-
PDF
- Split View
-
Views
-
Cite
Cite
Walter Magerl, Perry N. Fuchs, Richard A. Meyer, Rolf-Detlef Treede, Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia, Brain, Volume 124, Issue 9, September 2001, Pages 1754–1764, https://doi.org/10.1093/brain/124.9.1754
Close - Share Icon Share
Abstract
Polymodal nociceptors respond to mechanical, thermal and chemical stimuli. Whereas sensitivities to heat and to the irritant substance capsaicin have recently been linked via the properties of the vanilloid receptor type 1 receptor ion channel, sensitivity to noxious mechanical stimuli such as the pinpricks used in clinical neurology seems to be unrelated. We investigated the peripheral neural basis of pinprick pain using quantitative psychophysical techniques combined with selective conduction block by nerve compression and selective desensitization by topical capsaicin treatment. Complete A-fibre block by compression of the superficial radial nerve (criterion: loss of first pain sensation) lowered the stimulus–response function for pinprick pain (–82 ± 6% versus baseline). Topical pretreatment of the skin with a 10% capsaicin cream also lowered the pinprick stimulus–response function (–32 ± 10%), whereas laser-evoked heat pain was eliminated completely (–96 ± 2%). Under combined capsaicin desensitization and A-fibre blockade, pinprick pain was eliminated completely (–98 ± 1%). Intradermal injection of 40 μg capsaicin into normal skin between two skin areas that had been pretreated with either capsaicin cream or vehicle produced secondary hyperalgesia with a 260% enhancement of the stimulus–response function for pinprick pain in both areas. In contrast, axon reflexive flare spread only into the vehicle-treated area. These results suggest that capsaicin-sensitive afferents, including polymodal A-fibre and C-fibre nociceptors, make a small contribution to pinprick pain and that capsaicin-insensitive C-fibres do not contribute significantly to either mechanical or heat pain. Pinprick pain is mediated primarily by capsaicin-insensitive A-fibre nociceptors, which include high-threshold mechanoreceptors and type I mechano-heat nociceptors. In addition, central sensitization to input from these A-fibre nociceptors is the primary mechanism that accounts for the enhanced pain in response to punctate mechanical stimuli in the zone of secondary hyperalgesia. These capsaicin-insensitive A-fibre nociceptors may also mediate hyperalgesia in neuropathic pain.
Introduction
Application of the vanilloid capsaicin can lead to excitation and/or desensitization of a subgroup of small-diameter primary afferent nerve fibres, which are collectively called `capsaicin-sensitive afferents'. By the use of classical pharmacological techniques, these afferents have been shown to play important roles in pain sensation, hyperalgesia and neurogenic inflammation (for reviews, see Buck and Burks, 1986; Holzer, 1991). The molecular cloning of the vanilloid receptor type 1 (VR1) and its subsequent characterization as an ion channel that is activated by capsaicin, noxious heat and acids (for review, see Caterina and Julius, 1999) have supported the notion that capsaicin sensitivity is an important criterion to identify primary nociceptive afferents. At the level of the dorsal root ganglion neurone, for example, responses to noxious heat are inhibited by vanilloid receptor antagonists (Kirschstein et al., 1999; Nagy and Rang, 1999) and are eliminated in VR1 knockout mice (Caterina et al., 2000).
Not all nociceptive afferents, however, are sensitive to the actions of capsaicin. About 20% of small dorsal root ganglion neurones do not express VR1 mRNA (Michael and Priestley, 1999). Topical treatment of the skin with capsaicin leaves ~20% of epidermal afferents intact (Nolano et al., 1999). Mice lacking the VR1 gene show normal responses to noxious mechanical stimuli and exhibit only a moderate deficit in responses to noxious thermal stimuli (Caterina et al., 2000; Davis et al., 2000). Moreover, one of the classical nociceptor types, the Aδ-fibre high-threshold mechanoreceptor (HTM), is known to be resistant to the actions of capsaicin (Szolcsányi et al., 1988). In addition, type I A-fibre mechano-heat nociceptors are also insensitive to capsaicin (Ringkamp et al., 2001). This is consistent with the hypothesis that the VRL1 receptor, which is insensitive to capsaicin, mediates their heat response (Caterina et al., 1999).
We have suggested recently that A-fibre HTMs mediate the hyperalgesia to punctate mechanical stimuli (calibrated pinpricks) in the zone of secondary hyperalgesia surrounding an injury site (Treede and Cole, 1993; Cervero et al., 1994; Ziegler et al., 1999). In another study, we observed that hyperalgesia to a blade-shaped probe persisted in skin that was desensitized by topical treatment with capsaicin (Fuchs et al., 2000). On the other hand, a recent study found a close correlation between the size of secondary hyperalgesia and the size of flare detected by infrared thermography, which suggests that the same peptidergic nerve fibres that mediate flare may also mediate secondary hyperalgesia (Serra et al., 1998). In addition, a previous nerve block study reported that secondary hyperalgesia to punctate mechanical stimuli was mediated by C-fibres (Kilo et al., 1994). Resolution of this controversy will have important clinical implications, because capsaicin has been suggested as a topical agent for the treatment of neuropathic pain conditions, such as post-herpetic neuralgia (Watson et al., 1988; Lynn, 1990). Secondary hyperalgesia is considered to be a human surrogate model for neuropathic pain (Treede et al., 1992b; Liu et al., 1998), and capsaicin-insensitivity of an afferent fibre group involved in its mechanisms may explain the partial inefficacy of this treatment (Sindrup and Jensen, 1999).
The aim of this study was to quantify the contributions of capsaicin-sensitive and capsaicin-insensitive A-fibre and C-fibre nociceptors in signalling pain and secondary hyperalgesia to punctate mechanical stimuli. For this purpose, we refined and used two selective nerve fibre blocking techniques: nerve compression and topical capsaicin treatment of the skin. Secondary hyperalgesia to punctate mechanical stimuli was elicited by intradermal injections of capsaicin (LaMotte et al., 1991). In this model, hyperalgesia adjacent to the injection site is due to central sensitization in the spinal cord, which is induced by the discharges elicited by the capsaicin injection without changing primary afferent function in the zone of secondary hyperalgesia itself (Baumann et al., 1991; Simone et al., 1991; Sang et al., 1996).
