-
PDF
- Split View
-
Views
-
Cite
Cite
Roland Peyron, Luis García-Larrea, Marie-Claude Grégoire, Nicolas Costes, Philippe Convers, F. Lavenne, Francıois Mauguière, Daniel Michel, Bernard Laurent, Haemodynamic brain responses to acute pain in humans: Sensory and attentional networks, Brain, Volume 122, Issue 9, September 1999, Pages 1765–1780, https://doi.org/10.1093/brain/122.9.1765
Close - Share Icon Share
Abstract
Turning attention towards or away from a painful heat stimulus is known to modify both the subjective intensity of pain and the cortical evoked potentials to noxious stimuli. Using PET, we investigated in 12 volunteers whether pain-related regional cerebral blood flow (rCBF) changes were also modulated by attention. High (mean 46.6°C) or low (mean 39°C) intensity thermal stimuli were applied to the hand under three attentional conditions: (i) attention directed towards the stimuli, (ii) attention diverted from the stimuli, and (iii) no task. Only the insular/second somatosensory cortices were found to respond whatever the attentional context and might, therefore, subserve the sensory-discriminative dimension of pain (intensity coding). In parallel, other rCBF changes previously described as `pain-related' appeared to depend essentially on the attentional context. Attention to the thermal stimulus involved a large network which was primarily right-sided, including prefrontal, posterior parietal, anterior cingulate cortices and thalamus. Anterior cingulate activity was not found to pertain to the intensity coding network but rather to the attentional neural activity triggered by pain. The attentional network disclosed in this study could be further subdivided into a non-specific arousal component, involving thalamic and upper brainstem regions, and a selective attention and orientating component including prefrontal, posterior parietal and cingulate cortices. A further effect observed in response to high intensity stimuli was a rCBF decrease within the somatosensory cortex ipsilateral to stimulation, which was considered to reflect contrast enhancing and/or anticipation processes. Attentional processes could possibly explain part of the variability observed in previous PET reports and should therefore be considered in further studies on pain in both normal subjects and patients with chronic pain.
Introduction
According to current views, the pain experience results from a three-dimensional integration of sensory-discriminative, affective-motivational and cognitive-evaluative axes (Melzack and Casey, 1968; Melzack and Katz, 1994). The sensory-discriminative component subserves the ability to analyse location, intensity and duration of the stimulus, while the affective-emotional component gives rise to the unpleasant character of pain perception. The cognitive axis is involved in attention, anticipation and memory of past experiences (Guilbaud et al., 1994). In addition, the cognitive dimension is able to interact with the other two; for instance, both the intensity and the unpleasantness attributed to a painful stimulus are strongly modulated by the attention allotted to it (Miron et al., 1989).
In recent years, the brain haemodynamic response to both experimental and neuropathic pain has been assessed in a series of PET studies. A network of brain structures responding to pain with regional cerebral blood flow (rCBF) increases have been described, including consistently the second somatosensory (SII) and insular regions, the thalamus, and the anterior cingulate, parietal and prefrontal cortices. Less frequently, activation of the primary somatic area (SI), supplementary motor area, basal ganglia and cerebellum have also been described (see references in Tables 1 and 2). Variations in both the intensity and the distribution of rCBF changes have been observed according to the physical characteristics of the stimulus [i.e. heat versus cold (Casey et al., 1994, 1996; Craig et al., 1996); chemical versus electrical or laser (Svensson et al., 1997)], its intensity (Derbyshire et al., 1997), its duration [phasic versus tonic (Apkarian et al., 1992; Derbyshire and Jones, 1998], its mode [contact versus radiant (Svensson et al., 1997); stationary versus moving (Jones and Derbyshire, 1995)] and its site of application [(skin versus subcutaneous or muscles (Svensson et al., 1997)]. In previous literature, it is often implicitly accepted that some of the pain-related rCBF changes may index an anticipatory/attentional component (Jones et al., 1991; Derbyshire et al., 1994, 1997; Drevets et al., 1995; Casey et al., 1996; Hsieh et al., 1996; Svensson et al., 1997; Peyron et al. 1998), and some recent work has suggested that attention and pain might activate different sites within the anterior cingulate cortex (Davis et al., 1997; Derbyshire et al., 1998). However, selective manipulation of the attention alloted to a painful stimulus has not yet been specifically investigated with PET. Attention directed towards a painful stimulus, or away from it, has been shown to modify the magnitude of human electrocortical evoked potentials to thermal laser stimuli (Siedenberg and Treede, 1996; García-Larrea et al., 1997). Thus, it is likely that attentional changes may also influence the haemodynamic brain response. This is supported by recent observations that modifications of the affective component of pain (unpleasantness) by hypnotic suggestion induce specific rCBF changes (Rainville et al., 1997).
The present study was therefore designed to identify the effects of different attentional contexts on both pain perception and pain-related haemodynamic changes. Using 15O-labelled water injection, we investigated rCBF changes induced by a heat pain stimulation of the back of one hand in the three following contexts: (i) a neutral (N) situation, in the absence of any explicit attentional task, (ii) an attentional (A) context where the subject had to focus attention on the painful region, and (iii) a distractive (D) condition where the subject actively directed attention away from the painful stimulus. Our results suggest that, among the haemodynamic brain responses observed to painful stimuli, attention to pain is the major component while the encoding of thermal intensity per se concerns a very restricted cortical area.
Methods
PET procedure
After they provided written informed consent, 12 healthy volunteers were enrolled for the study, the procedure of which was accepted by the local ethics committee (Lyon).
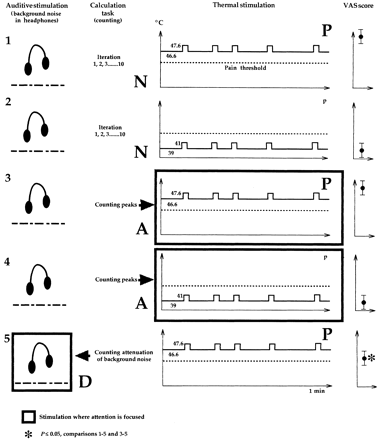
PET was recorded in the five following conditions (see Fig. 1).
Painful stimulation (P) without attention (a) task (neutral).
Non-painful stimulation (p) without attention (a) task (neutral).
Painful stimulation (P) with attention (A) directed to the painful stimulus.
Non-painful stimulation (p) with attention (A) directed to the stimulus.
Painful stimulation (P) with an auditive task diversive (D) away from pain.
