PDF
- Split View
-
Views
-
Cite
Cite
M. Dieterich, P. Bartenstein, S. Spiegel, S. Bense, M. Schwaiger, T. Brandt, Thalamic infarctions cause side-specific suppression of vestibular cortex activations, Brain, Volume 128, Issue 9, September 2005, Pages 2052–2067, https://doi.org/10.1093/brain/awh551
Close - Share Icon Share
Abstract
H2O15-PET was performed during caloric vestibular stimulation of the right and left external ears in eight right-handed patients with acute unilateral infarctions or haemorrhages of the posterolateral thalamus (four right, four left). The posterolateral thalamus is the relay station for ipsi- and contralateral ascending vestibular input to the multiple multisensory vestibular cortex areas. The aim of this study was to evaluate the differential effects of unilateral vestibular thalamic lesions on thalamo-cortical projections, right hemispheric dominance and reciprocal inhibitory visual-vestibular interaction, as well as perceptual and ocular motor consequences during caloric irrigation. The major findings of the group analyses of the patients with right-sided and those with left-sided lesions were as follows: (i) activation of the multisensory vestibular temporo-parietal cortex was significantly reduced in the hemisphere ipsilateral to the thalamic lesion when the ipsilesional or contralesional ear was stimulated; (ii) activation of multisensory vestibular cortex areas of the hemisphere contralateral to the irrigated ipsilesional ear was also diminished; and (iii) the right hemispheric dominance in right-handers described above was preserved in those with right and left thalamic lesions. Simultaneous deactivations were often restricted to only one hemisphere—the one contralateral to the stimulation and contralateral to the vestibular cortex areas activated. There was, however, one area in the inferior insula which was also activated by either right or left ear stimulation in the hemisphere ipsilateral to the lesion. This supports the assumption that there is a bilateral direct ascending vestibular projection from the vestibular nuclei to the inferior part of the insula, which bypasses the posterolateral thalamus and is stronger in the right hemisphere. The cortical asymmetry of the pattern of activation during horizontal semicircular canal stimulation by calorics was not associated with a significant direction-specific asymmetry of caloric nystagmus or perceived body motion. Thus, the data demonstrate the functional importance of the posterolateral thalamus as a unique relay station for vestibular input to the cortex, of the dominance of the right hemisphere in right-handedness, and of ipsilateral ascending pathways. Furthermore, the normal interaction between the two sensory systems—the vestibular and the visual—appears to be impaired.
Introduction
Vestibular pathways run bilaterally from the eighth nerve and the vestibular nuclei through ascending fibres such as the medial longitudinal fascicle, the ascending Deiters' tract or the brachium conjunctivum to the ocular motor nuclei and the supranuclear integration centres in the pontine and rostral mesencephalic brainstem. These fibres represent the vestibulo-ocular reflex (VOR), which transmits information from the vestibular endorgans to the ocular motor nuclei and the supranuclear coordination centres for eye and head integration. From there, they reach several multisensory vestibular cortex areas through projections via the posterolateral thalamic subnuclei to mediate the cortical functions of the vestibular system such as the perception of verticality and self-motion (Brandt and Dieterich, 1999).
The posterolateral thalamus—containing the subnuclei ventrocaudalis externus (Vce), ventro-oralis intermedius (Vim), dorsocaudalis (Dc), ventrocaudalis internus (Vci) and nucleus ventroposterior lateralis (VPLo) [human nomenclature of Hassler (1959) in the thalamus atlas of Van Buren and Borke (1972)]—is the afferent relay station for multiple multisensory vestibular cortex areas, as determined by vestibular stimulation in animal experiments (Sans et al., 1970; Deecke et al., 1973, 1974, 1977; Büttner and Henn, 1976). Via the subnuclei of this relay station, vestibular information in animals reaches several separate and distinct cortex areas such as the parieto-insular vestibular cortex (PIVC) in the posterior insula, adjacent retroinsular areas and the granular insular region (Grüsser et al., 1982, 1990a, b; Guldin and Grüsser, 1996), the visual temporal sylvian area VTS posterior to PIVC (Guldin and Grüsser 1996, 1998), area 3aV in the central sulcus (Schwarz et al., 1973; Ödkvist et al., 1974; Büttner and Lang, 1979), parts of area 7 in the inferior parietal lobe [Brodmann area (BA) 40] (Ventre and Faugier-Grimaud, 1986; Faugier-Grimaud and Ventre, 1989; Ventre-Dominey et al., 2003), and probably area 2v at the tip of the intraparietal sulcus (Fredrickson et al., 1966; Schwarz and Fredrickson, 1971; Büttner and Buettner, 1978). The oral portion of the VPLo and, to a lesser extent, the inferior portion (VPI) seem to play the most important role in the projection sites of ascending vestibular thalamocortical pathways in monkeys, according to neurophysiological and anterograde tracer studies that used labelling in area 3a (Büttner and Lang, 1979).
All the above-mentioned cortical areas are multisensory and respond not only to rotational vestibular but also to somatosensory and in part visual motion stimuli. Tracer studies in monkeys have shown that, anatomically, these cortical areas are closely connected to each other (Guldin et al., 1993; Guldin and Grüsser, 1996). Such studies in monkeys and cats also identified the multiple projections from the thalamus to several vestibular cortical representations, mainly the PIVC, area 7ant (2v) and 3aV (Akbarian et al., 1992, 1993, 1994; Guldin and Grüsser, 1996). The PIVC is the dominant multisensory vestibular cortex area, which was considered to be a ‘core region’ (Guldin and Grüsser, 1996). It receives its main input from the vestibular parts of the ventroposterior complex (Vce, Vci, nucleus ventro-oralis externus (Voe), Vim) and the medial pulvinar (Pum). The ‘proprioceptive vestibular area 3aV’ receives its major thalamic projection from the VPLo.
In the past 10 years, functional imaging studies using vestibular, somatosensory and visual optokinetic stimulation have reported evidence of similar locations and connections of these bilaterally organized multisensory vestibular cortical areas in humans. A complex network of areas predominantly in the temporo-insular and temporo-parietal cortex was found in both hemispheres (Bucher et al., 1998; Lobel et al., 1998; Bense et al., 2001; Suzuki et al., 2001; Fasold et al., 2002; Emri et al., 2003). The activation pattern of this network in both hemispheres, which was only recently identified in humans during vestibular stimulation, is not symmetrical but determined by three factors that are additive (Bense et al., 2003, 2004; Dieterich et al., 2003). The most important factor was the subject's handedness. The activation pattern revealed a dominance of the non-dominant hemisphere (i.e. the activation was stronger in temporo-insular-parietal areas of the right hemisphere in right-handers and of the left hemisphere in left-handers). Secondly, the side of the stimulated vestibular endorgan was another important determinant. Stronger activation was observed within the hemisphere ipsilateral to the stimulated ear (i.e. stronger activation within the right hemisphere than within the left during stimulation of the right vestibular endorgan). Thirdly, the direction of the induced vestibular symptoms was also relevant. Stronger activation occurred within the hemisphere ipsilateral to the fast phase of vestibular caloric nystagmus.
Since there is a vestibular thalamo-cortical network in both hemispheres, the question arose as to the consequences of a unilateral lesion of the ‘vestibular relay station’ in the posterolateral thalamus. Thus, the aim of this 15O-labelled H2O bolus PET study was to analyse the differential effects of unilateral caloric vestibular stimulation (right or left ear irrigation with warm water at 44°C) on the cortical and subcortical activation patterns of both hemispheres in patients suffering from an acute or subacute unilateral stroke of the posterolateral thalamus. Drawing on the three factors mentioned above that determine the activation pattern in healthy right-handers and the bilaterality of the pathways, we posed the following questions to elucidate data collected from two groups of right-handed patients: one group with a lesion of the right posterolateral thalamus and another group with a lesion of the left posterolateral thalamus.
Does an infarction of the right posterolateral thalamus influence the activation pattern during caloric irrigation of the right ear only within the right hemisphere or within both hemispheres?
Does an infarction of the right posterolateral thalamus influence the activation pattern during caloric irrigation of the left ear or does a normal pattern occur? If the pattern is affected, is it affected only within the right hemisphere?
Does an infarction of the left thalamus induce similar effects on the activation pattern as a right-sided infarction or are the effects less significant, since the left hemisphere is the non-dominant hemisphere for the vestibular cortical system?
Which areas of the multisensory vestibular cortical network are influenced by the lesion—only the areas in the insula or also areas in the temporo-parietal and frontal cortex?
Does the posterolateral thalamic lesion influence the clinical parameters of the VOR, e.g. the ocular motor (caloric nystagmus) and perceptual components?
Is there a correlation between cortical activation pattern and ocular motor and perceptual parameters? In other words, if there is no activation in the vestibular cortical areas of one hemisphere, does this cause a reduction of caloric nystagmus?
Patients and methods
Patients
Eight right-handed patients (four females, 23–62 years old, mean age 46 years; four males, 59–66 years old, mean age 63 years) presenting with a vascular lesion of the posterolateral thalamus participated in the study (Table 1) after giving their informed written consent in accordance with the Helsinki Declaration. The study was approved by the local Ethics Committee as well as by the radiation protection authorities of the Ludwig–Maximilians University and Technical University Munich. The aetiologies of the lesions were ischaemic infarctions in six (three right, three left) and circumscribed haemorrhages in two patients (one right, one left). All patients had CT and/or MRI scans to determine the aetiology, the exact site and the extent of the lesion. Patients were excluded from the study if they had received pharmacological medication (analgesic, anticonvulsant or sedative), had deficits from earlier brainstem events prior to the onset of disease, showed acute multiple lesions of the brainstem and/or the hemispheres, and if they were unable to keep in a supine position for at least three hours.