Methods
Subjects
After ethics committee approval had been obtained (Ethikkommission der Landesärztekammer Rheinland—Pfalz and JCCI of the Johns Hopkins Medical Institutions), experiments were performed in 16 volunteers (14 male, two female, age 21–52 years, mean age 31 years). Ten subjects participated in the A-fibre block study (Experiment 1) and 10 subjects in the study of secondary hyperalgesia (Experiment 2). Subjects were free from neurological or dermatological disorders and did not have skin lesions in the area to be tested. Subjects gave written informed consent according to the declaration of Helsinki. All subjects were comfortably seated in a reclining chair with their forearms on armrests.
Pain to punctate mechanical stimuli
Pain induced by punctate mechanical stimuli (pinprick pain) was tested by a series of seven punctate probes (a geometrical series spaced by factors of 2 from 8 to 512 mN) with flat tips that did not penetrate the skin. Each probe consisted of a stainless steel wire (diameter 0.2 mm) mounted on a plastic rod of defined weight. The rod moved freely within a hand-held tube that was positioned perpendicular to the skin surface, such that the weight of the rod rested solely on the wire tip (Chan et al., 1992; Ziegler et al., 1999). These punctate stimuli were adequate to excite cutaneous nociceptors (Andrew and Greenspan, 1999; Slugg et al., 2000).
All stimuli were applied for ~1 s at interstimulus intervals of 10 s. Each stimulus was applied once in pseudorandom order within a run, and each subject was tested with five runs for any given occasion of testing. To minimize stimulus interaction (sensitization or fatigue), care was taken to avoid application of successive stimuli at the same spot. Pain to mechanical stimuli was rated on a verbal numerical rating scale (0 = no pain, 100 = maximal imaginable pain).
Capsaicin desensitization
For topical application, capsaicin (10%) was dissolved in ethoxydiglycol and incorporated in a base compound of acetyl alcohol, stearic acid and fatty acid ester (hydrophilic base compound; capsaicin cream prepared by Dr Sam Georgiou, Professional Arts Pharmacy, Baltimore, Md., USA). This high concentration of capsaicin was chosen because lower concentrations, as used in commercially available formulations, provide incomplete block of capsaicin-sensitive nociceptor function even after weeks of application (Simone and Ochoa, 1991; Fuchs et al., 2000).
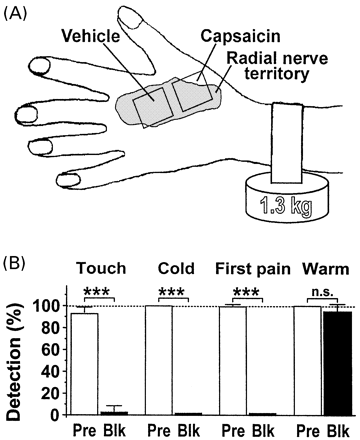
In Experiment 1 (A-fibre conduction blockade), two areas (25 × 25 mm) in the hairy skin of the left hand were pretreated for 6 h each on three successive days. One area was pretreated by topical application of 10% capsaicin cream and the other area was treated with vehicle alone (Fig. 1A). The two areas were located within the autonomous innervation territory of the superficial radial nerve and were separated by a 6 mm strip of untreated skin. In Experiment 2 (secondary hyperalgesia), two areas (25 × 25 mm) in the hairy skin of the left upper forearm (separated by a 10 mm strip of untreated skin) were pretreated in the same way for 6 h each on two successive days (Fig. 3A).
The areas were defined reproducibly by marking the skin, and each area was surrounded by a tape mask that was not penetrated by the cream. The cream depots were kept under occlusion throughout the application period. Capsaicin and vehicle were applied to the distal or proximal test areas in a balanced way, i.e. each to either the proximal or the distal area in half of the subjects. The efficacy of capsaicin desensitization on afferent nociceptor function (reduction of heat pain) and on efferent nociceptor function (elimination of flare) was assessed 1 day after treatments (see below).
Non-ischaemic A-fibre conduction blockade
In Experiment 1, differential conduction blockade of myelinated nerve fibres was achieved by applying pressure to the left superficial radial nerve (Fig. 1A). A 2.5 cm wide rubber band was put across the forearm just proximal to the wrist. The two ends were connected below the arm to a 1.3 kg weight. Myelinated fibre blockade was considered to be both complete and selective when A-fibre-related perceptions, including the first pain sensation, were completely lost but C-fibre-related perceptions were completely unaffected. The following criteria were used (for details, see Ziegler et al., 1999).
(i) Loss of light touch detection from a 4 mN von Frey hair (1.1 mm diameter), reflecting loss of action potential conduction in Aβ-fibre axons innervating low-threshold mechanoreceptors (Aβ-fibre LTMs).
(ii) Loss of innocuous cold detection, reflecting impaired action potential conduction in Aδ-fibre cold receptors. Cold detection was tested by touching the skin for ≤2 s with a water-filled glass tube kept at 7°C, which cooled the skin surface temperature by 7.3 ± 0.4°C, as tested in six subjects.
(iii) Loss of first pain sensation, reflecting impaired action potential conduction in Aδ-fibre nociceptors. First pain sensation was tested by measuring the reaction times to punctate mechanical stimuli from a weight-loaded needle tip (25 gauge needle, 125 mN force). The cut-off time for first pain reaction times was 500 ms; note that first pain detection is lost ~18 min later than all other A-fibre-related perceptions (Ziegler et al., 1999).
(iv) Preservation of second pain sensation, reflecting conduction in C-fibre nociceptors. Second pain sensation was tested using the same paradigm as for first pain, but counting reaction times >500 ms.
(v) Preservation of innocuous warmth detection, reflecting conduction in C-fibre warm receptors. Warmth detection was tested by touching the skin for ≤2 s with a water-filled glass tube kept at 57°C, which warmed the skin surface temperature by 8.6 ± 0.6°C, as tested in six subjects.
Two of the 10 subjects did not develop complete conduction blockade after 150 min, which is the longest time period we have chosen to use, for safety reasons. Data from these two subjects were not included in the analysis.
