-
PDF
- Split View
-
Views
-
Cite
Cite
Fardad T. Afshari, Jessica C. Kwok, Melissa R. Andrews, Bas Blits, Keith R. Martin, Andreas Faissner, Charles Ffrench-Constant, James W. Fawcett, Integrin activation or alpha9 expression allows retinal pigmented epithelial cell adhesion on Bruch’s membrane in wet age-related macular degeneration, Brain, Volume 133, Issue 2, February 2010, Pages 448–464, https://doi.org/10.1093/brain/awp319
Close - Share Icon Share
Abstract
Retinal pigment epithelial cell malfunction is a causative feature of age-related macular degeneration, and transplantation of new retinal pigment epithelial cells is an attractive strategy to prevent further progression and visual loss. However, transplants have shown limited efficacy, mainly because transplanted cells fail to adhere and migrate onto pathological Bruch’s membrane. Adhesion to Bruch’s membrane is integrin-mediated. Ageing of Bruch’s membrane leads to a decline in integrin ligands and, added to this, wet age-related macular degeneration leads to upregulation of anti-adhesive molecules such as tenascin-C. We have therefore investigated whether manipulation of integrin function in retinal pigment epithelial cells can restore their adhesion and migration on wet age-related macular degeneration-damaged Bruch’s membrane. Using spontaneously immortalized human retinal pigment epithelial cells (adult retinal pigment epithelium-19), we show that adhesion and migration on the Bruch’s membrane components is integrin-dependent and enhanced by integrin-activating agents manganese and TS2/16. These allowed cells to adhere and migrate on low concentrations of ligand, as would be found in aged Bruch’s membrane. We next developed a method for stripping cells from Bruch’s membrane so that adhesion and migration assays can be performed on its surface. Integrin activation had a moderate effect on enhancing retinal pigmented epithelial cell adhesion and migration on normal human and rat Bruch’s membrane. However, on Bruch’s membrane prepared from human wet age-related macular degeneration-affected eyes, adhesion was lower and integrin activation had a much greater effect. A candidate molecule for preventing retinal pigmented epithelial interaction with age-related macular degeneration-affected Bruch’s membrane is tenascin-C which we confirm is present at high levels in wet age-related macular degeneration membrane. We show that tenascin-C is anti-adhesive for retinal pigmented epithelial cells, but after integrin activation, they can adhere and migrate on it using alphaVbeta3 integrin. Alternatively, we find that transduction of retinal pigmented epithelial cells with alpha9 integrin, a tenascin-C-binding integrin, led to a large increase in alpha9beta1-mediated adhesion and migration on tenascin-C. Both expression of alpha9 integrin and integrin activation greatly enhanced the ability of retinal pigment epithelial cells to adhere to tenascin-rich wet age-related macular degeneration-affected Bruch’s membranes. Our results suggest that manipulation of retinal pigment epithelial cell integrins through integrin activating strategies, or expression of new integrins such as alpha9, could be effective in improving the efficacy of retinal pigment epithelial cell transplantation in wet age-related macular degeneration-affected eyes.
Introduction
Age-related macular degeneration is the leading cause of blindness in the developed world (Jager et al., 2008). Advanced age-related macular degeneration is characterized by focal deposits of amorphous material known as drusen, formation of choroidal new vessels, photoreceptor loss, retinal detachment, subretinal haemorrhage and visual loss (Fine et al., 1986). At least some of these changes can be ascribed to age-related malfunction of the retinal pigmented epithelium (RPE) and the underlying Bruch’s membrane. The RPE has multiple roles in the metabolic support of the retina and in phagocytosis of shed photoreceptor outer segment fragments.
Current treatment of wet age-related macular degeneration aims to halt the retinal damage due to neovascularisation. It includes removal of choroidal new vessels using techniques such as photodynamic therapy and photocoagulation (Gehrs et al., 2006). Replacement of the RPE is an attractive therapeutic option. In animal models of age-related macular degeneration, RPE transplantation has been shown to be effective in preventing progressive photoreceptor and visual loss (Li and Turner, 1988b; Castillo et al., 1997). This has led to several trials of RPE replacement in human patients (summarized in Del Priore et al., 2006; Binder et al., 2007; da Cruz et al., 2007) with transplantation of dissociated RPE cells or grafts of RPE attached to the underlying choroid. Partial success has been obtained in some trials, and more recently a combined strategy of removing the pathological choroidal new vessels followed by transplantation of RPE cells has been tried (Algvere et al., 1994; Binder et al., 2004, 2007; MacLaren et al., 2007). Where it has been possible to examine the fate of transplanted RPE cells, they have formed small aggregates, not attached to Bruch’s membrane and are therefore not positioned for maximal effect. Lack of adhesion of RPE cells to Bruch’s membrane is also detrimental to the survival of transplanted cells which undergo apoptosis if not attached to a surface (Tezel and Del Priore, 1997, 1999). Poor adhesion of RPE to Bruch’s membrane may also limit the migration of cells to achieve the repopulation of the denuded Bruch’s membrane exposed by surgery. For these reasons, transplants of patches of RPE attached to choroid or attached to a carrier substrate are probably the most promising current strategy (da Cruz et al., 2007).
There are several factors that affect RPE interactions with Bruch’s membrane. Ageing changes the membrane such that RPE cell adhesion is reduced (Del Priore and Tezel, 1998, 1999), and age also changes the pattern of gene expression and affects the capacity of RPE cells to phagocytose shed photoreceptor segments (Finnemann and Silverstein, 2001; Cai and Del Priore, 2006). In age-related macular degeneration, Bruch’s membrane undergoes multiple pathological changes due to inflammation, scarring, aberrant extracellular matrix production and neovascularization, also rendering it unable to support RPE cell adhesion (Zarbin, 2004). In addition to these pathological changes, treatments to remove choroidal new vessels may damage or remove the superficial basal lamina of Bruch’s membrane, exposing the underlying layers, which are less adhesive to RPE cells (Grossniklaus et al., 1994; Tezel et al., 1999; Tsukahara et al., 2002).
Various changes in the age-related macular degeneration-affected Bruch’s membrane affect its ability to support RPE cells, but of particular significance may be the upregulation of tenascin-C (TN-C). TN-C is an extracellular matrix glycoprotein that is anti-adhesive to many cell types, and which is upregulated in the damaged nervous system (Wehrle-Haller and Chiquet, 1993; Kiernan et al., 1996; Scholze et al., 1996; Zhang et al., 1997; Tang et al., 2003). In wet age-related macular degeneration, TN-C expression is increased in regions of angiogenesis and neovascularization, and it is deposited in Bruch’s membrane on the basal side of RPE cells (Zagzag et al., 1996; Zagzag and Capo, 2002; Fasler-Kan et al., 2005). In addition very high levels of TN-C have been detected in neovascularized membranes removed from wet age-related macular degeneration patients (Nicolo et al., 2000). TN-C is therefore a candidate for causing the failure of RPE adhesion in damaged and aged Bruch’s membrane.