Patient data
| Patient . | Age (years) . | Sex . | Day after onset . | Lesion side . | Aetiology . | Additional signs and symptoms . |
|---|---|---|---|---|---|---|
| BS | 59 | Male | 55 | Left | Haemorrhage | Hemiataxia right |
| EJ | 23 | Female | 35 | Left | Ischaemia | Hemiparesis right |
| HM | 63 | Male | 4 | Left | Ischaemia | Hemiparesis right |
| GW | 42 | Female | 19 | Left | Ischaemia | Hemiparesis right, gaze palsy upward |
| FK | 59 | Male | 18 | Right | Haemorrhage | Hemiparesis left |
| EW | 66 | Male | 28 | Right | Ischaemia | Hemiparesis left |
| HH | 62 | Female | 10 | Right | Ischaemia | Hemiparesis left |
| HM | 58 | Female | 10 | Right | Ischaemia | Hemiparesis left, gaze palsy upward |
| Patient . | Age (years) . | Sex . | Day after onset . | Lesion side . | Aetiology . | Additional signs and symptoms . |
|---|---|---|---|---|---|---|
| BS | 59 | Male | 55 | Left | Haemorrhage | Hemiataxia right |
| EJ | 23 | Female | 35 | Left | Ischaemia | Hemiparesis right |
| HM | 63 | Male | 4 | Left | Ischaemia | Hemiparesis right |
| GW | 42 | Female | 19 | Left | Ischaemia | Hemiparesis right, gaze palsy upward |
| FK | 59 | Male | 18 | Right | Haemorrhage | Hemiparesis left |
| EW | 66 | Male | 28 | Right | Ischaemia | Hemiparesis left |
| HH | 62 | Female | 10 | Right | Ischaemia | Hemiparesis left |
| HM | 58 | Female | 10 | Right | Ischaemia | Hemiparesis left, gaze palsy upward |
Patient data
| Patient . | Age (years) . | Sex . | Day after onset . | Lesion side . | Aetiology . | Additional signs and symptoms . |
|---|---|---|---|---|---|---|
| BS | 59 | Male | 55 | Left | Haemorrhage | Hemiataxia right |
| EJ | 23 | Female | 35 | Left | Ischaemia | Hemiparesis right |
| HM | 63 | Male | 4 | Left | Ischaemia | Hemiparesis right |
| GW | 42 | Female | 19 | Left | Ischaemia | Hemiparesis right, gaze palsy upward |
| FK | 59 | Male | 18 | Right | Haemorrhage | Hemiparesis left |
| EW | 66 | Male | 28 | Right | Ischaemia | Hemiparesis left |
| HH | 62 | Female | 10 | Right | Ischaemia | Hemiparesis left |
| HM | 58 | Female | 10 | Right | Ischaemia | Hemiparesis left, gaze palsy upward |
| Patient . | Age (years) . | Sex . | Day after onset . | Lesion side . | Aetiology . | Additional signs and symptoms . |
|---|---|---|---|---|---|---|
| BS | 59 | Male | 55 | Left | Haemorrhage | Hemiataxia right |
| EJ | 23 | Female | 35 | Left | Ischaemia | Hemiparesis right |
| HM | 63 | Male | 4 | Left | Ischaemia | Hemiparesis right |
| GW | 42 | Female | 19 | Left | Ischaemia | Hemiparesis right, gaze palsy upward |
| FK | 59 | Male | 18 | Right | Haemorrhage | Hemiparesis left |
| EW | 66 | Male | 28 | Right | Ischaemia | Hemiparesis left |
| HH | 62 | Female | 10 | Right | Ischaemia | Hemiparesis left |
| HM | 58 | Female | 10 | Right | Ischaemia | Hemiparesis left, gaze palsy upward |
All patients were examined neurologically, neuro-ophthalmologically and by electro-oculography. Furthermore, to measure the tonic vestibular signs, all underwent orthoptic examination, which included measurements of the subjective visual vertical (SVV) and of tonic ocular torsion by a scanning laser ophthalmoscope (for methods and normal values, see Dieterich and Brandt, 1993a). Static SVV was determined binocularly while the patients sat upright with their head fixed in front of a hemispheric dome (60 cm in diameter and covered with random dots). A central test edge had to be adjusted (means of 10 adjustments) from a random offset position to the subjective vertical, with the hemispheric dome stationary. Under these conditions, the normal range is ±2.5°. Ocular torsion was defined as the mean of four fundus photographs taken by the scanning laser ophthalmoscope with the head upright and fixed in the sitting position. Otoneurological examination and electronystagmography showed that no patient had peripheral vestibular dysfunction. The Laterality Quotient for right-handedness was +100 in six patients and +60 in the remaining two according to the 10-item inventory of the Edinburgh test (Oldfield, 1971; Salmaso and Longoni, 1985).
For group analyses with PET, the patients were subdivided into two groups of four patients, one subgroup with right-sided lesions (two females, two males; age 58–66 years; mean age 61 years) and the second subgroup with left-sided lesions (two females, two males; age 23–63 years; mean age 47 years). This was necessary because the activation pattern in normal subjects is dependent on handedness. The H2O15-PET scans were performed between day 4 and day 55 after lesion onset (day 10 was the mean time).
Caloric irrigation and electro-oculography in PET
During all conditions of PET data acquisition, the subject lay supine with eyes closed. Vestibular stimulation was performed by irrigating the right or left external ear canals with 100 ml of warm water at 44°C for ∼50 s. A 100-ml syringe was used in conjunction with a flexible plastic tube placed in the external auditory meatus of each ear. The head was slightly elevated (20°) for optimum stimulation of the horizontal canal. To monitor the effects, the horizontal DC electro-oculogram was recorded with the help of two silver–silver chloride electrodes placed at the lateral canthi of each eye (ground electrode over the glabella).
For calibration purposes, the subject was asked to look at targets 0°, 20° to the left and 20° to the right. The calibration saccades recorded were used for the analysis of the slow phase velocity (SPV) of caloric nystagmus, which was determined at the end of caloric irrigation and during the scanning period. After calibration, the subjects kept their eyes closed continuously during the control condition, and during and after caloric irrigation. After the scans, the subjects were asked to comment on their perception of motion. The subjective strength of the vestibular sensation was quantified by determining the perceived intensity of body rotation on a visual analogue scale (0–5). There were three stimulus conditions applied in random order with the eyes closed: (A) caloric irrigation of the right ear; (B) caloric irrigation of the left ear, and (C) the rest condition without vestibular stimulation.
PET scanning and image reconstruction
PET measurements were performed using a Siemens 951 R/31 PET scanner (CTI Knoxville, TN, USA) in 3D mode with a total axial field of view of 10.5 cm and no interplane dead space under standard resting conditions. Attenuation was corrected using a transmission scan with an external 68Ge/68Ga ring source obtained prior to the tracer injection. A semibolus injection of 7.5 mCi/run H2O15 was administered intravenously over 35 s using an infusion pump.
A dynamic acquisition protocol was performed starting with the infusion. It consisted of a sequence of eight short-duration frames: one 15-s frame followed by seven 10-s frames, covering a total scan time of 85 s. After corrections for randoms, dead time and scatter, images were reconstructed by filtered back-projection with a Hanning filter (cut-off frequency 0.4 cycles/projection element), resulting in 31 slices with a 128 × 128 pixel matrix (pixel size 2.0 mm) and interplane separation of 3.375 mm. Since time-activity curves of the whole brain showed initial tracer appearance during scan 4 and its maximum between scans 4 and 8 in all subjects, frames 4–8 were added to a single frame consisting of 50 s for further analysis. Each condition was repeated up to four times (typical configuration ABCCBAACBBCA). The initial stimulation condition was thus varied in random order. To avoid the influence of non-vestibular effects of caloric stimulation (somatosensory and acoustic sensations), scans that were sampled for 50 s were started 25 s after irrigation ended. Previous tests had revealed that nystagmus is still prominent at this time (for details see Wenzel et al., 1996; Dieterich et al., 2003).
Statistical PET analysis
Tracer counts were normalized proportionally to the global cerebral activity, which was arbitrarily set to 1000, in order to perform analysis on relative tissue regional cerebral blood flow (rCBF) activity (Fox and Raichle, 1984). An automated program (NEUROSTAT; University of Michigan, Ann Arbor, MI, USA) was used to co-register, reslice and transform the image arrays into the stereotactic space of Talairach and Tournoux (1988) as described previously (Minoshima et al., 1993, 1994). To eliminate individual differences in gyral anatomy, these images were further smoothed with a three-dimensional Gaussian filter to give an effective resolution of ∼12 mm (full width half maximum). Repeated control and activation images (four scans for each condition) were averaged for each within a subject by calculating the global mean for each voxel. Differences between control and activation images were then averaged across subjects and were expressed as voxel-by-voxel t-statistic values using a pooled variance estimated from the whole brain grey matter (Worsley et al., 1992). Since the resulting t-statistic map is known to approximate closely to a standard Gaussian distribution (Worsley et al., 1992), these values were described as Z-scores. To determine a threshold for significant activation on the resulting t-map, we calculated the image smoothness (Friston et al., 1991) and estimated a statistical threshold at a one-tail (positive) probability of P = 0.05 using a statistical model that adjusts multiple comparisons and inherent correlation of neighbouring pixels (Montreal threshold) (Worsley et al., 1992). Statistical parametric maps were defined as ‘activation maps’ using the contrasts A versus C and B versus C, and ‘deactivation maps’ using the contrasts C versus A and C versus B.
For the areas attributed to multisensory vestibular functions, for which we had a theory-driven, a priori hypothesis, a Z-score >3 in the respective regions was considered representative of a significant change in regional CBF (rCBF). This corresponds to t-values that, without correction for multiple comparisons, achieve a probability of P < 0.0001 (Kosslyn et al., 1994). Beside the temporo-insular and the temporo-parietal cortex, the activated areas included in the analysis based on the a priori hypothesis were: the anterior insula and adjacent inferior frontal gyrus; putamen and caudate nucleus; precuneus; precentral gyrus; medial, superior and diagonal frontal gyri; anterior cingulum; hippocampus; thalamus; and cerebellum (Lobel et al., 1998; Bense et al., 2001; Suzuki et al., 2001; Fasold et al., 2002; Dieterich et al., 2003; Emri et al., 2003).
Results
Psychophysical and ocular motor data
None of the patients complained of vertigo, dizziness or oscillopsia. All but one had mild contralateral hemiparesis and one had contralateral hemiataxia (see Table 1). Five of the eight patients had moderate difficulties in stance and gait, and exhibited a tendency to fall to one side. Seven patients presented with abnormal tilts of the SVV amounting to 4.2–10.1°, with a mean of 5.9° (normal range ±2.5°; Dieterich and Brandt, 1993a). The direction of SVV tilt was ipsilateral in three patients and contralateral in four patients (Table 2). The one patient (BS) with normal SVV measurements had a longer period after disease onset of 55 days, whereas the other seven patients had a range of 4 to 35 days (mean 18 days) after disease onset. From earlier studies, it is known that SVV tilts in thalamic lesions recover spontaneously within 30 to 40 days (Dieterich and Brandt, 1993b), thus explaining the normal data of patient BS.
Results of the intensity of motion perception and caloric nystagmus during caloric irrigation and adjustments of the subjective visual vertical
| Patient . | Lesion side (R/L) . | Intensity of motion perception (analogue scale 0–5)* . | . | Nystagmus (SPV in °/s)* . | . | SVV (°)# . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | Calorics . | . | Calorics . | . | . | ||
. | . | Ipsilesional . | Contralesional . | Ipsilesional . | Contralesional . | . | ||
| BS | L | 0.25 | 1.1 | 4 | 43 | −1.1 | ||
| EJ | L | 2.3 | 4.0 | 13 | 29 | −4.2 | ||
| HM | L | 0.5 | 0.5 | 34 | 38 | −5.0 | ||
| GW | L | 2.0 | 2.0 | 20 | 9 | +8.3 | ||
| FK | R | 0.75 | 1.0 | 40 | 49 | +5.0 | ||
| EW | R | – | – | – | – | −9.6 | ||
| HH | R | 0.5 | 0.7 | 42 | 42 | −4.2 | ||
| HM | R | 0.8 | 1.4 | 36 | 43 | −10.1 | ||
| Mean | 1.0 | 1.5 | 27.0 | 36.1 | 5.9 | |||
| SD | 0.8 | 1.2 | 14.8 | 13.4 | 3.1 | |||
| Patient . | Lesion side (R/L) . | Intensity of motion perception (analogue scale 0–5)* . | . | Nystagmus (SPV in °/s)* . | . | SVV (°)# . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | Calorics . | . | Calorics . | . | . | ||
. | . | Ipsilesional . | Contralesional . | Ipsilesional . | Contralesional . | . | ||
| BS | L | 0.25 | 1.1 | 4 | 43 | −1.1 | ||
| EJ | L | 2.3 | 4.0 | 13 | 29 | −4.2 | ||
| HM | L | 0.5 | 0.5 | 34 | 38 | −5.0 | ||
| GW | L | 2.0 | 2.0 | 20 | 9 | +8.3 | ||
| FK | R | 0.75 | 1.0 | 40 | 49 | +5.0 | ||
| EW | R | – | – | – | – | −9.6 | ||
| HH | R | 0.5 | 0.7 | 42 | 42 | −4.2 | ||
| HM | R | 0.8 | 1.4 | 36 | 43 | −10.1 | ||
| Mean | 1.0 | 1.5 | 27.0 | 36.1 | 5.9 | |||
| SD | 0.8 | 1.2 | 14.8 | 13.4 | 3.1 | |||
Of four stimulations of the right or left ear.