Secondary hyperalgesia
In Experiment 2, secondary hyperalgesia and a flare response were induced by intradermal injection of 40 μg of capsaicin (dissolved in 12.5 μl of 0.16% Tween 80 in normal saline) (for detail, see LaMotte et al., 1991) midway between the vehicle- and capsaicin-treated areas in the forearm (Fig. 3A). Intradermal capsaicin is a well-defined human pain model for producing secondary hyperalgesia that mimics the effect of tissue injury on nociceptors but avoids actual tissue damage. Typically, the capsaicin injection elicited pain that was maximal immediately upon injection and decayed rapidly (within 5 min). The flare response was identified visually and was marked 6 min after injection. The area of secondary hyperalgesia was mapped 6–10 min after capsaicin injection. Subsequently, the magnitude of pain to light touch and to punctate stimuli was tested 10–30 min after injection. No attempt was made to distinguish first and second pain in these ratings.
The area of secondary hyperalgesia was mapped with a 200 mN von Frey hair (diameter 0.45 mm) along eight different tracks at 45° angles, starting from the periphery well outside the hyperalgesic area and gradually moving towards the injection site. The border of the hyperalgesia zone was identified as the site at which there was a transition from normal to enhanced pain sensation. Reliability of the map was verified by testing at several adjacent spots along each track inside and outside the zone, close to its border.
The magnitude of hyperalgesia to light touch and to punctate stimuli was tested within the vehicle- and capsaicin-pretreated skin areas (i.e. 15 ± 5 mm from the capsaicin injection site). Hyperalgesia to light touch stimuli was tested with three different stimuli that are not painful in normal skin: a cotton wisp like those used clinically for testing corneal sensitivity (force 3 mN), a Q-Tip mounted on a flexible plastic strip (force 100 mN) and a soft make-up brush 1 cm wide (force 400 mN). All tactile stimuli were moved across the skin with short (1 cm) strokes at ~1 cm/s. Hyperalgesia to punctate stimuli was tested with the series of 0.2 mm diameter probes described above.
Pain to noxious heat stimuli
To document the desensitizing effects of topical capsaicin, heat pain sensitivity on the back of the hand was tested with suprathreshold stimuli from an infrared laser (Stimulaser; InPro, Norderstedt, Germany; a Cr,Tm-YAG laser, wavelength 2010 nm, energy 600 mJ, beam diameter 5 mm, duration 3 ms) in six subjects. The rapid increases in skin temperature produced by such laser heat stimuli lead to first pain mediated by excitation of capsaicin-sensitive A-fibre nociceptors (Beydoun et al., 1996; Treede et al., 1998; Magerl et al., 1999). Heat pain in the forearm was tested with a Peltier contact heat stimulator (TSA 2001; Medoc, Ramat Yishari, Israel; contact area 8 × 8 mm) with stimuli of 53°C, duration 4 s (with a temperature ramp of 10°C/s from a 35°C baseline at the start of the stimulus). Pain to either type of heat stimulus was rated on a verbal numerical rating scale (0 = no pain, 100 = maximal imaginable pain). These measurements were only performed at baseline, before induction of secondary hyperalgesia or A-fibre block.
Data analysis
For analysis of the rating data to mechanical pain stimuli (including light touch and punctate stimuli), all pain ratings of a given subject were normalized by dividing by the subject's average pain ratings to the strongest stimulus in both skin areas before pretreatment with capsaicin or vehicle (pre-drug controls). This normalization served to provide an equal weight of the data for every subject. Changes in pain perception after pretreatment or after induction of either A-fibre block or secondary hyperalgesia were calculated from the ratios of areas under the pain rating curves for group data and for every individual subject. The statistical significance of the changes was tested from these ratios with Student's t-test.
Results
Effects of A-fibre conduction blockade in normal and capsaicin-pretreated skin
In Experiment 1, we combined capsaicin pretreatment of the skin with a non-ischaemic compression block of the superficial radial nerve (Fig. 1A). Eight of the 10 subjects fulfilled the criteria indicating complete and selective A-fibre conduction blockade. The sensory status of these eight subjects after 101 ± 5 min of nerve compression indicated significant and complete loss of touch, cold and first pain sensation, whereas warmth sensation was fully intact (Fig. 1B). Data of the remaining two subjects had to be discarded because of incomplete conduction blockade of A-fibre nociceptors, as their first pain reaction times were not completely eliminated.
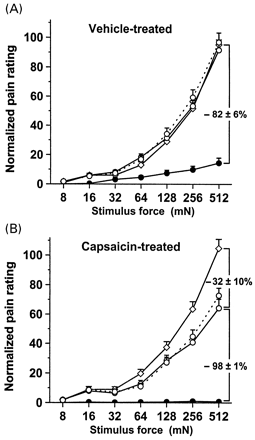
Both the vehicle-treated and the capsaicin-treated skin areas were partly included in the autonomous innervation territory of the radial nerve (Fig. 1B). Stimulus–response functions for pain evoked by the punctate probes were measured within the zones of overlap between pretreatment and the effects of conduction block (Fig. 2). Pretreatment of the skin with vehicle cream did not alter the perception of pain to punctate mechanical stimuli (Fig. 2A). In contrast, selective A-fibre conduction blockade dramatically reduced the magnitude of pain to punctate stimuli in normal skin (–82 ± 6% versus baseline, P < 0.001) (Fig. 2A). Thus, pain to punctate stimuli applied to normal skin is primarily mediated by A-fibre nociceptors. The A-fibre block uncovered a long-latency, burning pain percept that was mediated by C-fibre nociceptors. This pain was rated at a level that was ~18% of baseline.
In capsaicin-pretreated skin, the magnitude of pain to punctate mechanical stimuli was significantly reduced (–32 ± 10% versus baseline, P < 0.02) (Fig. 2B). Moreover, when selective A-fibre conduction blockade was established, the pain to punctate stimuli in the capsaicin-pretreated skin was eliminated completely (–98 ± 1% versus preblock pain ratings, P < 0.001). Thus, the pain to punctate mechanical stimuli in capsaicin-pretreated skin (~68% of baseline) was completely served by capsaicin-insensitive A-fibre nociceptors. Capsaicin-insensitive C-fibre nociceptors did not contribute to this type of pain sensation.