Attachment of cells to extracellular matrix structures is largely mediated via integrins, so manipulation of RPE integrins is a potential method for improving their adhesion and migration. Recent experiments in which integrins were manipulated before RPE transplantation support this idea. Prolonged culture of RPE cells, which leads to increased expression of integrin subunits (Zarbin, 2003; Gullapalli et al., 2004) or genetic modification of RPE cells to overexpress alpha6beta4 integrin (Fang et al., 2008) both led to increased adhesion of RPE cells to various layers of Bruch’s membrane. Apart from altered expression, integrin function can be modulated by modifying the activation state, taking them from a low affinity inactive state to a high affinity active state favouring ligand binding and downstream signalling (Humphries, 1996). Forced integrin activation has been shown to enhance cell adhesion and migration and axon growth under non-permissive conditions such as low ligand availability or the presence of inhibitory molecules (Ivins et al., 2000; Miao et al., 2000; Hu and Strittmatter, 2008).
In this study, we have asked whether various integrin-related interventions might enhance RPE cell adhesion and migration on normal and age-related macular degeneration-modified Bruch’s membrane and its components. We show that integrin activation increases RPE attachment and migration on the extracellular matrix molecules that constitute Bruch’s membrane; then, using a new Bruch’s membrane preparation, we confirm these findings on normal adult membranes. Having confirmed that TN-C is greatly upregulated in pathological human Bruch’s membrane from wet age-related macular degeneration patients, we show that RPE attachment and migration is inhibited by TN-C, but that this inhibition can be overcome by integrin activation or expression of the tenascin-binding integrin alpha9. Finally, we show that these manipulations can allow RPE cells to interact with age-related macular degeneration-affected Bruch’s membrane.
Materials and methods
Cell culture
Adult retinal pigmented epithelium-19 (ARPE-19) RPE cell line was purchased from American Type Culture Collection (ATCC-HB243). Cells were cultured in RPE medium containing Dulbecco's; Modified Eagle’s Medium: F12; 1:1 ratio (ATCC), supplemented with 10% foetal calf serum (Invitrogen), and penicillin–streptomycin–fungizone (Sigma, 1%). RPE cells were cultured on poly-d-lysine (Sigma; 20 µg/ml) coated flasks.
Antibodies and reagents
Antibodies used were: mouse monoclonal anti-RPE65 (1:100; Abcam), mouse monoclonal anti-cytokeratin18 (1:100; Abcam), rabbit polyclonal anti-laminin (1:100; Sigma), rabbit polyclonal anti-phospho-focal adhesion kinase tyr397 (1:100; Invitrogen), rat anti-β1 integrin (Clone9EG7,1:200; BD biosciences), β1 activating antibody TS2/16 (1:3 dilution from medium of ATCC-HB243), chicken polyclonal anti-α9 integrin (1:2000; Abcam), mouse monoclonal anti-α9β1 integrin (1:200; Abcam), mouse anti-human β1 integrin (1:200; Abcam), goat anti-human β1 integrin (1:500; R& D), rabbit polyclonal anti-focal adhesion kinase (1:100; Biosources), mouse anti-human β3 integrin (1:100, Abcam), mouse monoclonal anti-human TN-C (1:100; Abcam).
Di-I vibrant (Invitrogen, 5 μg/ml), chicken TN-C (Chemicon, 10 μg/ml, CC118), extracellular ligands laminin (L4544), fibronectin (F2006), collagen I (C5483) and collagen IV (C27 663) were all purchased from Sigma-Aldrich. Aphidicolin mitotic inhibitor (Sigma) was used at 1 µg/ml.
Migration assays
The inverted coverslip assay was as described in Wilby et al. (1999). Small fragments of poly-d-lysine-coated coverslips were covered with RPE cells (ARPE-19 cells obtained from ATCC) which were trypsinized briefly and 20 µl of dense cell suspension placed on each fragment. After 2 h RPE medium (Dulbecco’s Modified Eagle’s Medium: F12 1:1 ratio; 10% foetal calf serum; 1% penicillin–streptomycin–fungizone) was added to cover the fragments. When the RPE cells were confluent, they were inverted onto the test surface and migration assessed after 24–48 h in 1 µg/ml aphidicolin. Maximum distance from the edge of coverslip and the number of cells migrating from the edge of coverslip was measured. For assays on Bruch’s membrane, cells were suspended in medium containing 5 µl/ml of Di-I vibrant 555 for 25 min and then washed twice.
Adhesion assays
Surfaces were coated with laminin, fibronectin, collagen I and IV (1 µg/ml; Sigma) or TN-C (10 µg/ml) for 2 h and then washed twice with phosphate buffered saline. ARPE-19 cells were briefly trypsinized and washed and equal numbers of dissociated RPE cells were added to each 2 cm2 well. TS2/16 (1:3 dilution) or 500 µM manganese (Mn2+) were added to the required wells. The wells were agitated at 10 rpm (Luckham R300) for 1 h. After three washes, cultures were fixed in 4% paraformaldehyde for 15 min. Adhesion to 6 mm2 size squares of Bruch’s membrane was measured with cells labelled with Di-I vibrant 555. Human Bruch’s membrane samples were held flat with 1 cm diameter silicone rings.
Immunocytochemistry
For live staining, mouse anti-human anti-alpha9beta1 integrin (1:200; Abcam) or anti- αVβ3 integrin (1:1000; Abcam) was added to the medium for 15 min, and after three washes, secondary Alexa-Fluor conjugated goat anti-mouse antibody (1:500; Invitrogen) was added for 15 min, followed by three washes then fixation at −20°C in methanol for 4 min. The cells were rinsed and mounted in Fluorosave (Calbiochem). For immunostaining involving fixed cells, fixation was carried out in 4% paraformaldehyde. This was followed by washes and blocking in 0.2% Triton-X, 10% goat serum for 1 h. Primary antibodies were diluted in phosphate buffered saline—10% goat serum and incubated overnight.
To detect conformational changes of integrins, RPE cells were cultured with 500 µM of Mn2+. 9EG7 anti-β1 integrin antibody (2 µg/ml; BD biosciences) was added to the medium for 15 min. After washing, fluorescein isothiocyanate conjugated goat anti-rat antibody (1:500; Invitrogen) was added for 15 min. After washing and fixation in cold methanol, cells were mounted in Fluorosave (Calbiochem).
Immunohistochemistry
Eye tissue was embedded in Optimal Cutting Temperature compound (Raymond Lamb,UK) and frozen on dry ice. Fresh frozen eyes were then sectioned at 30 µm (transverse plane) on a cryostat, and mounted on slides (Superfrost Plus; VWR International). After blocking in 10% goat serum/0.3% TritonX-100 (Sigma-Aldrich) for 1 h, primary antibodies were added and incubated overnight at 4°C. Primary antibodies used included mouse monoclonal anti-RPE-65 (1:100; Abcam), anti-cytotokeratin-18 (1:100; Abcam), anti-laminin (1:100; Sigma) and anti-TN-C (1:100; Abcam). This was followed by thorough washes. Secondary antibodies were then added for 1 h, and after washing slides were mounted in Fluorosave (Calbiochem).
Scanning electron microscopy
Cells specimens were fixed in 4% gluteraldehyde in 0.1 M piperazine-N,N′-bis(2-ethanesulfonic acid buffer at pH 7.4 for 18 h at 4°C. They were rinsed in deionised water and treated with 4% osmium tetroxide at 4°C for 18 h. They were then rinsed in deionised water and dehydrated in an ascending series of ethanol solutions from 70% to 100%. Then they were placed in a Polaron critical point dryer (Quorum/Emitech, UK) where the ethanol was replaced with liquid carbon dioxide which was heated to 27°C where it changed state to its gaseous phase. The carbon dioxide gas was vented and the specimens were glued to scanning electron microscope stubs with double cellotape. The stubs were coated with 25 nm of gold in a Quorum/Emitech K575X sputter coater. They were viewed in an FEI-Philips XL30 FEG scanning electron microscope at 5 kv.
