-
PDF
- Split View
-
Views
-
Cite
Cite
Raghavan Raju, Goran Rakocevic, Ziwei Chen, Gerard Hoehn, Cristina Semino-Mora, Wei Shi, Richard Olsen, Marinos C. Dalakas, Autoimmunity to GABAA-receptor-associated protein in stiff-person syndrome, Brain, Volume 129, Issue 12, December 2006, Pages 3270–3276, https://doi.org/10.1093/brain/awl245
Close - Share Icon Share
Abstract
Stiff-person syndrome (SPS) is an autoimmune neurological disorder characterized by autoantibodies to glutamic acid decarboxylase (GAD), the enzyme responsible for the synthesis of inhibitory neurotransmitter GABA. To search for biomarkers that distinguish SPS from other neurological disorders (OND), we used surface enhanced laser desorption/ionization-time of flight (SELDI-TOF) mass spectrometry to obtain proteomic profile of sera from 25 GAD-positive SPS patients and 25 controls. A significant decrease was found in the level of a protein corresponding to GABAA-receptor-associated protein (GABARAP), which is responsible for the stability and surface expression of the GABAA-receptor. Up to 70% of the SPS sera examined, compared with 10% of the controls, immunoprecipitated GABARAP protein. Antibodies raised against GABARAP immunostained neuronal cell bodies as well as axonal and dendritic processes, as visualized by confocal microscopy. In vitro experiments demonstrated that the IgG from GABARAP antibody-positive patients, but not control IgG, significantly inhibited the surface expression of GABAA-receptor. We conclude that GABARAP is a new autoantigen in SPS. Because the patients' IgG inhibits the expression of GABAA-receptors, the circulating antibodies could impair GABAergic pathways and play a role in the clinical symptomatology of SPS patients.
Abbreviations
- GABARAP
GABAA-receptor-associated protein
- GAD
glutamic acid decarboxylase
- SPS
stiff-person sydrome
Introduction
Stiff-person syndrome (SPS) is an autoimmune CNS disorder characterized by rigidity of trunk and proximal limb muscles, intermittent superimposed spasms and heightened sensitivity to external stimuli (Levy et al., 1999; Dalakas et al., 2001b). The disease is also characterized by the presence of high titre autoantibodies against glutamic acid decarboxylase (GAD65), the rate-limiting enzyme for the synthesis of GABA (Kaufman et al., 1986; Solimena et al., 1998). Because GABA is the brain's major inhibitory neurotransmitter, impairment of GABAergic pathways by the autoantibodies may explain the patient's symptomatology. The autoantibodies to GAD65, which are also produced intrathecally, can inhibit the activity of GAD65 (Raju et al., 2005) and impair the synthesis of GABA (Dinkel et al., 1998) resulting in low GABA levels in the brain and CSF (Dalakas et al., 2001b; Levy et al., 2005). However, GAD65, like the two other autoantigens identified in paraneoplastic SPS, amphiphysin and gephyrin (De Camilli et al., 1993; Butler et al., 2000), are cytosolic and the mechanism by which antibodies recognize such intracellular targets is not clear. In SPS, the titres of GAD65 antibodies in the sera or CSF do not correlate with the severity of symptoms and GAD65 antibodies do not transfer the disease from mothers to infants (Rakocevic et al., 2004; Burns et al., 2005). Further, anti-GAD antibodies are not only seen in SPS but also in some patients with cerebellar ataxia, myoclonus, epilepsy and IDDM (Baekkeskov et al., 1990; Nemni et al., 1994; Vianello et al., 2003). At present, high-titre anti-GAD antibodies, as well as amphiphysin or gephyrin, remain excellent markers for SPS but their pathogenic role remains to be established. An autoantibody is however involved, as supported by the passive transfer of some SPS symptomatology from a patient with paraneoplastic SPS to experimental rats (Sommer et al., 2005).
