-
PDF
- Split View
-
Views
-
Cite
Cite
Justin M. Smith, Precious Lunga, David Story, Neil Harris, Janel Le Belle, Michael F James, John D. Pickard, James W. Fawcett, Inosine promotes recovery of skilled motor function in a model of focal brain injury, Brain, Volume 130, Issue 4, April 2007, Pages 915–925, https://doi.org/10.1093/brain/awl393
Close - Share Icon Share
Abstract
Recovery of function following traumatic brain injury (TBI) is partly through neuronal plasticity. However plasticity is limited in the adult CNS compared with young animals. In order to test whether treatments that enhance CNS plasticity might improve functional recovery after TBI, a new rat head injury model was developed, in which a computer-controlled impactor produced full thickness lesions of the forelimb region of the sensorimotor cortex. Behavioural deficits were seen in several sensorimotor tasks, most of which recovered spontaneously by 21 days. However, skilled paw reaching behaviour, a task that requires corticospinal function, was only ∼40% recovered by 28 days. In order to promote plasticity inosine was infused into the lateral ventricles for 28 days. This treatment produced an almost complete recovery of skilled paw reaching ability, associated with sprouting of the uninjured corticospinal axons across the midline into the territory of the lesioned pathway. In the cervical spinal cord the number of corticospinal axons originating from the uninjured cortex that innervated the contralateral cervical cord was five times that of controls, and in the red nucleus the number of contralaterally projecting axons was four times control values. Inosine treatment did not affect recovery in unskilled behavioural tasks, most of which recovered to normal levels by 28 days without treatment. Animals were placed in an enriched environment as an alternative method to promote plasticity. This resulted in more rapid recovery in several tasks including skilled paw function, but by 28 days normally housed animals had caught up to the same level of improvement.
Introduction
Current clinical care for traumatic brain injury (TBI) is focused on protection of the brain immediately after injury through maintenance of perfusion with oxygenated blood, and then, after the lesion has stabilized, on rehabilitation and physiotherapy (Nortje and Menon, 2004). Plastic changes in the brain have been shown to follow a variety of CNS lesions, and to be associated with recovery of function. After stroke or TBI functional mapping shows that functions that used to be situated in the damaged regions of the cortex may move to other parts of the brain (Cao et al., 1994; Xerri et al., 1998; Chu et al., 2000; Frost et al., 2003). The functions commonly shift to perilesional regions, but where the damage is more extensive they may become more widely distributed over larger distances. The timing of these plastic changes corresponds with functional recovery, and treatments that enhance structural plasticity also promote recovery of function after CNS lesions (Xerri et al., 1998; Raineteau et al., 2001; Bradbury et al., 2002; Bareyre et al., 2004; Papadopoulos et al., 2006; Dancause et al., 2005; Vavrek et al., 2006). A general observation in many parts of the CNS is that there is a critical period shortly after birth during which plasticity is much greater than in adulthood (Lorenz, 1958; Hensch, 2004). The most studied example is ocular dominance plasticity in the visual cortex, which terminates in rats at around 35 days, in humans at around 5 years (Pizzorusso et al., 2002, 2006; Hensch, 2005; Rittenhouse et al., 2006). After this time, the CNS becomes unable to correct for the loss of visual acuity and impaired visual behaviour associated with amblyopia. Digestion of chondroitin sulphate proteoglycans or inhibition of NogoA signalling reactivates ocular dominance plasticity in adult animals (Pizzorusso et al., 2002; McGee et al., 2005), and chondroitinase also promotes recovery from amblyopia (Pizzorusso et al., 2006). Young animals generally show a greater ability than adults to recover motor skills following CNS damage [known as the Kennard principle after Margaret Kennard who first demonstrated greater motor recovery in young compared with older primates (Finger and Wolf, 1988; Carroll et al., 2004)]. These and other observations demonstrate that plasticity is the mechanism behind functional compensation for CNS damage. However, plasticity can be maladaptive, as in children with head injuries in whom the profound re-organization of the cortex that may follow trauma can lead to cognitive deficits (Savage et al., 2005; Salmond et al., 2006). Rehabilitation of patients, particularly those with motor deficits, aims to drive plasticity in useful directions, and this can be enhanced by forcing patients to use affected limbs in constraint-induced movement therapy and other rehabilitation strategies (Mark and Taub, 2004). Similarly in animal models, recovery following stroke can be enhanced by environmental enrichment, and changes in the topography of the cortical motor map may be driven by forced use of limbs (Nudo et al., 1996; Schallert et al., 1997; Jones et al., 1999; Biernaskie and Corbett, 2001; Nudo, 2003; Johansson, 2004).