Bruch’s membrane preparations
For adult rat Bruch’s membrane, the posterior eye cup was dissected out in cold Hanks balanced salt solution (HBSS) and the retina removed. Four radial incisions were made to allow flattening of the eye cup, and then it was placed in water for 45 min to allow intact RPE lysis by hypotonicity. The remaining cell debris was removed by jets of water with a pipette. After further washes of the membrane in HBSS, they were stuck down in chamber slides (Nunc, LAB-TEK) using cyanoacrylate glue. Human Bruch’s membrane was prepared in the same way and held in chamber slides using silicon inserts. Human eyes were obtained from San Diego Human Eye Bank (Bank association of America, FDA registered, Registration no. 3002515992). The diagnosis of wet age-related macular degeneration was made by the physician in charge of the patient. Eyes were examined for drusen, to confirm the age-related macular degeneration specimens and to ensure that control subjects were drusen free. Immunohistochemistry for TN-C glycoprotein was then performed on sections from control and wet age-related macular degeneration samples to confirm further the diagnosis of wet age-related macular degeneration versus control non-age-related macular degeneration. Pictures showing the presence of TN-C in wet age-related macular degeneration eyes and its absence in controls are shown in Fig. 10. The protocol of study adhered to the tenets of the Declaration of Helsinki regarding research involving human tissue.
Western blots
RPE cells were trypsinized, centrifuged and pellets were lysed with radioimmunoprecipitation assay buffer (Roche), centrifuged at 15 000×g (10 min, 4°C) and the supernatant collected.
Samples were run on a 6% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel under reducing conditions and then proteins transferred to polyvinylidene fluoride, membrane (Amersham) at 4°C for 1.5 h at 100V. Membranes were washed twice in tris-buffered saline 0.05% Tween-20 then blocked with 5% skimmed milk and incubated overnight in chicken anti-human α9β1 integrin (1:2000, Abcam). Following washes to remove excess primary antibody and incubation with horseradish peroxidase-conjugated secondary antibody (1:10 000, Vector), proteins were visualized with chemiluminescence (ECL, Amersham).
Donor information
| . | Control . | Age-related macular degeneration . | ||||
|---|---|---|---|---|---|---|
| Patient number . | Age . | Sex . | Body refrigeration time . | Age . | Sex . | Body refrigeration time . |
| 1 | 91 | Male | 12 h | 91 | Male | 6 h 30 min |
| 2 | 84 | Male | 9 h 35 min | 93 | Female | 12 h 30 min |
| 3 | 89 | Male | 12 h 35 min | 91 | Male | 0 min |
| 4 | 69 | Male | 21 h 30 mins | 94 | Female | 1 h 25 min |
| . | Control . | Age-related macular degeneration . | ||||
|---|---|---|---|---|---|---|
| Patient number . | Age . | Sex . | Body refrigeration time . | Age . | Sex . | Body refrigeration time . |
| 1 | 91 | Male | 12 h | 91 | Male | 6 h 30 min |
| 2 | 84 | Male | 9 h 35 min | 93 | Female | 12 h 30 min |
| 3 | 89 | Male | 12 h 35 min | 91 | Male | 0 min |
| 4 | 69 | Male | 21 h 30 mins | 94 | Female | 1 h 25 min |
Viral preparation and transduction
For preparing third generation, self-inactivating lentiviral vectors containing either farnesylated (f) green fluorescent protein (GFP) or α9-integrin-internal ribosome entry site-fGFP under control of the cytomegalovirus (CMV) promoter, plasmids were generously provided by Dr L. Naldini (University of Torino). Lentiviral vectors were prepared as described in Andrews et al. (2009). Briefly, human embryonic kidney-293T cells were co-transfected using a calcium phosphate method to introduce two helper plasmids (pCMVdR84, containing lentiviral structural genes and pMD2.VSVG for pseudotyping) and the transfer plasmid (pRRLsin.PPT.CMV.fGFP.Wpre or pRRLsin.PPT.CMV.α-9-integrin-internal ribosome entry site-fGFP.Wpre, containing besides the transgenes, the HIV-1 cis-acting sequences, an expression cassette for the transgene driven by the CMV promoter, and the Woodchuck post-transcriptional regulatory element, Wpre). Following transfection, high titres of replication defective, self-inactivating vectors were released into the medium. The day after transfection, Iscove’s Modified Eagle Medium, supplemented with 10% fetal bovine serum and 50 µg/ml gentamicin (Gibco) was replaced. Medium was collected 48 h post-transfection and subjected to ultracentrifugation using an SW-28 rotor at 20 000 rpm. The transparent (yet yellowish-opaque appearing) pellet was resuspended in 500 μl phosphate buffered saline (pH 7.4), aliquoted and kept at −80°C until further use. Titration of the lentiviral vectors was performed using an enzyme-linked immunosorbent assay, directed against the p24 core protein (Perkin Elmer) according to the manufacturer’s protocol.
For transduction, RPE cells were plated on poly-d-lysine 24 h prior to transduction. The cells were then treated with either GFP control virus or α9 viruses for 15 h in the presence of polybrene (2 µg/ml; Sigma). The medium was then changed and replaced by fresh RPE medium (Dulbecco’s Modified Eagle’s Medium: F12-10% foetal calf serum-1% penicillin–streptomycin–fungizone). The cells were kept in culture for 72 h and the transduction efficiency was assessed by analyzing the number of GFP positive cells to 4′,6-diamidino-2-phenylindole positive nuclei.
Migration assay quantification
Under phase contrast the maximum distance of migration from the edge of the coverslip was measured and the number of cells per 100 µm2 at various distances counted. Each experimental condition contained at least three coverslips, and each experiment was repeated at least three times using independent samples.
Adhesion assay quantification
Under phase or epifluorescence random 500 µm square fields were counted. Counts were normalized to control non-coated coverslips. Experiments were repeated at least three times using independent samples. Each experiment contained at least three repeats/coverslips of the same condition. Cells in 20 random fields were counted in each coverslip. For morphological analysis cells with flat morphology were compared to the control group.
Quantification of immunostaining
In ImageJ software, cells were selected as areas of interest, then the integrated pixel density of cells in each group standardized to the surface area of the cell and the background signal intensity was subtracted.
Statistical analysis
Each experiment was repeated at least three times using independent samples and average was calculated. Each experiment contained at least three repeats/coverslips of the same condition. Cells in 20 random fields were counted in each coverslip in adhesion assays.
Inverted coverslip migration assays involving two groups. The maximum distance of migration was compared between the test and the control group. In addition, the number of cells migrating from the edge of the coverslip was compared with that of the control at the same distance. Students-t test (two-tailed) was carried out to assess the level of significance. Significance value were represented as *P < 0.05, **P < 0.01 and ***P < 0.001.
For comparisons involving more than two groups, one-way ANOVA was carried out followed by Bonferroni’s post-hoc test. Significance values were represented as *P < 0.05, **P < 0.01 and ***P < 0.001.