To search for molecules with biological relevance in the sera of SPS patients, we explored the spectrum of proteomic profile using surface enhanced laser desorption/ionization (SELDI), a method that utilizes chromatographic surfaces together with mass spectrometric detection of proteins retained by these surfaces (Rocken et al., 2004). We report that the molecular masses elucidated from the spectra of the studied patients distinguished SPS from the controls on the basis of reduced level of a serum protein identified as GABA-receptor-associated protein (GABARAP). This protein was subsequently demonstrated to be a target autoantigen recognized by IgG antibodies detected in the patients' sera.
Material and methods
Patients
We examined sera from 27 well-characterized SPS patients, evaluated under institutional review board-approved protocols at the Neuromuscular Diseases Section, National Institutes of Health, Bethesda, MD, from 1997 to 2004. All patients fulfilled the following strictly defined clinical criteria: rigidity of limb and axial (trunk) muscles prominent in the abdominal and thoracolumbar paraspinal areas; clinical and electrophysiological evidence of continuous contraction of agonist and antagonist muscles; episodic spasms precipitated by unexpected noises, tactile stimuli or emotional stress; absence of any other neurological basis that caused stiffness and rigidity; and presence of anti-GAD antibodies (Levy et al., 1999; Dalakas et al., 2001b).
Sera from 25 of the 27 SPS patients were tested by SELDI-TOF mass spectrometry and sera from all the 27 patients were tested by western blot. Among 27 studied SPS patients, 19 were women and 8 men, age 30 to 75 (mean 53). Four of them had also epilepsy, one encephalopathy, eight IDDM and five cerebellar ataxia. All SPS patients were treated with GABA-enhancing drugs at various combinations and doses; one of them was receiving intermittent IVIg infusions. All were symptomatic at the time of evaluation. For the SELDI experiments, we used as controls (patients with other neurological disorders; OND) 25 patients, 17 men and 8 women, age 22–82 (mean age 56); these included 10 patients with various myopathies, 2 with cramps, 6 with motor neuron disease, 4 with peripheral neuropathy, 2 with myelopathy and 1 with Parkinsonism. For western blot experiments we used more immune disease controls which included 10 men and 8 women [age 28–78 (mean 51]; 16 of those had acquired inflammatory myopathy (1 with thyroiditis) and 2 others muscular dystrophy. For the western blot experiments we also used sera from five healthy blood donors.
SELDI-TOF spectrometry and database mining
Proteomic profiling of sera was carried out using a Ciphergen SELDI-TOF mass spectrometer (Ciphergen, Freemont, CA). Briefly, serum samples (20 μl) from each patient, were thawed at 4°C, and centrifuged for 10 min at 14 000 g to remove any insoluble material. Specimens were then denatured with 30 μl of 9 M urea/2% CHAPS in a 96-well plate with vigorous shaking for 20 min and samples were diluted with an additional 150 μl of 1 M urea/0.2% CHAPS. Denatured and diluted serum samples (10 μl) were then added into the appropriate binding buffer according to manufacturer's recommendations (100 μl final volume). The samples were applied to either a SAX2, CM10, or IMAC30 ProteinChip array using a 96-well bioprocessor (Ciphergen Biosystems, Inc.) and run in duplicate. Following the addition of samples, ProteinChips were incubated with vigorous shaking for 1 h, washed three times in the appropriate binding buffer, twice with deionized water, and then air-dried for 15 min. Two applications of saturated sinapinic acid (0.5 μl each) prepared in 50% acetonitrile/0.5% trifluoroacetic acid, was added to each spot. A Ciphergen Protein Biological System II (PBSII) equipped with an automated chip loader was used to obtain mass spectra of serum proteins retained on the ProteinChip surfaces. Prior to all chip reading, the performance of the PBSII instrument was evaluated by measuring laser energy output, resolution, and sensitivity, and then calibrated using a broad range of calibrants spanning the user defined range of mass to charge ratio. Obtained spectra from each chip type were processed separately using consistent values for baseline subtraction and filtering, and total ion current normalization. A peak detection algorithm, using modifiable values for signal to noise and peak valley depth, was then employed to select peaks from a set of spectra from the same chip type. A Mann–Whitney non-parametric t-test was then used to compare the mean intensity value of each recognized peak between the controls (n = 25) and the SPS (n = 25) sera. All processing and analysis steps were done using CiphergenExpress server-client software. Molecular masses of statistically significant peaks (P < 0.05) were searched from a protein database using the TagIdent tool (Author Webpage) to generate lists of potentially interesting proteins.
