-
PDF
- Split View
-
Views
-
Cite
Cite
Tamara Shiner, Ben Seymour, Klaus Wunderlich, Ciaran Hill, Kailash P. Bhatia, Peter Dayan, Raymond J. Dolan, Dopamine and performance in a reinforcement learning task: evidence from Parkinson’s disease, Brain, Volume 135, Issue 6, June 2012, Pages 1871–1883, https://doi.org/10.1093/brain/aws083
Close - Share Icon Share
Abstract
The role dopamine plays in decision-making has important theoretical, empirical and clinical implications. Here, we examined its precise contribution by exploiting the lesion deficit model afforded by Parkinson’s disease. We studied patients in a two-stage reinforcement learning task, while they were ON and OFF dopamine replacement medication. Contrary to expectation, we found that dopaminergic drug state (ON or OFF) did not impact learning. Instead, the critical factor was drug state during the performance phase, with patients ON medication choosing correctly significantly more frequently than those OFF medication. This effect was independent of drug state during initial learning and appears to reflect a facilitation of generalization for learnt information. This inference is bolstered by our observation that neural activity in nucleus accumbens and ventromedial prefrontal cortex, measured during simultaneously acquired functional magnetic resonance imaging, represented learnt stimulus values during performance. This effect was expressed solely during the ON state with activity in these regions correlating with better performance. Our data indicate that dopamine modulation of nucleus accumbens and ventromedial prefrontal cortex exerts a specific effect on choice behaviour distinct from pure learning. The findings are in keeping with the substantial other evidence that certain aspects of learning are unaffected by dopamine lesions or depletion, and that dopamine plays a key role in performance that may be distinct from its role in learning.
Introduction
Dopamine is strongly implicated in reward signalling, playing a central role in reward learning in animals (Wise and Rompre, 1989; Schultz et al., 1997; Schultz, 1998; Wise, 2004; Bayer and Glimcher, 2005) and humans (Pessiglione et al., 2006). Accumulating evidence from pharmacological interventions in healthy subjects (Pessiglione et al., 2006) and patients with Parkinson’s disease studied ON and OFF medication (Frank et al., 2004, 2007b) indicate that manipulating dopamine neurotransmission in humans influences reward-related reinforcement learning and decision-making. An assumption arising from these data is that dopamine exerts a direct effect on instrumental learning, a form of learning that links actions and their outcomes. At a mechanistic level, activity in dopaminergic neurons express a prediction error believed to mediate learning and updating the reward value of predictive stimuli (Schultz et al., 1997). The idea that prediction error-based learning is computationally implemented via activity patterns within the dopaminergic system is supported by a substantial body of experimental work across a range of species (Haber and Knutson, 2010).
However, the functions of dopamine are known to extend beyond reinforcement learning. First, considerable evidence points to a contribution to the control of Pavlovian approach behaviour (Ikemoto and Panksepp, 1999; Parkinson et al., 2002; Day and Carelli, 2007) as well as in motivational engagement and vigour (Ahlenius et al., 1977; Beninger and Phillips, 1981; Berridge and Robinson, 1998; McClure et al., 2003; Niv, 2007; Niv et al., 2007; Bardgett et al., 2009; Lex and Hauber, 2010; Boureau and Dayan, 2011). These influences are distinct from learning (Yin et al., 2008), even in cases where they arise from a signal that actually reports a prediction error (McClure et al., 2003). Secondly, many aspects of appetitive learning can progress normally in the absence of dopamine, most dramatically in the case of genetically engineered dopamine-deficient mice (Palmiter, 2008). Thirdly, a number of previous studies that investigated the effect of dopamine in humans could not easily distinguish between action learning and action performance (Frank et al., 2004, 2007b; Pessiglione et al., 2006). Consequently, in assessing dopamine’s impact on behaviour, it is necessary to distinguish influences on learning from influences on the modulation of the expression of learning, i.e. an effect on actual choice behaviour or performance.
Parkinson’s disease is a common neurological disorder characterized by neuronal loss in the substantia nigra (Edwards et al., 2008), which leads to depleted levels of striatal dopamine (Koller and Melamed, 2007). Parkinson’s disease results in deficits across several cognitive domains, including probabilistic learning and classification tasks (Knowlton et al., 1996; Graef et al., 2010), with dopamine replacement therapy having distinct effects on these behaviours. For example, when Parkinson’s disease patients are OFF dopamine replacement therapy, it is reported that their expression of learning from positive feedback is impaired (Frank et al., 2004, 2007b), while when ON dopamine replacement therapy they show impaired performance in learning from negative outcomes (Frank et al., 2004; Bodi et al., 2009). This behavioural pattern has been attributed to increased levels of striatal dopamine when patients are ON medication boosting prediction error signals resulting in enhanced learning from positive outcomes. In contrast, a prevention of dips in dopaminergic activity, as occurs with omission of expected outcomes, is suggested to worsen learning from negative outcomes (Frank et al., 2004; Frank, 2007b; Maia and Frank, 2011).
Here, we sought to dissociate dopaminergic effects on learning from effects on choice (performance) by acquiring neuroimaging data during a reinforcement learning task in patients with Parkinson’s disease. We employed a two-stage learning task that involves separate phases of (i) acquisition and (ii) a subsequent performance testing involving generalization of learning. This task has previously been shown to provide an effective means of examining the neural mechanisms underlying cognitive deficits in Parkinson’s disease (Frank et al., 2004, 2007b). These previous studies focused on learning, while here we also probed the effect of dopaminergic status (ON medication, and OFF medication) on test ‘performance’, i.e. on the expression of learning during behavioural extinction. Crucially, this dissociation between learning and performance has not been explicitly explored in previous human investigations.
Materials and methods
The study and its procedures were approved by the National Research Ethics Service, The Joint UCL/UCLH Committees on the Ethics of Human Research (Committee A).
Participants
Fourteen early-to-moderate stage (Hoehn and Yahr stage: mean 1.69, SE 0.26) patients with idiopathic Parkinson’s disease (10 males) (as per UK Brain Bank criteria) aged between 44 and 81 years (mean 61.8 years, SE 3.3 years) participated in and completed the study. Patients were recruited from the movement disorder clinic at the National Hospital for Neurology and Neurosurgery.