All five conditions recorded in visually deprived subjects included a basal continuous and pre-determined thermal stimulation (low intensity, p, mean 39°C; high intensity, P, 46.6°C; 1 min duration) on which five peaks were randomly added (2 s duration for each peak: p, mean 41°C; P, mean 47.6°C). The stimulation was delivered on the back of one hand (right, n = 7; left, n = 5) by means of a thermode (3 × 3 cm) controlled by a quantified sensory tester (Medoc®, TSApain 2001). Instructions for identification and counting of both the rise and the descent of the temperature curve during the thermal peaks were given to recruit attention (A) towards the stimulated hand (conditions 3 and 4).
Instructions of identification (spotting) and counting random attenuations of a background noise delivered in headphones were given to engage the subject in an auditive task, diversive (D) from pain (condition 5).
In the neutral (N) conditions (1 and 2), the subject was asked to perform a repetitive iteration from 1 to 10 (so that a mental calculation task was present in all conditions) and to pay no attention to thermal changes and background noise attenuations.
The paired condition associating a non-painful stimulation (p) and a diversive (D) task could not be recorded, because of considerations on the radiation dosimetry.
After subjects had been trained for each one of the five conditions and after a 1-min test for habituation of the subject to the experimental procedure and to avoid the effect of first stimulation, a personalized thermoformable mask was adjusted to minimize head movements. Then a 20-min transmission scan was performed prior to any injection. After injection of a 9 mCi dose of H215O in the left antecubital vein, 60-s scans were recorded. Stimulations and attentional tasks began 10 s after injection, with an inter-condition interval of 10 min. The order of conditions was randomized within a cluster of five which were repeated a further three times.
Pain assessement
The subjective pain intensity was assessed after each recording using a Visual Analogue Scale (VAS) for the three following parameters: the average pain sensation during the 60 s recording, the maximal pain sensation during peaks of temperature and the average sensation of unpleasantness.
PET data analysis
Acquisitions were performed with a PET scanner (HR+, Siemens®) which generates sixty-three 2.425 mm-thick slices. Images were reconstructed with a Hanning filter providing a spatial resolution of 7 mm at the centre of the field of view. Attenuation and scatter correction were performed and residual activity was subtracted. As no arterial catheter was used the reconstructed images were not converted to rCBF. However, on the tested range, blood flow has been shown to be linearly related to the observed activity (Herscovitch et al., 1983). Therefore, the responses reported here are changes in linear radioactive distribution but will be referred to as changes in rCBF.
Data analysis was performed using the Statistical Parametric Map (SPM96) software developed at the Functional Imaging Laboratory, London, UK.
Patient movements between scans were corrected by a realignment procedure. Then all data were spatially normalized (Friston et al., 1995a) according to a stereotaxic space (Talairach and Tournoux, 1988) to allow inter-individual pooling onto the MNI (Montréal National Institute, Canada) standard brain. Images were then smoothed with a Gaussian filter (full-width half-maximum 15 mm) to account for anatomical-functional variability.
The effect of global activity changes was removed by proportional scaling. The analysis was based on the estimation of the covariates introduced in the general linear model (Friston et al., 1995b) for each and every pixel exceeding 80% of the global mean value. Inference was performed through linear comparisons or contrasts based on a t test. The resulting set of voxel values (t map) was then transformed to the unit normal distribution (Z map) and thresholded (3.09). Significance judgement was based on the combination of spatial extent and peak intensity of cluster of voxels exceeding the threshold of 3.09 (Poline et al., 1997). The effect related to the repetition of conditions (including the effect of time) was included in the model as a confounding covariate for the analyses.
In a preliminary study, using three subtraction analyses, each one of the three painful (P) conditions (1, 3 and 5) was successively compared with the minimal condition of the study [2, non-painful heat (p) no task] which was used as a reference. Thus, in each one of these three pre-determined contrasts, we isolated the rCBF changes reflecting brain responses to pain (P versus p conditions) plus activity possibly related to the attentional context (i.e. A, N, D).
Then, the first step of our study was to categorize rCBF changes into the two relevant components of our factorial design (Table 1).
(i) A first component labelled intensity, which isolated the rCBF changes related to the differences of thermal intensity between P and p conditions, regardless of the attentional context. It was assessed by the subtraction of paired painful (P, 1 and 3) and non-painful conditions (p, 2 and 4; Table 1, left column; Fig. 2). To minimize the participation of the general features of attention, this intensity coding component was also approached using a conjunction analysis (Price and Friston, 1997) of the three pre-determined contrasts (1 versus 2, 3 versus 2, and 5 versus 2; Table 1, middle column).
(ii) A second component, labelled selective attention, contained the rCBF changes specifically related to the turning of attention to the stimulated hand. It was assessed by the comparison of the two attentional (A, 3 and 4) with the two non-attentional (N, 1 and 2) conditions, regardless of thermal intensity (Table 1, right column; Fig. 2).
In a second step, the interactions between the intensity and the attentional components were evaluated. Comparison of conditions 3 and 4 versus 1 and 2 allowed investigation of the intensity-related responses in an attentional versus a non-attentional context. Comparison of conditions 3 and 1 versus 4 and 2 allowed investigation of the attention-related responses to a painful versus a non-painful stimulus.
Finally, in a further comparison of the three pre-determined contrasts (1 versus 2, 3 versus 2, and 5 versus 2), we qualitatively assessed the variability of brain responses to pain according to the attentional context (Fig. 3; Table 2). The effect of auditive diversion was assessed by the contrast subtracting condition 1 (pain, no task) to 5 (pain, diversion).
All the previous comparisons were performed on two data sets. In the first, images of subjects who were stimulated on the left side were flipped in order to homogenize data for the side of stimulation before normalization and inter-individual pooling (data set I). In the second (data set II), images were not flipped to determine brain activities regardless of the side where the stimulus was applied. Then, for each contrast, in a multi-study performed on unflipped data, we compared the responses of subjects stimulated on the right with the responses of subjects stimulated on the left side.
Results
Behavioural aspects
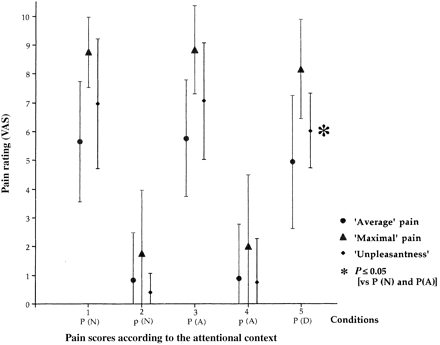
Rating of pain sensation was parallel for each one of the three scoring methods (i.e. average pain, maximal pain, unpleasantness; Fig. 4). VAS was significantly lower for non-noxious (p, 2 and 4) than for noxious stimuli (P, 1, 3 and 5). Subjective pain intensity did not significantly differ (paired t test, P ≥ 0.7) in neutral (1) and attentional (3) conditions but VAS was significantly lower in the diversive (5) context than in both neutral and attentional conditions (P ≥ 0.05; Figs 1 and 4).
rCBF: lateralization
No significant difference was observed between the two populations of subjects, those stimulated on the left and those stimulated on the right side for the successive comparisons which were performed as shown above. Using non-flipped images (data set II) subjects who were stimulated on the left side showed isolated right-sided hemispheric responses for attentional responses in the prefrontal and the parietal cortices (i.e. responses which were independent of pain and side of stimulation, Table 1).