A potential therapeutic method for improving the function of patients following traumatic brain injury or stroke is to deliver treatments that bring back plasticity to the adult CNS. However, in order to avoid the possible adverse effects of enhancing plasticity globally (Anderson et al., 2005), it will be advantageous to target the treatment to specific brain regions.
In previous studies inosine has been shown to promote axon regeneration and axonal sprouting after CNS damage, and to promote recovery in a stroke model associated with corticospinal tract sprouting (Benowitz et al., 1999; Schallert et al., 2000; Chen et al., 2002; Shen et al., 2006). In the present study we investigated its ability to improve recovery following a focal traumatic brain injury. Our aims were to create a novel model of focal traumatic brain injury involving the forelimb area of the sensorimotor cortex, develop a set of behavioural tests for evaluating functional deficits after injury, then examine the effect of inosine on recovery of function. We show that inosine promotes anatomical sprouting of the corticospinal tract and behavioural recovery in a task that tests corticospinal function in the rat. In addition we show that environmental enrichment can speed recovery in several tasks after focal brain injury.
Material and methods
Surgical procedures
The experimental work was carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and within guidelines of the local animal ethics committee. The experimental design of the controlled cortical impaction (CCI) injury was modified from a previous study (Chen et al., 2003). Male Lister Hooded rats (200–300 g) were anaesthetized with 3% isoflurane in 70% N2O/30% O2 and then maintained with 2% isoflurane during surgery. The head was fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA), and the scalp retracted. A drill-trephine was used to make a 7 mm craniotomy over the right parietal cortex, 0.5 mm posterior to the coronal suture and 3 mm lateral to the sagittal suture while continuously perfusing saline over the area to prevent damage to the underlying brain. Care was taken to avoid injury to the underlying dura when removing the bone flap. Core body temperature was monitored throughout the procedure using a rectal probe and maintained at 37°C with a homeothermic blanket (Harvard Apparatus, Ltd, Edenbridge, Kent, UK).
In a preliminary study we investigated the recovery profile from two different severities of injury. Animals received a CCI injury that was produced using a pneumatic piston with a rounded metal tip, 2.5 mm in diameter. The piston was angled at 22.5° to the vertical so that the tip was perpendicular with the brain surface at the centre of the craniotomy. An impact velocity of either 2 or 3 m/s (n = 5 per group) and a deformation depth below the dura of 2 mm was used. Immediately after injury, the bone flap was replaced and sealed with dental cement, and the animals allowed to recover from anaesthesia. In the second experiment, animals were divided into three surgical groups. In the inosine treatment (n = 9) and vehicle control groups (n = 7), animals received a CCI injury as described above, at 3 m/s velocity and 2 mm travel. Sham-operated animals received a craniotomy as before but no CCI (n = 9). Immediately following CCI, or sham surgery, a 1 mm burr hole was drilled stereotaxically through the contralateral cranium (−0.9 mm from bregma, 1.5 mm laterally from the midline) through which a brain infusion cannula (Brain Infusion Kit II, Alzet, Durect Corporation, Cupertino, CA) was inserted 5 mm below the cranial surface, penetrating into the left cerebral ventricle. Cannulae were held in place using a dental cement (Dyract AP, Dentsply DeTrey, GmbH, Germany) in conjunction with two stainless steel dental screws (Semat International Ltd, Herts, UK) that were screwed partial thickness into the cranium, ∼2 mm anterior and posterior to the infusion cannula. Animals were implanted with two osmotic mini-pumps (Model 2004, Alzet, Durect Corporation, Cupertino, CA, USA) that were joined together using a T-piece, which were placed subcutaneously along the back. Mini-pumps contained either inosine (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany; 10 mM in sterile artificial CSF) as administered to the treatment group, or sterile artificial CSF (Harvard Apparatus Ltd, Edenbridge, Kent, UK) as administered to control and sham-operated groups. Osmotic pumps were then connected to the infusion cannula to give a combined flow rate of 0.5 µl per hour, over a 28-day period. Following the implantation of brain infusion cannula and osmotic pumps, the scalp wound was sutured closed. Rats were placed in a heated cage to maintain body temperature during recovery from anaesthesia. A single injection of Rimadyl (carprofen 5%, Pfizer Animal Health, Tadworth, Surrey, UK; 5 mg/kg dose) was administered i.p. for postoperative analgesia.
In a third experiment, we compared the recovery of skilled forelimb function in animals that were housed in normal cages following surgery compared with those housed in activity cages that contained objects that they could manipulate with their forelimbs (see subsequently). All animals received an impactor injury as described earlier, at 3 m/s velocity and 2 mm travel.