Of 10 adjustments (+ tilt to the right, – tilt to the left).
Results of the intensity of motion perception and caloric nystagmus during caloric irrigation and adjustments of the subjective visual vertical
| Patient . | Lesion side (R/L) . | Intensity of motion perception (analogue scale 0–5)* . | . | Nystagmus (SPV in °/s)* . | . | SVV (°)# . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | Calorics . | . | Calorics . | . | . | ||
. | . | Ipsilesional . | Contralesional . | Ipsilesional . | Contralesional . | . | ||
| BS | L | 0.25 | 1.1 | 4 | 43 | −1.1 | ||
| EJ | L | 2.3 | 4.0 | 13 | 29 | −4.2 | ||
| HM | L | 0.5 | 0.5 | 34 | 38 | −5.0 | ||
| GW | L | 2.0 | 2.0 | 20 | 9 | +8.3 | ||
| FK | R | 0.75 | 1.0 | 40 | 49 | +5.0 | ||
| EW | R | – | – | – | – | −9.6 | ||
| HH | R | 0.5 | 0.7 | 42 | 42 | −4.2 | ||
| HM | R | 0.8 | 1.4 | 36 | 43 | −10.1 | ||
| Mean | 1.0 | 1.5 | 27.0 | 36.1 | 5.9 | |||
| SD | 0.8 | 1.2 | 14.8 | 13.4 | 3.1 | |||
| Patient . | Lesion side (R/L) . | Intensity of motion perception (analogue scale 0–5)* . | . | Nystagmus (SPV in °/s)* . | . | SVV (°)# . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | Calorics . | . | Calorics . | . | . | ||
. | . | Ipsilesional . | Contralesional . | Ipsilesional . | Contralesional . | . | ||
| BS | L | 0.25 | 1.1 | 4 | 43 | −1.1 | ||
| EJ | L | 2.3 | 4.0 | 13 | 29 | −4.2 | ||
| HM | L | 0.5 | 0.5 | 34 | 38 | −5.0 | ||
| GW | L | 2.0 | 2.0 | 20 | 9 | +8.3 | ||
| FK | R | 0.75 | 1.0 | 40 | 49 | +5.0 | ||
| EW | R | – | – | – | – | −9.6 | ||
| HH | R | 0.5 | 0.7 | 42 | 42 | −4.2 | ||
| HM | R | 0.8 | 1.4 | 36 | 43 | −10.1 | ||
| Mean | 1.0 | 1.5 | 27.0 | 36.1 | 5.9 | |||
| SD | 0.8 | 1.2 | 14.8 | 13.4 | 3.1 | |||
Of four stimulations of the right or left ear.
Of 10 adjustments (+ tilt to the right, – tilt to the left).
Measurements of tonic ocular torsion of both eyes were available in seven patients; all were within the normal range.
Caloric irrigation in PET
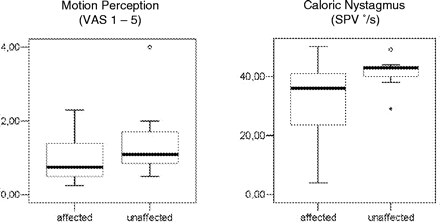
Warm-water vestibular stimulation of one ear caused a sensation of being tilted toward the other ear and a caloric nystagmus to the contralateral ear (direction of the slow phase). Measurements of intensity of motion sensation and caloric nystagmus were made during each stimulation period in PET, i.e. four times in each patient for each ear. The mean intensity of motion sensation was 1.0 for all patients when the ipsilesional ear was stimulated and 1.5 when the contralesional ear was stimulated (for details, see Table 2). The difference was not significant between the ipsilesional and the contralesional ear, nor for left- and right-sided lesions (paired t-test: 0.067) (Fig. 1). Mean slow phase velocity of the caloric nystagmus was 27.0°/s during stimulation of the ipsilesional ear and 36.1°/s during stimulation of the contralesional ear. This trend to a stronger effect if the ear contralateral to the lesion was stimulated also showed no statistical significance as determined by one-way ANOVA (analysis of variance).
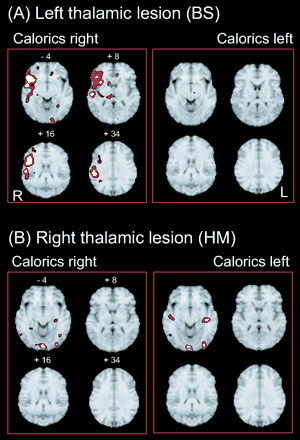
(A) Areas activated during caloric stimulation of the right or left ear in a single right-handed patient BS, who had a left posterolateral thalamic lesion (I < 0.001). Activations were found during caloric irrigation of the right ear—unaffected contralateral side (left panel); e.g. there was one large cluster in the posterior and anterior insula, inferior frontal gyrus, superior temporal gyrus, inferior parietal lobule, superior parts of the parietal lobule, hippocampus, posterolateral thalamus, putamen and medial frontal gyrus of the right hemisphere. There were only a few small activations within the left hemisphere in the anterior cingulate gyrus, the gyrus rectus and the occipital gyrus. Caloric irrigation of the affected ipsilateral left side (right panel) led to no significant activation within the right hemisphere and only minimal activation of the midbrain and basal ganglia within the left hemisphere. (B) Areas activated during caloric stimulation of the right or left ear in another right-handed patient HM with a right posterolateral thalamic lesion. During caloric irrigation of the ipsilateral affected side (left panel), activations were found in the hippocampus of the left hemisphere and bilaterally in the temporo-occipital (corresponding to MT/V5) and occipital visual cortex. Stimulation of the contralateral, unaffected side (right panel) led to activations of inferior parts of the insula and the adjacent temporal gyri bilaterally as well as of areas in the temporo-occipital and occipital visual cortex bilaterally. No activations were found in the multisensory vestibular cortex areas bilaterally during both stimulation conditions.
PET: cortical areas activated during caloric irrigation
Patients with lesions of the left posterolateral thalamus
In the four patients with left-sided lesions, stimulation of the ipsilesional left ear caused activations within the left hemisphere located in the caudate nucleus, the lingual gyrus (BA 18), the anterior and medial parts of the insula, the subcallosal gyrus (BA 25), the medial temporal gyrus (BA 21/37), and the superior frontal gyrus (BA 10) (Table 3; Figs 2 and 3). The only activations of the right side were found in the cerebellar vermis and the anterior cingulate gyrus. Thus, the activation pattern within the left hemisphere was reduced and impaired, showing no activity of the posterior insula, superior temporal gyrus, or inferior and superior parietal lobe. Instead, there were activations of areas within the left visual cortex (BA 18), which are deactivated in normal subjects. Furthermore, apart from very small activations in the anterior cingulate gyrus and cerebellar vermis (both of which belong functionally to the activations within the left hemisphere), simultaneous activations of the right hemisphere were completely lacking.
Box plots (median, 25% and 75% percentile, and minimal and maximal values) of the perceived motion sensation (visual analogue scale 1–5) and SPV (in °/s) of caloric nystagmus in the patients during caloric irrigation of the ear ipsilateral (affected) or contralateral (unaffected) to the thalamic lesion (one way ANOVA). t-Test and variation analysis were not significant for either parameter.
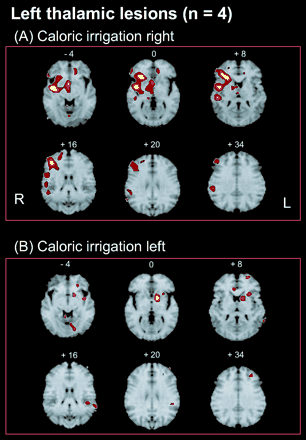
Activated areas during caloric stimulation of the right ear (A) or left ear (B) in patients with a left-sided posterolateral thalamic lesion (group analyses; each group n = 4; P < 0.001). (A) Activations were found for the left-sided lesions during right calorics (non–affected side) as large clusters in the posterior and anterior insula, inferior frontal gyrus, superior temporal gyrus, inferior parietal lobule, superior parts of the parietal lobule, hippocampus, paramedian thalamus and midbrain, nucleus ruber, putamen, medial and superior frontal gyrus, and cerebellar vermis of the right hemisphere. Activations of the left hemisphere were found in only the anterior cingulate gyrus and diagonal frontal gyrus. (B) Caloric irrigation of the affected left side was associated with smaller activations, predominantly within the left hemisphere, anterior and median parts of the insula, inferior frontal gyrus, putamen, caudate nucleus, superior frontal gyrus, medial temporal gyrus/inferior parietal lobule and lingual gyrus. Activations within the right hemisphere were only in the anterior cingulate gyrus and the cerebellar vermis.