We obtained written informed consent from all subjects and transport costs were reimbursed.
Subjects were interviewed for psychiatric and neurological history as well as current and past medication. They were also examined by a clinician and asked to complete several questionnaires, including a health questionnaire, a Mini-Mental State Examination and an impulse control disorder screening questionnaire (Supplementary Table 1).
One subject had difficulty understanding the task demands and adopted an incorrect strategy for stimulus selection, whereby he explicitly believed the incorrect stimulus to be correct and continued to select it despite ongoing negative feedback resulting in significantly worse than chance performance. Data from this subject are not included in any analyses. Another subject was excluded from the imaging analysis due to an incidental finding of abnormally large ventricles, compromising normalization of this data set to a standard coordinate space. Hence, 13 subjects were analysed behaviourally and 12 subjects were analysed in the functional MRI study.
Twelve of the subjects were right-handed and one was left-handed. All were fluent English speakers. The duration of Parkinson’s disease varied from 1 to 10 years from the time of initial diagnosis (mean 4.9 years, SE 0.96 years). Subjects had no history of other major neurological or psychiatric disease. Patients were all on levodopa/carbidopa combinations; eight patients were also on dopamine agonists; total daily dose of levodopa/carbidopa varied from 50/12.5 mg to 1000/255 mg (mean 400/100 mg, SE 74.4/18.6 mg) (Supplementary Table 2). We did not recruit patients on trihexyphenidyl, benzhexol or high-dose tolterodine due to possible confounding effects of high-dose anti-cholinergic medication, or patients on amantadine due to its effect on multiple neuromodulators.
Stimuli
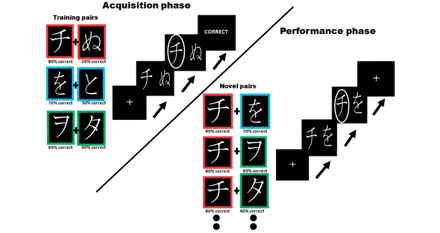
We used a version of the generalization task introduced by Frank et al. (2004, 2007b). Stimuli consisted of Hiragana symbols presented in white fonts on a black background where each stimulus had a different probability of being correct when selected. These probabilities ranged from 80% to 20%. In the first, or acquisition, stage of the task, the symbols were paired to form three sets: the 80% stimulus was paired with the 20% stimulus; the 70% stimulus was paired with the 30% stimulus and the 60% stimulus with the 40% stimulus. The sets were presented in a randomized order. In the second, or performance phase, along with all the training pairings, the best stimulus (the one with 80% chance of being correct) and the worst stimulus (the one with only 20% chance of being correct) were presented in novel pairings with all the other stimuli (Fig. 1).
Task. Stimuli consisted of Hiragana symbols which were presented in white fonts on a black background. Each stimulus had a different probability of being correct when selected. In the first, or acquisition stage of the task, symbols were paired to form three ‘training pairs’ that remained the same throughout this phase: the 80% stimulus was paired with the 20% stimulus (highlighted for illustration purposes with a red border); the 70% stimulus was paired with the 30% stimulus (blue border) and the 60% stimulus with the 40% stimulus (green border). Subjects selected the left or right stimulus by button presses and, during the acquisition phase, also received information about the outcome (correct/incorrect). In the second, or performance phase, along with all the training pairings, the best stimulus (the one with 80% chance of being correct) and the worst stimulus (the one with only 20% chance of being correct) were presented in novel pairings with all the other stimuli. During this phase subjects did not receive information about the outcome of their choice. During this phase, subjects were also presented again with the three sets of ‘training pairs’, which were interspersed with the novel pairs.
Procedure
Overview
Each patient participated in three separate sessions on different days, which were a minimum of 1 week apart (i.e. a within-subject design). Each session involved different Hiragana symbols (Fig. 1). All patients performed the task in three different drug states (Supplementary Table 3): acquisition and performance in the ON state (ON–ON), acquisition and performance in the OFF state (OFF–OFF) and acquisition of the stimulus contingencies in the OFF state but performance in the ON medication state (OFF–ON). The order of the different drug states in which patients performed the task was randomized. The OFF state in two of the conditions was achieved by a minimum of 12 h withdrawal from all dopaminergic medication and omission of all slow release preparations for a minimum of 18 h. On the remaining day (ON–ON), patients were asked to take their morning dopaminergic medication as usual. We were unable to test patients in the ON–OFF state, i.e. acquisition in the ON state and performance in the OFF state, due to the half-life of levodopa/carbidopa combinations, which would require a minimum of 7.5 h to be metabolized and excreted resulting in too long an interval between the acquisition and performance phases. All patients were tested at similar times in the morning to equalize washout times and to control for diurnal symptom fluctuations.
To familiarize subjects with the structure of the task, we undertook a short practice block before the first scanning session. During the practice session, patients worked on an identical task as in the main study except for the fact they were presented with different Hiragana symbols. The main session began with two functional scans (Scans 1 and 2, acquisition sessions). Most subjects completed a third acquisition session on a laptop. In OFF–ON condition, patients took their medication following this training. All patients then waited for 45–60 min before undergoing a third functional scanning session (Scan 3, performance session) for performance testing.
On one of the 3 days, after the training and performance stages were complete, the patients also underwent a structural scan, a Mini-Mental State Examination and completed questionnaires as detailed above.
Acquisition phase of the task: Scans 1 and 2
Scan sessions 1 and 2 (acquisition phase of the task), lasted ∼16 min, and consisted of 120 trials of 8 s each. On each trial, two Hiragana characters appeared on the screen side by side, presented via a mirror mounted on the head coil. Subjects’ task was to select one of the characters on each trial by pressing either the right or the left key on a button box. The stimuli remained on the screen for 4 s, followed by presentation of the outcome (either ‘correct’ or ‘incorrect’) for 2 s. The likelihood of being correct or incorrect was probabilistically determined for each stimulus (see above). If subjects did not respond within 4 s that the stimuli were on the screen the message ‘no key pressed’ was presented and the trial was excluded from the analysis. A fixation cross was presented for 2 s during the intertrial interval.