The results are generated from the inter-individual pooling of datasets flipped for subjects stimulated on the left side and unflipped for subjects stimulated on the right side (data set I). This procedure was chosen to take into account the side of stimulation, given that the responses in the two populations did not differ and that (right) hemispheric responses have been previously identified.
Intensity and attentional components
The main statistical comparisons were designed to dissect the effects of the intensity coding and the selective attentional components on rCBF changes (see Methods).
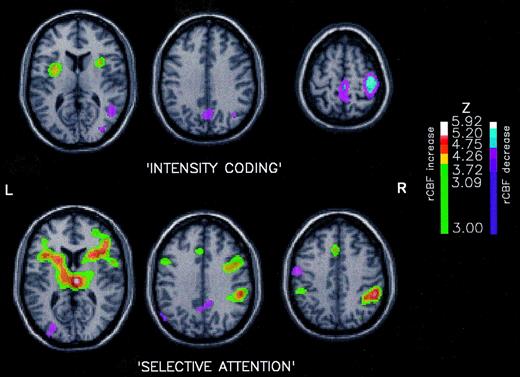
The rCBF increases associated with the intensity factor (once the general features of attention had been averaged out), were restricted to the anterior insula/SII regions, bilaterally (Fig. 2; Table 1, left column). On the other hand, rCBF decreases were observed in the hemisphere ipsilateral to pain, in the primary somatosensory cortex, paracentral lobule [Brodmann area (BA) 7], parieto-occipital cortex (BA 19 and 39) and hippocampal formation (Fig. 2; Table 1, left column). No rCBF change was found in anterior cingulate cortex. The same changes (concerning both increased and decreased rCBF) plus a significant thalamic activation contralateral to stimulation were observed when intensity coding was assessed by a conjunction analysis of the three pre-determined contrasts.
The rCBF changes associated with selective attention, irrespective of stimulus intensity (conditions 3 and 4 versus conditions 1 and 2) demonstrated a widely extended cortico-thalamo-mesencephalic network (Fig. 2; Table 1, right column). Increases in rCBF associated with attention were observed in both thalami and in prefrontal (BA 44, 45), parietal (BA 40) and anterior cingulate (BA 24) cortices. Prefrontal (BA 44) and posterior parietal (BA 40) rCBF increases were found to be lateralized on the right hemisphere, regardless of the side where the stimulus was applied. This was confirmed by statistical analyses performed successively in both sub-populations of subjects, those stimulated on the right hand, and those stimulated on the left hand. No left-sided activity was evidenced. Decreases in rCBF were found in the primary motor and the temporo-occipital cortices contralateral to stimulation and in the posterior cingulate cortex (BA 31).
Interactions between intensity and attentional components
The interactions between the intensity and the attentional rCBF effects (i.e. conditions 3 and 4 versus 1 and 2, and 3 and 1 versus 4 and 2) were not significant. Thus, the functional activation maps related to intensity coding and selective attention appeared to be superimposed rather than to interact.
Variability of brain responses to pain
In the further statistical analysis, the brain responses to pain were shown to be different in the three attentional contexts (Fig. 3; Table 2). A common denominator in all attentional contexts (A, N, D) was the rCBF increase in the anterior insula/SII cortex contralateral to pain (Fig. 3B). Additional rCBF increases were observed bilaterally in the insula and thalamus and in the cerebellar vermis in the conditions where the subject was asked to perform an attentional task, whether directed or not towards the stimulus (A, D; Fig. 3A and C).
In the attentional (A) condition (Fig. 3A), increases of rCBF were also observed bilaterally in the prefrontal cortices (BA 9, 11, 44, 46) and in the posterior parietal cortex (BA 40) ipsilateral to stimulation. Stereotaxic coordinates were similar to those observed for the selective attentional component (Table 2, right column).
In the diversive (D) condition (Fig. 3C), a dissociation was observed between significantly decreased VAS scores and the rCBF increase in the mid part of anterior cingulate cortex (BA 24). Compared with the cingulate rCBF increase, as a part of the selective attentional component which is located anteriorly and rostrally, this activity appeared different without any overlapping of activated areas (Fig. 5). In the condition D, which included an auditive discrimination task, a rCBF increase was also observed (below the statistical threshold), in the temporal neocortex (BA 22) immediately posterior to Heschl's gyrus. When the effect of auditive diversion was isolated (comparison of condition 5, P, D with 1, P, N) there was an increased rCBF in thalami and the temporal neocortex but it was unmodified in insulae/SII cortices and anterior cingulate.
The primary somatosensory cortex ipsilateral to pain showed significant rCBF decrease across the three comparisons. The size and the significance of blood flow changes increased with the level of attention to the thermal stimulus, i.e. they were minimal in the diversive and maximal in the attentional conditions. There was also a decrease in rCBF in the posterior cingulate (BA 31), only in the attentional condition, and in the paracentral lobule (BA 7) in both the attentional and the diversive conditions of pain.
Discussion
The increases in rCBF observed in our subjects have all been previously reported as `pain-related' responses in functional imaging studies (see references in Tables 1 and 2), suggesting that they are truly dependent upon pain or pain-associated processes. However, as pain sensation is known to result from multi-dimensional integrations (Melzack and Casey, 1968; Melzack and Katz, 1994), our study was designed to discriminate between the sensory and attentional-cognitive components of the brain response to a painful stimulus. Our approach allowed us to distinguish, within the previously reported `pain-activated' areas, an intensity coding matrix superimposed on an attentional network. The intensity coding matrix was comprised of the anterior insula and SII cortices bilaterally and the contralateral thalamus, and the attentional network which was much more extended, involving both thalami, the posterior parietal and prefrontal cortices, and the anterior cingulate gyrus.
rCBF increases associated with intensity coding (Fig. 2)
Averaging out of the attentional component allowed isolation of the nociceptive or the intensity coding map of the brain response to our noxious stimulation. This was restricted to the anterior insula/SII cortices and the contralateral thalamus. Given the number of studies in which similar activations in these structures were reported (see Table 1), their relationship with pain-related activity does not seem questionable. Conversely, we did not find any verification of the hypothesis (Craig et al., 1996; Rainville et al., 1996) that the anterior cingulate cortex also encodes the intensity of a thermal stimulation. Indeed, the anterior cingulate cortex appeared to belong to the second functional map detected in our study—i.e. the selective attentional matrix—suggesting that this structure is primarily involved in attentional processes associated with pain sensation rather than in the encoding of stimulus intensity.