Signal increases (Z-score >3.0)
| Thalamic infarction right . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation right (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation left (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Areas . | R/L . | BA . | x . | y . | z . | Z-score . | Areas . | R/L . | BA . | x . | y . | z . | Z-score . | |||||||||||||
| Posterior insula/LPi | L | −39 | −24 | 16 | 4.02 | Posterior insula | L | −51 | −22 | 9 | 3.89 | |||||||||||||||
| Posterior insula/putamen | L | −33 | −15 | 9 | 3.39 | Putamen/posterior insula | L | −28 | −4 | 4 | 4.34 | |||||||||||||||
| Anterior insula | L | −35 | 21 | 4 | 3.89 | |||||||||||||||||||||
| GTs | L | 22 | −62 | −28 | 18 | 3.17 | GTs | L | 47/38 | −39 | 10 | −20 | 3.20 | |||||||||||||
| GTm/GOm | L | 37/39 | −48 | −62 | 7 | 3.23 | ||||||||||||||||||||
| Subcallosal gyrus | L | 25 | −10 | 21 | −14 | 3.51 | ||||||||||||||||||||
| GTm/GOm | R | 19/37 | 51 | −55 | −7 | 3.96 | ||||||||||||||||||||
| GTi/hippocampus | R | 34 | 17 | 1 | −20 | 3.87 | Hippocampus/inferior insula | R | 37 | −17 | −7 | 4.03 | ||||||||||||||
| Cerebellum/GH | R | 35 | 17 | −37 | −9 | 3.15 | ||||||||||||||||||||
| Cerebellar vermis, culmen | R | 26 | −46 | −11 | 3.63 | |||||||||||||||||||||
| Amygdaloid body | R | 28 | −6 | −16 | 3.63 | |||||||||||||||||||||
| Posterior cingulate gyrus | R | 23/29 | 8 | −42 | 22 | 3.60 | ||||||||||||||||||||
| Gyrus rectus/GFi | R | 11 | 1 | 32 | −20 | 3.73 | ||||||||||||||||||||
| Thalamic infarction right . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation right (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation left (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Areas . | R/L . | BA . | x . | y . | z . | Z-score . | Areas . | R/L . | BA . | x . | y . | z . | Z-score . | |||||||||||||
| Posterior insula/LPi | L | −39 | −24 | 16 | 4.02 | Posterior insula | L | −51 | −22 | 9 | 3.89 | |||||||||||||||
| Posterior insula/putamen | L | −33 | −15 | 9 | 3.39 | Putamen/posterior insula | L | −28 | −4 | 4 | 4.34 | |||||||||||||||
| Anterior insula | L | −35 | 21 | 4 | 3.89 | |||||||||||||||||||||
| GTs | L | 22 | −62 | −28 | 18 | 3.17 | GTs | L | 47/38 | −39 | 10 | −20 | 3.20 | |||||||||||||
| GTm/GOm | L | 37/39 | −48 | −62 | 7 | 3.23 | ||||||||||||||||||||
| Subcallosal gyrus | L | 25 | −10 | 21 | −14 | 3.51 | ||||||||||||||||||||
| GTm/GOm | R | 19/37 | 51 | −55 | −7 | 3.96 | ||||||||||||||||||||
| GTi/hippocampus | R | 34 | 17 | 1 | −20 | 3.87 | Hippocampus/inferior insula | R | 37 | −17 | −7 | 4.03 | ||||||||||||||
| Cerebellum/GH | R | 35 | 17 | −37 | −9 | 3.15 | ||||||||||||||||||||
| Cerebellar vermis, culmen | R | 26 | −46 | −11 | 3.63 | |||||||||||||||||||||
| Amygdaloid body | R | 28 | −6 | −16 | 3.63 | |||||||||||||||||||||
| Posterior cingulate gyrus | R | 23/29 | 8 | −42 | 22 | 3.60 | ||||||||||||||||||||
| Gyrus rectus/GFi | R | 11 | 1 | 32 | −20 | 3.73 | ||||||||||||||||||||
| Thalamic infarction left . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation left (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation right (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Posterior insula | R | 42 | −1 | −4 | 4.88 | |||||||||||||||||||||
| Anterior insula | R | 30 | 26 | 4 | 4.75 | |||||||||||||||||||||
| GTs/posterior insula | R | 22 | 51 | −26 | 9 | 3.93 | ||||||||||||||||||||
| LPi | R | 40 | 53 | −31 | 32 | 3.35 | ||||||||||||||||||||
| GFi | R | 44/45 | 55 | 41 | 2 | 3.68 | ||||||||||||||||||||
| GFi/GFm | R | 45/46 | 39 | 32 | 16 | 4.48 | ||||||||||||||||||||
| GFm/GFi | R | 10 | 53 | 46 | 0 | 3.57 | ||||||||||||||||||||
| GFm/GFs | R | 8 | 44 | 39 | 38 | 3.23 | ||||||||||||||||||||
| GFs | R | 9/10 | 21 | 46 | 16 | 3.29 | ||||||||||||||||||||
| Caudate nucleus | R | 3 | 10 | −2 | 4.03 | |||||||||||||||||||||
| Cerebellar vermis, culmen | R | 6 | −42 | −11 | 4.29 | Cerebellar vermis, culmen | R | 8 | −42 | −11 | 3.82 | |||||||||||||||
| Middle/anterior insula | L | −39 | 3 | −7 | 3.46 | |||||||||||||||||||||
| GTm | L | 21/37 | −66 | −49 | 7 | 3.10 | ||||||||||||||||||||
| GFs | L | 10 | −24 | 57 | 11 | 3.10 | GFd | L | 11 | −17 | 39 | −11 | 3.52 | |||||||||||||
| Subcallosal gyrus | L | 25 | −15 | 12 | −11 | 3.22 | ||||||||||||||||||||
| Caudate nucleus | L | −12 | 8 | 4 | 3.73 | |||||||||||||||||||||
| Lingual gyrus | L | 18 | −15 | −76 | −7 | 3.56 | ||||||||||||||||||||
| Thalamic infarction left . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation left (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation right (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Posterior insula | R | 42 | −1 | −4 | 4.88 | |||||||||||||||||||||
| Anterior insula | R | 30 | 26 | 4 | 4.75 | |||||||||||||||||||||
| GTs/posterior insula | R | 22 | 51 | −26 | 9 | 3.93 | ||||||||||||||||||||
| LPi | R | 40 | 53 | −31 | 32 | 3.35 | ||||||||||||||||||||
| GFi | R | 44/45 | 55 | 41 | 2 | 3.68 | ||||||||||||||||||||
| GFi/GFm | R | 45/46 | 39 | 32 | 16 | 4.48 | ||||||||||||||||||||
| GFm/GFi | R | 10 | 53 | 46 | 0 | 3.57 | ||||||||||||||||||||
| GFm/GFs | R | 8 | 44 | 39 | 38 | 3.23 | ||||||||||||||||||||
| GFs | R | 9/10 | 21 | 46 | 16 | 3.29 | ||||||||||||||||||||
| Caudate nucleus | R | 3 | 10 | −2 | 4.03 | |||||||||||||||||||||
| Cerebellar vermis, culmen | R | 6 | −42 | −11 | 4.29 | Cerebellar vermis, culmen | R | 8 | −42 | −11 | 3.82 | |||||||||||||||
| Middle/anterior insula | L | −39 | 3 | −7 | 3.46 | |||||||||||||||||||||
| GTm | L | 21/37 | −66 | −49 | 7 | 3.10 | ||||||||||||||||||||
| GFs | L | 10 | −24 | 57 | 11 | 3.10 | GFd | L | 11 | −17 | 39 | −11 | 3.52 | |||||||||||||
| Subcallosal gyrus | L | 25 | −15 | 12 | −11 | 3.22 | ||||||||||||||||||||
| Caudate nucleus | L | −12 | 8 | 4 | 3.73 | |||||||||||||||||||||
| Lingual gyrus | L | 18 | −15 | −76 | −7 | 3.56 | ||||||||||||||||||||
GFd = diagonal frontal gyrus; GFi = inferior frontal gyrus; GFm = middle frontal gyrus; GFs = superior frontal gyrus; GH = parahippocampal gyrus; GOm = middle occipital gyrus; GTi = inferior temporal gyrus; GTm = middle temporal gyrus; GTs = superior temporal gyrus; LPi = inferior parietal lobule.
Signal increases (Z-score >3.0)
| Thalamic infarction right . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation right (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation left (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Areas . | R/L . | BA . | x . | y . | z . | Z-score . | Areas . | R/L . | BA . | x . | y . | z . | Z-score . | |||||||||||||
| Posterior insula/LPi | L | −39 | −24 | 16 | 4.02 | Posterior insula | L | −51 | −22 | 9 | 3.89 | |||||||||||||||
| Posterior insula/putamen | L | −33 | −15 | 9 | 3.39 | Putamen/posterior insula | L | −28 | −4 | 4 | 4.34 | |||||||||||||||
| Anterior insula | L | −35 | 21 | 4 | 3.89 | |||||||||||||||||||||
| GTs | L | 22 | −62 | −28 | 18 | 3.17 | GTs | L | 47/38 | −39 | 10 | −20 | 3.20 | |||||||||||||
| GTm/GOm | L | 37/39 | −48 | −62 | 7 | 3.23 | ||||||||||||||||||||
| Subcallosal gyrus | L | 25 | −10 | 21 | −14 | 3.51 | ||||||||||||||||||||
| GTm/GOm | R | 19/37 | 51 | −55 | −7 | 3.96 | ||||||||||||||||||||
| GTi/hippocampus | R | 34 | 17 | 1 | −20 | 3.87 | Hippocampus/inferior insula | R | 37 | −17 | −7 | 4.03 | ||||||||||||||
| Cerebellum/GH | R | 35 | 17 | −37 | −9 | 3.15 | ||||||||||||||||||||
| Cerebellar vermis, culmen | R | 26 | −46 | −11 | 3.63 | |||||||||||||||||||||
| Amygdaloid body | R | 28 | −6 | −16 | 3.63 | |||||||||||||||||||||
| Posterior cingulate gyrus | R | 23/29 | 8 | −42 | 22 | 3.60 | ||||||||||||||||||||
| Gyrus rectus/GFi | R | 11 | 1 | 32 | −20 | 3.73 | ||||||||||||||||||||
| Thalamic infarction right . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation right (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation left (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Areas . | R/L . | BA . | x . | y . | z . | Z-score . | Areas . | R/L . | BA . | x . | y . | z . | Z-score . | |||||||||||||
| Posterior insula/LPi | L | −39 | −24 | 16 | 4.02 | Posterior insula | L | −51 | −22 | 9 | 3.89 | |||||||||||||||
| Posterior insula/putamen | L | −33 | −15 | 9 | 3.39 | Putamen/posterior insula | L | −28 | −4 | 4 | 4.34 | |||||||||||||||
| Anterior insula | L | −35 | 21 | 4 | 3.89 | |||||||||||||||||||||
| GTs | L | 22 | −62 | −28 | 18 | 3.17 | GTs | L | 47/38 | −39 | 10 | −20 | 3.20 | |||||||||||||
| GTm/GOm | L | 37/39 | −48 | −62 | 7 | 3.23 | ||||||||||||||||||||
| Subcallosal gyrus | L | 25 | −10 | 21 | −14 | 3.51 | ||||||||||||||||||||
| GTm/GOm | R | 19/37 | 51 | −55 | −7 | 3.96 | ||||||||||||||||||||
| GTi/hippocampus | R | 34 | 17 | 1 | −20 | 3.87 | Hippocampus/inferior insula | R | 37 | −17 | −7 | 4.03 | ||||||||||||||
| Cerebellum/GH | R | 35 | 17 | −37 | −9 | 3.15 | ||||||||||||||||||||
| Cerebellar vermis, culmen | R | 26 | −46 | −11 | 3.63 | |||||||||||||||||||||
| Amygdaloid body | R | 28 | −6 | −16 | 3.63 | |||||||||||||||||||||
| Posterior cingulate gyrus | R | 23/29 | 8 | −42 | 22 | 3.60 | ||||||||||||||||||||
| Gyrus rectus/GFi | R | 11 | 1 | 32 | −20 | 3.73 | ||||||||||||||||||||
| Thalamic infarction left . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation left (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation right (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Posterior insula | R | 42 | −1 | −4 | 4.88 | |||||||||||||||||||||
| Anterior insula | R | 30 | 26 | 4 | 4.75 | |||||||||||||||||||||
| GTs/posterior insula | R | 22 | 51 | −26 | 9 | 3.93 | ||||||||||||||||||||
| LPi | R | 40 | 53 | −31 | 32 | 3.35 | ||||||||||||||||||||
| GFi | R | 44/45 | 55 | 41 | 2 | 3.68 | ||||||||||||||||||||
| GFi/GFm | R | 45/46 | 39 | 32 | 16 | 4.48 | ||||||||||||||||||||
| GFm/GFi | R | 10 | 53 | 46 | 0 | 3.57 | ||||||||||||||||||||
| GFm/GFs | R | 8 | 44 | 39 | 38 | 3.23 | ||||||||||||||||||||
| GFs | R | 9/10 | 21 | 46 | 16 | 3.29 | ||||||||||||||||||||
| Caudate nucleus | R | 3 | 10 | −2 | 4.03 | |||||||||||||||||||||
| Cerebellar vermis, culmen | R | 6 | −42 | −11 | 4.29 | Cerebellar vermis, culmen | R | 8 | −42 | −11 | 3.82 | |||||||||||||||
| Middle/anterior insula | L | −39 | 3 | −7 | 3.46 | |||||||||||||||||||||
| GTm | L | 21/37 | −66 | −49 | 7 | 3.10 | ||||||||||||||||||||
| GFs | L | 10 | −24 | 57 | 11 | 3.10 | GFd | L | 11 | −17 | 39 | −11 | 3.52 | |||||||||||||
| Subcallosal gyrus | L | 25 | −15 | 12 | −11 | 3.22 | ||||||||||||||||||||
| Caudate nucleus | L | −12 | 8 | 4 | 3.73 | |||||||||||||||||||||
| Lingual gyrus | L | 18 | −15 | −76 | −7 | 3.56 | ||||||||||||||||||||
| Thalamic infarction left . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation left (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation right (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Posterior insula | R | 42 | −1 | −4 | 4.88 | |||||||||||||||||||||
| Anterior insula | R | 30 | 26 | 4 | 4.75 | |||||||||||||||||||||
| GTs/posterior insula | R | 22 | 51 | −26 | 9 | 3.93 | ||||||||||||||||||||
| LPi | R | 40 | 53 | −31 | 32 | 3.35 | ||||||||||||||||||||
| GFi | R | 44/45 | 55 | 41 | 2 | 3.68 | ||||||||||||||||||||
| GFi/GFm | R | 45/46 | 39 | 32 | 16 | 4.48 | ||||||||||||||||||||
| GFm/GFi | R | 10 | 53 | 46 | 0 | 3.57 | ||||||||||||||||||||
| GFm/GFs | R | 8 | 44 | 39 | 38 | 3.23 | ||||||||||||||||||||
| GFs | R | 9/10 | 21 | 46 | 16 | 3.29 | ||||||||||||||||||||
| Caudate nucleus | R | 3 | 10 | −2 | 4.03 | |||||||||||||||||||||
| Cerebellar vermis, culmen | R | 6 | −42 | −11 | 4.29 | Cerebellar vermis, culmen | R | 8 | −42 | −11 | 3.82 | |||||||||||||||
| Middle/anterior insula | L | −39 | 3 | −7 | 3.46 | |||||||||||||||||||||
| GTm | L | 21/37 | −66 | −49 | 7 | 3.10 | ||||||||||||||||||||
| GFs | L | 10 | −24 | 57 | 11 | 3.10 | GFd | L | 11 | −17 | 39 | −11 | 3.52 | |||||||||||||
| Subcallosal gyrus | L | 25 | −15 | 12 | −11 | 3.22 | ||||||||||||||||||||
| Caudate nucleus | L | −12 | 8 | 4 | 3.73 | |||||||||||||||||||||
| Lingual gyrus | L | 18 | −15 | −76 | −7 | 3.56 | ||||||||||||||||||||
GFd = diagonal frontal gyrus; GFi = inferior frontal gyrus; GFm = middle frontal gyrus; GFs = superior frontal gyrus; GH = parahippocampal gyrus; GOm = middle occipital gyrus; GTi = inferior temporal gyrus; GTm = middle temporal gyrus; GTs = superior temporal gyrus; LPi = inferior parietal lobule.