Anatomical studies
Four weeks after the initial CCI injury, all animals from each group were anaesthetized, and the brain infusion needle removed. Following a craniotomy, biotinylated dextran amine (BDA, moleular probes: 10% w/v solution in sterile PBS) was injected stereotaxically at a depth of 1.5 mm at three sites distributed over the sensorimotor cortex contralateral to the injury (Stereotaxic coordinates AP + 2, ML + 3; AP + 0, ML + 2 and AP − 2, ML + 2; 0.5 µl per injection). Two weeks after intraparenchymal injections of BDA, animals were trancardially perfused with PBS–4% paraformaldehyde. Brain tissue was postfixed for 24 h then impregnated with 30% (w/v) sucrose before being frozen and cut coronally at 40 μm. Spinal cord tissue was cut coronally at 40 μm using a vibratome. Free floating brain sections and semi-free floating spinal cord sections were incubated sequentially in TBS plus 0.5% Triton X-100 (three times for 30 min), avidin–biotin–peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories Ltd, UK; 1.5 h), 50 mM Tris–HCl with 0.4% (NH4)2Ni(SO4)2 (10 min) to which 0.015% diaminobenzidine was added plus 20 μl of 20% H2O2 (per 100 μl of incubation solution). Finally sections were rinsed in Tris–HCl (three times for 30 min) before being mounted and counterstained with cresyl violet to visualize anatomical landmarks.
Contusion volumes were estimated for animals in the inosine treatment and control CCI groups. Contusion area was calculated (NIH image software, Ver. 1.6.2) from all cresyl violet-stained sections that contained contused brain and a volume measurement computed by summation of areas multiplied by the interslice distance.
BDA-labelled axons crossing the midline were counted at the level of the red nucleus and in the cervical spinal cord. At the level of the red nucleus the number of BDA-labelled axons that crossed to the denervated side was counted in three coronal sections per case that included the red nuclei. Results are from at least four animals from each group, selected at random. For counting axons in the spinal cord that crossed the midline, the central canal and dorsal median fissure were used as landmarks with the number of labelled axons projecting to the denervated side being counted in five sections per case. Results are from at least four animals from each group.
Environmental enrichment
Following surgery animals were housed in groups of six in an activity cage three stories high that contained a running wheel, ladders, climbing ropes and balls. Food requiring digital manipulation was provided in the form of seeds and pasta. Control animals were housed in standard plastic boxes and fed with animal chow pellets in the roof container.
Behavioural tests assessment of sensorimotor function
In the preliminary study, sensorimotor function was assessed over a 25-day period, using the staircase test, tapered beam and ladder walking tasks. An appropriate period of training was given in the staircase test to ensure that the animals had achieved a plateau in their ability to perform the task before surgery was carried out. For the other behavioural tasks, animals were habituated with the apparatus prior to initial behavioural assessment.
In the second study, behavioural tests included the staircase test, limb-use asymmetry (cylinder) test, tapered beam and ladder walking tests. Any animal demonstrating a significant bias in fore-paw use (asymmetry) prior to surgery was eliminated from the study. Following surgery, behavioural tests were carried out at 3, 7, 14, 21 and 28 days.
In the third study, skilled paw reaching (staircase), beam and ladder walking tests were used to examining the effects of housing animals in activity cages versus normal cages at 4, 7, 9, 11, 16, 18, 21, 23 and 25 days post-injury.
‘The staircase test’ (Montoya et al., 1991) provides an objective measure of skilled forepaw use by assessing maximum forepaw extension and grasping ability. In this test rats were placed in a Perspex box in which a baited double staircase was presented bilaterally (Campden Instruments Ltd, Leicester, UK). Each staircase consisted of seven graded stages or ‘stairs’, into which a well of 8 mm depth had been drilled, with increasing distance from the rat and therefore increasing reaching difficulty; each well was baited with two food pellets (Noyes Rodent Precision Pellets, Research Diets Inc., NJ, USA). Throughout the experiment, rats were fed once a day with 15 g of standard rat chow, always being fed once testing had been completed. On testing days, rats were tested in the staircase box for a period of 15 min each. After each test, the numbers of pellets successfully grasped and eaten using each forepaw were counted. Additionally, the number of displaced pellets and maximum distance reached were determined. Asymmetry scores were calculated by subtracting the number of pellets retrieved by the affected (left) limb from those retrieved by the right. Animals showing a significant asymmetry of paw use in this task during initial training were not used for further studies.
Limb-use asymmetry test (cylinder)
To assess forelimb use during an explorative activity rats were placed in a transparent Perspex cylinder (20 cm diameter and 30 cm height). By virtue of its shape the cylinder encourages rearing activity, i.e. vertical exploration of the walls with the forelimbs.