Activation of the contralateral insular and SII cortices was also the only common denominator of the rCBF response across the three attentional contexts (N, A, D) associated with heat pain stimulation (Fig. 3; Table 2). This is consistent with recent results, obtained using evoked potentials to noxious CO2-laser stimuli, which showed that the lateralized early component NP160, generated in or near SII (Valeriani et al., 1996; Frot et al., 1999), is a stable response resistant to attentional modulation (Garcıa-Larrea et al., 1997). The localization of the insular/SII response in our subjects is congruent with those reported in previous PET studies (Casey et al., 1994, 1996; Coghill et al., 1994; Craig et al., 1996; Hsieh et al., 1996; Vogt et al., 1996; Derbyshire et al., 1997; Rainville et al., 1997; Svensson et al., 1997; Xu et al., 1997; Iadarola et al., 1998; May et al., 1998), even though insula and SII responses are not easily differentiated from each other using PET because of the limited spatial resolution of the technique, the need for group analysis, the inter-individual variability in the rostrocaudal distribution of SII (Mauguière et al., 1997) and the anatomical proximity of the two structures. Notwithstanding, the stereotaxic localization of SII/insular rCBF changes in our subjects fits accurately with that of the responses to CO2-laser stimuli recorded in the insula and SII cortices by intra-cerebral electrodes (Frot et al., 1999). Therefore, as previously suggested (Casey et al., 1996; Craig et al., 1996; Derbyshire et al., 1997), this activity may be essential to the encoding of thermal discrimination between warm and painful heat temperature.
rCBF increases related to selective attention (Fig. 2): cognitive aspects of pain perception
Increases in rCBF in the posterior parietal (BA 40), anterior cingulate (BA 32), dorsolateral prefrontal (BAs 44 and 45) and thalamic regions (Fig. 2), have all been previously reported as `pain-related' activities in studies where the attentional component of pain was not specifically investigated (see Table 1). In parallel, these same structures have also been reported as belonging to functional attentional networks in both visual and somatosensory modalities (Pardo et al., 1991; Corbetta et al., 1993; Posner, 1994; Lewin et al., 1996; Fink et al., 1997; McCarthy et al., 1997; Nobre et al., 1997). This large cortical and thalamo-mesencephalic network is therefore activated both in attentional contexts and when a subject is undergoing pain. We suggest that in this latter case, such a network reflects in part, the attentional-cognitive activity triggered by the noxious stimulus. Interestingly, the neocortical components of the attentional network to pain predominated in the right hemisphere, as has been reported in previous attentional studies. In particular, right-sided rCBF responses in dorsolateral prefrontal and posterior parietal cortices, identical to those observed in our subjects, have been specifically described as `attention-related' activities (Pardo et al., 1991; Corbetta et al., 1993; Gitelman et al., 1996).
Relationships between attention and intensity coding (Fig. 2)
When gathered together into one integrative functional map, overlapping of the selective attention and intensity coding networks closely matched the previously reported `pain-related' activities (Table 1). No evidence of significant interactions was found between the intensity coding and the attentional matrices—i.e. the magnitude and distribution of the attentional responses were not influenced by the stimulus intensity and vice versa (see Results). This suggests that, under our experimental conditions, the attentional and intensity maps were strictly superimposed on each other, the sum of the two contributing to the subjective pain experience. Of course, we cannot exclude (and it is indeed likely) that under other conditions of attentional load, particularly if they are sufficient to modify the VAS rating, the intensity coding and the selective attentional maps may interact.
Particular aspects of the attentional matrix: selective attention versus arousal
As shown in Fig. 3, pairwise comparisons using the minimal condition (no pain/no attention) as a reference showed striking similarities between the attentional and diversive contexts. Notably, the thalamus and upper brainstem exhibited significant rCBF increases whether attention was directed towards (A) or away (D) from the thermal stimulus. Conversely, the right prefrontal and posterior parietal cortices had enhanced rCBF exclusively when attention was directed toward the stimulated hand (A) while the auditory associative cortex (supratemporal plane) and the anterior cingulate rCBF were increased only during auditory attention. We may thus hypothesize that the attentional network disclosed by the factorial SPM analysis might be further decomposed into two components, one of which would be non-specific and common to all conditions requiring the active detection of sensory targets, whatever their origin (i.e. somatosensory or auditive). Such a component, involving both thalami and upper brainstem regions (Fig. 3A and C versus B), may be assimilated to arousal and has been previously identified in different kinds of attentional tasks (Posner, 1994; Posner and Dehaene, 1994; Fredrikson et al., 1995). It is supposed to involve thalamoreticular structures which might support the concept of amplification of the relevant information which is addressed to specialized cortical areas (Posner, 1994). Further to this arousal network, other activated areas might reflect the selective components of attention, which are spatial and modality-dependent. Thus, as previously reported (Pardo et al., 1991; Posner, 1994; Fink et al., 1997; Nobre et al., 1997), the prefrontal and posterior parietal effects (Fig. 3A) appear to be more specifically linked to the spatial components of selective attention while the activation of the temporal associative cortex immediately posterior to Heschl's gyrus (Fig. 3C) would reflect tonal discrimination processes (Binder et al., 1996; O'Leary et al., 1997).
It is noteworthy that neither the arousal, nor the selective attentional components were detected in the no-task condition where participants had been asked to pay no attention to the stimuli. In this condition, the brain response was reduced to strictly discriminative aspects (insula/SII; Fig. 3B). This is surprising if we consider that, by default, a noxious heat stimulus should have prompted an attentional reaction from the subject, even in the absence of an explicit task. The absence of such attentional drive may be explained in our subjects by their intensive pre-experimental training, introduced to ensure that the no task situation was as neutral a condition as possible. This finding further illustrates the importance of a strict control of the attentional context and of the degree of subjects' training to the experimental paradigm.