Caloric irrigation of the contralesional right ear induced widespread activations only within the right hemisphere. The largest clusters were located in the posterior (Z-score = 4.88) and anterior (Z-score = 4.75) insula and adjacent superior temporal gyrus (BA 22), followed by activations in the inferior/medial frontal gyrus (BA 45/46), caudate nucleus, paramedian thalamus, hippocampus, cerebellar vermis, inferior frontal gyrus (BA 44/45), inferior/medial frontal gyrus (BA 10, 47), inferior and superior parietal lobule (BA 40), superior frontal gyrus (BA 9/10) and medial/superior frontal gyrus (BA 8) (Table 3; Figs 1 and 3).
This activation pattern of the right hemisphere represents the typical one seen in healthy right-handed volunteers when identical methods are used for vestibular stimulation of the right ear only for the right hemisphere (for comparison, see Dieterich et al., 2003). The only activated area within the left hemisphere of the patients was found in the superior frontal gyrus/anterior cingulate gyrus (BA 11). The latter is typically located contralateral to significant activations of multisensory vestibular cortex areas and therefore belongs to the circuitry of vestibular cortical areas (Guldin and Grüsser, 1996) of the right hemisphere. Such a severely reduced activation within the left hemisphere (functionally no activation at all) was not found in healthy volunteers subjected to identical stimulation conditions. Thus, the activation pattern during stimulation of the non-affected right side appeared normal for the right hemisphere, but was completely missing for the affected left hemisphere.
These widespread and strong activations within the right hemisphere allow the further conclusion that the diminished activation patterns during the other stimulation conditions were not caused artificially by too few patients or an inappropriate patient selection; they seem to be reliable results.
Patients with lesions of the right posterolateral thalamus
Warm-water vestibular stimulation of the ipsilesional right ear caused regional blood flow increases of the right hemisphere in the following areas: the medial temporal gyrus adjacent to the medial occipital gyrus (BA 19/37; area MT/V5); the hippocampus/inferior temporal gyrus (BA 34); the amygdaloid body; posterior cingulate gyrus (BA 23/29); the inferior frontal gyrus; and the cerebellum. No activations were found in the insular region of the right hemisphere. Activations of the left hemisphere were located in the posterior insula and retroinsular region, reaching superiorly into the inferior parietal lobule (Z-score = 4.0) and more inferiorly into the putamen, the medial temporal gyrus adjacent to the medial occipital gyrus (BA 37/39; upper parts adjacent to MT/V5), and the superior temporal gyrus (BA 22) (Table 3; Figs 2 and 4). Thus, compared with healthy volunteers (Dieterich et al., 2003), the activation pattern of the right hemisphere was severely reduced, while that of the left hemisphere was moderately reduced, showing some but not all of the multisensory vestibular areas.
Activations in patients with right-sided lesions during caloric irrigation of the ipsilesional right side (A) were located in the posterior insula (transverse temporal gyrus), inferior frontal gyrus, hippocampus and medial temporal gyrus/medial occipital gyrus of the right hemisphere. Activations within the left hemisphere were found in the posterior insula and retroinsular region, putamen, inferior parietal lobule and medial temporal/medial occipital gyri. (B) Calorics of the left unaffected ear led to activations of the posterior and anterior insula, putamen, superior temporal gyrus, inferior frontal gyrus, medial frontal gyrus and medial temporal gyrus within the left hemisphere. In contrast, there were few activations within the right hemisphere, i.e. only in the inferior posterior insula, the midbrain and gyrus rectus.
Caloric irrigation of the contralesional left ear led to activations only of the hippocampus, the inferior posterior insula and the gyrus rectus (BA 11) within the right hemisphere, and to activations of the anterior and posterior insulae and the adjacent inferior frontal gyrus, putamen, subcallosal gyrus (BA 25) and superior temporal gyrus (BA 47/38) of the left hemisphere (Table 3; Figs 2 and 4). Thus, the activation pattern included a few of the areas within the left temporo-insular region known from healthy subjects, but it lacked others in the upper parts of the left temporo-parietal cortex, and was severely reduced within the right hemisphere.
PET: Cortical areas deactivated during caloric irrigation
Patients with lesions of the right posterolateral thalamus
During caloric irrigation of the ipsilesional right ear areas of rCBF decrease (i.e. deactivations) were located in the left lingual gyrus (BA 18/19; Z-score = 4.53) and left cuneus (BA 18), the medial temporal gyrus (BA 21) bilaterally, and the culmen of the right cerebellar vermis (Table 4). Thus, deactivations within the visual cortex did not appear bilaterally, which is also the case in healthy volunteers (Wenzel et al., 1996; Bense et al., 2001), but mainly in the hemisphere contralateral to the lesion.
Signal decreases (Z-score >3.0)
| Thalamic infarction right . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation right (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation left (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Areas . | R/L . | BA . | x . | y . | z . | Z-score . | Areas . | R/L . | BA . | x . | y . | z . | Z-score . | |||||||||||||
| Lingual gyrus | L | 18/19 | −24 | −62 | −2 | 4.53 | ||||||||||||||||||||
| GTm | L | 21 | −46 | 1 | −14 | 4.32 | ||||||||||||||||||||
| Cuneus/precuneus | L | 18 | −1 | −69 | 20 | 3.57 | Precuneus | L | 31 | −12 | −64 | 22 | 3.28 | |||||||||||||
| Posterior cingulate gyrus | L | 29 | −1 | −46 | 4 | 3.57 | ||||||||||||||||||||
| GTs | R | 38 | 44 | 10 | −18 | 3.52 | ||||||||||||||||||||
| GTm | R | 21 | 46 | 10 | −18 | 4.08 | GTm/GOm | R | 19 | 51 | −78 | 11 | 3.33 | |||||||||||||
| GOm | R | 19 | 33 | −91 | 7 | 3.13 | ||||||||||||||||||||
| Cerebellar vermis, culmen | R | 3 | −51 | −7 | 3.87 | Cerebellar vermis, culmen | R | 6 | −51 | −4 | 3.29 | |||||||||||||||
| Thalamic infarction right . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation right (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation left (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Areas . | R/L . | BA . | x . | y . | z . | Z-score . | Areas . | R/L . | BA . | x . | y . | z . | Z-score . | |||||||||||||
| Lingual gyrus | L | 18/19 | −24 | −62 | −2 | 4.53 | ||||||||||||||||||||
| GTm | L | 21 | −46 | 1 | −14 | 4.32 | ||||||||||||||||||||
| Cuneus/precuneus | L | 18 | −1 | −69 | 20 | 3.57 | Precuneus | L | 31 | −12 | −64 | 22 | 3.28 | |||||||||||||
| Posterior cingulate gyrus | L | 29 | −1 | −46 | 4 | 3.57 | ||||||||||||||||||||
| GTs | R | 38 | 44 | 10 | −18 | 3.52 | ||||||||||||||||||||
| GTm | R | 21 | 46 | 10 | −18 | 4.08 | GTm/GOm | R | 19 | 51 | −78 | 11 | 3.33 | |||||||||||||
| GOm | R | 19 | 33 | −91 | 7 | 3.13 | ||||||||||||||||||||
| Cerebellar vermis, culmen | R | 3 | −51 | −7 | 3.87 | Cerebellar vermis, culmen | R | 6 | −51 | −4 | 3.29 | |||||||||||||||
| Thalamic infarction left . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation left (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation right (contralesional) . | . | . | . | . | . | . | |||||||||||||
| GH | R | 28/35 | 17 | −17 | −14 | 3.50 | ||||||||||||||||||||
| GH | R | 35 | 21 | −35 | −7 | 3.48 | ||||||||||||||||||||
| Lingual gyrus | R | 18 | 19 | −71 | −9 | 3.21 | ||||||||||||||||||||
| GOm | R | 19/18 | 44 | −76 | 11 | 3.17 | ||||||||||||||||||||
| GTm | L | 21 | −53 | −46 | −2 | 3.15 | ||||||||||||||||||||
| GTm | L | 39 | −48 | −58 | 11 | 3.12 | ||||||||||||||||||||
| LPi | L | 40 | −48 | −40 | 40 | 3.17 | ||||||||||||||||||||
| LPi | L | 40 | −46 | −31 | 36 | 3.14 | ||||||||||||||||||||
| Parietal white matter | L | −28 | −40 | 45 | 3.20 | |||||||||||||||||||||
| GFi | L | 46 | −39 | 35 | 9 | 4.45 | ||||||||||||||||||||
| GFi | L | 47 | −46 | 37 | −11 | 3.56 | ||||||||||||||||||||
| GFm | L | 10 | −44 | 50 | 7 | 4.53 | ||||||||||||||||||||
| GFm | L | 9 | −29 | 23 | 27 | 3.90 | ||||||||||||||||||||
| GFm | L | 11 | −39 | 32 | −9 | 3.83 | ||||||||||||||||||||
| Frontal white matter | L | −24 | 17 | −7 | 3.61 | |||||||||||||||||||||
| Thalamic infarction left . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation left (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation right (contralesional) . | . | . | . | . | . | . | |||||||||||||
| GH | R | 28/35 | 17 | −17 | −14 | 3.50 | ||||||||||||||||||||
| GH | R | 35 | 21 | −35 | −7 | 3.48 | ||||||||||||||||||||
| Lingual gyrus | R | 18 | 19 | −71 | −9 | 3.21 | ||||||||||||||||||||
| GOm | R | 19/18 | 44 | −76 | 11 | 3.17 | ||||||||||||||||||||
| GTm | L | 21 | −53 | −46 | −2 | 3.15 | ||||||||||||||||||||
| GTm | L | 39 | −48 | −58 | 11 | 3.12 | ||||||||||||||||||||
| LPi | L | 40 | −48 | −40 | 40 | 3.17 | ||||||||||||||||||||
| LPi | L | 40 | −46 | −31 | 36 | 3.14 | ||||||||||||||||||||
| Parietal white matter | L | −28 | −40 | 45 | 3.20 | |||||||||||||||||||||
| GFi | L | 46 | −39 | 35 | 9 | 4.45 | ||||||||||||||||||||
| GFi | L | 47 | −46 | 37 | −11 | 3.56 | ||||||||||||||||||||
| GFm | L | 10 | −44 | 50 | 7 | 4.53 | ||||||||||||||||||||
| GFm | L | 9 | −29 | 23 | 27 | 3.90 | ||||||||||||||||||||
| GFm | L | 11 | −39 | 32 | −9 | 3.83 | ||||||||||||||||||||
| Frontal white matter | L | −24 | 17 | −7 | 3.61 | |||||||||||||||||||||
GFi = inferior frontal gyrus; GFm = middle frontal gyrus; GH = parahippocampal gyrus; GOm = middle occipital gyrus; GTm = middle temporal gyrus; GTs = superior temporal gyrus; LPi = inferior parietal lobule.