In contrast to the small reduction in mechanically induced pain, topical pretreatment of the skin with 10% capsaicin cream for 3 days resulted in nearly complete abolition of laser-evoked heat pain. Pain ratings dropped from 40.3 ± 10.3 on the visual analogue scale (VAS) for vehicle-treated skin to 1.7 ± 1.1 for capsaicin-treated skin (i.e. –96 ± 2%, n = 6, P < 0.005, data not shown). These findings indicate that heat-sensitive A- and C-fibre nociceptors were functionally eliminated by topical capsaicin.
Secondary hyperalgesia in normal and capsaicin-pretreated skin
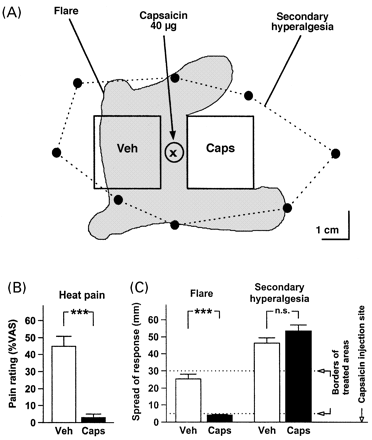
In Experiment 2, we combined topical capsaicin pretreatment of the skin with intradermal capsaicin injections to elicit secondary hyperalgesia (Fig. 3A). One day after topical pretreatment of skin on the volar forearm with 10% capsaicin cream for 2 days, the pain to 53°C contact heat stimuli applied for 4 s (3.0 ± 2.1 on the VAS) was significantly lower than in the vehicle-pretreated area (44.9 ± 5.9; i.e. –95 ± 3%, n = 10, P < 0.001) (Fig. 3B), indicating that most heat-sensitive nociceptors in this skin area were functionally eliminated after 2 days of pretreatment.
One day after pretreatment, capsaicin (40 μg in 12.5 μl) was injected intradermally midway between the pretreatment areas into the 10 mm strip of untreated skin to elicit secondary hyperalgesia. The capsaicin injection also elicited a clearly visible flare reaction surrounding the injection site. Whereas the flare spread into and sometimes beyond the vehicle area, it always stopped at the border of the capsaicin area (Fig. 3A). The average axial extent of the flare was 25 ± 3 mm towards the vehicle area but only 4.2 ± 0.4 mm towards the capsaicin area (note that a 5 mm border of untreated skin existed on either side of the injection site; P < 0.001) (Fig. 3C). Thus, a blockade of the efferent function of capsaicin-sensitive nociceptors was demonstrated by the lack of neurogenic vasodilatation following adjacent capsaicin injection.
The capsaicin injection elicited an intense burning pain upon injection (83 ± 6 on the VAS), which subsided within 10 min and was followed by pronounced tenderness to light touch and to punctate mechanical stimuli in the skin surrounding the injection site (secondary hyperalgesia). Typically, the zone of secondary hyperalgesia had an oval shape, with its longer extension in the proximal–distal axis of the forearm, in contrast to flare, which often had an irregular shape. In contrast to the flare reaction, secondary hyperalgesia was found in both vehicle- and capsaicin-pretreated skin (Fig. 3A). The axial extent of the secondary hyperalgesia zone on the capsaicin-pretreated side (53.5 ± 3.5 mm) did not differ from the extent on the vehicle-pretreated side (46 ± 3 mm) (Fig. 3C).
Quantitative psychophysical testing with mechanical stimuli further confirmed that secondary hyperalgesia developed normally in both pretreatment areas. Stroking the skin by means of light tactile stimuli elicited pain in seven out of 10 subjects (`allodynia'), which never occurred in normal skin. Notably, the hyperalgesia to light touch was similar in vehicle- and capsaicin-pretreated skin (P > 0.40 for all test stimuli) (Fig. 4A and B).
Pretreatment of the skin with vehicle cream did not alter the pain perception to punctate mechanical stimuli (Fig. 4A). Following adjacent injection of capsaicin, the pain ratings in the vehicle pretreatment area were increased to 262 ± 54% of control (P < 0.02). Pretreatment of the skin with capsaicin cream slightly reduced the pain to punctate mechanical stimuli (–27 ± 12% versus control; P = 0.06) (Fig. 4B), which corroborated the findings in the back of the hand (Fig. 2B). After adjacent injection of capsaicin, pain ratings in the capsaicin pretreatment area were also slightly lower than in the vehicle pretreatment area (–25 ± 9% versus control, P < 0.05). Thus, capsaicin injection elicited an increase in pain to punctate mechanical stimuli in the capsaicin-pretreated part of the secondary hyperalgesia zone (259 ± 53% of control, P < 0.02) (Fig. 4B), which was about the same amount as in the vehicle pretreatment area. Both pretreatment areas thus exhibited robust and quantitatively identical hyperalgesia to punctate mechanical stimuli, whereas flare and heat pain were eliminated in capsaicin-pretreated skin.
Discussion
These data demonstrate that A-fibre nociceptors play the dominant role in signalling the pain to punctate mechanical stimuli (pinprick pain) applied to normal skin, and that C-fibre nociceptors make only a small contribution to this sensation. Once the A-fibre conduction had been blocked, a long-latency, burning pain was uncovered that was mediated by C-fibres, the perceived magnitude of which was about one-fifth of the overall pain perceived with intact A-fibre innervation. Previous studies have shown that pinprick pain also loses its sharp quality during A-fibre blockade (MacKenzie et al., 1975). The mechanisms of integration of first and second pain are not fully understood yet, but there is some evidence that C-fibre-mediated second pain may be largely suppressed by preceding A-fibre-mediated first pain (Bromm and Treede, 1984; Magerl et al., 1999). Thus, the perceived pain intensity of pinprick pain in normal skin may be mediated almost completely by A-fibre nociceptors.
The capsaicin desensitization experiments revealed that at least two-thirds of the A-fibre-mediated pain came from capsaicin-insensitive nociceptors. Thus, capsaicin-insensitive A-fibre nociceptors play the major role in signalling pain to punctate mechanical stimuli. The fact that the combined A-fibre block and capsaicin desensitization completely eliminated pain to mechanical stimuli suggests that capsaicin-insensitive C-fibre afferents do not contribute to pain perception to punctate mechanical probes.