Results
Integrin activation promotes retinal pigment epithelial cell adhesion and migration
Adhesion and migration assays on Bruch’s membrane components
To test the hypothesis that increasing integrin activation can increase RPE cell adhesion and migration, we investigated the behaviour of the ARPE-19 RPE cell line on defined extracellular matrix molecule substrates. ARPE-19 cells are spontaneously immortalized human RPE cells that form polarized epithelial monolayers, are accepted as a model of RPE behaviour and have been used in many recent studies. However, ARPE-19 cells have a few differences in gene expression compared with cultured adult RPE cells (Tezel et al., 2004; Cai and Del Priore, 2006; Sun et al., 2007; Fang et al., 2008).
Integrin activation has been shown to enhance adhesion and migration in other cell types (Lin et al., 1993; Ivins et al., 2000). Activation of integrins can be achieved by application of extracellular Mn2+ (at 500 µM) and by application of conformation-specific monoclonal antibodies, the most commonly used being TS2/16 (Lin et al., 1993; Mould et al., 1995; Tsuchida et al., 1997; Miao et al., 2000; Hu and Strittmatter, 2008) The integrin activation state can be detected with other conformation-specific (but non-activating) anti-β1 antibodies such as 9EG7, or by measuring levels of phosphorylated focal adhesion kinase (Bazzoni et al., 1995; Wennerberg et al., 1998; Sawai et al., 2005; Hu and Strittmatter, 2008).
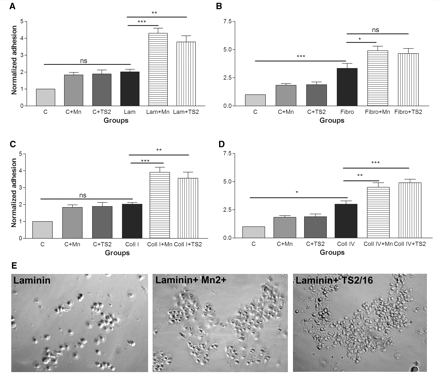
The composition of various extracellular matrix molecules in each layer of the normal Bruch’s membrane has previously been characterized and has been shown to include laminin, fibronectin, collagen I and collagen IV (Das et al., 1990). We have therefore used these extracellular matrix molecules in assessing the adhesion and migration of RPE cells in our experiments. To investigate the effects of integrin activation on RPE adhesion, 1 h adhesion assays were carried out on various matrix components of Bruch’s membrane including laminin, fibronectin, collagen I and collagen IV (all at 1 µg/ml) in the absence or presence of the integrin activators Mn2+ or TS2/16. The adhesion promoting properties of the substrates ranked in the order fibronectin > collagen IV > laminin> collagen I > non-coated, in agreement with previous studies (Ho and Del Priore, 1997). We treated the cultures with either Mn2+ or TS2/16 to find out whether increased integrin activation increased the adhesion of RPE cells to these substrates. As shown in Fig. 1, we found that integrin activation by either Mn2+ or the antibody increased adhesion to all the substrates. RPE cells produce laminin, and therefore over time deposit their own extracellular matrix substrate. This may explain why addition of Mn2+ and TS/16 consistently produced a small increase in adhesion of the cells to tissue culture plastic (Supplementary Fig. 1).
Effect of integrin activation on RPE cell adhesion to extracellular matrix molecules. Integrin activation with Mn2+ (500 µM) and TS2/16 antibody increases RPE adhesion to laminin (A), fibronectin (B), collagen I (C) and collagen IV (D). Extracellular matrix molecules support the adhesion of RPE cells in the order of fibronectin > collagen IV > laminin > collagen I > non-coated. n = 3. All values are normalized to control adhesion (non-coated glass). (ANOVA test: *P < 0.05, **P < 0.01, ***P < 0.001). (E) shows adhesion assays of RPE cells in control laminin only, laminin+Mn2+ and laminin+TS2/16 groups.
We next asked whether integrin activation can increase the rate of RPE cell migration on defined extracellular matrix substrates. RPE cell migration assays were performed using the inverted coverslip assay (Fok-Seang et al., 1995; Wilby et al., 1999) in which coverslip fragments covered with RPE cells are inverted onto the test surfaces. The maximum distance of migration and the number of RPE cells per unit distance were then quantified. Migration assays were carried out over 24 h and in the presence of a mitotic inhibitor to eliminate any effect of proliferation.
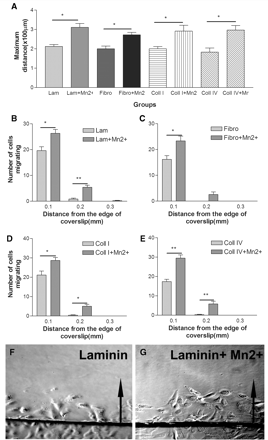
The test surfaces were laminin, fibronectin, collagen I and collagen IV (all at 1 µg/ml) in the absence or presence Mn2+ or TS2/16. Mn2+ increased the maximum distance of migration and the number of RPE cells migrating from the edge of coverslip significantly on all the four components of Bruch’s membrane (Fig. 2). TS2/16 increased the number of cells migrating from the edge of coverslip to a similar extent to Mn2+ although it had no effect on the maximum distance of migration (Supplementary Fig. 2).
Effect of integrin activation on RPE cell migration on extracellular matrix molecules. (A) Integrin activation with Mn2+ (500 µM) significantly increases maximum distance of migration of RPE cells on all the substrates tested. Integrin activation significantly increases the number of cells migrating from the edge of coverslip compared to control cells on laminin (B), fibronectin (C), collagen I (D) and collagen IV (E). n = 3 (t-test: *P < 0.05, **P < 0.01). (F and G) Inverted coverslip migration assay showing RPE cell migration on laminin (F) and laminin+Mn2+ group (G). Arrows point to the direction of cell migration away from the edge of coverslip.
Manganese activates integrins in retinal pigment epithelial cells
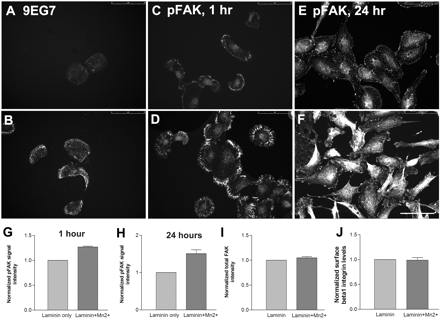
To confirm that Mn2+ changes the conformation of integrins in the RPE cells, we used the monoclonal antibody (9EG7) that detects an epitope on β1 integrin that is exposed after application of integrin activating agents such as Mn2+ (Bazzoni et al., 1995). RPE cells were plated overnight on laminin (1 µg/ml) in the presence or absence of Mn2+ (500 µM). Analysis of the antibody binding demonstrated that in the absence of Mn2+, the 9EG7-binding epitope was detected at very low levels, but in Mn2+ treated cells, there was increased binding of the antibody (Fig. 3). These changes were not due to an increase in the level of β integrin on the cell surface, because immunostaining for β1 in non-permeabilized cells was not affected by Mn2+. We assessed whether Mn2+ application leads to a change in downstream signalling associated with integrin activation by immunostaining for tyr397-phosphorylated focal adhesion kinase, a phosphorylation site associated with integrin activation (Crowe and Ohannessian, 2004; Sawai et al., 2005). Quantification showed that RPE cells exposed to Mn2+ exhibit increased phosphorylated focal adhesion kinase levels both at 1 h (adhesion assay time point) and at 24 h (migration assay time point) in comparison to control non-treated cells (Fig. 3). There was no change in overall focal adhesion kinase immunoreactivity using an antibody that binds to both activated and non-activated forms of the molecule.