GABARAP protein and anti-GABARAP and other polyclonal antibodies
Full-length GABARAP was expressed in Escherichia coli and purified by glutathione-agarose affinity chromatography (Wang and Olsen, 2000). Polyclonal antibodies to GABARAP were generated in rabbits using affinity purified GST-GABARAP as the antigen. The anti-GABARAP antibodies (E2) showed significant titres at dilutions over 1:100 000 in an ELISA using GST-GABARAP as an antigen, after subtracting absorbance due to binding to GST alone. Polyclonal antibody to KAL-1 protein was generated by immunizing rabbits with a peptide of KAL-1, CSHLKHRHPHHYKPSPERY conjugated to KLH. The bleed was tested for specificity by enzyme-linked immunosorbent assay (ELISA) and in western blot using total human brain (Biochain, Hayward, CA).
Immunoprecipitation and western blot
Aliquots of 15 μl serum from SPS or OND control patients were incubated with 50 μl of protein A-agarose (Pierce, Rockford, IL), washed and incubated with 10 μg rat cerebellum total protein extract. The immunoprecipitates were washed and eluted using gel loading buffer under reducing conditions. The eluates were resolved on a 4–12% Nupage gel, transferred onto nylon membrane and immunoblotted with the E2 antibody at 1:20 000 dilution. Peroxidase conjugated anti-rabbit IgG (Santa-Cruz Biotechnology, CA) was used as secondary antibody at 1:6000 dilution. The proteins were detected by chemiluminescence using ECL reagent (Santa-Cruz Biotechnology, CA). The bands obtained were quantified using the QuantityOne (Bio-Rad, Hercules, CA) software. Each of the sera was tested at least three times and the mean band intensity was used for analysis. One of the SPS sera, which was strongly positive for GABARAP, served as an internal positive control in all the experiments. The intensity of the bands immunoprecipitated by the tested sera was normalized against the average intensity of the positive control.
Immunohistochemistry
Frozen sections (5 μm) of rat cerebellum were fixed with cold acetone for 5 min at room temperature. After washing with Tris buffer (1 M Tris, 5 M NaCl and 925 ml d-H20, pH 7.5), the sections were incubated with blocking solution (2% BSA, 5% normal horse serum, 0.01% Tween-20 and 1% milk) for 45 min. After blocking, the sections were incubated with the E2 polyclonal antibody at 1:250 dilution followed by mouse biotinylated anti-rabbit IgG (1:100) for 30 min and avidin–Texas red (1:100) for 30 min. The immunostatining was examined using confocal microscopy.
Effect of SPS IgG on cultured hippocampal neurons
Low-density hippocampal neurons from E18 Sprague–Dawley rats were prepared by papain-dissociation system (Worthington Biochem) and cultured with neurobasal medium with B27 serum-free supplement with penicillin and streptomycin (Gibco). The cells were plated at a density of 50 000 cells per well on to 12 mm round cover-glasses that had been coated with poly-d-lysine (0.05 mg/ml) and washed with H2O. The neurons were grown in a humidified atmosphere of 5% CO2–95% air at 37°C, and fed every 7 days by exchange of 50% of the medium. Cultured cells were treated with medium alone, with IgG from three OND subjects (20 μg/ml) or with the IgG (20 μg/ml) from two SPS patients for 4 h (optimal incubation time was obtained after several pilot experiments). Subsequently, the cells were fixed, washed with PBS and incubated with a primary antibody against N-terminus of γ2 subunit of GABAA-receptor (1:100) (Chemicon, Temecula, CA; Cat no. AB5954) for 1 h followed by secondary antibody conjugated with Alexo Fluro 488. The density of immunostained neurons and neuronal processes was analysed quantitatively using MetaVue (Universal Imaging). The pixel intensity for each neuron was averaged for each group (n = 16 for cell bodies and n = 35 for processes in each group) and analysed statistically using one-way ANOVA by Prism software. The specificity of autoantibodies to GABARAP was further verified by pre-adsorption experiments. GST-GABARAP-Affi-Gel 10 beads were made by coupling 10 mg GST-GABARAP with 500 μl Affi-Gel 10 beads (Bio-Rad, Hercules, CA; Cat no. 1536099) as per the manufacturer's directions. For each experiment 10 μl of the gel was used for each experiment. The human IgGs were incubated with GST-GABARAP-Affi-Gel 10 beads at 4°C overnight. The beads were spun down and the supernatant was added to cultured hippocampal neurons. The preadsorbed IgGs were incubated with the cultured neurons for 4 h before immunostaining, as described above.