Three classes of A-fibre nociceptors have been identified in the primate that differ with respect to their capsaicin sensitivity as well as their responses to mechanical and heat stimuli (Treede et al., 1998). Type II A-fibre mechano-heat-sensitive nociceptors (type II AMHs) have relatively low heat thresholds (<50°C). Type II AMHs subserve first pain to heat stimuli, which is eliminated by capsaicin desensitization. Thus, type II AMHs appear to be capsaicin-sensitive (cf. Beydoun et al., 1996; et al., 2001). Type I AMHs have high heat thresholds (>50°C) that may be related to expression of the VRL1 receptor, a non-selective cation channel that can be activated by intense heat stimuli but not by capsaicin (Caterina et al., 1999; Nagy and Rang, 1999). Type I AMHs appear to be insensitive to capsaicin (Ringkamp et al., 2001). The third class of A-fibre nociceptors, the HTMs (high-threshold mechanoreceptors), do not respond to heat or to capsaicin (Szolcsányi et al., 1988). Thus, the capsaicin-insensitive A-fibre nociceptors that predominantly mediate pinprick pain consist of type I AMHs and HTMs. These two classes of A-fibre nociceptors constitute about two-thirds of all A-fibre nociceptors (Treede et al., 1998).
Pain to punctate mechanical stimuli is strongly enhanced in the zone of secondary hyperalgesia (e.g. following an adjacent injury), whereas heat pain is either unchanged or reduced (Raja et al., 1984; Dahl et al., 1993; Ali et al., 1996; Brennan et al., 1996). Since the response properties of the classical primary afferent nociceptors are not changed within the area of secondary hyperalgesia (for review, see Treede et al., 1992b), secondary hyperalgesia is considered to be due to enhanced efficacy of synaptic transmission in the central nervous system (central sensitization). In recordings from rat or monkey spinal cord neurones, this enhanced synaptic efficacy was found to be specific for mechanically evoked input, whereas synaptic efficacy for heat-evoked input was unchanged or even reduced (Simone et al., 1991; Dougherty et al., 1998; Pertovaara, 1998). In contrast, Serra and colleagues recently proposed that a small but distinct subgroup of nociceptors, namely capsaicin-sensitive widely branching C-fibres, may become sensitized (peripheral sensitization) and be responsible for both the flare and secondary hyperalgesia to punctate stimuli. This proposal was based on the observation that the area of flare, as determined by thermography, coincided with the area of punctate hyperalgesia (Serra et al., 1998). Our findings contradict this hypothesis, since we demonstrated that the two phenomena were readily dissociated by the use of topical capsaicin, which eliminated the flare but preserved the secondary hyperalgesia. The different findings are unlikely to be attributable to differences in methods, since the test stimuli for hyperalgesia were similar and the flare was also substantially reduced when measured by laser Doppler, which has a sensitivity similar to that of thermography (Fuchs et al., 2000).
The mechanisms of mechanical hyperalgesia depend strongly on the shape and mode of application of the mechanical test stimuli (Kilo et al., 1994). Mechanisms range from peripheral sensitization of C-fibre nociceptors (static hyperalgesia to blunt pressure confined to the site of injury) to central sensitization of Aβ-fibre LTM input (dynamic hyperalgesia or allodynia outside the site of injury). Nevertheless, secondary hyperalgesia to the punctate probes used in the present study and to a blade-shaped probe (Fuchs et al., 2000) both persisted in capsaicin-pretreated skin. Thus, secondary hyperalgesia to those mechanical stimuli that, by their shape, are adequate to activate nociceptive afferents (Andrew and Greenspan, 1999; Slugg et al., 2000) is mediated predominantly by the same capsaicin-insensitive A-fibre nociceptors that mediate pinprick pain.
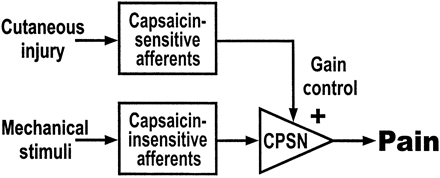
Our results suggest that the subdivision of nociceptive primary afferents according to their capsaicin sensitivity has important functional implications. Capsaicin-sensitive afferents include most of the unmyelinated fibres (polymodal, heat-sensitive and/or chemosensitive C-fibres) and some of the Aδ fibres (type II AMHs). These afferents respond to the capsaicin injection and are responsible for inducing a state of sensitization in central nociceptive neurones. Capsaicin-insensitive afferents include most of the myelinated nociceptors (HTMs and type I AMHs). These afferents do not respond to the capsaicin injection, but they are responsible for signalling the mechanical hyperalgesia induced by the capsaicin injection. Moreover, in additional nerve block experiments on secondary hyperalgesia (Ziegler et al., 1999), we found that A-fibre nociceptors did not contribute significantly to the induction of central sensitization by intradermal capsaicin, which was fully accounted for by C-fibre input. Conversely, at the same time, mechanically evoked C-fibre input was not facilitated itself when secondary hyperalgesia was established. Thus, the facilitating pathways (capsaicin-sensitive afferents) and the facilitated pathways (capsaicin-insensitive afferents) in secondary hyperalgesia consist of two functionally distinct populations of primary nociceptive afferents (Fig. 5).
These two populations project to different parts of the dorsal horn of the spinal cord. A-fibre HTMs terminate in lamina I (the marginal zone), whereas C-fibres terminate primarily in lamina II (Light and Perl, 1979; Sugiura et al., 1986; Maxwell and Réthelyi, 1987). This comprises the substantia gelatinosa, which has been recognized as a nucleus of interaction and modulation of cutaneous sensory information (Cervero and Iggo, 1980; Petersen-Zeitz and Basbaum, 1999). Thus, C-fibre terminals are in a strategic anatomical and functional position to modify input from other classes of nociceptors. The present findings suggest that capsaicin-sensitive nociceptors (primarily C-fibres) exert a gain-control function in the induction and maintenance of secondary hyperalgesia (Fig. 5). Specifically, we propose that activity in capsaicin-sensitive C-nociceptors amplifies the transmission of a separate class of mechano-sensitive but capsaicin-insensitive primary afferents (nociceptive A-fibres). The mechanisms of this heterosynaptic modulation of spinal transmission are not delineated precisely. They may involve spinal sensitization by primary afferent input (McMahon et al., 1993; Meller, 1994; Urban et al., 1994), descending facilitation originating in the rostral ventral medulla (Li and Zhuo, 1998; Urban and Gebhart, 1999), spinal disinhibition (Yaksh, 1989; Sivilotti and Woolf, 1994; Lin et al., 1996) or the combined action of several of these mechanisms (Pertovaara, 1998). Heterosynaptic modulation, similar to that postulated here, is known to play an important role in nocifensive reflexes in Aplysia (Bailey et al., 2000).