Performance phase of the task: Scan 3
Scanning session 3 (performance phase) was 10-min long and consisted of 110 trials of 6 s each. Similar to the acquisition phase, two Hiragana characters were presented side by side on each trial and subjects had to select one of the characters by pressing either the right or the left key. As before, characters remained on screen for 4 s. This time subjects did not receive feedback after making a response and the trial instead progressed immediately to the presentation of a fixation cross during the 2 s intertrial interval.
Importantly, in addition to the stimulus pairs used during training (80% with 20%; 70% with 30% and 60% with 40%), the symbols were shown in eight novel pairings. Four of the pairings had the ‘best’ stimulus paired with all other stimuli (80% with 70%; 80% with 60%; 80% with 40% and 80% with 30%), and the other four pairings compared the ‘worst’ stimulus to all other stimuli (20% with 70%; 20% with 60%; 20% with 40% and 20% with 30%). All pairs were presented 10 times each in randomized order, resulting in 110 pairs overall (Fig. 1).
Magnetic resonance imaging
The study was conducted at the Wellcome Trust Centre for Neuroimaging at University College London using a 3 T (Siemens TRIO) scanner equipped with a Siemens 12-channel phased array head coil. Anatomical images were acquired using modified equilibrium Fourier transform T1 gradient echo scans, which were followed by 1-mm thick axial slices parallel to the anterior commissure–posterior commissure plane. Functional scans used a gradient echo sequence; repetition time, 2.04 s; echo time 30 ms; flip angle 90°; matrix size 64 × 64; field of view 192 mm; slice thickness, 2 mm. A total of 30 axial slices were sampled. The in-plane resolution was 2 × 2 mm.
Functional imaging data were analysed using statistical parametric mapping software (SPM5; Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm). During preprocessing, images were realigned with the first volume (after discarding six volumes to allow for T1 equilibration effects), and unwarped. For each subject, the mean functional image was coregistered to a high resolution T1 structural image. This image was then spatially normalized to standard Montreal Neurological Institute (MNI) space using the ‘unified segmentation’ algorithm available within SPM5 (Ashburner and Friston, 2005) with the resulting deformation field applied to the functional imaging data. These data were then spatially smoothed using an isotropic 6-mm full-width half-maximum Gaussian kernel.
Data analysis
Behavioural analysis
Acquisition sessions 1–3
All subjects reached at least 65% accuracy for the easiest pairing or after completion of three acquisition sessions had a minimum accuracy of 60% over all training pairs before proceeding to the performance phase. Accuracy levels in the acquisition phase were then separately computed for each drug state by averaging the overall accuracy across all acquisition sessions on that day. Accuracy was defined as percentage of trials on which the correct stimulus, i.e. the stimulus with the highest probabilistic contingency in each training pair was selected. We then compared overall accuracy during acquisition in the ON condition to overall accuracy in the two OFF medication states using paired t-tests and a linear mixed model to detect differences in accuracy in the acquisition phase between different drug states. We also tested for differences in the acquisition rate between the different drug states by comparing learning rates in a reinforcement learning model (see below). For this test, we individually fitted the parameters of the reinforcement learning model to subjects’ choices in the ON and OFF medication condition, comparing the resulting learning rates using a paired t-test.
Performance session
Data from the performance session were separated into trials in which the ‘best’ stimulus (80% chance of being correct) was presented, and trials in which the ‘worst’ stimulus (20% chance of being correct) was presented. We calculated the percentage of times subjects picked the best stimulus and the percentage of times the subjects avoided the worst stimulus in these novel pairings and tested for any differences in performance between the different medication conditions.
Reinforcement learning model
We used a simple prediction error-based reinforcement learning model (Sutton and Barto, 1998) to estimate a trial-by-trial measure of stimulus value, and thus an outcome prediction error δ defined as the difference between the actual observed outcome R (correct/incorrect = 1/0) and the current expected value of the chosen stimulus.
For each pair of stimuli A and B, the model estimates the expected values of choosing A, (QA) and choosing B (QB), on the basis of individual sequences of choices and outcomes. The expected values were set to zero before learning. After every trial t > 0 the value of the chosen stimulus (e.g. ‘A’) was updated according to the rule QA(t + 1) = QA(t) + α × δ(t). The outcome prediction error is the difference between the actual and the expected outcome, δ(t) = R(t) − QA(t) with the actual outcome being either ‘Correct’ or ‘Incorrect’ (1 or 0). Values of stimuli that were not shown on a trial were not updated.
Given the expected values, the probability (or likelihood) of the observed choice was estimated using the softmax rule: PA(t) = exp[QA(t)/β]/{exp[QA(t)/β] + exp[QB(t)/β]}. The parameters α (learning rate) and β (temperature) were adjusted to maximize the likelihood of the actual choices under the model, for all subjects. Trial-by-trial outcome prediction errors estimated by the model were then used as parametric regressors in the imaging data.
We also considered an alternative reinforcement learning model, which allowed for separate learning rates α+ on positive updates (increasing the predicted value) and α− on negative updates (decreasing the predicted value). We then compared model likelihoods of the models with separate learning rates and the original reduced model on an individual subject level using Bayesian Information Criterion (BIC), which corrects for the different complexity in models (smaller values indicate better fit) (Schwarz, 1978), and population level using Bayesian model comparison (Stephan et al., 2009).
Functional magnetic resonance imaging: whole-brain general linear model parametric analysis
Acquisition session
Functional MRI time series were regressed onto a composite general linear model containing four regressors: trial onset time (the appearance of the hiragana characters), outcome onset time, motor response time and fixation cross presentation time. The outcome onset was parametrically modulated by the prediction error as estimated by the reinforcement learning model. We also composed another general linear model in which there were four regressors: correct trial onset time, incorrect trial onset time, motor response time and fixation cross presentation time. The actual value of the chosen cue in each trial was entered as a parametric modulator of the two trial onset regressors.
Performance session
Four regressors were entered into the functional MRI model: correct trial onset time, incorrect trial onset time, motor response time and fixation cross presentation time. The actual value of the chosen cue in each trial was entered as a parametric modulator of the two trial onset regressors.
The regressors were convolved with the canonical haemodynamic response function, and low frequency drifts were excluded with a high-pass filter (128-s cut-off). Short-term temporal autocorrelations were modelled using an autoregressive [AR(1)] process. Motion correction regressors estimated from the realignment procedure were entered as covariates of no interest. Statistical significance was assessed using linear compounds of the regressors in the general linear model, generating statistical parametric maps of t-values across the brain for each subject and contrast of interest. These contrast images were then entered into a second-level random-effects analysis using a one-sample t-test against zero.