Results
Identification of biomarkers by proteomics
SELDI-TOF mass spectrometry has the flexibility to use different surface matrices (cationic, anionic or metal affinity) allowing the exploration of proteins with specific characteristics to be fractionated prior to laser ionization. In spite of the large number of different proteins present in the neat sera and the preponderant abundance of certain proteins such as albumin and globulin, we were able to identify within the obtained spectra a very small number of protein peaks distinctly different in SPS from the controls. Although an inherent weakness of this technique relates to the sensitivity and molecular masses, the difference of the obtained peaks between SPS and control were so clear that we did not have to use filtering methods to select among the hundreds of differentially expressed proteins.
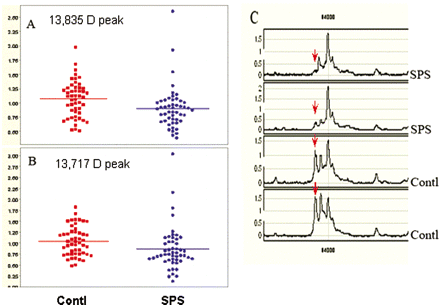
Using three different protein chip surfaces, IMAC (immobilized metal affinity capture), SAX2 (strong anion exchange array) and CM-10 (weak cation exchange array with carboxylate functionality), we resolved a large number of protein peaks in the sera of SPS and OND controls. Univariate analysis comparing the mean intensity of each peak from the SPS group with the OND control group revealed seven peaks among the 290 peaks detected with a statistically significant P-value <0.05. Four of the seven peaks had a molecular size ranging from 2.6 to 3.3 kDa representing peptides of ∼21–28 residues. These sizes were not further analysed as they probably represent proteolysed fragments of larger proteins. Two peaks of molecular masses, 13 835 Da and 13 717 Da were examined from among ∼100 hits. The seventh marker with molecular mass 17 657 Da did not relate to proteins relevant to the disease. The size of two peaks, 13 835 Da and 13 717 Da, were significantly (P < 0.05) lower in SPS compared with the OND controls (Fig. 1A and B). The spectra of two representative SPS and control sera demonstrating decreased abundance of 13 717 Da marker in the SPS patients is shown in Fig. 1C (arrows). Database searches of proteins that show these molecular masses by mass spectra yielded scores of hits in Expasy database. Among the more than fifty proteins that emerged as candidate proteins for the 13 835 molecular mass, only one protein, the GABARAP, had an apparent relationship to the pathobiology of the disease. It was also a rare coincidence to find a paralogue of the same protein, GABARAPL2, to be among a similar list of proteins with 13 717 Da mass, when the same database was parsed. This unexpected finding helped us to focus our further investigations on GABARAP. GABARAP is a polypeptide that interacts with gephyrin and enables the assembly of GABAA-receptor into plasma membrane (Wang and Olsen, 2000; Rocken et al., 2004). We hypothesized that the significantly lower levels of GABARAP in the SPS patients' sera was associated with the presence of autoantibodies against this protein.
Proteomic profiling differentiates SPS from control group (A and B). The mean protein peak intensities of two different molecular sizes one at 13 835 kDa (A) and the other at 13 717 kDa (B) were found to be significantly lower (p < 0.05) in SPS compared to controls. Each spot represents a single patient, each run in duplicate. The mass spectrometric tracing in C shows the two identified protein peaks, 13 835 kDa and 13 717 kDa from two representative SPS patients compared with two controls. The peak at 13 717 kDa is marked with a red arrow.