Confirmation of integrin activation by Mn2+. RPE cells plated on laminin (1 µg/ml) in the absence (A) and presence (B) of Mn2+. 9EG7 binding to β1 integrin is increased following Mn2+ treatment. Immunostaining for phosphorylated focal adhesion kinase (pFAK) in RPE cells in the absence (C and E) and presence (D and F) of Mn2+ after 1 h and 24 h. (G and H) Quantification of phosphofocal adhesion kinase levels in RPE cells after Mn2+ treatment. All values normalized to the levels in the absence of Mn2+. No difference in total focal adhesion kinase levels (I) and surface β1 integrin levels (J) were observed following Mn2+ treatment. n = 3. Scale bar = 75 µm.
Integrin activation increases retinal pigment epithelial cell adhesion and migration on normal Bruch’s membrane
Rat intact Bruch’s membrane model
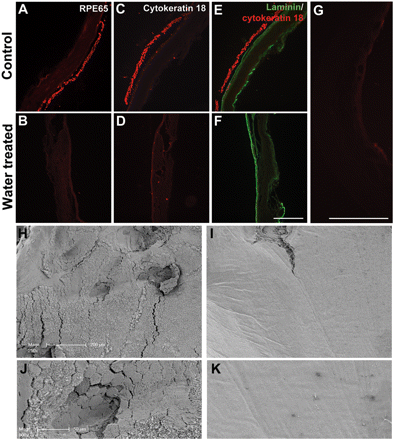
Having shown that RPE cell behaviour is changed by integrin manipulation, we needed to see whether these modified behaviours were also seen on Bruch’s membrane. For these experiments, we developed a new model for assessment of RPE cell interactions with intact normal Bruch’s membrane. Squares of RPE–Bruch’s–choroid–sclera complex were dissected from normal young adult rat eyes. In order to study RPE cell adhesion and migration, the endogenous RPE cells must be removed, which was achieved with water lysis followed by forceful flushing with a pipette, as used in previous studies of glia extracellular matrix biology (Smith-Thomas et al., 1994; Fidler et al., 1999) To demonstrate that endogenous RPE cells were removed by this method, treated membranes were cryo-sectioned and stained for two specific RPE markers; RPE-65 and cytokeratin18. This showed that the RPE layer was removed by water treatment but not by incubation in isotonic HBSS (Fig. 4). Serial sections at 30 µm intervals were analysed through the whole eye to demonstrate that the water treatment removed RPE cells efficiently (Supplementary Fig. 3). To demonstrate that this RPE removal method does not damage the underlying basal lamina of Bruch’s membrane, water treated specimens were stained for laminin, revealing that the prominent laminin-positive basement membrane was intact in both water treated and HBSS-treated samples (Fig. 4). Scanning electron microscopy of the eyecups showed that untreated or HBSS-treated eyes have an intact continuous layer of regular cobblestone-like RPE cells. After water treatment none of these cells were seen, but instead there was a smooth exposed Bruch’s membrane (Fig. 4).
Rat Bruch’s membrane preparation and confirmation of RPE layer removal. Immunostaining for RPE-65, demonstrating the presence of the RPE layer in control HBSS-treated sample (A) and its absence in a water treated sample (B). (C and D) Immunostaining for cytokeratin 18 demonstrating efficient removal of the RPE layer after water treatment. Immunostaining for laminin and cytokeratin 18 shows the presence of intact laminin rich basement membrane in both control treated (E) and water treated samples (F). Scale bar = 250 µm. (G) Staining negative control for RPE-65 and cytokeratin 18. Scanning electron micrographs of the subretinal surface in HBSS control treated (H) and water treated (I) samples. Water treatment leads to complete removal of the hexagonal shaped RPE cell monolayer exposing the smooth Bruch’s membrane underneath. Scale bar = 200 µm. High magnification scanning electron micrographs of HBSS control treated (J) and water treated (K) samples. Scale bar = 50 µm.
Integrin activation increases retinal pigment epithelial cell adhesion and migration on Bruch’s membrane
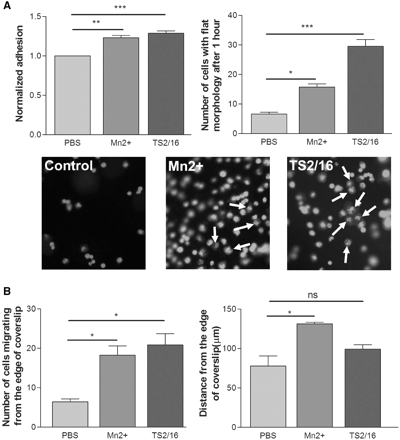
Using the same adhesion and migration assays described above, RPE behaviour on Bruch’s membrane was assessed. To do this, squares of RPE-free Bruch’s membrane were stuck to the bottom of the chamber slide wells. ARPE-19 cells were labelled using vibrant Di-I, which labelled 99% of the cells allowing them to be visualized by fluorescence microscopy. To explore the role of integrin activation in RPE adhesion to the intact Bruch’s membrane, adhesion assays were carried out in the presence of Mn2+ and TS2/16. The number of cells attached and the percentage of cells with flat morphology were analysed after 1 h. Both Mn2+ and TS2/16 increased the number of RPE cells attached to the Bruch’s membrane, and greatly increased the percentage of cells that had spread out with a flat morphology (Fig. 5A). RPE migration on Bruch’s membrane was assessed using the inverted coverslip assay, performed in the presence of a mitotic inhibitor to eliminate the effects of cell proliferation. Integrin activation increased the maximum distance of migration and the number of cells migrating from the edge of coverslip compared to the control samples (Fig. 5B). These results suggest that increasing integrin activation can increase the interaction of RPE cells with the extracellular matrix molecules in Bruch’s membrane, enhancing the adhesion and migration of the cells.
Effect of integrin activation on RPE adhesion and migration on rat Bruch’s membrane. (A) Mn2+ and TS2/16 application both increase the number of cells attached and the percentage of cells with flat morphology on Bruch’s membrane. White arrows point to examples of cells with flat morphology in each group. n = 3 (ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001). Scale bar = 250 µm. (B) Mn2+ and TS2/16 increase the number of cells migrating from the edge of coverslips. Mn2+ also increased the maximum distance of migration although TS2/16 had no significant effect on this parameter. n = 3 (ANOVA: *P < 0.05).