Anatomical localization was carried out by overlaying the t-maps on a normalized structural image averaged across subjects, and with reference to an anatomical atlas (Naidich et al., 2009). All coordinates are reported in MNI space (Mazziotta et al., 1995).
Region of interest analysis
We extracted data for all region of interest analyses using a cross-validation leave-one-out procedure: we re-estimated our main second-level analysis 12 times, always leaving out one subject. Starting at the peak voxel for the chosen cue value signal in ventromedial prefrontal cortex and nucleus accumbens, which was identified by looking over all correct trials (in both the ON and OFF drug states), we selected the nearest maximum in these cross-validation second-level analyses. Using that new peak voxel, we then extracted the data from the left-out subject and calculated a representative timecourse for each region of interest as first eigenvariate from data in all voxels within a 4-mm sphere around that peak. We then performed a small volume correction on the striatal activations in the putamen using an anatomical region of interest defined according to the Talairach Daemon atlas (Lancaster et al., 1997) using the SPM WFU PickAtlas tool (Maldjian et al., 2003).
Results
We used a within-subject design to study a single group of patients with Parkinson’s disease [early-to-moderate stage (Hoehn and Yahr stage: mean 1.69, SE 0.26)] in a generalization task introduced by Frank et al. (2004, 2007b) in three separate drug states (Fig. 1). We employed a within-subject design given the inherent difficulty in accurately matching patients with Parkinson’s disease with different disease severity. We also believe that this design allowed us to minimize and control, as far as possible, for individual cognitive and genetic differences that may exist in our cohort, allowing us to look at the within-subject effects of drug on behaviour. In parallel with our behavioural analysis, we also acquired neural data using functional MRI. Thus, this design enabled us to explore the effect of dopamine on behaviour and on the brain by testing patients in three different drug states: acquisition and performance ON medication; acquisition and performance OFF medication and acquisition in an OFF medication state and performance in an ON medication state. The inclusion of the latter condition specifically enabled us to probe whether dopamine’s effects are expressed during task acquisition (learning) or task performance.
Acquisition phase
Behavioural results
At the end of the acquisition phase, average choice accuracy on the training pairs did not differ between groups across the different drug states [paired t-tests: comparing ON–ON with OFF–ON: t(1,12) = 0.15, P = 0.87; comparing ON–ON with OFF–OFF: t(1,12) = 0.095, P = 0.92; comparing OFF–ON with OFF–OFF: t(1,12) = −0.079, P = 0.93] (Supplementary Table 4). Similarly, we found no significant difference in learning rates between patients when they were in an OFF compared to ON medication state [ON: mean 0.25, SE 0.02; OFF: mean 0.24, SE 0.01; paired t-test t(1,12) = 0.117, P = 0.90] (Supplementary Table 5), or in the number of sessions required to reach criteria [ON: mean 1.23, SE 0.12; OFF: mean 1.38, SE 0.16 paired t-test t(1,12) = −0.69, P = 0.50]. When the three types of training pairs were examined separately, there were no differences in choice accuracy between the different drug states. When we compared log evidences from a learning model with separate learning rates for positive and negative updates (posneg) with a single learning rate model (single), we found that the more complex model did not explain behaviour any better than the simple model (average BICsingle = 260.9 versus BICposneg = 261.4; posterior probability Psingle = 0.72; exceedance probability single versus posneg model P > 0.99). Thus, subjects’ behaviour could not be explained better with separate learning rates for positive and negative updating.
Neuroimaging data
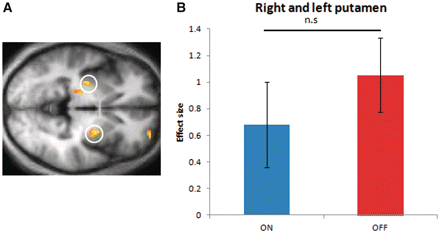
Here, we examined brain responses that correlated with outcome prediction errors computed from a reinforcement learning model, fit to subjects’ behaviour during the acquisition phase. We found that bilateral responses in the striatum (central coordinates right putamen x = 26, y = 0, z = −4; left putamen x = −28, y = −12, z = −2) (Fig. 2) strongly correlated with reward prediction errors [small volume corrected using anatomical region of interest for false discovery rate (FDR); left putamen P = 0.007, right putamen P = 0.006], consistent with many previous results (McClure et al., 2003; O'Doherty et al., 2003; Schonberg et al., 2010). However, similar to our behavioural findings, we found no differences in prediction error-related brain activation between the different drug states during acquisition [paired t-test ON compared with OFF: t(1,11) = −0.076, P = 0.46] (see Supplementary Table 6 for a whole-brain activation table for prediction error-related activity across all drug conditions). We separately examined neural responses to positive and negative prediction errors but did not find any differences between the different drug states [paired t-tests comparing positive prediction errors ON compared with OFF: t(1,11) = −0.083, P = 0.42; and comparing negative prediction errors ON with OFF: t(1,11) = −0.051, P = 0.614]. Perhaps most surprisingly, at the time of cue onset, we did not observe any correlation between brain activity and the value of the chosen cue in any of the drug states.
Prediction error-related activity during acquisition. (A) Brain activity in putamen correlated with magnitude of outcome prediction errors across all trials during the acquisition phase. Activations are thresholded at P < 0.001 uncorrected. (B) Correlation between outcome prediction errors and blood oxygen level-dependent activity in the two different drug states. Data in the ‘ON’ state was averaged across the two acquisition sessions performed in the scanner under this medication state and data in the ‘OFF’ state across the four acquisition sessions performed in this medication state. Error bars represent SEM. n.s. = not significant.