Immunoprecipitation and western blot
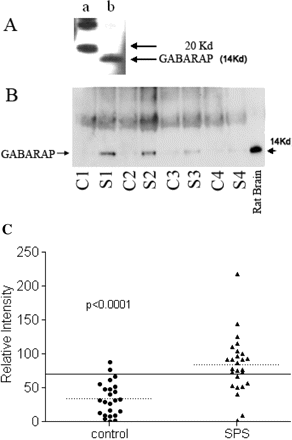
We searched for anti-GABARAP autoantibodies in sera of 27 SPS patients and 24 control subjects using immunoprecipitation and western blot. The immunoprecipitated GABARAP was detected on Western blots using our E2 antibodies, which recognize the 14 kDa GABARAP as shown in Fig. 2A. A polyclonal rabbit antibody against an extracellular matrix protein KAL-1, reported previously (Raju and Dalakas, 2005), that we used as control, did not recognize GABARAP. Each serum was tested three to seven times and the average band intensity was used for comparative analysis, against the strong positive control used consistently throughout all experiments. A large number of sera from the SPS patients and a small number of sera from the controls immunoprecipitated a band of 14 kDa size, detected by anti-GABARAP polyclonal antibody, confirming the presence of autoantibodies to GABARAP in SPS patients (Fig. 2B). A total of 19 of the 27 (70%) SPS sera demonstrated band intensities higher than the cut-off level defined as twice the mean intensity of the controls (Fig. 2C). Among the OND controls, only two patients, one with inclusion body myositis and thyroiditis and another with autoimmune fascitis and goitre, immunoprecipitated GABARAP significantly, but still close to the cut-off level.
(A) Polyclonal antibody specificity to GABARAP. The polyclonal antibody to GABARAP developed in rabbit recognized the protein of expected 14 kDa size (lane b) in a western blot. Lane a represents molecular size markers. (B) SPS sera immunoprecipitate GABARAP. Sera from SPS and controls were used to immunoprecipitate GABARAP from rat cerebellum total protein extract. Western blots show GABARAP bands detected by the polyclonal antibody E2 from four representative SPS patients (S) and four controls (C). Total cerebellum protein was loaded in the last well (rat brain) and immunobloted with E2 antibody. (C) High prevalence of GABARAP antibodies in SPS sera. A total of 27 SPS sera and 19 controls were used to immunoprecipiate GABARAP from rat cerebellar total protein. The immunoprecipitates were resolved and the bands obtained on immunoblots were quantified using QuantityOne software. The reference sera used to normalize the band intensities of all the sera used was assigned an intensity value of 100. The dotted lines represent respective group means and the solid line indicates two times the mean of the control samples. A total of 19 of the 27 SPS samples and 2 of the 19 control samples had GABARAP antibodies above the cut-off (two times the mean of the controls).
Confocal microscopy using anti-GABARAP antibodies and patient sera
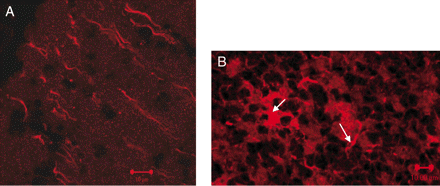
Immunohistochemistry using the anti-GABARAP polyclonal antibody, E2 and examination of the staining by confocal microscopy showed immunolocalization of GABARAP in the axonal processes in the molecular layer (Fig. 3A) as well as on a large number of cells in the granular layer (Fig. 3B). In the granular layer, immunostaining was observed on both cell bodies as well as dendritic processes (Fig. 3B, see arrows). Immunoreactivity of GABARAP was also observed on Purkinje cells. The patients' sera showed a similar pattern (data not shown) but it has been difficult to distinguish the immunoreactivity related to GABARAP from that of GAD.
Confocal microscopy of anti-GABARAP immunoreactivity on cerebellar neurons. Immunostaining of rat cerebellum sections using E2 antibody demonstrates GABARAP immunoreactivity on the axons in the molecular layer (A) as well as neuronal cell bodies and processes (see arrows) in the granular layer (B). Polyclonal rabbit pre-immune serum was used as a negative control.