Integrin activation increases retinal pigment epithelial cell adhesion to wet age-related macular degeneration human Bruch’s membrane
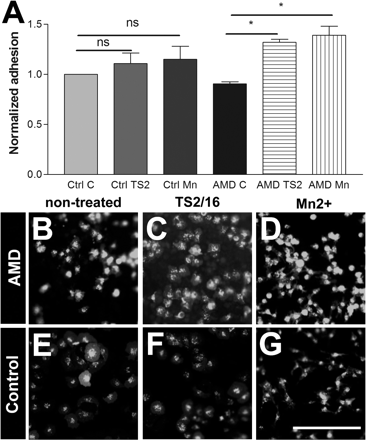
The composition of Bruch’s membrane when damaged by age-related macular degeneration is very different to that of the normal eye. We therefore asked whether RPE cells can adhere to Bruch’s membrane taken from patients with wet age-related macular degeneration, and whether this adhesion is increased by integrin activation. Four control human eyes, and four eyes from patient donors who had been diagnosed with the wet form of age-related macular degeneration were used for RPE cell adhesion assays. As with the rat Bruch’s membrane preparation, squares of RPE–Bruch’s–choroid were dissected from the eye and treated with water and pipette washing to lyse and remove RPE cells. Adhesion assays were performed as in the preceding experiments. Mn2+ and TS2/16 did not significantly increase the already high adhesion to the normal Bruch’s membrane. However, the level of adhesion to age-related macular degeneration-damaged Bruch’s membrane was improved with integrin activation using either Mn2+ or TS2/16 (Fig. 6). This result suggests that the normal Bruch’s membrane has a sufficient level of integrin ligands, but in wet age-related macular degeneration, either the level of ligands is lower or there are inhibitory molecules that partially block RPE adhesion. The effects can be overcome by integrin activation. We repeated the assay using a 24 h adhesion assay which gave very similar results to those obtained using the short time point 1 h adhesion assay (Supplementary Fig. 4).
Effect of integrin activation on RPE adhesion on human Bruch’s membrane. (A) Integrin activation with Mn2+ and TS2/16 increases RPE adhesion to pathological Bruch’s membrane from wet age-related macular degeneration patients. n = 3 (ANOVA test:*P < 0.05). RPE adhesion to Bruch’s membrane from wet age-related macular degeneration (AMD) samples in non-treated (B), TS2/16 treated (C) and Mn2+ treated (D) group. (E–G) RPE adhesion to Bruch’s membrane from control samples in non-treated (E), TS2/16 treated (F) and Mn2+ treated (G) group. Scale bar = 250 µm.
TN-C and retinal pigment epithelial cell adhesion and migration
Because of the anti-adhesive and non-permissive nature of TN-C, and because it has been reported to be upregulated at the basal side of RPE cells and in association with neovascularisation in Bruch’s membrane in wet age-related macular degeneration (Nicolo et al., 2000; Fasler-Kan et al., 2005), we investigated adhesion and migration of RPE cells on this molecule. Our hypothesis was that upregulation of TN-C in the age-related macular degeneration-affected Bruch’s membrane is a major factor in blocking RPE adhesion and migration.
TN-C inhibits retinal pigment epithelial cell adhesion and migration
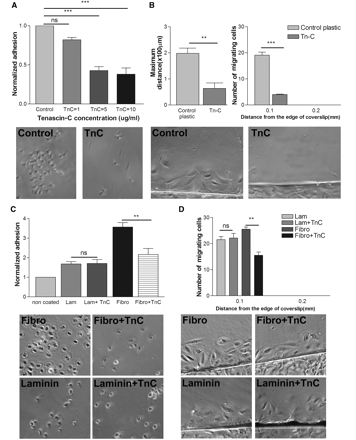
RPE adhesion assays were carried out on TN-C coated coverslips at 1, 5 and 10 µg/ml. The number of cells attached at 1 h was counted and compared to control non-coated surfaces. TN-C reduced the number of RPE cells attached at all concentrations, with maximal reduction in adhesion seen at 10 µg/ml (Fig. 7A). We investigated the role of TN-C on RPE migration using the inverted coverslip assay. TN-C inhibited RPE migration significantly at 10 µg/ml, with reductions in the maximum distance of migration and the number of cells migrating compared to plain non-coated plastic (Fig. 7B). To determine whether TN-C is actually inhibitory rather than just non-permissive, we investigated adhesion and migration on mixtures of TN-C with laminin and fibronectin. The presence of TN-C in the mixture inhibited adhesion and migration on fibronectin, although it had no effect on similar processes on laminin, supporting an inhibitory role for TN-C (Fig. 7C and D).
Effect of TN-C on RPE cell adhesion and migration. (A) TN-C inhibits RPE cell adhesion in a dose responsive manner. Maximal inhibition is achieved at 10 µg/ml of TN-C. Control corresponds to adhesion on non-coated glass coverslips. (ANOVA: *P < 0.05, ***P < 0.001). (B) TN-C inhibits the number of cells and the maximum distance of migration from the edge of coverslip (t-test *P < 0.05, ***P < 0.001). (C) TN-C mixed with laminin (Lam) or fibronectin (Fibro). TN-C inhibits adhesion to fibronectin although it has no effect on adhesion to laminin. (D) TN-C mixed with laminin or fibronectin. TN-C inhibits migration on fibronectin although it has no effect on migration on laminin. n = 3 (ANOVA: *P < 0.05, **P < 0.01).
Inhibition of TN-C is overcome by integrin activation
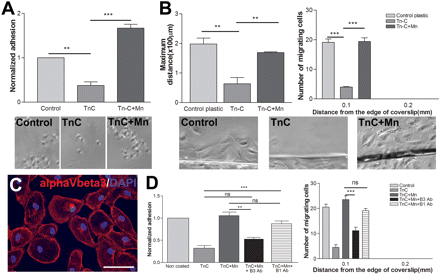
To assess whether integrin activation can overcome the inhibitory effect of TN-C, adhesion and migration assays were performed on a high concentration TN-C (10 µg/ml) with or without Mn2+. In both adhesion and migration assays, the presence of Mn2+ returned RPE behaviour to control levels or greater (Fig. 8A and B). This reversal of inhibition is likely to be due to activation of αVβ3 integrin, the only integrin known to be expressed by RPE cells that is capable of interacting with tenascin. We demonstrated this directly by showing that the RPE cells express αVβ3, and by showing that a β3-blocking antibody inhibits the adhesion and migration of Mn2+-activated RPE cells to TN-C. Blocking β1 integrins had no effect (Fig. 8D). Also the integrin β1-activating antibody TS2/16 had no effect on RPE cell adhesion or migration on TN-C confirming that RPE cells do not express an α integrin subunit capable of binding to β1 integrin to allow interaction with TN-C (Supplementary Fig. 5).
Effect of integrin activation on RPE cell behaviour on TN-C. (A) Integrin activation by application of Mn2+ enabled RPE cells to adhere to a TN-C coated surface. Non-coated glass coverslip was used for the control. n = 3, ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001. (B) Integrin activation with Mn2+ restored the ability of RPE cells to migrate on TN-C. The number of migrating cells and the maximum distance of migration of RPE cells go back to control levels. n = 3, ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001. (C) Immunostaining for αVβ3 integrin in RPE cells showing surface expression of this integrin dimer. Scale bar = 75 µm. (D) Application of β3-blocking antibody reduces the adhesion and migration of Mn2+-activated RPE cells on TN-C. This shows that αVβ3 is activated by Mn2+, increasing adhesion and migration on TN-C. N = 3, (ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001).
Inhibition by TN-C is overcome by expression of the tenascin-binding integrin alpha9
The preceding results demonstrate that RPE cells, at least in the absence of a treatment to activate integrins, lack a functional receptor to allow attachment or migration on TN-C. We therefore investigated whether RPE cells could adhere and migrate on TN-C if transduced to express a tenascin-binding integrin. α9β1 binds to the non-alternatively spliced fibronectin-III domain of TN-C (Yokosaki et al., 1994, 1998). RPE cells contain a large pool of β1 integrins; therefore expression of the α9 subunit is sufficient to allow formation of α9β1 heterodimers capable of interaction with TN-C. Lentiviral vectors were used to transduce RPE cells with human α9 integrin subunit or GFP as a control. The α9 integrin construct also contains GFP sequence under an internal ribosomal entry site to allow α9-transduced cells to be identified.