When the no pain/no attention condition was used as a reference, the mid part of anterior cingulate cortex appeared to undergo the most important rCBF changes during the auditory discriminative task, i.e. when participants' attention was driven away (D) from the thermal stimulus. This activity was not found to be related to auditive attention. It was associated with lowered pain scores, and thus underscored the dissociation between anterior cingulate rCBF and the encoding of pain intensity. It could not be attributed to selective attention since it was located caudally (Fig. 5), and previous studies showed rostral and mid-cingulate activities in relation to attention and pain processes, respectively (Davis et al., 1997; Derbyshire et al., 1998). Conversely, our findings are in accordance with the notion that anterior cingulate activity (i) strongly depends on the intrusive nature of a stimulus and its ability to capture awareness (Posner, 1994), and (ii) is enhanced under conditions of divided attention (Pardo et al., 1990; Corbetta et al., 1991; Bench et al., 1993). These two characteristics were indeed present especially during our diversive condition, where the participants' attention, disturbed by the peaks of temperature, iteratively shifted between the auditory modality (main detection task) and the peaks of pain. Such attentional shift (including orientating and/or reply to peaks of pain) could have subserved the mid-cingulate activity observed in this condition, and perhaps also in previous studies where the attentional component of pain was not controlled. The lower mid-cingulate activation in the attentional context (A; Fig. 3A) could be explained by both a decrease of attentional shifting and an easier thermal detection task for noxious temperatures than for innocuous stimulations. Indeed, anterior cingulate activity is known to be lowered during simple or repetitive tasks (Grafton et al., 1994; Posner, 1994; Posner and Dehaene, 1994; Davis et al., 1997; Jueptner et al., 1997; Bush et al., 1998) and to be inhibited when, as in our subjects, a sustained attention increases activity in the prefrontal cortex (Van Hoesen et al., 1993; Posner, 1994). Additionally, the variability of the cingulate response across conditions in our subjects and the known poor functional specificity of this multi-integrative structure (Grafton et al., 1994; Devinsky et al., 1995; Fredrikson et al., 1995; Gitelman et al., 1996; Murtha et al., 1996; Picard and Strick, 1996; Warburton et al., 1996; Davis et al., 1997; Jueptner et al., 1997; Morris et al., 1998) suggest complex interactions between the different components of attention and probably also with several additional parameters such as emotion, motor planning and memory which were not adressed in this study.
rCBF decrease in primary sensory areas: anticipation of pain?
Neither the intensity coding, nor the attentional networks disclosed in this study implicated increased rCBF in the primary somatosensory area (SI). Previous reports on pain-related rCBF changes have been notoriously inconclusive about the possible existence of consistent SI responses. Thus, while a number of studies have reported significant pain-related rCBF increases in SI (Talbot et al., 1991; Casey et al., 1994, 1996; Coghill et al., 1994; Craig et al., 1996; Hsieh et al., 1996; Rainville et al., 1997; Iadarola et al., 1998), a similar number of other reports have failed to do so (Jones et al., 1991; Apkarian et al., 1992; Derbyshire et al., 1994; Hsieh et al., 1995; Vogt et al., 1996; Derbyshire et al., 1997; Svensson et al., 1997; Xu et al., 1997; May et al., 1998; Peyron et al., 1998, 1999). A general trend that can be inferred from previous literature is that SI rCBF tended to be enhanced when the painful stimulus was a moving one, while no change occurred in cases of immobile stimuli (Jones 2et al., 1995). This has led to the hypothesis that most of SI rCBF changes are related to activity in lemniscal pathways, rather than the spinothalamic system (Peyron et al., 1998). In accordance with previous results, in our present study, which used fixed stimuli, no increase in rCBF was detected in the SI area contralateral to the heat stimulus. In contrast, a very robust rCBF decrease was observed in the SI region ipsilateral to the stimulated hand (Figs 2 and 3). A similar decrease in blood flow in the SI area ipsilateral to the stimulus was described by Drevets and colleagues (Drevets et al., 1995) during an experiment where subjects awaited a noxious stimulus which in fact never came. Decreased synaptic activity in the sensory cortex which is not directly involved in the processing of the expected painful stimulus was considered to reflect pain anticipation, and a similar interpretation may be applied to our results. However, in our patients, decreased rCBF in SI appeared to pertain also to the intensity factor, i.e. it was significant when the attentional component had been eliminated (Fig. 2). Thus, independently of the cognitive construct labelled anticipation, the reduction of activity in cortical areas ipsilateral to the stimulus may be seen as a sensory adaptative mechanism to enhance the functional contrast between homologous SI cortices and thus the detection of intensity differences. It is noteworthy that several other systems apparently based on contrast-enhancing mechanisms have been described in the context of pain processing, notably the descending noxious inhibitory control (DNIC) (Le Bars et al., 1979a, b). Thus, contrast-enhancing mechanisms linked to anticipation, stimulus repetition or both, may also contribute to the intensity coding processes detected in this study.
Other sites of rCBF changes whose classification remains uncertain
Decreases in rCBF of uncertain interpretation were observed in the hippocampal formation and the primary motor and the parieto-occipital cortices (Fig. 2). A rCBF decrease in the hippocampal formation during pain experiments has been previously reported (Derbyshire et al., 1997; Kupers et al., 1998). With the exception of insular activity, this was the only rCBF change in our study that might pertain to the affective-emotional response to pain. This component of pain processing has very seldom been investigated in previous PET studies and is likely to have been minimized by the instruction and intensive training of our subjects. Since very little information is available on the rCBF counterparts of affective-motivational components and because our study design was not aimed at assessing this particular axis of pain processing, we prefer not to engage in further speculative discussion.
As in other studies on pain (Derbyshire et al., 1994, 1997; Svensson et al., 1997; Peyron et al., 1998), we observed that rCBF decreases bilaterally in temporo-occipital and parieto-occipital cortices. The significance of these findings remains unknown as does their relation to pain since similar results have also been reported during a variety of tasks including vestibular stimulations (Wenzel et al., 1996) and semantic tasks (Warburton et al., 1996). A common interpretation of such peri-occipital deactivation is a shift of activity from brain areas not involved in the task to functionally activated cortices (Peyron et al., 1998). However, the possibility for these poorly explained rCBF decreases to be a part of cortical networks involved in some aspect of pain representation processes cannot be ruled out, especially if we consider the additional rCBF decreases that we observed in other cortical areas. Particularly, the focal rCBF decrease in the primary motor cortex might reflect inhibition and/or control of motor activity when the subjects are paying attention to their stimulated hand. Balance and interactions between structures of large networks involved in selective attention, pain or motor control could account for these focal rCBF decreases. The anterior cingulate and its reciprocal connections with motor, visuospatial, attentional and affective-emotional systems (Devinsky et al., 1995) may be considered as a possible interface between these different systems.
In conclusion, our findings suggest that the haemodynamic brain response to pain, as assessed by PET, combines at least three components. The contralateral insula/SII cortex appears to be constantly activated during noxious heat, regardless of the attention assigned to the stimulus; the insula/SII interface cortices may thus contribute to the sensory-discriminative processing of pain. A major attentional component was also found to contribute to rCBF pain-related changes, and to involve a distributed cortico-subcortical network. The combination of arousal mechanisms and selective attention toward the stimulated hand activated a large network involving mesencephalon, thalamus and prefrontal, posterior parietal and anterior cingulate cortices, primarily on the right hemisphere. The anterior cingulate cortex was mainly activated in conditions of strong orientating to intrusive stimuli, and is likely to integrate several cognitive-evaluative aspects. The possible cingulate contribution to the affective dimension of pain experience was not assessed in this study. Finally, a rCBF decrease in primary sensory areas ipsilateral to pain may contribute to a mechanism of intensity contrast enhancement and perhaps reflect some anticipatory components of the pain response.