The modality-specific facilitation of a mechanically sensitive sensory channel in secondary hyperalgesia results in increased painfulness of contact with sharp objects in the vicinity of an injury site. This type of increased gain in the somatosensory system is likely to promote functionally adequate guarding behaviour that prevents the access of potentially injurious mechanical stimuli to the actual injury site and thus avoids interference with the progress of wound healing. The functional properties of secondary hyperalgesia are those of a warning system in adjacent uninjured tissue. The functional segregation of the warning system (capsaicin-insensitive A-fibres) and the system signalling the injury (capsaicin-sensitive C-fibres) would effectively prevent self-facilitation and the maintenance of central sensitization by the facilitated pathway alone.
The proposed gain control system may, however, may become maladaptive, when either the capsaicin-sensitive pathway develops sustained ongoing activity (Devor et al., 1992; Gracely et al., 1992; Treede et al., 1992a) or when the capsaicin-insensitive pathway develops the capacity to produce self-facilitation (Ma and Woolf, 1996; Neumann et al., 1996; Baba et al., 1999). These mechanisms may lead to the hyperalgesic form of chronic neuropathic pain. Notably, the symptoms of neuropathic pain (i.e. mechanical hyperalgesia and allodynia) match the hallmark signs of secondary hyperalgesia, which has therefore been used as an experimental model to understand some of the mechanisms (Treede et al., 1992b; Treede and Magerl, 2000). For example, neuropathic pain states are often not sufficiently ameliorated by opioid treatment (Arnér and Meyerson, 1988; Jadad et al., 1992; Dellemijn, 1999), which is consistent with the limited efficacy of opiates to reduce capsaicin-induced secondary hyperalgesia (Park et al., 1995). This opiate insensitivity becomes explicable by our observation that secondary hyperalgesia to punctate mechanical stimuli is mediated by A-fibre nociceptors, which, in contrast to C-fibre nociceptors, lack presynaptic opioid receptors (Taddese et al., 1995; Schulz et al., 1998). Likewise, our model predicts that the utility of topical capsaicin as a treatment for neuropathic pain will be limited to cases in which the facilitating and maintaining focus [the capsaicin-sensitive `irritable' C-nociceptor (cf. Fields et al., 1998; Rowbotham et al., 1998)] is accessible for inactivating treatment. Thus, understanding the diverse roles of anatomically and functionally distinct nociceptive subsystems will enable a more rational and mechanism-based treatment of neuropathic pain syndromes (Woolf et al., 1998).
Conclusions
The combined effects of two selective nerve fibre blocking techniques (nerve compression and topical capsaicin treatment) on the stimulus–response functions of pain elicited by punctate mechanical probes indicate that capsaicin-insensitive A-fibre nociceptors play the major role in signalling pain and secondary hyperalgesia to pinpricks. This group of nociceptors consists of two classes of afferents that are known from monkey skin (HTMs and type I AMHs). Capsaicin-sensitive polymodal nociceptors (both A- and C-fibres) make a minor but significant contribution to pinprick pain. C-fibre nociceptor input, however, is not facilitated centrally in secondary hyperalgesia; for polymodal A-fibre nociceptors (type II AMH), central facilitation may occur but has not been shown conclusively. Capsaicin-insensitive C-fibres, which make up ~20% of small-diameter afferents, do not contribute to mechanical pain sensation. These psychophysical data provide further evidence that sets HTMs apart from polymodal nociceptors. They subserve important functions in mediating pinprick pain, which is the modality assessed in clinical sensory testing, and in secondary hyperalgesia, which shares mechanisms with some types of neuropathic pain.
Effects of selective A-fibre conduction blockade in normal versus capsaicin-pretreated skin. (A) Arrangement for testing the effects of topical capsaicin pretreatment and of nerve compression on the perception of pain to punctate stimuli in the innervation territory of the superficial radial nerve. Capsaicin = 10% capsaicin cream, 6 h each on three successive days; Vehicle = vehicle cream base. (B) Verification of complete and selective A-fibre blockade in vehicle-pretreated skin (n = 8). After 101 ± 5 min of nerve compression, detection of touch (Aβ-fibres) and cold (Aδ-cold fibres) was lost. Likewise, first pain (nociceptive Aδ-fibres) was eliminated and reaction times had shifted from first pain (<500 ms, average reaction time 180 ms) to second pain reaction times (>500 ms, average reaction time 1200 ms). In contrast, C-fibre-mediated warmth detection was unchanged. Pre = before A-fibre blockade; Blk = during A-fibre blockade.
Pain to punctate mechanical stimuli is mediated mainly by capsaicin-insensitive A-fibre nociceptors. (A) Effect of A-fibre conduction blockade on perception of mechanically induced pain in vehicle-pretreated skin (n = 8). Pain to punctate stimuli increased as a function of stimulus force before topical pretreatment (open diamonds), and was not altered by topical pretreatment with the vehicle (open circles, solid line). Mechanically induced pain was substantially diminished in A-fibre-blocked skin (–82 ± 6%; closed circles). C-fibre-mediated pain was uncovered when the A-fibres were blocked. The stimulus–response function of pricking pain returned to baseline values 10 min after block release (open circles, broken line). (B) Effect of A-fibre conduction blockade on perception of mechanically induced pain in capsaicin-pretreated skin (n = 8). Before topical pretreatment, the stimulus–response function of pricking pain to punctate stimuli (open diamonds) matched that in vehicle-pretreated skin. Pain to punctate stimuli was reduced after topical pretreatment with 10% capsaicin cream (–32 ± 10%; open circles, solid line). During the selective A-fibre conduction blockade, mechanically induced pain was eliminated (closed circles), suggesting that capsaicin-insensitive C-fibre nociceptors do not contribute to mechanically induced pain. In capsaicin-pretreated skin, stimulus–response functions of pricking pain also returned to baseline values 10 min after block release (open circles, broken line).