Effect of SPS IgG on GABAA-receptor assembly
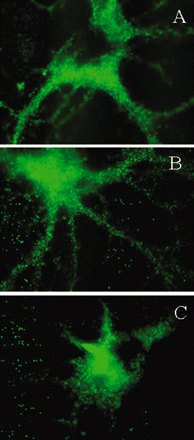
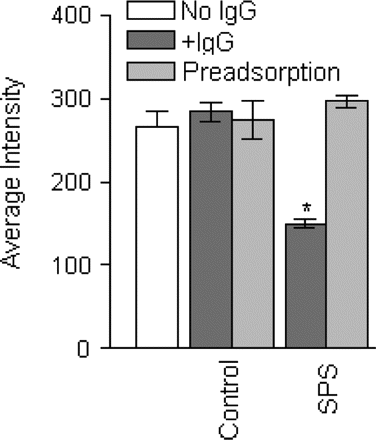
The GABARAP antibody-positive IgG from two SPS patients, compared with controls, significantly downregulated the density of GABAA-receptors on neuronal processes, as assessed by immunolocalization of GABAA-receptor γ2 subunit (Fig. 4A–C). In some neurons, the inhibition of GABAA-receptor expression was so prominent that the processes became almost invisible (seeFig. 4C); the extent of inhibition however varied between the two sera. In contrast, the IgG from the three OND controls had no effect (Fig. 4B). The grey-scale analysis of GABAA-receptor γ2 subunit staining in a total of 35 processes from the SPS IgG-treated neurons and 35 processes from control IgG-treated neurons, demonstrated significant reduction in the neuronal processes only in the SPS IgG-treated cells. When the serum from the two SPS patients or one OND control was preincubated with the GABARAP fusion protein, the preadsobed serum was unable to alter the surface expression of GABAA-receptor γ2 subunit (Fig. 5).
Inhibition of cell-surface expression of GABAA-receptor by SPS sera. Rat hippocampal neurons were treated in vitro with medium alone (A), with control IgG (B) or with an SPS patient IgG (C) for 4 h (magnification ×100). Immunostaining of GABAA-receptor γ2 subunit on representative cell body and neuronal processes demonstrates significant inhibition of the surface expression of GABAA-receptor on neuronal processes (C). Reduction of GABAA-receptor expression was also observed on the cell bodies of some neurons. The IgG purified from an OND subject that did not have detectable GABARAP antibodies, had no effect (B).
Pre-adsorption of GABARAP antibodies abolishes the inhibition of GABAA-receptor by SPS sera. A total of 15 neurons were analysed in each group by grey-scale analysis for GABAA-receptor surface expression. A significant reduction of GABAA-receptor γ2 subunit surface expression was noted in the neurons treated with the IgGs from the SPS patients (*P < 0.0001; dark grey bar, SPS) compared with those treated with cell culture medium (open bar). The neurons treated with the control IgGs (dark grey bar, control) did not show any effect. When the IgG from a control or the SPS patients was preadsorbed with GABARAP-GST fusion protein, the preadsorbed SPS IgG did not inhibit GABAA-receptor surface expression (light grey bar, SPS).
Discussion
GABARAP is a 117 residue polypeptide interacting with gephyrin and enabling the assembly of GABAA-receptor into plasma membrane (Essrich et al., 1998; Kneussel and Betz, 2000). Both gephyrin and GABARAP are microtubule-binding proteins and are believed to be important for clustering and anchoring GABAA-receptors by facilitating binding to the cytoskeleton (Essrich et al., 1998; Wang and Olsen, 2000). GABARAP in vitro interacts with gephyrin and when co-expressed with GABAA-receptors in quail fibroblasts, modulates GABAA-receptor channel kinetics (Essrich et al., 1998; Kneussal and Betz, 2000). GABARAP may play a role in intracellular GABAA-receptor transport but not synaptic anchoring, via its ability to interact with N-ethylmaleimide-sensitive factor, a protein critical for intracellular membrane trafficking events (Chen et al., 2000, 2005; Leil et al., 2004). Absence of GABARAP has been shown to prevent the assembly and surface expression of GABAA-receptors (Chen et al., 2000). Whether the circulating autoantibodies are responsible for the reduced level of GABARAP detected with the mass spectroscopy remains unclear. It is possible that anti-GABARAP antibodies in the serum bind to GABARAP present in the blood and reduce the availability of free protein to bind to the appropriate chromatographic surface on the protein chip. The reduced GABARAP level found in the serum of SPS patients along with the effect of serum IgG to inhibit the expression of GABAA-receptor, support the view that in SPS patients there is impairment of the GABAergic system.