To determine forelimb-use asymmetry, independent use of the left or right forelimb versus the simultaneous use of both forepaws was assessed for: (i) the initial contact of the cylinder wall during a full rear; (ii) all weight bearing lateral movements along the wall. Limb use was assessed during over 10 independent rears. If there was doubt when determining whether one limb was being used independently or simultaneously, that movement was not scored. This test was run under red light conditions.
Ladder and beam tests
Horizontal ladder and tapered beam tests were run on an illuminated glass walkway which allowed for unambiguous detection of paw-placement errors (Vrinten and Hamers, 2003). A mirror placed at 45° beneath the glass walkway, allowed the tests to be video-recorded for subsequent analysis. The ladder test consisted of a 115 cm long horizontal ladder, with a distance of 3.5 cm between successive rungs. The ladder was placed at a height of 3 cm above the glass walkway. Sugar pellets were placed as a reward in a blacked out box at one end of the apparatus. Animals were then run across the apparatus three times per testing session with the number of paw-placement errors quantified each time from video tape. The tapered beam test was run in a similar way; the 115 cm long beam tapered from a maximum width of 3 cm to a minimum width of 1.5 cm.
All behavioural scores were evaluated using a two-way repeated measure ANOVA to detect significant differences between and among treatment groups; Student–Newman–Keuls post hoc analysis was used to determine where those differences occurred (SigmaStat 3.11, Systat Software Inc.).
Results
Impact parameters
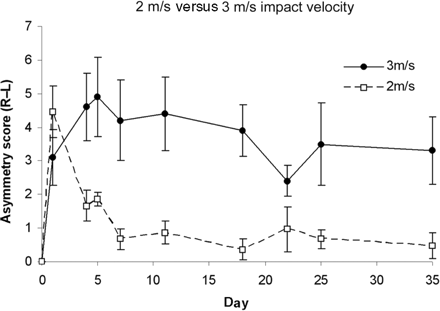
Preliminary data were gathered in order to determine the most appropriate impact parameters for studying functional recovery following CCI. A 2 m/s velocity and 2 mm excursion impact produced a short lived behavioural deficit following which animals regained complete function by day 5 following injury. Histological damage was confined to cortical grey matter with sub cortical white matter being left intact. In comparison, an impact velocity of 3 m/s and excursion of 2 mm produced a sustained behavioural deficit (Fig. 1). A previous study demonstrated that a 4 m/s impact produced a considerable contusion cavity with damage also evident in the hippocampus and thalamus (Chen et al., 2003). It was therefore decided to conduct behavioural experiments using an impact velocity of 3 m/s.
Recovery of skilled forepaw use after differing severities of injury. Animals receiving a CCI of 2 m/s velocity and 2 mm depth demonstrated a short-lived behavioural deficit as measured using the staircase (pellet retrieval) test, in which almost complete function was regained by day 5 following injury. In comparison, animals receiving a CCI of 3 m/s velocity and 2 mm depth demonstrate a more sustained behavioural deficit lasting beyond day 35 after injury.
Contusion volume
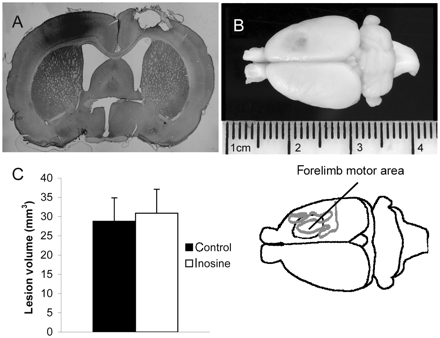
The 3 m/s impaction velocity used in this study produced a full thickness lesion to the cortex, with damage extending part way through the subcortical white matter, but leaving the hypothalamus intact (Fig. 2A and B). Lesion position was checked at the end of the experiment, when preparing tissue for histology. Three animals were excluded from the study on the basis of incorrect lesion location, the lesions being >2 mm from the correct location. Measurement of contusion volume revealed no significant differences (P ≥ 0.59) between inosine-treated animals (30.9 ± 6.22 mm3; mean ± SEM) and controls (28.7 ± 6.01 mm3; Fig. 2C).
Lesion characteristics and size. (A) Coronal section through rat brain (×5 magnification) showing the characteristics of a typical lesion produced by an impaction injury of 3 m/s velocity and 2 mm impaction depth. The damage to the corticex is full thickness, with partial damage to underlying subcortical white matter. There is an injection of BDA to the left cortex. (B) Photograph of a rat brain showing a typical lesion over the right motor cortex, affecting fore-limb use. Underneath is a line drawing showing the approximate position of the forelimb sensorimotor cortex relative to the lesion. Much of the sensorimotor activity is on the lateral aspect of the cortex. (C) Average lesion volumes for control and inosine-treated animals are not significantly different (P = 0.59).