Three days after the application of the lentiviruses, 95% of cells expressed α9 integrin (as identified by internal ribosome entry site-GFP) in the α9-transduced group and 94% of cells in the control group were transduced with control GFP. To confirm the expression of the α9 protein and its presence on the surface of RPE cells, live cells without permeabilization were stained with an antibody to α9β1, revealing its presence in over 95% of lentivirus-treated cells. Expression of α9 was also confirmed by western blotting (Supplementary Fig. 6).
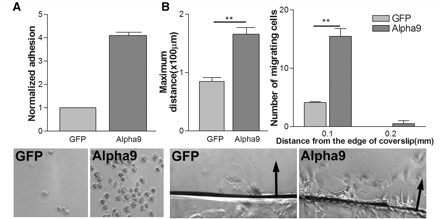
To assess the effect of α9 expression on the adhesion of RPE cells to TN-C, we performed our adhesion and migration assays on a high concentration of TN-C (10 µg/ml) comparing α9-transduced cells with GFP-transduced cells. This showed that α9 considerably increased the adhesion of the cells to TN-C (Fig. 9A). We also found that α9-transduced cells migrated further and in greater number than GFP cells in the migration assay (Fig. 9B). This result confirms that RPE cells are able to adhere and migrate on TN-C if they express an appropriate integrin.
Effect of α9 integrin expression on RPE cell adhesion and migration on TN-C. (A) Alpha9 expression increases RPE cell adhesion compared to GFP-expressing control RPE cells. Adhesion levels normalized to control GFP cells. n = 3 (t-test *P < 0.05, ***P < 0.001). (B) α9 expression increases the maximum distance of migration and the number of cells migrating from the edge of coverslip compared to GFP control cells. Arrows point to the direction of cell migration away from the edge of coverslip. n = 3 (t-test: *P < 0.05, **P < 0.01).
Alpha9 expression increases adhesion of retinal pigment epithelial cells to pathological human Bruch’s membrane
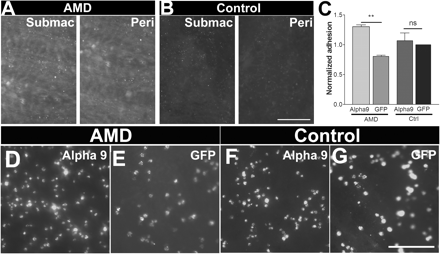
To assess adhesion on human Bruch’s membrane, specimen preparations were made from four control non-age-related macular degeneration human eyes and four eyes from wet age-related macular degeneration patients. Samples from the eyes were stained for TN-C, revealing a high concentration in the wet age-related macular degeneration eyes on the surface of Bruch’s membrane both in submacular and peripheral zones (Fig. 10A) in agreement with previous studies (Fasler-Kan et al., 2005). We tested the adhesion of α9-transduced and GFP-transduced RPE cells to normal and age-related macular degeneration-affected Bruch’s membrane preparations. The control GFP-transduced cells adhered to control Bruch’s membrane better than age-related macular degeneration-affected membrane. Expression of α9 integrin had a marginal effect on adhesion to normal Bruch’s membrane, which does not contain much TN-C. However, in the Bruch’s membrane derived from wet age-related macular degeneration eyes the α9-expressing cells showed a large increase in adhesion compared to control, consistent with the large amount of TN-C present on Bruch’s membrane in the age-related macular degeneration membranes (Fig. 10C–G). We repeated the assay using a 24 h adhesion assay which gave a very similar result to the 1 h adhesion assay (Supplementary Fig. 7).
Effect of α9 integrin expression on RPE cell adhesion to human Bruch’s membrane. Immunostaining for TN-C on Bruch’s membranes derived from patients with wet age-related macular degeneration (AMD) (A) and control subjects (B). Both submacular (Submac) and peripheral (Peri) areas in wet age-related macular degeneration show raised TN-C levels compared to similar areas in the control non-age-related macular degeneration membranes. Scale bar = 100 µm. (C) Integrin α9 expression increases RPE cell adhesion to wet-age-related macular degeneration membranes while having no effect on control membranes. n = 3 (ANOVA: *P < 0.05, **P < 0.01). RPE adhesion of α9 expressing cells. (D) compared to GFP control cells (E) on age-related macular degeneration-derived membranes. RPE adhesion of α9 expressing cells (F) compared to GFP control cells (G) on control non-age-related macular degeneration membranes. Scale bar = 250 µm.
Discussion
RPE cell transplantation has great promise for the treatment of age-related macular degeneration, but so far clinical experience has been disappointing due to the early death of transplanted cells and their tendency to form aggregates at the injection site rather than spreading as a sheet around the back of the retina. Both of these problems may be related to the inability of the cells to adhere and interact with the aged and pathologically and/or surgically damaged Bruch’s membrane (Li and Turner, 1988,a, b; Algvere et al., 1994; Castillo et al., 1997; Binder et al., 2004; Gullapalli et al., 2005; MacLaren et al., 2007). Lack of adhesion of RPE cells has previously been shown to be associated with cell death (Tezel and Del Priore, 1997).
Various changes in Bruch’s membrane mitigate against the adhesion and migration of RPE cells. As the eye ages, there are age-related changes in Bruch’s membrane including a reduction in integrin ligands (Pauleikhoff et al., 1990), such that even young RPE cells adhere poorly to aged Bruch’s membrane (Gullapalli et al., 2005). In addition, in eyes affected by age-related macular degeneration, there is accumulation of various inhibitory molecules which can affect RPE cell adhesion, including TN-C and sulphated proteoglycans (Hewitt et al., 1989; Zarbin, 2004; Fasler-Kan et al., 2005). These changes may be compounded by exposure of the deeper layers of the membrane during surgery to remove choroidal new vessels (Tezel and Del Priore, 1999; Tsukahara et al., 2002; Wang et al., 2003; Tezel et al., 2004). These deep layers are relatively non-adhesive to RPE cells compared with the basement membrane of Bruch’s membrane, and are likely to be rendered less adhesive due to upregulation of TN-C and chondroitin sulphate proteoglycans as part of the post-surgery injury reaction (Wang et al., 2003). Removal of neovascularized membranes has been shown to lead to damage to the basement membrane and exposure of deeper non-adhesive layers. In this study, we have used the basement membrane of the Bruch’s membrane as a model to explore the effect of integrin manipulation on RPE cell behaviour on the basement membrane between the control non-age-related macular degeneration versus wet age-related macular degeneration eyes.
Adhesion of cells to basal laminae and extracellular matrix is mediated via integrins which, in polarized cells such as RPE cells, are usually concentrated in the basal aspect of the cell. RPE cells express various integrin subunits known to bind to extracellular ligands in the normal Bruch’s membrane (Zarbin, 2003; Hoffmann et al., 2005) As the ability of Bruch’s membrane to support RPE cell adhesion declines with age, provision of appropriate integrins becomes critical. Moreover, as anti-adhesive TN-C levels increase in pathological eyes, it becomes essential for the cells to express an integrin that binds to this molecule. Previous studies have demonstrated the presence of αVβ3 which has some TN-C-binding ability, but our experiments indicate that this integrin will only mediate RPE cell adhesion to TN-C when it is artificially activated by Mn2+.