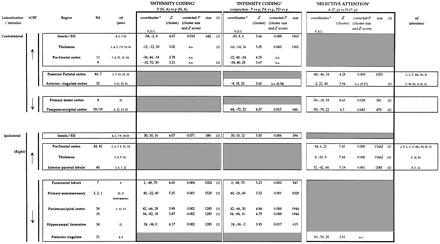
Schematic evaluation of brain response to pain. Stimulus intensity-related (P versus p conditions, left and middle column) and selective attention-related (A versus N conditions, right column) rCBF changes. There were no overlapping peaks of rCBF changes, suggesting that the comparisons accurately categorized two different activities. Overall, the two methods which were used to assess the intensity coding component provided similar results except for thalamic rCBF increase contralaterally to pain which did not reach significance in the subtraction analysis (left column) while it was significant in the conjunction analysis (middle column). * = Talairach and Tournoux atlas, 1988; n.s. = non-significant (P > 0.05, corrected); BA = Brodmann area; size = number of connected voxels exceeding the Z hreshold of 3.09; [1] = regions which were significantly activated in the study using unflipped data i.e. regions which are activated bilaterally or regardless of the side of stimulation). References of previous PET studies are listed according to the following numbers for pain studies and as alphabetical letters for the studies on attention. 1 = (Talbot et al., 1991); 2 = (Jones et al., 1991); 3 = (Derbyshire et al., 1994); 4 = (Coghill et al., 1994); 5 = (Casey et al., 1994); 6 = (Hsieh et al., 1995); 7 = (Hsieh et al., 1996); 8 = (Vogt et al., 1996); 9 = (Casey et al., 1996); 10 = (Craig et al., 1996); 11 = (Rainville et al., 1997); 12 = (Derbyshire et al., 1997); 13 = (Svensson et ., 1997); 14 = (Xu et al., 1997); 15 = (May et al., 1998); 16 = (Iadarola et al., 1998). C = (Corbetta et al., 1993); F = (Fredrikson et al., 1995); G = (Grafton et al., 1994); Gi = Gitelman et al., 1996); J = (Jueptner et al., 1997); L = (Lewin et al., 1996); M = (Murtha et al., 1996); Mc = (McCarthy et al., 1997); N = (Nobre et al., 1997); P = (Pardo et al., 1991); Po = (Posner, 1994; Posner and Dehaene, 1994); W = (Warbuton et l.,1996).

Schematic evaluation of brain response to pain. Stimulus intensity-related (P versus p conditions, left and middle column) and selective attention-related (A versus N conditions, right column) rCBF changes. There were no overlapping peaks of rCBF changes, suggesting that the comparisons accurately categorized two different activities. Overall, the two methods which were used to assess the intensity coding component provided similar results except for thalamic rCBF increase contralaterally to pain which did not reach significance in the subtraction analysis (left column) while it was significant in the conjunction analysis (middle column). * = Talairach and Tournoux atlas, 1988; n.s. = non-significant (P > 0.05, corrected); BA = Brodmann area; size = number of connected voxels exceeding the Z hreshold of 3.09; [1] = regions which were significantly activated in the study using unflipped data i.e. regions which are activated bilaterally or regardless of the side of stimulation). References of previous PET studies are listed according to the following numbers for pain studies and as alphabetical letters for the studies on attention. 1 = (Talbot et al., 1991); 2 = (Jones et al., 1991); 3 = (Derbyshire et al., 1994); 4 = (Coghill et al., 1994); 5 = (Casey et al., 1994); 6 = (Hsieh et al., 1995); 7 = (Hsieh et al., 1996); 8 = (Vogt et al., 1996); 9 = (Casey et al., 1996); 10 = (Craig et al., 1996); 11 = (Rainville et al., 1997); 12 = (Derbyshire et al., 1997); 13 = (Svensson et ., 1997); 14 = (Xu et al., 1997); 15 = (May et al., 1998); 16 = (Iadarola et al., 1998). C = (Corbetta et al., 1993); F = (Fredrikson et al., 1995); G = (Grafton et al., 1994); Gi = Gitelman et al., 1996); J = (Jueptner et al., 1997); L = (Lewin et al., 1996); M = (Murtha et al., 1996); Mc = (McCarthy et al., 1997); N = (Nobre et al., 1997); P = (Pardo et al., 1991); Po = (Posner, 1994; Posner and Dehaene, 1994); W = (Warbuton et l.,1996).
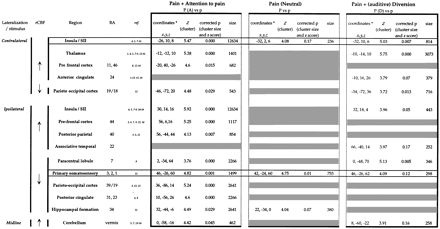
All comparisons performed versus the minimal reference condition [2, non-painful heat (p), no task, N]. In each column the rCBF changes related to pain in a neutral condition of attention (middle column), pain + attention to pain (left column) or pain + attention away from pain (right column). * = Talairach and Tournoux atlas, 1988; n.s. = non-significant (P > 0.05, corrected); BA = Brodmann area; size = number of connected voxels exceeding the Z threshold of 3.09; boxed insert = regions with common rCBF changes to all three conditions. For references see footnotes to Table 1.

All comparisons performed versus the minimal reference condition [2, non-painful heat (p), no task, N]. In each column the rCBF changes related to pain in a neutral condition of attention (middle column), pain + attention to pain (left column) or pain + attention away from pain (right column). * = Talairach and Tournoux atlas, 1988; n.s. = non-significant (P > 0.05, corrected); BA = Brodmann area; size = number of connected voxels exceeding the Z threshold of 3.09; boxed insert = regions with common rCBF changes to all three conditions. For references see footnotes to Table 1.
Summary of the PET procedure. In each of the five conditions were performed: (i) a thermal continuous stimulation of high (painful, P) or low (non-painful, p) intensity on which five peaks (1°C higher, 2 s) were randomly added, (ii) an auditory stimulation (background white noise with random attenuations), neglected in all conditions except in condition 5 where it was diversive (D) from pain, and (iii) a counting task: either repetitive iteration from 1 to 10 (conditions 1 and 2), counting of temperature peaks in attentional task (A, conditions 3 and 4) or counting of background noise attenuations in diversive auditive task (D, condition 5). Large arrows indicate to where attention is directed. The mean VAS scores of each condition is indicated on the right.