GABARAP plays a critical role in the function of post-synaptic signal reception of GABAergic neurons and as a post-synaptic protein, an immune response to GABARAP is expected to target post-synaptic sites. Unlike gephyrin, the other post-synaptic cellular protein involved in paraneoplastic SPS (Butler et al., 2000), GABARAP is not only expressed within the intracellular compartment but also on the axonal processes (Fig. 3E). Although previous studies had shown localization of GABARAP to the plasma membrane and neuronal processes, it had not been determined whether GABARAP is exposed to the extracellular environment. Our observation that the patient IgG inhibits the surface expression of GABAA-receptor on the neuronal processes suggests that the patients' antibodies are of functional significance. Whether these antibodies in vivo enter the intracellular compartment and inhibit GABARAP, i.e. to interfere with the traffic and surface expression of GABAA-receptor, or act directly on the neuronal processes remains unclear. When the blood–brain barrier is compromised, circulating IgG can be internalized by cerebellar Purkinje cells (Greenlee et al., 1995) suggesting that under certain circumstances an antibody response directed against Purkinje cell antigens may reach their targets. The observation that the patients' IgG caused in vitro inhibition of GABAA-receptors mostly on the processes and to a lesser degree on the cell bodies, is intriguing. This may be related to the gradient of GABAA-receptors across the length of the neuronal processes, the number of antibody molecules in the sera, the affinity of antibodies to GABARAP and the accessibility of intracellular compartments by the GABARAP IgG antibodies.
The prevalence of autoantibodies to GABARAP in SPS is much higher than that observed against amphiphysin or gephyrin, though less than that against GAD65. Whether clinically proven SPS patients without GAD65 antibodies are GABARAP-positive remains unclear as we have not yet tested such patients. Low titres of anti-GABARAP antibodies were also found in some control sera probably related to the normal immune repertoire, as is also observed for various other autoantibodies. In SPS patients however, the anti-GABARAP antibody titres were significantly elevated in up to 70% of the patients with more than twice the mean band intensity of the controls (Fig. 2C). The titre of an autoantibody required to elicit autoimmunity under native conditions remains still unclear.
Among the 17 of the 27 SPS patients with significantly high anti-GABARAP antibodies, five of them also had signs of cerebellar dysfunction. Because GABARAP was prominent in the axonal processes of Purkinje cells and GABA is rich in the cerebellum, the anti-GABARAP antibodies may interfere with the feedback inhibition exerted on these cells by other inhibitory interneurons, resulting in overactivation and abnormal cerebellar output. Whether anti-GABARAP antibodies are also seen in other cerebellar disorders as is the case with anti-GAD antibodies (Nemni et al., 1994; Vianello et al., 2003), remains to be determined. Based on the validated scales of stiffness index (Dalakas et al., 2001a), 12 SPS patients with high anti-GABARAP antibody levels had clinically more severe disease, compared with eight other SPS patients with anti-GABARAP titres less than the cut-off level, suggesting that these antibodies may identify a more severe clinical phenotype. The GABARAP antibody titres were not studied serially to demonstrate if in a given patient the titres correlate with disease severity.
The pathogenic target antigen in SPS remains still elusive. The present observation offers the possibility that GABARAP may be an autoantigen of pathogenic significance because GABARAP-positive IgG can inhibit GABAA-receptor expression on the axonal processes. Such an inhibition may be sufficient to impair the stability of GABAA-receptors and provide a rational explanation for the patients' stiffness and heightened sensitivity owing to dysfunction of inhibitory GABAergic pathways.
This study was supported by the intramural research program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD. The authors also thank all members of the Neuromuscular Diseases Section for helpful discussion and Mr Tom Baginski from the Bioinstrumentation Center, USUHS, Bethesda, MD for technical assistance with confocal microscopy.