Signal decreases (Z-score >3.0)
| Thalamic infarction right . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation right (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation left (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Areas . | R/L . | BA . | x . | y . | z . | Z-score . | Areas . | R/L . | BA . | x . | y . | z . | Z-score . | |||||||||||||
| Lingual gyrus | L | 18/19 | −24 | −62 | −2 | 4.53 | ||||||||||||||||||||
| GTm | L | 21 | −46 | 1 | −14 | 4.32 | ||||||||||||||||||||
| Cuneus/precuneus | L | 18 | −1 | −69 | 20 | 3.57 | Precuneus | L | 31 | −12 | −64 | 22 | 3.28 | |||||||||||||
| Posterior cingulate gyrus | L | 29 | −1 | −46 | 4 | 3.57 | ||||||||||||||||||||
| GTs | R | 38 | 44 | 10 | −18 | 3.52 | ||||||||||||||||||||
| GTm | R | 21 | 46 | 10 | −18 | 4.08 | GTm/GOm | R | 19 | 51 | −78 | 11 | 3.33 | |||||||||||||
| GOm | R | 19 | 33 | −91 | 7 | 3.13 | ||||||||||||||||||||
| Cerebellar vermis, culmen | R | 3 | −51 | −7 | 3.87 | Cerebellar vermis, culmen | R | 6 | −51 | −4 | 3.29 | |||||||||||||||
| Thalamic infarction right . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation right (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation left (contralesional) . | . | . | . | . | . | . | |||||||||||||
| Areas . | R/L . | BA . | x . | y . | z . | Z-score . | Areas . | R/L . | BA . | x . | y . | z . | Z-score . | |||||||||||||
| Lingual gyrus | L | 18/19 | −24 | −62 | −2 | 4.53 | ||||||||||||||||||||
| GTm | L | 21 | −46 | 1 | −14 | 4.32 | ||||||||||||||||||||
| Cuneus/precuneus | L | 18 | −1 | −69 | 20 | 3.57 | Precuneus | L | 31 | −12 | −64 | 22 | 3.28 | |||||||||||||
| Posterior cingulate gyrus | L | 29 | −1 | −46 | 4 | 3.57 | ||||||||||||||||||||
| GTs | R | 38 | 44 | 10 | −18 | 3.52 | ||||||||||||||||||||
| GTm | R | 21 | 46 | 10 | −18 | 4.08 | GTm/GOm | R | 19 | 51 | −78 | 11 | 3.33 | |||||||||||||
| GOm | R | 19 | 33 | −91 | 7 | 3.13 | ||||||||||||||||||||
| Cerebellar vermis, culmen | R | 3 | −51 | −7 | 3.87 | Cerebellar vermis, culmen | R | 6 | −51 | −4 | 3.29 | |||||||||||||||
| Thalamic infarction left . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation left (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation right (contralesional) . | . | . | . | . | . | . | |||||||||||||
| GH | R | 28/35 | 17 | −17 | −14 | 3.50 | ||||||||||||||||||||
| GH | R | 35 | 21 | −35 | −7 | 3.48 | ||||||||||||||||||||
| Lingual gyrus | R | 18 | 19 | −71 | −9 | 3.21 | ||||||||||||||||||||
| GOm | R | 19/18 | 44 | −76 | 11 | 3.17 | ||||||||||||||||||||
| GTm | L | 21 | −53 | −46 | −2 | 3.15 | ||||||||||||||||||||
| GTm | L | 39 | −48 | −58 | 11 | 3.12 | ||||||||||||||||||||
| LPi | L | 40 | −48 | −40 | 40 | 3.17 | ||||||||||||||||||||
| LPi | L | 40 | −46 | −31 | 36 | 3.14 | ||||||||||||||||||||
| Parietal white matter | L | −28 | −40 | 45 | 3.20 | |||||||||||||||||||||
| GFi | L | 46 | −39 | 35 | 9 | 4.45 | ||||||||||||||||||||
| GFi | L | 47 | −46 | 37 | −11 | 3.56 | ||||||||||||||||||||
| GFm | L | 10 | −44 | 50 | 7 | 4.53 | ||||||||||||||||||||
| GFm | L | 9 | −29 | 23 | 27 | 3.90 | ||||||||||||||||||||
| GFm | L | 11 | −39 | 32 | −9 | 3.83 | ||||||||||||||||||||
| Frontal white matter | L | −24 | 17 | −7 | 3.61 | |||||||||||||||||||||
| Thalamic infarction left . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloric irrigation left (ipsilesional) . | . | . | . | . | . | . | Caloric irrigation right (contralesional) . | . | . | . | . | . | . | |||||||||||||
| GH | R | 28/35 | 17 | −17 | −14 | 3.50 | ||||||||||||||||||||
| GH | R | 35 | 21 | −35 | −7 | 3.48 | ||||||||||||||||||||
| Lingual gyrus | R | 18 | 19 | −71 | −9 | 3.21 | ||||||||||||||||||||
| GOm | R | 19/18 | 44 | −76 | 11 | 3.17 | ||||||||||||||||||||
| GTm | L | 21 | −53 | −46 | −2 | 3.15 | ||||||||||||||||||||
| GTm | L | 39 | −48 | −58 | 11 | 3.12 | ||||||||||||||||||||
| LPi | L | 40 | −48 | −40 | 40 | 3.17 | ||||||||||||||||||||
| LPi | L | 40 | −46 | −31 | 36 | 3.14 | ||||||||||||||||||||
| Parietal white matter | L | −28 | −40 | 45 | 3.20 | |||||||||||||||||||||
| GFi | L | 46 | −39 | 35 | 9 | 4.45 | ||||||||||||||||||||
| GFi | L | 47 | −46 | 37 | −11 | 3.56 | ||||||||||||||||||||
| GFm | L | 10 | −44 | 50 | 7 | 4.53 | ||||||||||||||||||||
| GFm | L | 9 | −29 | 23 | 27 | 3.90 | ||||||||||||||||||||
| GFm | L | 11 | −39 | 32 | −9 | 3.83 | ||||||||||||||||||||
| Frontal white matter | L | −24 | 17 | −7 | 3.61 | |||||||||||||||||||||
GFi = inferior frontal gyrus; GFm = middle frontal gyrus; GH = parahippocampal gyrus; GOm = middle occipital gyrus; GTm = middle temporal gyrus; GTs = superior temporal gyrus; LPi = inferior parietal lobule.
Caloric irrigation of the contralesional left ear led to deactivations of the posterior cingulate gyrus (BA 29) and the precuneus (BA 31) of the left hemisphere, whereas more deactivations were seen within the right hemisphere, e.g. in the superior temporal gyrus (BA 38), medial temporal and medial occipital gyrus (BA 19), and culmen of the cerebellar vermis (Table 4).
Patients with lesions of the left posterolateral thalamus
During caloric irrigation of the ipsilesional left ear, deactivations were located in the hippocampus (BA 28/35) and visual cortex of only the contralateral right hemisphere [the hippocampal gyrus, lingual gyrus (BA 18) and medial occipital gyrus (BA 19/18)] (Table 4). Caloric irrigation of the contralesional right ear led to deactivations primarily within the left hemisphere only; they were located in the medial and inferior frontal gyri, caudate nucleus, lingual gyrus, anterior and medial part of the insula, medial temporal gyrus and inferior parietal lobule (Table 4).
Discussion
Vestibular dysfunction in patients with thalamic lesions
We know from patients with acute unilateral thalamic infarctions that only lesions of the posterolateral region cause transient vestibular signs and symptoms such as perceptual deficits with ipsi- or contralateral tilts of the subjective visual vertical and corresponding deviations of stance and gait. However, they cause no ocular motor deficits (Dieterich and Brandt, 1993; 2001). These signs and symptoms of vestibular imbalance are probably identical with the syndrome called earlier ‘thalamic astasia’, a condition of irresistible falls without paresis or sensory or cerebellar signs (Masdeu and Gorelick, 1988). Like these earlier findings of a tonic vestibular deficit in patients with posterolateral infarctions, our patients also presented with ipsi- or contralateral tilts of the SVV and postural imbalance. In addition, seven of eight patients had mild contralateral hemiparesis and one contralateral hemiataxia, signs of which were due to ischaemia or oedema of the adjacent internal capsule, which is regularly seen in combination with the vestibular signs (Barth et al., 2001; Bogousslavsky and Caplan, 2001; Pullicino, 2001). Thus, this combination of signs and symptoms was compatible with a lesion of the vestibular relay station within the posterolateral thalamus, the function of which was described earlier in humans and animals.
Neurophysiological data and tracer studies of the posterolateral thalamus in cats and monkeys describe these subnuclei as a relay station for vestibular cortex areas (Sans et al., 1970; Deecke et al., 1973, 1974; Büttner and Henn, 1976; Büttner and Lang, 1979), since vestibular stimulation of the animals elicited corresponding responses in their neurons. Most ascending vestibular fibres in the subnuclei such as VPLo were in close contact, intermingled with proprioceptive relay cells, and also responded to optokinetic stimuli (Büttner and Lang, 1979); this finding argues for an integration of vestibular and proprioceptive information at the thalamic level. Electrical stimulation of the posterolateral thalamic subnucleus Vim (nucleus ventro-oralis intermedius corresponding to VPLo) in humans elicited a rotation or spinning of the body, head or eyes either counterclockwise (more often) or clockwise (Hassler, 1959; Tasker and Organ, 1971; Tasker et al., 1982). These signs during stimulation of thalamic neurons were in agreement with the human ‘vestibular thalamic deficits’ described earlier in patients with posterolateral thalamic infarctions (Dieterich and Brandt, 1993b) as well as the patients in our current study. However, anatomical differences and divergent nomenclature make it difficult to correlate human and animal thalamic structures directly. Jones (1990) compared monkey and human data and proposed a carefully revised nomenclature for the ventral nuclei, according to which the ‘vestibular’ thalamic nuclei in humans were considered to be the ventral parts of nucleus ventrolateralis, pars posterior (VLp), nucleus ventroposterior lateralis, pars posterior (VPLp) and nucleus ventroposterior inferior (VPI) (for details, see Dieterich and Brandt, 1993b). The preferably affected subnuclei in the current study were the VPLp and dorsal and ventral parts of the VLp.