Performance phase
Behavioural results
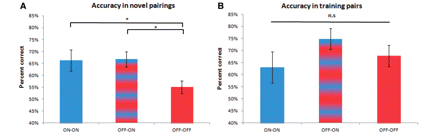
In the performance phase, after patients had acquired the task contingencies, along with all the training pairings, we presented the best (the one with 80% chance of being correct) and worst stimulus (the one with only 20% chance of being correct) in novel pairings with all the other stimuli (Fig. 1). We found that patients ON their dopamine replacement therapy performed significantly better than patients OFF dopamine replacement therapy [main effect comparing accuracy of the mean of ON–ON/OFF–ON with OFF–OFF, paired t-test, t(1,12) = 2.8, P = 0.01]. Crucially, a separate examination of the three drug states revealed a main effect of drug on performance but not on acquisition (Fig. 3A). Subjects who acquired the contingencies in an OFF medication state and received their dopamine replacement therapy after the acquisition phase, but before the performance phase, had the same level of overall accuracy as subjects who both acquired the contingencies ON medication and performed ON medication [paired t-test comparing ON–ON with OFF–ON, t(1,12) = −0.03, P = 0.97]. Both the ON–ON and OFF–ON groups were significantly more accurate than the OFF–OFF group [paired t-test comparing ON–ON with OFF–OFF, t(1,12) = 2.17, P = 0.05; and comparing OFF–ON with OFF–OFF, t(1,12) = 2.28 P = 0.04]. A mixed effects linear model showed a significant effect of drug state on performance accuracy at the performance phase [F(1,36) = 5.38, P = 0.02] but not at the acquisition phase [F(1,36) = 0.002, P = 0.96].
Accuracy during performance phase. (A) Accuracy in novel pairings was significantly higher when subjects were ON dopamine replacement therapy during the performance phase than when they were OFF. This effect is independent of drug state during the previous acquisition phase. Shown is the combined accuracy in selecting the best stimulus and avoiding the worst stimulus over the three drug states when subjects had to pick the stimulus with the highest likelihood of being correct when presented in novel parings. ON–ON session (blue), when patients took their usual dopamine replacement therapy; OFF–ON session (blue/red stripe), when patients took their dopamine replacement therapy only after completing the acquisition phase; OFF–OFF session (red), when patients abstained from their dopamine replacement therapy throughout the task. (B) Accuracy in selecting the better stimulus among the training pairs during the performance phase did not differ between drug states. n.s. = not significant.
In addition to the novel pairings, we also presented subjects with the three stimulus pairs on which they had been trained during acquisition. Interestingly, we found no difference in accuracy levels on these training pairs across the different drug states [paired t-tests comparing ON–ON with OFF–ON, t(1,12) = −1.36, P = 0.19; comparing ON–ON with OFF–OFF, t(1,12) = −0.64, P = 0.52; comparing OFF–ON with OFF–OFF t(1,12) = 1.26, P = 0.23] (Fig. 3B), even when the three types of training pairs were examined separately. Indeed, there was no difference in the accuracies for training pairs versus novel pairs in ON–ON or OFF–ON drug states [paired t-tests comparing training pair accuracy with novel pair accuracy in ON–ON, t(1,12) = 0.61, P = 0.55 and OFF–ON drug states, t(1,12) = −1.75, P = 0.10]. However, as expected, in the OFF–OFF drug state, accuracy for training pairs was significantly higher than for novel pairs [t(1,12) = −2.28, P = 0.04]. Note that our results cannot be explained by a faster extinction effect in the patients when they were OFF dopamine replacement therapy. An effect of this sort would predict an overall gradual performance decrement over time. Instead, we found that patients OFF medication maintained their performance in the training pairs, which were randomly interspersed with the novel pairs, throughout the test session. One difference between our study and that of Frank et al. (2004, 2007b) is that in our study there was an added time delay between acquisition and transfer, whilst l-DOPA took effect. Since dopamine influences processes such as working memory (Sawaguchi and Goldman-Rakic, 1991; Watanabe et al., 1997; Fuster, 2001; Stuss and Knight, 2002) and enhancing dopamine signalling will have had an effect on these processes (Sawaguchi, 2001; Wang et al., 2004; Gibbs and D'Esposito, 2005; Cools and D’Esposito, 2011), one could argue that the poor generalization in the OFF group could stem from the delay. However, we believe this is unlikely given evidence that the OFF group performed just as well as the ON group on training pairs.
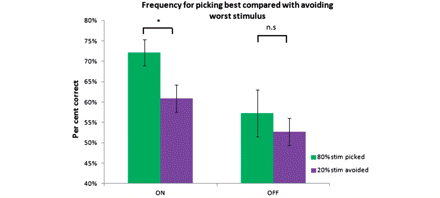
We next tested for differential performance in selecting the best, and avoiding the worst, stimulus within the novel pairings. Interestingly, being in the ON dopamine replacement therapy state during the performance phase selectively improved accuracy in selecting the best stimulus compared to avoiding the worst stimulus for novel stimuli pairs [paired t-tests comparing ON accuracy for picking the best compared with avoiding worse stimulus t(1,12) = 2.16, P = 0.05]. This performance difference between selecting the best and avoiding the worst stimulus was not evident when subjects both acquired and performed the task in the OFF medication state [paired t-tests comparing OFF accuracy for picked best compared with avoiding worse stimulus t(1,12) = 0.58, P = 0.56], although their overall performance was worse (Fig. 4). Of note, there was no interaction between the medication status (ON versus OFF) during performance and the ability to pick the best compared with avoiding the worst stimulus as has previously been reported (Frank et al., 2004, 2007b). We only found this selective improvement in picking the best stimulus compared with avoiding the worst stimulus within the ON group. We observed the same pattern when analysing the ON–ON and OFF–ON groups separately. However, although a trend was evident this did not reach full significance [paired t-tests comparing ON accuracy for picking the best compared with avoiding worse stimulus in the ON–ON group alone, t(1,12) = 1.5, P = 0.15; and in the OFF–ON group alone, t(1,12) = 1.68, P = 0.11]. When we separately compared accuracy for picking the best stimuli, and for avoiding the worst stimuli, across groups there were no significant differences [paired t-tests comparing ‘pick best’ accuracy; ON–ON with OFF–ON, t(1,12) = −0.26, P = 0.98; comparing ON–ON with OFF–OFF, t(1,12) = 1.79, P = 0.09; comparing OFF–ON with OFF–OFF, t(1,12) = 1.73, P = 0.1; paired t-tests comparing ‘avoid worse’ accuracy: ON–ON with OFF–ON, t(1,12) = −0.07, P = 0.94; comparing ON–ON with OFF–OFF, t(1,12) = 1.27, P = 0.22; comparing OFF–ON with OFF–OFF, t(1,12) = 1.6, P = 0.13]. This indicates that when subjects were ON dopaminergic medication there was an asymmetry in performance between picking the best compared with avoiding the worst stimuli. Our data do not show that medication selectively improves generalization only for the best stimuli but rather that it affects the ability to generalize learnt information overall. When we examined reaction times, we found that across all groups there was a significant difference in reaction times between pick best and avoid worse stimuli trials [paired t-test, t(1,12) = −2.52, P = 0.027] with subjects being faster for the pick best trials. There were, however, no significant between group differences in reaction times.