Inosine promotes sprouting of uninjured corticospinal tract
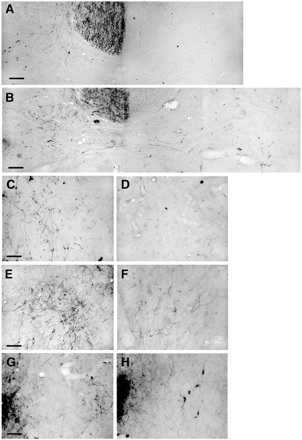
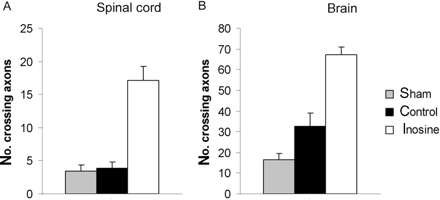
BDA was injected to label axons originating from the forelimb region of the uninjured sensorimotor cortex by injecting it into the left sensorimotor cortex (Fig. 2A) at the end of the behavioural testing period (i.e. at 4 weeks post TBI). Two weeks were allowed for the BDA to be transported to distal axons before the animals were prepared for histology. The corticospinal tract was strongly labelled contralateral to the injection site, with some axons crossing the midline to innervate structures ipsilateral to the injection. These BDA-labelled fibres crossing the midline were counted in the cervical spinal cord and from the intact to the denervated red nucleus. Results from the cervical spinal cord demonstrated that sham and control animals had few crossing axons. Animals receiving an infusion of inosine showed a marked increase in the number of BDA-labelled axons crossing the midline (Fig. 3A–F). This could be seen at all levels of the cervical spinal cord with a 4–5-fold increase in the number of crossing axons compared with sham or control animals (Student's t-test, control versus treatment, P = 0.004, Fig. 4).
Pictures of anterograde BDA tracing from the cortex contralateral to the injury. (A) The cervical spinal cord from an animal that received an injury and an infusion of artificial CSF. Axons are seen in the corticospinal tract and branching into the ipsilateral grey matter, but very few cross the midline. (B) A similar view of an animal treated with inosine. There is a heavy projection to the ipsilateral grey matter, and axons can also be seen crossing the midline and throughout the contralateral grey matter. C and D show high power pictures from the ipsilateral and contralateral grey matter of the cord from an animal that received a control infusion, while E and F show equivalent views from an animal that received inosine. (G) The red nucleus of an animal that received inosine, showing axons crossing the midline and forming neuropil in the contralateral red nucleus on the right of the picture. (H) A control animal with fewer red nucleus axons crossing the midline and no contralateral neuropil. Bars 50 µm in A, B, G, H and 25 µm in C–F.
Axon sprouting in the cervical spinal cord and red nucleus. (A) Quantification of BDA-labelled axons projecting to the denervated side of the cervical spinal cord (average from four sections per case). Animals receiving inosine treatment showed a marked increase in the number of BDA-labelled axons crossing the midline compared with sham or control animals (Student's t-test, control versus treatment, P ≤ 0.01). (B) Quantification of axons crossing from the uninjured side of the brain to the denervated red nucleus (averaged from three sections per case). Inosine treatment increased the number of crossing axons by an additional 2-fold over the level seen in the control group. (Student's t-test, P ≤ 0.001).
Data from the location of the red nucleus showed the number of crossing fibres in sham animals to be low, but greater after CCI, even in animals treated with vehicle alone (artificial CSF). Inosine treatment increased the number of crossing axons 4–5-fold above the number seen in sham animals (Student's t-test, P ≤ 0.0001), i.e. an additional 2-fold over the level seen in injured animals receiving artificial CSF (Student's t-test, P = 0.0008) (Figs 3G–H and 4).
Inosine improves recovery in a test of corticospinal function
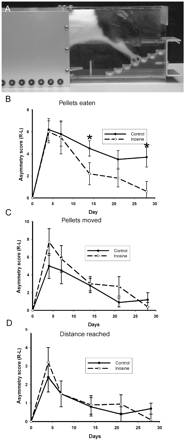
We assessed skilled forelimb use in rats through use of a paw-reaching task (Fig. 5A). Previous data have shown that lesions to the corticospinal tract greatly degrade performance in this task (Li et al., 1998; Farr and Whishaw, 2002; Piecharka et al., 2005). The number of pellets successfully retrieved is a measure of skilled limb use. However, animals with ‘clumsy’ limbs can still displace pellets from the wells, but not pick them up. Pellets displaced and maximum distance reached therefore give measures of unskilled limb control. As demonstrated by the staircase test, animals showed a marked reduction in their ability to retrieve pellets with the affected (left) forepaw contralateral to the lesion in the initial days after CCI injury (Fig. 5B). Skilled forepaw ability showed some improvement over time, even in the absence of inosine treatment. Thus control animals treated with artificial CSF showed a 40.3% improvement in asymmetry of pellet retrieval by day 28 post-injury. In contrast, inosine treated animals showed significantly improved recovery by day 28 compared with controls (P = 0.037), achieving an improvement in recovery of 89.1%. Two-way repeated measures ANOVA showed the inosine treatment group to be overall statistically different from the control group (P ≤ 0.05), and post hoc analysis showed that the 15- and 28-day differences were significant. Sham animals showed an initial deficit in left forepaw use following surgery, which fully recovered by 7 days.