The ability of integrins to mediate cell adhesion and intracellular signalling is controlled by activation (Humphries, 1996). Many cell functions such as adhesion, migration and neurite outgrowth are modulated through changes in integrin activation (Lin et al., 1993; Ivins et al., 2000). Agents such as Mn2+ ions (Mould et al., 1995, 2002) and integrin activating antibodies as used in our experiments (Humphries, 2004) are useful experimental tools for converting integrins into the high affinity-binding state. Mn2+ binds to the extracellular domain of integrins, favouring the activated configuration of integrins which support binding to ligands and signalling into the cell (Mould et al., 1995). Integrin activating antibodies such as TS2/16 bind to integrins and stabilize the activated conformation by locking them in the high affinity state. (Humphries, 1996, 2004). In normal physiology, activation is mainly modulated by inside-out signalling, through binding of activated talin and other molecules to the intracellular domain (Tadokoro et al., 2003; Nieswandt et al., 2007; Banno and Ginsberg, 2008; Moser et al., 2009).
The aim of the current study was to investigate whether modulating integrin function in RPE cells, through controlling integrin activation or through expression of a TN-C-binding integrin, might increase the adhesion and migration of RPE cells on normal and pathological Bruch’s membrane. Through adhesion and migration assays, we show that RPE adhesion and migration is strongly influenced by integrin activation. This was particularly the case at low ligand concentrations, such as in aged Bruch’s membrane (Pauleikhoff et al., 1990; Grossniklaus et al., 1994; Tsukahara et al., 2002). Integrins are not just adhesion molecules, but adhesion also triggers downstream signalling events that affect cell survival and movement. The integrin activation treatments that we used increase the phosphorylation of focal adhesion kinase in RPE cells, which is one of the main downstream signalling pathways associated with integrin activation (Sawai et al., 2005). Increasing integrin activation could therefore be a way to improve adhesion and accelerate the integration of cells to Bruch’s membrane following transplantation.
In addition, we have investigated the role of the inhibitory molecule TN-C in the prevention of cell adhesion and migration. TN-C is upregulated in the wet/exudative type of age-related macular degeneration on the basal side of RPE cells (Fasler-Kan et al., 2005) and in association with neovascularization (Nicolo et al., 2000). We show that TN-C is inhibitory for both RPE cell adhesion and migration. These findings are in agreement with other studies finding a non-adhesive or inhibitory role for TN-C in other cell types (McKeon et al., 1991; Wehrle-Haller and Chiquet, 1993). The mechanism by which TN-C mediates its non-adhesive or inhibitory effect is not clearly understood but previous studies have shown that TN-C can disrupt integrin function in other cell types (Probstmeier and Pesheva, 1999; Huang et al., 2001), and other inhibitory molecules such as NogoA may have part of their effect through integrin inactivation (Miao et al., 2000; Hu and Strittmatter, 2008).
In our assays, addition of Mn2+ completely reversed the inhibition of RPE cell adhesion and migration caused by TN-C suggesting that integrin activation can overcome the inhibition caused by this molecule. However, the β1-activating antibody TS2/16 had no effect on TN-C inhibition, suggesting that Mn2+ works in this situation by activating previously inactive αVβ3, which is present in RPE cells and has some tenascin-binding ability (Taga et al., 2002; Hoffmann et al., 2005). This is supported by our data showing that the tenascin-binding activity of activated RPE cells is reversed by a β3-blocking antibody. The preceding experiments were performed on purified matrix molecules and rat Bruch’s membrane. While extracellular matrix glycoproteins generally have the same integrin-binding properties between human and rodent species, there are some data to suggest human RPE cells behave differently on rat versus human matrix molecules (Wang et al., 2006). In addition there may be differences in the composition of Bruch’s membrane between rat and human. We therefore tested our hypotheses on human tissue. We tested the ability of integrin-activating treatments to modify cell adhesion and migration on both normal control and wet age-related macular degeneration-affected Bruch’s membrane. On normal non-pathological rat membrane, integrin activation had a significant although not very large effect on both adhesion and migration. We obtained samples of wet age-related macular degeneration-affected eyes, and on these surfaces, rich in TN-C, integrin activation had a marked effect (greater than that on normal Bruch’s membrane) on increasing the adhesion of RPE cells.
A possible alternative to activating integrins artificially in RPE cells is to express a new integrin that is appropriate for the environment of the cells. In the age-related macular degeneration-affected eye, the appropriate integrin would bind to TN-C. α9β1 is an integrin that binds to one of the non-alternatively spliced fibronectin-III domains of TN-C (Yokosaki et al., 1994, 1998), and which has been shown to promote axon growth and cell migration on TN-C (Andrews et al., 2009). We therefore examined the effects of overexpressing α9 on RPE cell behaviour. On TN-C containing surfaces, α9 expression was a powerful stimulus to RPE cell adhesion and migration. We therefore looked at the effects of α9 expression on RPE interactions with both normal and wet age-related macular degeneration-affected Bruch’s membrane. We demonstrated a modest increase in adhesion to normal Bruch’s membrane (which contains little TN-C) but a much greater increase in adhesion to the tenascin-rich Bruch’s membrane which we prepared from the eyes of patients with wet age-related macular degeneration.
We show that integrin activation and α9 expression improved adhesion to the pathological Bruch’s membrane. TN-C has been reported in association with neovascularisation in wet age-related macular degeneration. More work is needed to elucidate the role of integrin manipulation in the dry form of age-related macular degeneration.
Integrin activating cations such as Mn2+ are not practicable for therapeutic use, and monoclonal antibodies such as TS2/16 would have to be continuously infused, and would affect other cell types in the eye. Ex vivo integrin manipulation can be carried out prior to transplantation to optimize the RPE cell adhesion to the Bruch’s membrane. For in vivo therapy, development of suitable viral vectors will be necessary.
In summary, our experiments suggest two ways in which modification of integrin function in RPE cells might be used to improve adhesion and migration after transplantation to the age-related macular degeneration-affected retina. Integrin activation enhances the adhesion and migration of cells on low concentrations of ligand, as found in aged Bruch’s membrane, and permits them to adhere to TN-C, which is greatly upregulated in wet age-related macular degeneration. Alternatively or in addition, expression of a new TN-C-binding integrin, α9, allows the cells to bind to TN-C, thereby converting an inhibitory molecule into an adhesive and migration-promoting molecule. Together, these interventions should make it possible for RPE cells to maximize their interaction with the limited positive cues available and overcome the inhibitory molecules that accumulate in age-related macular degeneration. It seems probable that stem-cell derived RPE cells will be used for future therapy and modification of these cells to promote integrin activation and/or express α9 integrin will increase their effectiveness.
Funding
Medical Research Council; Henry Smith Charity; John and Lucille van Geest foundation; European Union Framework 6 network of excellence NeuroNE.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors are grateful to David Story and Dr Mariangela Iovino for their kind help and input in this study.
Abbreviations
- ARPE-19
adult retinal pigment epithelium-19
- CMV
cytomegalovirus
- HBSS
Hanks balanced salt solution
- GFP
green fluorescent protein
- Mn2+
manganese
- RPE
retinal pigmented epithelium
- TN-C
tenascin-C