Differences in accuracy at picking best compared with avoiding worst stimuli. ON state during performance phase selectively improved accuracy for picking the best stimulus (the 80%) compared to avoiding the worst stimulus (the 20% stimulus) in novel pairings. The data in the ‘ON’ state comes from the two performance bouts performed in this medication state and the data in the ‘OFF’ state comes from the single performance bout performed in this medication state.
Neuroimaging data
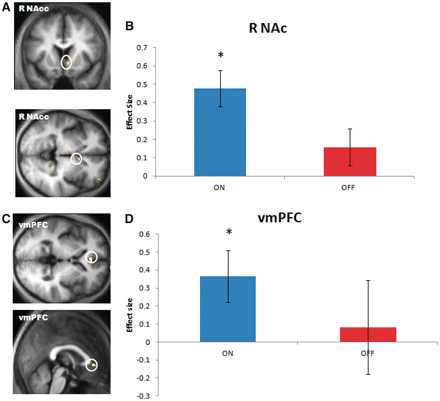
To investigate possible neural mechanisms underlying the observed behavioural effects during the performance phase, we next tested for differences in the degree at which functional MRI blood oxygen level-dependent activity correlated with decision variables between different drug states. We tested whether neural representations of stimulus values at the time of cue presentation differed between drug states. We found that blood oxygen level-dependent activity in nucleus accumbens (central coordinates x = 8, y = 12, z = −4) correlated with the value of the chosen cue, but this effect was only evident in the ON medication state for correct novel trials [one sample t-test, t(1,11) = 2.7, P = 0.01]. Cue-evoked blood oxygen level-dependent activity did not correlate with the value of the chosen cue when patients were OFF their dopamine replacement therapy [one sample t-test, t(1,11) = 0.98, P = 0.34] or made an incorrect choice [one sample t-test ON incorrect, t(1,11) = −2.12, P = 0.06; OFF incorrect t(1,11) = −0.06, P = 0.94] (Fig. 5A and B). We found an identical effect in ventromedial prefrontal cortex (x = −2, y = 38, z = 0), where blood oxygen level-dependent activity varied with the value of the chosen cue when patients were both ON medication and made the correct choice [one sample t-test, t(1,11) = 2.52, P = 0.02], but not when they were OFF their dopamine replacement therapy [one sample t-test, t(1,11) = 0.31, P = 0.76], or made an incorrect choice [one sample t-test ON incorrect, t(1,11) = −1.28, P = 0.22; OFF incorrect t(1,11) = 0.76, P = 0.46] (Fig. 5C and D). These findings show that activity in nucleus accumbens and ventromedial prefrontal cortex successfully reflect the values of the most rewarding cue only in an ON medication state, a characteristic that precisely mirrors patients’ improved performance in this state. We found that this effect was not driven solely by the ON–ON group. When the OFF–ON group are examined separately, cue-evoked blood oxygen level-dependent activity correlated with the value of the chosen cue with patients made the correct choice both in the nucleus accumbens [one sample t-test, t(1,11) = 2.86, P = 0.015] and in the ventromedial prefrontal cortex [one sample t-test, t(1,11) = 2.93 P = 0.014], however, when we directly compared the activations in OFF–ON with the OFF–OFF group this did not reach statistical significance [paired t-test comparing OFF–ON with OFF–OFF, nucleus accumbens t(1,11) = 1.62, P = 0.13, ventromedial prefrontal cortex t(1,11) = 1.02, P = 0.32].
Brain activity correlating with the value of the chosen cue during performance phase. (A) Brain activity in right nucleus accumbens (R NAc) correlated with the value of the chosen cue. Analysis performed over all correct trials (both ON and OFF) in a context where novel parings are presented. (B) A differential analysis between drug states reveals this correlation was selective to the ON state. (C) Brain activity in ventromedial prefrontal cortex (vmPFC) also correlated with the value of the chosen cue. Whole brain analysis performed over all correct trials (both ON and OFF). (D) Similar to activity in nucleus accumbens, the correlation between blood oxygen level-dependent values in ventromedial prefrontal cortex and the value of the chosen cue was only evident in ON but not in OFF state. The error bars represent SEM. Thresholds in statistical parametric map images set to P < 0.005 uncorrected.
Akin to the neuroimaging findings from the acquisition phase, when we examined value-related neural activity at the time of cue onset during presentation of the training pairs at the performance phase, we did not find a significant correlation between blood oxygen level-dependent activity and the value of the chosen cue in either of the drug states.
Discussion
We show a striking effect of dopamine replacement therapy on the ability of patients with Parkinson’s disease to select the correct stimulus in a probabilistic reinforcement learning task. Importantly, our data show that medication status at the acquisition task phase does not impact successful task learning. Instead, the data show that the critical factor is medication status at the performance phase, by which time stimulus values must already have been successfully acquired. The findings challenge a proposal that the impact of dopaminergic status on this form of decision-making solely reflects its involvement in learning.
Our key observation was that patients who were OFF dopamine during the second task phase performed significantly worse when stimuli occurred in novel pairings. However, dopaminergic drug state did not impact their ability to choose when confronted with pairs on which they had been trained in the first phase of the task. This indicates that the subjects OFF medication could successfully retrieve learnt contingencies but were unable to use this knowledge to make correct choices when they had to select between novel stimulus pairings. There was no difference in learning rates or accuracy during the acquisition phase between the different drug conditions, indicating that dopamine did not affect the ability to learn stimulus values. Consequently, our data indicate that dopamine replacement therapy influenced the ability to generalize, in a context, where subjects needed to select the best stimulus in a state characterized by presentation of novel pairings.