Forepaw reaching following cortical injury. (A) A side view of a rat in the paw-reaching apparatus. The right forearm is stretching to reach a sugar pellet in the fourth stair. (B) Pellets retrieved (eaten) provides a measure of skilled forelimb use. Control animals show a sustained deficit. Animals treated with inosine showed significantly improved recovery by day 28 compared with controls (P ≤ 0.05), post hoc analysis showed significant effects at 14 and 21 days. (C) Pellets displaced and (D) maximum distance reached provides measures of unskilled limb control. Both tests show similar reductions in initial unskilled use of the denervated forepaw and reveal a similar time course and extent of recovery between inosine-treated animals and controls.
Similar reductions in the initial unskilled use of the denervated forepaw were observed when examining ‘pellets moved’ (Fig. 5C) and ‘maximum distance reached’ (Fig. 5D). However, both tests revealed a similar time-course and extent of recovery between inosine-treated animals and controls. Repeated measures ANOVA did not reveal any overall effect of inosine treatment (pellets moved, P = 0.407; distance reached, P = 0.762). Therefore, inosine treatment produced marked recovery in skilled limb behaviour, while unskilled limb movements were unaffected by treatment.
Other motor tests
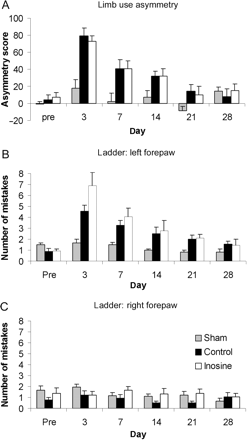
To further assess motor function, the cylinder and ladder tests, which have been widely used in experimental models of stroke, were used. In the cylinder task, primarily a test of neglect, animals showed initial asymmetry (Fig. 6A). This recovered to almost baseline levels in control and inosine-treated animals over the 28-day experimental time course. No significant differences were observed between groups (P = 0.86).
Limb use asymmetry and sensorimotor function tests. (A) Animals showed an initial deficit in left forepaw use in the cylinder test, as indicated by an increase in asymmetry score following CCI. This recovered spontaneously to near baseline levels in both control and inosine-treated animals by 28 days, with no significant group effect being found (P = 0.86). (B) To assess sensorimotor function the ladder test was used. This was scored as the total number of mistakes made by left fore- and hind-limbs and right fore- and hind-limbs. Animals demonstrated a deficit in left-sided limb use immediately after injury which recovered to normal levels by 21 days after contusion. No differences in recovery were observed between treated and untreated groups (P = 0.19). (C) Right-sided limb use in the ladder test was completely unaffected by CCI.
The ladder test, which is designed to assess sensory–motor function, particularly local spinal reflex control of limb movement, showed that animals had a deficit immediately after injury (Fig. 6B and C). This generally recovered to normal levels by 21 days after contusion. There were no differences in recovery rated between treated and untreated groups (P = 0.19).
The effects of environmental enrichment on skilled forepaw function
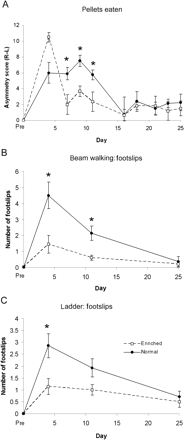
Environmental enrichment has been shown to drive functional recovery after various CNS lesions. Intense physiotherapy is used in human patients after stroke affecting upper limb function, in order to optimize hand use. We therefore compared recovery of skilled forelimb function in animals that were kept in normal cages without objects that they could manipulate with their forelimbs, with animals kept multiply in activity cages which included ladders, a treadmill, climbing ropes and small food objects. In the skilled paw reaching, beam and ladder tasks animals kept in activity cages showed a more rapid recovery than those kept in unenriched conditions, but there was no difference in the recovery levels by 28 days, at which time the animals kept in unenriched conditions had caught up (Fig. 7).