Proposed intensity coding and selective attention components of rCBF changes. `Intensity coding' (P versus p conditions, top row): when compared with low-intensity (p) conditions (2 and 4), the high intensity (P) scans (1 and 3) showed significantly higher rCBF in insula/SII bilaterally. Significant rCBF decrease was observed ipsilaterally to pain in SI cortex. `Selective attention' (A versus N conditions, bottom row): the comparison of attentional (3 and 4) versus non-attentional conditions (1 and 2), irrespective of stimulus intensity, showed a large network of attention-related rCBF increase involving anterior cingulate cortex, thalami, prefrontal and posterior parietal cortices bilaterally. Significantly decreased rCBF were observed in primary motor cortex contralaterally to the stimulated hand, in the parieto-occipital cortex and the posterior cingulate. In each comparison, data were thresholded for Z > 3.09 and P corrected for cluster size and Z score was P < 0.05 (Poline et al., 1997).
Variations of pain-related rCBF according to the attentional context. Each of the three conditions using noxious stimuli (P, 1, 3 and 5) was succesively compared with the reference condition [2, non-painful heat (p), no task]. The context associated with the noxious stimuli conditions was: (A) attention to the stimulus, (B) no task, and (C) attention away from the stimulus (diversive). The only region of rCBF increase common to the three comparisons was the insula/SII response contralateral to pain (conjunction analysis, P ≤ 0.0001, corrected). The bilateral rCBF increase in thalamus (A and C) was seen in both attentional conditions and may be considered as a marker of non-specific attention or arousal. When attention was focused to the stimulated hand (A) rCBF changes in prefrontal and posterior parietal cortices were disclosed, with a localization similar to the selective attentional network (Fig. 2; Table 1). The size and the significance of decreased rCBF in SI ipsilaterally to stimulation (conjunction analysis, P = 0.001, corrected) increased with the level of attention to pain and was assumed to reflect anticipation. Auditory attention (diversive from pain) showed rCBF increase in the temporal neocortex, immediately posterior to Heschl's gyrus (C). Increased rCBF in the anterior cingulate gyrus was observed when the subject's attention was directed away from pain (C) suggesting an alerting effect, orienting to pain or attentional shift. In each omparison, data were thresholded for Z > 3.09 and P corrected for cluster size and Z score was P < 0.05.
Relative localization of increased rCBF within anterior cingulate cortex: the role of retort to pain and `selective attention'. Increased rCBF in anterior cingulate cortex was observed in two comparisons, one resulting from subtraction of reference from diversive condition (green colour scale) and the other reflecting the sum of attentional activities (`selective attention', blue colour scale). The results of the two comparisons were superimposed on the MRI of the template to determine the accurate localization of each one of these two functions. Data were thresholded for Z > 3.09 and P correlated for cluster size and Z score was P < 0.05. There was no overlap between these two activations, the `selective attention' activity being localized anteriorly.
Relative localization of increased rCBF within anterior cingulate cortex: the role of orienting response to pain and selective attention. Increased rCBF in anterior cingulate cortex was observed in two comparisons, one resulting from subtraction of reference condition [2, non-painful heat (p), no task] from diversive condition (green colour scale) and the other reflecting the sum of attentional activities (selective attention, blue colour scale). The results of the two comparisons were superimposed on the MRI of the template to determine the accurate localization of each one of these two functions. Data were thresholded for Z > 3.09 and P corrected for cluster size and Z score was P < 0.05. There was no overlap between these two activations, the selective attention activity being localized anteriorly.
We wish to thank the Institut UPSA® de la douleur for financial support, the 12 volunteers who `suffered' for this study and the CERMEP team for PET data acquisition.
References
Apkarian AV, Stea RA, Manglos SH, Szeverenyi NM, King RB, Thomas FD. Persistent pain inhibits contralateral somatosensory cortical activity in humans.
Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, et al. Investigations of the functional anatomy of attention using the Stroop Test.
Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Function of the left planum temporale in auditory and linguistic processing.
Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting stroop: an interference task specialized for functional neuroimaging. Validation study with functional MRI.
Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli.
Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain.
Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, et al. Distributed processing of pain and vibration by the human brain.
Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color and speed: functional anatomy by positron emission tomography.
Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention.
Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain [see comments].
Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex.
Derbyshire SW, Jones AK. Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography.
Derbyshire SW, Jones AK, Devani P, Friston KJ, Feinmann C, Harris M, et al. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography.
Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity.
Derbyshire SW, Vogt BA, Jones AK. Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex.
Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. [Review].
Drevets WC, Burton H, Videen TO, Snyder AZ, Simpson JR Jr, Raichle ME. Blood flow changes in human somatosensory cortex during anticipated stimulation [see comments].
Fink GR, Dolan RJ, Halligan PW, Marshall JC, Frith CD. Space-based and object-based visual attention: shared and specific neural domains.
Fredrikson M, Wik G, Fischer H, Andersson J. Affective and attentive neural networks in humans: a PET study of Pavlovian conditioning.
Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images.
Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach.
Frot M, Rambaud L, Guénot M, Mauguière F. Intracortical recordings of early pain-related CO2-laser potentials in the human second somatosensory (SII) area.
García-Larrea L, Peyron R, Laurent B, Mauguière F. Association and dissociation between laser-evoked potentials and pain perception.
Gitelman DR, Alpert NM, Kosslyn S, Daffner K, Scinto L, Thompson W, et al. Functional imaging of human right hemispheric activation for exploratory movements.
Grafton ST, Woods RP, Tyszka M. Functional imaging of procedural motor learning: relating cerebral blood flow with individual subject performance.
Guilbaud G, Bernard JF, Besson JM. Brain areas involved in nociception and pain. In: Wall PD, Melzack R, editors. Textbook of pain. 3rd ed. Edinburgh: Churchill Livingstone; 1994. p. 113–28.
Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis.
Hsieh JC, Hägermark O, Ståhle-Bäckdahl M, Nordell B, Ericson K, Eriksson L, et al. The urge to scratch represented in the human cerebral cortex during itch: PET and fMRI.
Hsieh JC, Ståhle-Bäckdahl M, Hägermark Ö, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study.
Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, et al. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET.
Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography.
Jones AK, Derbyshire SWG. Cortical and thalamic imaging in normal volunteers and patients with chronic pain. In: Besson JM, Guilbaud G, Ollat H, editors. Forebrain areas involved in pain processing. Paris: John Libbey Eurotext; 1995. p. 229–38.
Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error.
Kupers RC, Svensson P, Jensen TS, Arendt-Nielsen L, Gjedde A. Cerebral responses to experimentally induced muscle pain in the facial region measured by positron emission tomography [abstract].
Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat.
Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications.
Lewin JS, Friedman L, Wu D, Miller DA, Thompson LA, Klein SK, et al. Cortical localization of human sustained attention: detection with functional MR using a visual vigilance paradigm.
Mauguière F, Merlet I, Forss N, Vanni S, Jousmäki V, Adeleine P, et al. Activation of a distributed somatosensory cortical network in the human brain. A dipole modelling study of magnetic fields evoked by median nerve stimulation. Part I: location and activation timing of SEF sources.
McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI.
May A, Kaube H, Büchel C, Eichten C, Rijntjes M, Jüptner M, et al. Experimental cranial pain elicited by capsaicin: a PET study.
Melzack R, Casey KL. Sensory, motivational, and central control determinants of pain. In Kenshalo DR, editor. The skin senses. Springfield (IL): Charles C. Thomas; 1968. p. 423–39.
Melzack R, Katz J. Pain measurement in persons in pain. In: Wall PD, Melzack R, editors. Textbook of pain. 3rd ed. Edinburgh: Churchill Livingstone; 1994. p. 337–51.
Miron D, Duncan GH, Bushnell MC. Effects of attention on the intensity and unpleasantness of thermal pain.
Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions.
Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A. Anticipation causes increased blood flow to the anterior cingulate cortex.
Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography.
O' Leary DS, Andreasen NC, Hurtig RR, Torres IJ, Flashman LA, Kesler ML, et al. Auditory and visual attention assessed with PET.
Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm.
Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography.
Peyron R, García-Larrea L, Grégoire MC, Convers P, Lavenne F, Veyre L, et al. Allodynia after lateral-medullary (Wallenberg) infarct. A PET study.
Peyron R, García-Larrea L, Grégoire MC, Convers P, Richard A, Manet L, et al. Parietal and cingulate processings in central pain. A positron emission tomography (PET) study of one original case. Pain. In press 1999.
Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. [Review].
Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging.
Posner MI. Attention: the mechanisms of consciousness. [Review].
Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments.
Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex.
Siedenberg R, Treede RD. Laser-evoked potentials: exogenous and endogenous components.
Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Cerebral processing of acute skin and muscle pain in humans.
Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex [see comments].
Valeriani M, Rambaud L, Mauguière F. Scalp topography and dipolar source modelling of potentials evoked by CO2 laser stimulation of the hand.
Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus: a comprehensive handbook. Boston: Birkhäuser; 1993. p. 249–84.
Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging.
Warburton E, Wise RJ, Price CJ, Weiller C, Hadar U, Ramsay S, et al. Noun and verb retrieval by normal subjects. Studies with PET. [Review].
Wenzel R, Bartenstein P, Dieterich M, Danek A, Weindl A, Minoshima S, et al. Deactivation of human visual cortex during involuntary ocular oscillations. A PET activation study.


![Variations of pain-related rCBF according to the attentional context. Each of the three conditions using noxious stimuli (P, 1, 3 and 5) was succesively compared with the reference condition [2, non-painful heat (p), no task]. The context associated with the noxious stimuli conditions was: (A) attention to the stimulus, (B) no task, and (C) attention away from the stimulus (diversive). The only region of rCBF increase common to the three comparisons was the insula/SII response contralateral to pain (conjunction analysis, P ≤ 0.0001, corrected). The bilateral rCBF increase in thalamus (A and C) was seen in both attentional conditions and may be considered as a marker of non-specific attention or arousal. When attention was focused to the stimulated hand (A) rCBF changes in prefrontal and posterior parietal cortices were disclosed, with a localization similar to the selective attentional network (Fig. 2; Table 1). The size and the significance of decreased rCBF in SI ipsilaterally to stimulation (conjunction analysis, P = 0.001, corrected) increased with the level of attention to pain and was assumed to reflect anticipation. Auditory attention (diversive from pain) showed rCBF increase in the temporal neocortex, immediately posterior to Heschl's gyrus (C). Increased rCBF in the anterior cingulate gyrus was observed when the subject's attention was directed away from pain (C) suggesting an alerting effect, orienting to pain or attentional shift. In each omparison, data were thresholded for Z > 3.09 and P corrected for cluster size and Z score was P < 0.05.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/122/9/10.1093_brain_122.9.1765/1/m_b0902.f3.jpeg?Expires=1716366868&Signature=4DItWgkJDfWPyiVQvYRHvwHaYoZp22YlfiUnAKEzmufjCFObnYcX-Rt1e9WeSkR7qvLSaDrqKCsds-K-CwHFjxpqytrxXN-ac2MtHLCqDLOuKzME~m51sjc7n15NJAJfzikAhxEqypvO9UzNjBZzfDt0c7ORUubLcykXE4lYWKeTdAPiYkjaD237VWkZKRwtCr4C2YwBQgXegOTKqVBK63Ty1RE7Qfvg9i4rjahoV6Py6ekiz5ETr0x9cDMxiMLXa83RZfLRsQ6IC-dbRdWKPC87XYejsQv~wSBIchMlI0isymS8kRz17FN0UUSOmHjQ9Ktrbnus3eykDaeDbQIjRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Relative localization of increased rCBF within anterior cingulate cortex: the role of orienting response to pain and selective attention. Increased rCBF in anterior cingulate cortex was observed in two comparisons, one resulting from subtraction of reference condition [2, non-painful heat (p), no task] from diversive condition (green colour scale) and the other reflecting the sum of attentional activities (selective attention, blue colour scale). The results of the two comparisons were superimposed on the MRI of the template to determine the accurate localization of each one of these two functions. Data were thresholded for Z > 3.09 and P corrected for cluster size and Z score was P < 0.05. There was no overlap between these two activations, the selective attention activity being localized anteriorly.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/122/9/10.1093_brain_122.9.1765/1/m_b0902.f5.jpeg?Expires=1716366868&Signature=qBDvVH68T~wRoyl4076sAsBwmmV-uHEaBv9mjwvR-Abix5Ge4p~VfeHMZ9nlhJlIfSqz22KXIDfE3i3b0qjToda6Bcd34ugiKamFwcUT6JFPmg5IYwDuHS5Xc47HgkLmFKOb7udlsaOL4KBkVYoUf4x74GjC6avdWQ~vO3hbxCnPPbBx~esWXD3Wp~iaTio3Cq1JV6rkid~NV7WzVP-w8SdXO9dlQ~T9zjf0zDAJf8GuM8pMNrG4pX6Utm8m2f2MRu1gMgKCPM0-f6GGEhOc5DDX1gwDG~2HZJFjfUPFvjI~C0cvyb8Vw2XIR6TGiBag481y463YCESkHbwgy3cY4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