Secondary hyperalgesia to punctate mechanical stimuli persists in capsaicin-pretreated skin. (A) Arrangement for testing the effects of topical capsaicin pretreatment of the volar forearm (Caps = 10% capsaicin cream, 6 h each on two successive days; Veh = vehicle cream base) on secondary hyperalgesia elicited by intradermal capsaicin injection (40 μg in 12.5 μl). Following intradermal capsaicin injection (injection site marked by `x') into the strip of normal skin separating the pretreatment areas, a flare response (shaded area) developed that spread into the vehicle-pretreated area but not into the capsaicin-pretreated area (results from a typical subject). In contrast, secondary hyperalgesia developed symmetrically across both treatment areas (dotted line). (B) Inhibition of the afferent function of capsaicin-sensitive nociceptors was verified by almost complete abolition of heat-evoked pain (contact heat 53°C for 4 s, n = 10) in the capsaicin pretreatment area. (C) Inhibition of the efferent function of capsaicin-sensitive nociceptors was verified by almost complete abolition of capsaicin-evoked flare in the capsaicin pretreatment area. In contrast, secondary hyperalgesia to punctate stimuli developed symmetrically on either side (spatial extent of response along longitudinal axis of the forearm, n = 10).
Secondary hyperalgesia to light touch and to punctate stimuli in vehicle versus capsaicin pretreated skin (n = 10). (A) Capsaicin injection induced secondary hyperalgesia to light touch (`allodynia') as assessed with light tactile stimuli [cotton wisps (Cw), soft make-up brush (Br) and Q-Tip (Qt) at test sites 15 ± 5 mm from the injection] in vehicle-pretreated skin (left panel). After capsaicin injection, these normally non-painful stimuli (open circles) elicited mild burning pain (closed circles). Secondary hyperalgesia to punctate stimuli was assessed with force-controlled stimuli applied with a 0.2 mm diameter probe (right panel). Pain ratings to these punctate stimuli in normal skin increased as a function of stimulus intensity. Pain ratings were similar before (open diamonds) and after (open circles) vehicle pretreatment, but increased significantly (by a factor of 2.6) after adjacent capsaicin injection (closed circles). (B) In capsaicin-pretreated skin, adjacent capsaicin injection induced secondary hyperalgesia to light touch indistinguishable from that in vehicle-pretreated skin (left panel). After topical capsaicin pretreatment, pain to punctate stimuli (right panel) was significantly reduced (–27%, open circles) compared with pre-drug controls (open diamonds). Following capsaicin injection, the pain to punctate stimuli increased significantly by a factor of ~2.6 (closed circles). Thus, similar degrees of hyperalgesia to light touch and to punctate stimuli were present in capsaicin- and vehicle-pretreated skin.
Gain control model for secondary hyperalgesia. Capsaicin-insensitive nociceptive afferents project to central pain signalling neurones (CPSN). Mechanical stimulation of the nociceptor terminals of these afferents leads to the perception of pinprick pain from normal skin. Activity in unmyelinated capsaicin-sensitive afferents (e.g. due to an adjacent injury or capsaicin injection) leads to an increase in the gain (or facilitation) of these CPSNs. Now, mechanical stimulation of the terminals of capsaicin-insensitive afferents leads to enhanced pain (secondary hyperalgesia). A similar augmentation occurs for capsaicin-insensitive, low-threshold mechanoreceptors, leading to allodynia. Potential mechanisms of the gain control include heterosynaptic facilitation in the spinal cord, disinhibition and descending facilitation.
Present address: Department of Psychology, University of Texas at Arlington, Box 19528, Arlington TX 76019, USA
These authors contributed equally to this work
We wish to thank Dr J. Sandkühler for helpful discussions. This study was supported by NATO collaborative research grant 95032540495 and NIH grant NS-14447.
References
Ali Z, Meyer RA, Campbell JN. Secondary hyperalgesia to mechanical but not heat stimuli following a capsaicin injection in hairy skin.
Andrew D, Greenspan JD. Peripheral coding of tonic mechanical cutaneous pain: comparison of nociceptor activity in rat and human psychophysics.
Arnér S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain.
Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Aβ fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord.
Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory?
Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia.
Beydoun A, Dyke DB, Morrow TJ, Casey KL. Topical capsaicin selectively attenuates heat pain and A delta fiber-mediated laser-evoked potentials.
Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain.
Bromm B, Treede R-D. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation.
Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. [Review].
Caterina MJ, Julius D. Sense and specificity: a molecular identity for nociceptors. [Review].
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat.
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor.
Cervero F, Iggo A. The substantia gelatinosa of the spinal cord. A critical review.
Cervero F, Meyer RA, Campbell JN. A psychophysical study of secondary hyperalgesia: evidence for increased pain to input from nociceptors.
Chan AW, MacFarlane IA, Bowsher D, Campbell JA. Weighted needle pinprick sensory thresholds: a simple test of sensory function in diabetic peripheral neuropathy.
Dahl JB, Brennum J, Arendt-Nielsen L, Jensen TS, Kehlet H. The effect of pre- versus postinjury infiltration with lidocaine on thermal and mechanical hyperalgesia after heat injury to the skin.
Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia.
Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction.
Dougherty PM, Willis WD, Lenz FA. Transient inhibition of responses to thermal stimuli of spinal sensory tract neurons in monkeys during sensitization by intradermal capsaicin.
Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. [Review].
Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin.
Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input.
Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. [Review].
Jadad AR, Carroll D, Glynn CJ, Moore RA, McQuay HJ. Morphine responsiveness of chronic pain: double-blind randomised crossover study with patient-controlled analgesia.
Kilo S, Schmelz M, Koltzenburg M, Handwerker HO. Different patterns of hyperalgesia induced by experimental inflammation in human skin.