The data from the current PET study involve various aspects of vestibular connectivity, vestibular–visual interactions and interhemispheric interactions of the cortex (Fig. 5). These are discussed separately as to their anatomy and function below.
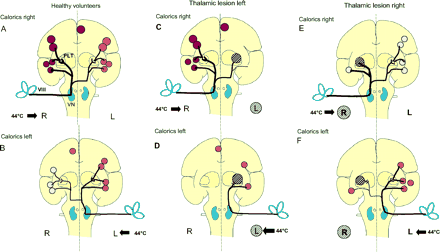
Schematic drawing of hypothetical ascending bilateral vestibular pathways from the vestibular nerve (VIII) via the vestibular nuclei (VN) of the medullary brainstem through the midbrain and posterolateral thalamus (PLT) to the temporo-insular region. Projections from the vestibular nuclei to the PIVC of the posterior insula are known to be stronger on the ipsilateral side (thicker line). Schematic depiction of the most consistent activations in the temporo-parieto-insular regions obtained from our data during caloric irrigation. The schematic coronal section through the insula summarizes all activations in the anterior-posterior direction from the frontal to parietal lobe. These coloured areas represent, from top to bottom, the anterior cingulum contralateral to stimulation, the inferior parietal lobule (BA 40), the posterior insula and retroinsular regions, the superior temporal gyrus (BA 22) and the inferior insula. The intensity of activation is represented by the intensity of the red colour and the size of the coloured areas. (A, B) These areas show, first, the strongest activations on the hemisphere ipsilateral to caloric irrigation (compare top and bottom) and, secondly, a hemispheric dominance of the right non-dominant hemisphere (top). The schematic transmission of vestibular input from the vestibular nerve via the vestibular nuclei to the posterolateral thalamus is based on the assumption that bilateral pathways cross in the upper pontine brainstem and the hypothesis generated by our study data that activation of the inferior insula bypasses the thalamic projection by direct ipsilateral and contralateral pathways. Schematic depiction of bilateral fronto-parietal and insular activations during caloric irrigation in posterolateral thalamic lesions left (C, D) and right (E, F), according to those obtained in healthy volunteers. In patients with a left thalamic lesion, caloric irrigation of the contralesional right ear (C) activated all ipsilateral multisensory vestibular cortex areas within the right hemisphere as depicted in normals (A) and the contralateral anterior cingulum. There were no vestibular cortical activations on the contralateral left hemisphere. During caloric irrigation of the ipsilesional left ear (D), the only activated cortical vestibular area was that of the left inferior insula. Thus, there was no significant activation of vestibular cortex areas contralateral to caloric irrigation in the right hemisphere, despite preserved ascending vestibular pathways on the right side. In patients with right thalamic lesions and ipsilesional caloric irrigation (E), there is a distinct activation of only the inferior part of the insula and a significant activation of the multisensory vestibular cortex areas of the left hemisphere via the preserved contralateral ascending pathways. Irrigation of the contralesional left ear (F) elicited a pattern similar to that of right ear stimulation.
Differential effects of unilateral caloric irrigation in patients with unilateral vestibular thalamic lesions
A unilateral lesion of the vestibular relay station caused the following effects within the multisensory cortical network of both hemispheres involved in the processing of vestibular information during caloric stimulation. Our major findings of the group analyses of patients with right-sided and left-sided thalamic lesions in H2O15-PET were fourfold.
The activation of multisensory vestibular cortex areas was significantly reduced in the ipsilesional hemisphere when the ear ipsilateral to the thalamic lesion was stimulated.
The activation of multisensory vestibular cortex areas of the hemisphere contralateral to the irrigated ipsilesional ear was also diminished but to a lesser extent.
Right hemispheric dominance was preserved in the patients with right-sided and left-sided lesions.
Compared with the deactivation pattern in healthy volunteers during vestibular stimulation, which is always located bilaterally and mainly in the visual and somatosensory cortex areas (Wenzel et al., 1996; Bense et al., 2001), the deactivations in our study were remarkable. They were reduced and predominantly located within only one of the hemispheres, the one contralateral to the stimulated ear and therefore contralateral to the activations, which were mainly ipsilateral to the stimulated ear. Accordingly, posterolateral thalamic lesions in our patients not only affected the patterns of activation in both hemispheres, but also the patterns of simultaneous deactivation.
During irrigation of the ipsilesional ear, the activations of contralateral vestibular cortex areas were most often diminished. Single patients (e.g. BS) even showed no activation of the two hemispheres during vestibular stimulation of the ear ipsilateral to the lesion site (Fig. 2), and caloric nystagmus and perception of rotation were either absent or minimal. Furthermore, the activation within the left hemisphere in patients with left-sided thalamic lesions was significantly diminished when the contralateral right ear was stimulated, although the activation pattern within the right hemisphere appeared normal. The hemispheric asymmetry of the cortical activation pattern during vestibular stimulation of the horizontal semicircular canals, however, was not associated with a significant directional asymmetry of caloric nystagmus or motion perception for the entire group (t-tests not significant). There was only a trend of the caloric nystagmus to be stronger during stimulation of the ear contralateral to the lesion side. Any further functional interpretation should proceed cautiously as only eight patients were involved in this study.
There was a striking contrast between the significant hemispheric differences in the mediation of vestibular input (activations) and the minimal vestibular signs and symptoms of the patients. One explanation could be that the calorically induced vestibular nystagmus appears to be mainly mediated by a subthalamic brainstem VOR circuitry and the vestibular cerebellum rather than by thalamo-cortical structures. This was also reflected by the absence of spontaneous vestibular nystagmus and rotational vertigo in our patients with acute and subacute lesions of the vestibular thalamus. The unaffected perception of body motion may be associated with the bilateral activation of visual motion-sensitive cortical areas in the temporo-occipital cortex (V5), which were largely activated independently of the lesions in both hemispheres (Fig. 3A).
The right hemispheric dominance in right-handers described above was preserved in those with right-sided or left-sided thalamic lesions. The absence of activation in both hemispheres was more pronounced in patients with right-sided than with left-sided lesions. Thus, the data demonstrate both the functional importance of the dominance of ipsilateral ascending pathways and the vestibular dominance of the non-dominant hemisphere (here right hemisphere in right-handers) (Fig. 5). Both factors were shown to be relevant for the processing of vestibular information.
Our data on the patients with posterolateral thalamic lesions fit the concept recently reported for healthy right-handers and left-handers during caloric vestibular stimulation (Dieterich et al., 2003). The importance of ipsilateral projections for the bilateral ascending vestibular pathways was a basic, and especially novel, finding in this lesion study, which further elucidated the role of the projections in normal subjects. If there had been an acute disruption of one projection of the bilateral pathways, theoretically one would expect all information to instead travel immediately via the preserved projection, even if it were the weaker one under normal conditions. However, this did not seem to be the case in our patients.
An exceptional finding was the preserved activation of an area in the inferior part of the insula (Fig. 1b, 4A: Z = −4) which, in normal volunteers, is confluent with the activation of the posterior insula, e.g. the PIVC. This inferior insula was also activated in the hemisphere ipsilateral to the lesion when the ipsilesional ear was irrigated. This supports the assumption that there is a bilateral direct vestibular projection from the vestibular nuclei to the inferior part of the insula, which bypasses the posterolateral thalamus (Fig. 5).
Alternatively, non-thalamic transcallosal interhemispheric transmission might be involved. This is less likely, however, because in such a case one would expect activation of all vestibular cortical areas within the lesioned hemisphere due to their intimate connections as described in the Introduction. The functional significance of this preserved area requires further investigations, especially to determine if it contributes to the relatively mild and transient vestibular symptoms of these patients.
Caloric irrigation in healthy volunteers not only activates vestibular cortex areas but at the same time deactivates visual cortex areas bilaterally (Wenzel et al., 1996; Bense et al., 2001). In the current study, deactivations of visual cortex areas were most often found in only one hemisphere, namely in the hemisphere contralateral to the stimulation and contralateral to activated vestibular cortex areas. Thus, the normal interaction between the two sensory systems, the vestibular and the visual, described earlier as a reciprocal inhibitory interaction (Brandt et al., 1998a), is impaired in our patients. Since the activations were mainly located in one hemisphere ipsilateral to the stimulated ear, the deactivations were located in the other hemisphere contralateral to the activations. One can thus speculate that the inhibitory interaction between the visual and the vestibular systems is organized by pathways that cross the hemispheres and which were preserved in our patients. Further functional interpretations appear premature at this stage.
Particular importance of ipsilateral ascending pathways for vestibular function
Animal studies have identified a network of cortical and subcortical multisensory areas responsible for the processing of vestibular information, which has strong bilateral connections to an ‘inner vestibular circle’, i.e. PIVC, area 2v (7ant), and area 3aV (Akbarian et al., 1993; Guldin and Grüsser, 1996). The vestibular nuclei in the medullary brainstem receive monosynaptic input from all these cortex regions, and their cortico-vestibular projections run bilaterally to the ipsilateral and contralateral vestibular nuclei. Interestingly, the contribution of ipsilateral and contralateral pathways varies for the different cortex areas (Guldin and Grüsser, 1996). The areas of the posterior insular region—PIVC, VTS and granular insula—generally project to and from the ipsilateral vestibular nuclei, whereas the premotor area 6, the somatosensory areas 3aV and 3aH, and the area 2v have more pronounced projections to the contralateral vestibular nuclei (Akbarian et al., 1993; 1994; Guldin et al., 1993). Thus, the ipsilateral projection to and from the PIVC, adjacent VTS, and the granular insula can explain why the side of the stimulated ear is important in healthy volunteers as well as in our patients with posterolateral thalamic lesions (Fig. 5).
Particular importance of handedness for vestibular function
The other highly relevant factor for the processing of vestibular information is the dominance of the non-dominant hemisphere as defined recently in detail in right-handed and left-handed healthy volunteers during identical stimulation conditions (Dieterich et al., 2003). The cortex areas with vestibular input were activated bilaterally in all right-handers, but there was a significant preponderance of the non-dominant right hemisphere. This dominance of the non-dominant hemisphere was even more pronounced in the healthy volunteers with strong left-handedness. A significant right hemispheric dominance for vestibular cortex areas had been assumed earlier in right-handers in the temporo-insular-parietal region with optokinetic stimulation (Dieterich et al., 1998; Brandt and Dieterich, 1999) and vestibular stimulation (Bense et al., 2001; Suzuki et al., 2001; Fasold et al., 2002). Furthermore, in right-handed healthy volunteers who performed a line bisection task with and without galvanic stimulation of the right or left vestibular nerve, the relevant cortical areas for the processing of vestibular information were shown to be the posterior insula bilaterally, but right more than left. The relevant cortical areas for egocentric perception of space and body orientation could be ascribed to the parietal lobule and the prefrontal cortex, both only within the right hemisphere (Fink et al., 2003). Our study on right-handed patients with posterolateral thalamic lesions underlines the dominance of the non-dominant right hemisphere; this dominance is maintained even when damage occurs within the bilateral cortical network at the thalamic level.