Effects of environmental enrichment on recovery of forelimb function. Skilled forepaw reaching as evaluated using the staircase test (A), and sensorimotor function as measured using the beam walking (B) and ladder (C) tests show a more rapid recovery in animals housed in activity cages (enriched conditions). However, by day 28, no differences in forepaw function can be observed between animals housed in enriched versus normal conditions.
Discussion
Using a computer-controlled impactor, we have been able to make a reproducible lesion to the forelimb region of the rat sensorimotor cortex. This lesion produces a deficit which can be measured using a number of behavioural tasks. The animals showed considerable spontaneous recovery in most tasks, but retained a sustained deficit in skilled forepaw function which has been shown in previous studies to be a good measure of corticospinal function.
Cortical injury size correlates with behavioural deficits
The speed of impact and the travel of our impactor device can be varied, leading to reproducible changes in the size and depth of the cortical lesions. The lesions in our experiments were restricted to the cortex with little subcortical damage. We found that lesions that only affect the superficial layers produced a short-lived behavioural deficit which recovered in 7 days or less. However full thickness lesions of the cortex produced long-lasting behavioural deficits, particularly in skilled forelimb function, which did not fully recover in 28 days.
Injury to the cortex produces behavioural deficits with different recovery rates
We assessed a variety of behavioural tasks for monitoring the effects of the cortical lesions, and rate of recovery. We were particularly interested in tasks that would demonstrate activity in the corticospinal tract, since in humans this tract is the major controller of spinal motor action. In rodents the corticospinal tract is small relative to humans, and many of its axons connect to the cervical cord. We therefore focused on tests of forelimb function, which are particularly affected by corticospinal lesions in rats (Whishaw et al., 1998; Piecharka et al., 2005). There were deficits in many tests following cortical lesions, including beam walking, grid walking, cylinder rearing and skilled forepaw reaching. In our initial experiments we found that there was very rapid recovery in some tasks, particularly walking over a narrow raised beam. However, recovery in this task and some other tasks appeared to be because animals were learning to carry out the task despite their motor deficit because of fear of falling. We therefore changed our assessment methods to tasks in which animals did not feel in danger. We substituted the raised beam with a tapered beam resting on a flat glass surface, so that when animals’ paws missed the beam they were supported by the underlying surface (Zhao et al., 2005a). Animals showed longer lasting deficits in this task, presumably because they were not trying to circumvent it. When we had excluded tasks that animals were circumventing, most tasks showed that recovery of function started by 7 days and was largely finished by 14–21 days. However the animals did not show complete recovery in all tasks over the timescale of our experiments. In particular they did not regain their previous ability at skilled forepaw function. This indicates that the sensorimotor cortex is involved in the performance of this task, and that the spontaneous level of plasticity in the adult rat CNS is insufficient to compensate for the loss of its function.
Effects of enriched environment on recovery rates
There are many examples of enhanced or accelerated neurological recovery resulting from environmental enrichment, and intense behavioural treatment such as constraint-induced movement therapy for arm function which is used successfully to treat human patients after stroke (Grotta et al., 2004; Mark and Taub, 2004). We compared recovery rates in animals kept in standard cages with recovery of animals kept in groups of six in a multi-level cage with a running wheel, ladders and other features. These animals showed faster recovery of function in the skilled paw reaching, ladder and beam walking tasks, although in this model the control animals caught up in all the tests by 28 days. Previous investigators have investigated the mechanism by which behavioural enhancement can promote recovery, and have shown enhanced neurogenesis, increased dendritic sprouting, axonal sprouting and remapping of the cortical motor map (Nudo et al., 1996; Jones et al., 1999; Van et al., 1999; Biernaskie and Corbett, 2001; Kleim et al., 2003; Johansson, 2004; Zhao et al., 2005b).
Inosine promotes sprouting of the corticospinal tract
Inosine acts through a direct intracellular mechanism to stimulate axon growth in several types of neurons (Benowitz et al., 1998; Zurn et al., 1988; Irwin et al., 2006). Recent studies show that its target is Mst3b, a signal transduction kinase that also mediates axon outgrowth in response to trophic factors (Irwin et al., 2006). It has been shown to promote axon regeneration, sprouting of the corticospinal tract and behavioural recovery after stroke and spinal cord injury in rats (Chen et al., 2002). In our experiments we infused inosine continuously over 28 days into the lateral ventricle contralateral to the cortical lesion. Anatomical tracing of the unlesioned contralateral corticospinal tract revealed extensive sprouting into the territory of the lesioned tract at the level of the red nucleus and spinal cord compared with saline-infused controls. This widespread sprouting response suggests that the inosine diffuses throughout the brain and spinal cord. A similar sprouting response was seen following inosine treatment in a stroke model (Chen et al., 2002).