Kirschstein T, Greffrath W, Büsselberg D, Treede R-D. Inhibition of rapid heat responses in nociceptive primary sensory neurons of rats by vanilloid receptor antagonists.
LaMotte RH, Shain CN, Simone DA, Tsai E-FP. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms.
Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord.
Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers.
Lin Q, Peng YB, Willis WD. Inhibition of primate spinothalamic tract neurons by spinal glycine and GABA is reduced during central sensitization.
Liu MW, Max MB, Robinovitz E, Gracely RH, Bennett GJ. The human capsaicin model of allodynia and hyperalgesia: sources of variability and methods for reduction.
Lynn B. Capsaicin: actions on nociceptive C-fibres and therapeutic potential. [Review].
Ma Q-P, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord.
MacKenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block.
Magerl W, Ali Z, Ellrich J, Meyer RA, Treede R-D. C- and A-fiber components of heat-evoked cerebral potentials in healthy human subjects.
Maxwell DJ, Réthelyi M. Ultrastructure and synaptic connections of cutaneous afferent fibres in the spinal cord.
McMahon SB, Lewin GR, Wall PD. Central hyperexcitability triggered by noxious inputs. [Review].
Meller ST. Thermal and mechanical hyperalgesia: a distinct role for different excitatory amino acid receptors and signal transduction pathways?
Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy.
Nagy I, Rang HP. Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin.
Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons.
Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation.
Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects.
Pertovaara A. A neuronal correlate of secondary hyperalgesia in the rat spinal dorsal horn is submodality selective and facilitated by supraspinal influence.
Petersen-Zeitz KR, Basbaum AI. Second messengers, the substantia gelatinosa and injury-induced persistent pain. [Review].
Raja SN, Campbell JN, Meyer RA. Evidence for different mechanisms of primary and secondary hyperalgesia following heat injury to the glabrous skin.
Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors.
Rowbotham MC, Petersen KL, Fields HL. Is postherpetic neuralgia more than one disorder?
Sang CN, Gracely RH, Max MB, Bennett GJ. Capsaicin-evoked mechanical allodynia and hyperalgesia cross nerve territories. Evidence for a central mechanism.
Schulz S, Schreff M, Schmidt H, Handel M, Przewlocki R, Hollt V. Immunocytochemical localization of somatostatin receptor sst2A in the rat spinal cord and dorsal root ganglia.
Serra J, Campero M, Ochoa J. Flare and hyperalgesia after intradermal capsaicin injection in human skin.
Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans.
Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, et al. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons.
Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. [Review].
Sivilotti L, Woolf CJ. The contribution of GABA(A) and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord.
Slugg RM, Meyer RA, Campbell JN. Response of cutaneous A- and C-fiber nociceptors in the monkey to controlled-force stimuli.
Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin.
Szolcsányi J, Anton F, Reeh PW, Handwerker HO. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin.
Taddese A, Nah S-Y, McCleskey EW. Selective opioid inhibition of small nociceptive neurons.
Treede R-D, Cole JD. Dissociated secondary hyperalgesia in a subject with a large-fibre sensory neuropathy.
Treede R-D, Magerl W. Multiple mechanisms of secondary hyperalgesia. In: Sandkühler J, Bromm B, Gebhart GF, editors. Nervous system plasticity and chronic pain. Prog Brain Res, Vol. 129. Amsterdam: Elsevier; 2000. p. 331–41.
Treede R-D, Davis KD, Campbell JN, Raja SN. The plasticity of cutaneous hyperalgesia during sympathetic ganglion blockade in patients with neuropathic pain.
Treede R-D, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. [Review].
Treede R-D, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat response properties.
Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. [Review].
Urban L, Thompson SW, Dray A. Modulation of spinal excitability: co-operation between neurokinin and excitatory amino acid neurotransmitters. [Review].
Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, et al. Towards a mechanism-based classification of pain? [editorial].
Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists.


![Secondary hyperalgesia to light touch and to punctate stimuli in vehicle versus capsaicin pretreated skin (n = 10). (A) Capsaicin injection induced secondary hyperalgesia to light touch (`allodynia') as assessed with light tactile stimuli [cotton wisps (Cw), soft make-up brush (Br) and Q-Tip (Qt) at test sites 15 ± 5 mm from the injection] in vehicle-pretreated skin (left panel). After capsaicin injection, these normally non-painful stimuli (open circles) elicited mild burning pain (closed circles). Secondary hyperalgesia to punctate stimuli was assessed with force-controlled stimuli applied with a 0.2 mm diameter probe (right panel). Pain ratings to these punctate stimuli in normal skin increased as a function of stimulus intensity. Pain ratings were similar before (open diamonds) and after (open circles) vehicle pretreatment, but increased significantly (by a factor of 2.6) after adjacent capsaicin injection (closed circles). (B) In capsaicin-pretreated skin, adjacent capsaicin injection induced secondary hyperalgesia to light touch indistinguishable from that in vehicle-pretreated skin (left panel). After topical capsaicin pretreatment, pain to punctate stimuli (right panel) was significantly reduced (–27%, open circles) compared with pre-drug controls (open diamonds). Following capsaicin injection, the pain to punctate stimuli increased significantly by a factor of ~2.6 (closed circles). Thus, similar degrees of hyperalgesia to light touch and to punctate stimuli were present in capsaicin- and vehicle-pretreated skin.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/124/9/10.1093/brain/124.9.1754/2/m_b0903f4.gif?Expires=1716356095&Signature=daOuNd5Ewin8rq6oEYfrUyDNdRNq0vF1Ds4vckAfszjqDEcVi1bC4k03bxtnCzo4urMq3N52daC2hhJ4-qdhTJQEx00Qgb8H8Bm1D0MWaoox5wNxV2Pb4u3-ykTKo7Hnpm3a54eQV8mwsIVtqeqwD7fUxy9egg6Ut-~Vw7S2eXsBMBu0oF8a5lrwslu2BrgCCRzUy3pOBdIPWvF3YCeVkuMYoedsbU8YK9yvceR7xxoVTAcPJQx3N6v-xTT2m4i1RIiGVocGYrRY88fMsMd7ZqzKBEMhyoy~OoqJt822AQGowYVuPTtHgxiyw3Gm4ARGmDgnIsPZZs66QcYQIDOc9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)