A mechanistic basis for our behavioural findings is provided by our functional MRI data, which specifically addressed the neural representation of stimulus value during the performance phase. Even when subjects had learned stimuli OFF dopamine replacement therapy, and were only given their dopamine replacement therapy after learning had occurred, activity in nucleus accumbens and ventromedial prefrontal cortex encoded the value of the chosen stimulus during the performance phase, allowing the brain to compare those values in novel pairings. This suggests that, in contrast to previous accounts (Schultz et al., 1997; O’Doherty et al., 2003; Bayer and Glimcher, 2005; Pessiglione et al., 2006), reduced dopamine availability during learning did not impair value acquisition. Instead, our data show that decreased dopamine during performance resulted in an impoverished neural representation of stimulus value. This we suggest impaired an ability to compare cue values in novel pairings, in other words to generalize from learnt information. On the other hand, non-novel pairings (i.e. the acquisition pairs) could still be answered correctly if a stimulus–response association had been learned; this would not depend directly on value representations [and could for example be achieved by fixed stimulus–response associations, or explicit (episodic) memory retrieval], and could therefore operate successfully even when dopamine levels were low. This explanation best accounts for why subjects who were OFF medication throughout the task were equally successful at choosing the best stimuli in the context of the training pairs. It is of interest that the two structures highlighted in our data, the nucleus accumbens and ventromedial prefrontal cortex, are strongly associated with various forms of value prediction and prediction errors in reinforcement learning contexts (Matsumoto et al., 2003; Day and Carelli, 2007; Luk and Wallis, 2009). The pattern of findings we observed, whereby stimulus value correlated with activity in these two regions in the ON state, implies that these brain areas can successfully represent the reward value of cues when patients are ON medication enabling successful performance for novel pairings. However, when this signal is degraded as seen in the OFF state, performance is impaired.
The fact that patients in all three drug states performed equally well when they were selecting the best cue for sets on which they had been previously trained further indicates that dopamine did not influence patients’ accuracy by a direct influence on learning. Levodopa medication in patients with Parkinson’s disease has previously been shown to have a positive effect on generalization of learnt information in novel contexts; however, those observations were on a background of impaired learning and therefore crucially different from our current findings (Myers et al., 2003; Shohamy et al., 2006). Of course, many different systems are likely to be involved in learning, only some of which depend directly on dopamine (Beninger, 1983; Dickinson et al., 2000; Daw et al., 2005; Palmiter, 2008), and we cannot discount the possibility that a more complex learning task, such as one involving sequences of choices, might be necessary to fully reveal effects of dopamine on learning.
Beyond its putative role in learning, dopamine is implicated in a number of distinct processes related to motivation, including the control of Pavlovian conditioned responses and motivational vigour (Dickinson et al., 2000; Parkinson, et al., 2002; Salamone et al., 2003; Berridge, 2007; Mazzoni et al., 2007; Niv, 2007; Bardgett et al., 2009; Beeler et al., 2010; Boureau and Dayan, 2011). The strong influence of dopamine on performance, separate to that on learning, is well known from animal data. For example, dopamine-deficient mice retain the ability to pick the most rewarding drink (sucrose compared with water) when presented in a discrimination task (Cannon and Palmiter, 2003). Genetic dopamine-deficient mice when tested in a maze task appears initially impaired but when subsequently treated with l-DOPA (Robinson et al., 2005), or caffeine (Hnasko et al., 2005) can be shown to have learned, consistent with an effect of dopamine on the expression of learning rather than on learning itself. In addition, dopamine is implicated in controlling movement rate and vigour (Ungerstedt, 1971; Salamone et al., 2003; Cagniard et al., 2006) with dopamine depletion causing decreased motivation to work for rewards under demanding reinforcement schedules (Salamone and Correa, 2002; Niv, 2007).
Importantly, these other roles remain consistent with the fact that phasic activity of dopamine neurons codes for an appetitive prediction error (McClure et al., 2003). However, our study has enabled us to disentangle these effects from a mere effect on learning in a manner that provides compelling evidence that dopamine has a specific role on the expression of learning that is distinct from any effect it may have on learning itself. We are, however, unable to comment on whether the drug manipulation, which included the withdrawal and then reinstatement of both l-DOPA and dopamine agonists, primarily exerted its main effect on tonic or phasic levels of dopamine although we infer that it is likely to have an effect on both.
The involvement of the nucleus accumbens during successful performance is particularly notable, since this structure is well known to control the immediate effects of dopamine on numerous aspects of performance (Berridge and Robinson, 1998; Ikemoto and Panksepp, 1999; Berridge, 2009; Lex and Hauber, 2010). The nucleus accumbens is a site where the predicted values of stimuli are transformed into preparatory Pavlovian responses under a modulatory influence of dopamine (Berridge and Robinson, 1998). We suggest that a preparatory response of approach is likely to be a key substrate for the behavioural patterns we observed in our task (Dayan et al., 2006). This provides another reminder of the complexities inherent in a single neuromodulator (dopamine) supporting two apparently independent roles, namely reporting on appetitive prediction errors and influencing motivation and vigour (Ikemoto and Panksepp, 1999; Niv et al., 2007; Boureau and Dayan, 2011; Cools et al., 2011).
A further important finding is the engagement of ventromedial prefrontal cortex in a context in which subjects made the correct choice between novel pairings of stimuli in the ON state, but not when subjects made incorrect choices in the ON state. This region is strongly implicated in valuation (Gottfried et al., 2003; Seymour and McClure, 2008; Boorman et al., 2009; Kable and Glimcher, 2009; Fitzgerald et al., 2010; Plassmann et al., 2010) across a range of experimental manipulations, with mounting evidence pointing to a specific role when subjects have to choose between distinct options with different values (Padoa-Schioppa and Assad, 2006; FitzGerald et al., 2009; Wunderlich et al., 2010). This fits neatly with our observation that this region was engaged when subjects generated correct choices based upon an assessment of a learnt value difference between novel pairings. However, our data are intriguing in suggesting that the integrity of a dopamine input to this region is important for this form of value-based decision.