Interhemispheric connections
The fact that caloric irrigation of the unaffected right ear of patients with left-sided thalamic lesions led to a ‘normal’ activation pattern within the right hemisphere but no activation within the lesioned left hemisphere, except for the anterior cingulum in the frontal lobe (Fig. 1A), argues for the thalamus being a gate to the whole hemisphere. This finding could mean that there are no interhemispheric connections outside the thalamus between the temporal, insular and parietal cortices or between larger parts of the frontal cortex. However, there is one exception, i.e. the activation of the anterior cingulum in the superior frontal lobe. Activation occurs regularly within the hemisphere contralateral to the stimulated ear (Fig. 3A), even if this hemisphere has the lesioned thalamus (Fig. 1A). This exception can probably be attributed to crossed thalamo-cortical projections to the frontal cortex, which were shown in tracer studies in mice to represent bilateral components of the fronto-thalamic system (Carretta et al., 1996). Furthermore, the anterior cingulum belongs to the circuitry of vestibular cortical areas as described by Guldin and Grüsser (1996) in monkeys.
The only relevant interhemispheric projection in our study appeared between both temporo-occipital areas representing the motion-sensitive area MT/V5, i.e. between parts of the visual cortex bilaterally. During calorics of the right ear of patients with right-sided thalamic lesions, a significant activation occurred within the posterior insula and the parietal lobule within the left hemisphere. This was associated with an activation of temporo-occipital areas (MT/V5) bilaterally but not with an activation of vestibular areas within the ipsilateral right hemisphere (Fig. 4A). The activation of MT/V5 was unexpected, since there was no visual motion stimulation in the patients, who kept their eyes closed in darkness during caloric irrigation. A direct callosal connection between right and left middle temporal/medial superior temporal areas (MT/V5) was assumed (Brandt et al., 1998b), because sustained extrastriate cortical activation of MT/V5 without visual awareness had been found in functional MRI (fMRI) studies of hemianopic patients (Brandt et al., 1998b; Goebel et al., 2001). The bilateral MT/MST activation may be based on direct ipsilateral or transcallosal visual input. Transcallosal connections between areas MT/MST (V5) have been described in monkeys (Maunsell and van Essen, 1987). On the other hand, ipsilateral visual information can bypass V1 along the superior colliculus-pulvinar route and the lateral geniculate body nucleus route to the prestriate cortex as described in monkeys (Fries, 1981; Girard et al., 1992). However, these latter pathways were not involved in our study since there was no visual stimulation during caloric irrigation with the eyes closed. Thus, apart from the transcallosal projection between both temporo-occipital areas, there seem to be no further relevant interhemispheric connections of the vestibular system that could allow for compensation of the posterolateral thalamic lesions.
In a series of studies, we have tried to elucidate the pathways and function of the vestibular system by imaging cortical activity in humans with acute unilateral vestibular lesions at the vestibular nerve (acute vestibular failure by vestibular neuritis: Bense et al., 2004), the vestibular nuclei (Wallenberg's syndrome by medullary infarction: Dieterich et al., 2005) and the posterolateral thalamus level. Further studies are required on strategic lesions of the vestibular cerebellum, the medial longitudinal fasciculus (MLF) and vestibular cortex areas, especially of the PIVC in the posterior insula, in order to construct a more conclusive model of central vestibular disorders and their repair by compensation and substitution over time.
We wish to thank Judy Benson for her critical reading of the manuscript. This work was supported by the Wilhelm Sander-Stiftung and the German Research Foundation (DFG: Di 379/4–2; Br 639/6–2).
References
Akbarian S, Grüsser O-J, Guldin WO. Thalamic connections of the vestibular cortical fields in the squirrel monkey (Saimiri sciureus).
Akbarian S, Grüsser O-J, Guldin WO. Corticofugal projections to the vestibular nuclei in the squirrel monkey: further evidence of multiple cortical vestibular fields.
Akbarian S, Grüsser O-J, Guldin WO. Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the macaque monkey.
Barth A, Bogousslavsky J, Caplan LR. Thalamic infarcts and haemorrhages. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2nd ed. New York: Cambridge University Press;
Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI).
Bense S, Bartenstein P, Lutz S, Stephan T, Schwaiger M, Brandt T, et al. Three determinants of vestibular hemispheric dominance during caloric stimulation.
Bense S, Bartenstein P, Lochmann M, Schlindwein P, Brandt T, Dieterich M. Metabolic changes in vestibular and visual cortices in acute vestibular neuritis.
Brandt T, Dieterich M. The vestibular cortex: its locations, functions, and disorders.
Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction: visual motion stimulation deactivates the parieto-insular vestibular cortex.
Brandt T, Bucher SF, Seelos KC, Dieterich M. Bilateral functional MRI activation of the basal ganglia and middle temporal/medial superior temporal motion-sensitive areas. Optokinetic stimulation in homonymous hemianopia.
Bucher SF, Dieterich M, Wiesmann M, Weiss A, Zink R, Yousry T, et al. Cerebral functional MRI of vestibular, auditory and nociceptive areas during galvanic stimulation.
Büttner U, Henn V. Thalamic unit activity in the alert monkey during natural vestibular stimulation.
Büttner U, Buettner UW. Parietal cortex area 2 V neuronal activity in the alert monkey during natural vestibular and optokinetic stimulation.
Büttner U, Lang W. The vestibulocortical pathway: neurophysiological and anatomical studies in monkey.
Carretta D, Sbriccoli A, Santarelli M, Pinto F, Granato A, Minciacchi D. Crossed thalamo-cortical and cortico-thalamic projections in adult mice.
Deecke L, Schwarz DWF, Fredrickson JM. Single unit recordings in the vestibular thalamus (VPI area) of the macaque. Pflügers Arch
Deecke L, Schwarz DWF, Fredrickson JM. Nucleus ventroposterior inferior (VPI) as the thalamic relay in the rhesus monkey. I. Field potential investigation.
Deecke L, Schwarz DWF, Fredrickson JM. Vestibular responses in the rhesus monkey ventroposterior thalamus. II. Vestibulo-proprioceptive convergence at thalamic neurons.
Dieterich M, Brandt Th. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs.
Dieterich M, Brandt T. Thalamic infarctions: Differential effects on vestibular function in the roll plane (35 patients).
Dieterich M, Brandt T. Vestibular syndromes and vertigo. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2nd ed. New York: Cambridge University Press;
Dieterich M, Bucher SF, Seelos KC, Brandt T. Horizontal or vertical optokinetic stimulation activates visual motion-sensitive, ocular motor, and vestibular cortex areas with right hemispheric dominance: an fMRI study.
Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Brandt T, et al. Dominance for vestbular cortical function in the non-dominant hemisphere.
Dieterich M, Bense S, Stephan T, Schwaiger M, Bartenstein P, Brandt T. Medial vestibular nucleus lesions in Wallenberg's syndrome cause decreased activity of the contralateral vestibular cortex.
Emri M, Kisely M, Lengyel Z, Balkay L, Marian T, Miko L, et al. Cortical projection of peripheral vestibular signaling.
Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, et al. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging.
Faugier-Grimaud S, Ventre J. Anatomic connections of inferior parietal cortex (Area 7) with subcortical structures related to vestibulo-ocular function in a monkey (Macaca fascicularis).
Fink GR, Marshall JC, Weiss PH, Stephan T, Grefkes C, Shah NJ, et al. Performing allocentric visuospatial judgements with induced distortion of the egocentric reference frame: an fMRI study with clinical implications.
Fox PT, Raichle ME. Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography.
Fredrickson JM, Figge U, Scheid P, Kornhuber HH. Vestibular nerve projection to the cerebral cortex of the rhesus monkey.
Fries W. The projection from the lateral geniculate nucleus to the prestriate cortex of the macaque monkey.
Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: the assessment of signal change.
Girard P, Salin PA, Bullier J. Response selectivity of neurons in area MT of the macaque monkey during reversible inactivation of area V1.
Goebel R, Muckli L, Zanella FE, Singer W, Stoerig P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients.
Grüsser OJ, Pause M, Schreiter U. Neuronal responses in the parieto insular vestibular cortex of alert Java monkeys (Macaca fascicularis). In: Roucoux A, Crommelink M, editors. Physiological and pathological aspects of eye movements. The Hague: Dr W Junk Publishers;
Grüsser OJ, Pause M, Schreiter U. Vestibular neurons in the parieto-insular cortex of monkeys (Macaca fascicularis). Visual and neck receptor responses.
Grüsser OJ, Pause M, Schreiter U. Localization and responses of neurons in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis).
Guldin WO, Grüsser OJ. The anatomy of the vestibular cortices of primates. In: Collard M, Jeannerod M, Christen Y, editors. Le cortex vestibulaire. Edition IRVINN. Paris: Ipsen;
Guldin WO, Mirring S, Grüsser O-J. Connections from the neocortex to the vestibular brain stem nuclei in the common marmoset.
Hassler R. Anatomy of the thalamus. In: Schaltenbrand G, Bailey P, editors. Introduction to stereotaxis with an atlas of the human brain. Vol. 1. Stuttgart: Thieme;
Jones EG. Correlation and revised nomenclature of ventral nuclei in the thalamus of human and monkey.
Kosslyn SM, Alpert NM, Thompson WL, Chabris CF, Rauch SL, Anderson AK. Identifying objects seen from different viewpoints. A PET investigation.
Lobel E, Kleine JF, Le Bihan D, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation.
Masdeu JC, Gorelick PB. Thalamic astasia: inability to stand after unilateral thalamic lesions.
Maunsell JH, van Essen DC. Topographic organization of the middle temporal visual area in the macaque monkey: representational biases and the relationship to callosal connections and myeloarchitectonic boundaries.
Minoshima S, Koeppe RA, Mintum MA, Berger KL, Taylor SF, Frey KA. Automated detection of the intercommisural line for stereotactic loaclization of functional brain images.
Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory.
Ödkvist LM, Schwarz DWF, Fredrickson JM, Hassler R. Projection of the vestibular nerve to the area 3a arm field in the squirrel monkey (Saimiri sciureus).
Pullicino P. Lenticulostriate arteries. In: Bogousslavsky J, Caplan LR, editors Stroke syndromes. 2nd ed. New York: Cambridge University Press;
Sans A, Raymond J, Marty R. Response thalamiques et corticales a la stimulation electrique du nerf vestibulaire chez le chat.
Schwarz DWF, Fredrickson JM. Rhesus monkey vestibular cortex: a bimodal primary projection field.
Schwarz DWF, Deecke L, Fredrickson JM. Cortical projection of group I muscle afferents to areas 2, 3a and the vestibular field in the rhesus monkey.
Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogama T, et al. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging.
Tasker RR, Organ LW. Mapping of somatosensory and auditory pathways in upper midbrain and thalamus of man. In: Neurophysiology studied in man. International Congress Series 253. Amsterdam: Excerpta Med;
Tasker RR, Organ LW, Hawrylyshyn PA. The thalamus and midbrain of man. A physiological atlas using electrical stimulation. Springfield (IL): Charles C Thomas;
Van Buren JM, Borke RC. Variations and connections of the human thalamus 2. Variations of the human diencephalon. Berlin: Springer;
Ventre J, Faugier-Grimaud S. Effects of posterior parietal lesions (area 7) on VOR in monkeys.
Ventre-Dominey J, Nighoghossian N, Denise P. Evidence for interacting cortical control of vestibular function and spatial representation in man.
Wenzel R, Bartenstein P, Dieterich M, Danek A, Weindl A, Minoshima S, et al. Deactivation of human visual cortex during involuntary ocular oscillations. A PET activation study.
Author notes
Departments of 1Neurology and 2Nuclear Medicine, Johannes Gutenberg University, Mainz, 3Department of Neurology, Ludwig Maximilians University and 4Department of Nuclear Medicine, Technical University, Munich, Germany