Inosine produces functional recovery in skilled paw control
The behavioural recovery produced by inosine was very specific. In our experiments we focussed particularly on forelimb behaviour, and were particularly interested in skilled forepaw control because previous experiments have indicated that the corticospinal tract is involved, and because we found that this behaviour showed only partial spontaneous recovery after sensorimotor cortex lesions. In the staircase forepaw reaching task it is possible to distinguish between fine forepaw control and gross limb movement (Montoya et al., 1991; Whishaw et al., 1997). Fine forepaw control is required for animals to pick-up sugar pellets and put them in their mouth. The food wells are small and deep, and examination of videos of animals retrieving pellets shows them using their digits to winkle the pellets out of wells and pick them up. When animals have recovered gross forelimb movement but lack fine digit control their determined attempts to grab the pellets can lead to them being flicked out of the wells, but the animals are seldom able to grasp them and bring them to their mouths. The number of pellets successfully eaten is therefore a good measure of skilled paw function, the number of wells cleared of pellets or the maximum distance reached are a measure of gross limb function, and the ratio of pellets retrieved to pellets removed from well is a measure of whether paw function is skilled or clumsy. After cortical lesions there was an initial decrease in both skilled and gross forelimb function. However gross limb movement largely recovered in both treated and control animals. Skilled paw use continued to show a considerable deficit in control animals, but recovered almost to normal in inosine-treated animals. We consider that the anatomical sprouting of the corticospinal tract that we observed in inosine-treated animals and their recovery of skilled paw function are likely to be related, providing further evidence that skilled paw use is a useful measure of corticospinal control in rodents. Sprouting in other pathways could also have contributed.
Plasticity-inducing treatments in the treatment of CNS damage
Many imaging, physiological and anatomical studies have demonstrated plastic changes and axonal sprouting in the damaged CNS (Darian-Smith and Gilbert, 1994; Stroemer et al., 1995; Benowitz et al., 1999; Chu et al., 2000; Chen et al., 2002). These changes have been linked to the partial return of function that generally follows CNS damage. There are several indications that enhanced plasticity in the adult CNS can lead to greater degrees of recovery. Several treatments have been shown to promote sprouting and plasticity, including chondroitinase (Bradbury et al., 2002; Caggiano et al., 2005), antibodies to NogoA (Buchli and Schwab, 2005) and in some situations neurotrophins (Lynskey et al., 2006). The present and previous studies (Benowitz et al., 1999; Chen et al., 2002; Shen et al., 2006) show that inosine also promotes plasticity. Applications of these treatments to the injured CNS, particularly spinal cord injury and stroke models, have been shown to promote recovery of function (Grill et al., 1997; Bradbury et al., 2002; Schwab, 2002; Wiessner et al., 2003; Hagg et al., 2005). The various experiments with plasticity-inducing treatments have not fully addressed the optimum timing of treatments and have not identified the part of the CNS in which they have their greatest effect. The most effective site of action could be in the brain, the spinal cord or both. Lesions of the motor cortex remove corticospinal innervation to part of the spinal cord, and it is probable that an effective way to bring back lost function is to promote sprouting of the remaining corticospinal axons in the spinal cord. Plasticity within the indirect motor pathways at various levels of the CNS may also promote recovery. Inosine infused into the lateral ventricles is probably transported into the spinal canal, as indicated by its effects on corticospinal sprouting that we and a previous study (Chen et al., 2002) observed within the cord. It is also possible that sprouting in the cord was a consequence of inosine effects in neuronal cell bodies in the brain. As discussed earlier, promotion of plasticity in the cortex is an important mechanism in functional recovery, and sprouting of connections and dendrites is seen in the cortex after injury (Schallert et al., 1997; Dancause et al., 2005). In order to assess the importance of plasticity in different parts of the CNS it will be useful in the future to apply treatments to localized areas in various injury models. It will also be important to determine the therapeutic time window for treatments that promote plasticity. In principle a treatment that promotes plasticity could be of benefit even long after the initial lesion. Experiments on plasticity in the damaged non-human primate CNS have demonstrated that plastic changes in central projections need to be driven by behaviours to which the animal is devoting attention (Jenkins et al., 1990; Byl et al., 1996; Liu and Rouiller, 1999; Dancause et al., 2005; Barbay et al., 2006). It is probable that in human patients the most effective use of plasticity-inducing treatments will be to open a window during which intensive rehabilitation can drive plasticity in useful directions (Nudo, 2003; Hummel and Cohen, 2005).
Acknowledgements
This work was funded by grants from GlaxoSmithKline, the Medical Research Council, the Wellcome Trust, European Union Network of Excellence NeuroNE and the Henry Smith Charity. The authors are grateful for expert technical assistance from Jill Shaw.
References
Abbreviations:
Author notes
*These authors contributed equally to this work.