Of course, we cannot be certain as to dopamine’s precise role in our task. However, two possibilities are immediately apparent: dopamine is either necessary for a stable value representation that can support generalization, or alternatively, for taking the difference between the values of the available stimuli in order to choose. We were unable to dissociate whether the neural value correlates were precursors to choice (stimulus values) or the output of the choice process (chosen values) (Wunderlich et al., 2009). It remains an open question for future research as to whether the deficit is due to a misrepresentation of pre-choice values that are fed into a decision comparator, or reflect a problem at the value comparison stage itself or indeed a combination of both.
Our study involved testing patients with Parkinson’s disease, which although providing the best human model of dopamine depletion, there is by necessity the problem of whether observations in this population can be generalized to the healthy population. Despite this caveat, our findings do lend support to the hypotheses (Berridge and Robinson, 1998; Berridge, 2007) and animal studies (Ahlenius et al., 1977; Beninger and Phillips, 1981; Wyvell and Berridge, 2000; Cannon and Palmiter, 2003; Denenberg et al., 2004; Robinson et al., 2005) that stress a major role for dopamine outside of learning.
A significant finding from our study is that when patients were ON their dopamine replacement therapy, they were worse at avoiding stimuli with the poorest probabilistic contingencies than at choosing the stimuli with the best probabilistic outcomes. This is in keeping with previous research showing a similar outcome valence performance asymmetry, whereby patients ON their dopamine replacement therapy are impaired at avoiding the least rewarding stimuli (Frank et al., 2004, 2007b). It has been postulated that this worsening in performance is due to ‘overdosing’ of the striatum, which interferes with the dips in dopamine that express negative prediction errors (Frank et al., 2004, 2007b). However, in our study, as in several others (de Wit et al., 2011; Jocham et al., 2011), we did not find a direct effect of medication on learning, and we postulate that the worsened performance may reflect some other mechanism, perhaps an impaired expression of avoidance behaviour in a high dopamine state.
Of note, we did not find the interaction between medication state and picking the best compared with avoiding the worst stimulus that has been reported in some previous studies (Frank et al., 2004, 2007b; Voon et al., 2010). We found an overall improvement in performance when subjects were ON medication and an asymmetry in this performance accuracy between picking the best compared with avoiding the worst stimuli within this group.
We did not find a significant difference between picking the best stimulus in the ON state and picking the best stimulus in the OFF state. Thus, although we can conclude that when subjects were ON dopaminergic medication, there was an asymmetry in performance between picking the best compared with avoiding the worst stimuli, we cannot be certain whether this improvement in performance in the ON compared with the OFF group is solely due to an improved ability to select the most rewarding stimuli. Since the OFF–OFF patients generalized so poorly, there remains a possibility that we were unable to detect an asymmetry in performance in the OFF group due to floor effects. However, the clear asymmetry observed in the ON group is of interest as it provides a hint as to where dopamine may exert an important influence.
Several studies have also found differences in striatal activations in response to wins and losses when comparing patients with Parkinson’s disease with compulsive behaviour ON medication to patients with idiopathic Parkinson’s disease (Steeves et al., 2009; Voon et al., 2010) or in healthy subjects who were given dopaminergic modulating drugs (Pessiglione et al., 2006; Cools et al., 2007). We did not find this pattern in our study, possibly because of the unique feature of our design in making within-subject comparisons in patients with idiopathic Parkinson’s disease. In particular, acute pharmacological manipulations in healthy volunteers may have very different effects to those found in patients previously exposed to dopaminergic agents. Another potential explanation for these differences is that in contrast to the studies that found differences in striatal activations in response to gains and losses (Pessiglione et al., 2006; Bodi et al., 2009; Palminteri et al., 2009; Voon et al., 2010), we did not have actual losses as outcomes, only stimuli that were probabilistically more or less likely to be correct. We caution against a conclusion that our data indicate that prediction errors do not play an important role in learning. Indeed, we observed prediction errors during the task acquisition. We were, however, unable to detect differences in the magnitude of these activations when dopaminergic drugs were given to this patient group, consistent with a suggestion that for successful choice, it is critical that dopamine levels are high during actual performance. We also cannot discount the fact that there might remain some residual activity within the dopaminergic system of patients with Parkinson’s disease, resulting in adequate levels of dopamine to signal reward prediction errors in some structures, but not enough to form adequate cue value representations to allow successful generalization in those, or other, structures. Furthermore, in some contexts the relationship between dopamine appears to follow an inverted U-shaped function whereby the optimum level of performance exists at a certain level of dopaminergic stimulation and moving off that peak, either by reducing or increasing the levels of dopamine, leads to worsened task performance (Robbins, 2000; Rowe et al., 2008; Cools and D'Esposito, 2011) rendering dopaminergic manipulations crucially dependant on baseline levels. There is a possibility that high level of cognitive processing, such as working memory, required for this task may obscure differences in striatal activations. Indeed, differing performance in tasks of this type has been shown in genetic studies to have a differential impact on prefrontal and striatal dopamine (Frank et al., 2007a; Klein et al., 2007). However, in our task if dopamine had in fact boosted the prediction error magnitude in a way that impacted learning, we would have expected to see this in improved behaviour in the performance phase. This would result in the ON–ON group performing the best, which was in fact not the case.
By teasing apart learning and performance in patients with Parkinson’s disease, we found that dopaminergic medication impacted the latter, but not the former. At the neural level, this improved performance in the ON medication state was associated with enhanced nucleus accumbens and ventromedial prefrontal cortex activity for the chosen cue value, an effect that was absent in the OFF medication state. Thus, the improved performance in patients ON medication cannot solely be attributed to an effect on learning and must reflect some other effect of dopamine, perhaps Pavlovian appetitive approach or a modulation of the motivational impact of cues associated with improved neural representation of cue value in a high dopamine state. By isolating the processes on which dopamine has the greatest impact, our findings point to likely mechanisms that underlie common behavioural deficits seen in patients with Parkinson’s disease, both clinically and in various laboratory tasks, as well as providing a basis for future cognitive-oriented therapies.
Funding
Wellcome Trust [Ray Dolan programme grant number 078865/Z/05/Z], an MRC grant (to T.S.), Gatsby Charitable Foundation (to P.D.), Wellcome Trust (091593/Z/10/Z).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank the reviewers for their insightful and helpful comments. They also thank Tali Sharot, Rosalyn Moran and the emotion group for their helpful input on this work.