-
PDF
- Split View
-
Views
-
Cite
Cite
Laura Avanzino, Davide Martino, Isadora Martino, Elisa Pelosin, Carmelo M. Vicario, Marco Bove, Gianni Defazio, Giovanni Abbruzzese, Temporal expectation in focal hand dystonia, Brain, Volume 136, Issue 2, February 2013, Pages 444–454, https://doi.org/10.1093/brain/aws328
Close - Share Icon Share
Abstract
Patients with writer’s cramp present sensory and representational abnormalities relevant to motor control, such as impairment in the temporal discrimination between tactile stimuli and in pure motor imagery tasks, like the mental rotation of corporeal and inanimate objects. However, only limited information is available on the ability of patients with dystonia to process the time-dependent features (e.g. speed) of movement in real time. The processing of time-dependent features of movement has a crucial role in predicting whether the outcome of a complex motor sequence, such as handwriting or playing a musical passage, will be consistent with its ultimate goal, or results instead in an execution error. In this study, we sought to evaluate the implicit ability to perceive the temporal outcome of different movements in a group of patients with writer’s cramp. Fourteen patients affected by writer’s cramp in the right hand and 17 age- and gender-matched healthy subjects were recruited for the study. Subjects were asked to perform a temporal expectation task by predicting the end of visually perceived human body motion (handwriting, i.e. the action performed by the human body segment specifically affected by writer’s cramp) or inanimate object motion (a moving circle reaching a spatial target). Videos representing movements were shown in full before experimental trials; the actual tasks consisted of watching the same videos, but interrupted after a variable interval (‘pre-dark’) from its onset by a dark interval of variable duration. During the ‘dark’ interval, subjects were asked to indicate when the movement represented in the video reached its end by clicking on the space bar of the keyboard. We also included a visual working memory task. Performance on the timing task was analysed measuring the absolute value of timing error, the coefficient of variability and the percentage of anticipation responses. Patients with writer’s cramp exhibited greater absolute timing error compared with control subjects in the human body motion task (whereas no difference was observed in the inanimate object motion task). No effect of group was documented on the visual working memory tasks. Absolute timing error on the human body motion task did not significantly correlate with symptom severity, disease duration or writing speed. Our findings suggest an alteration of the writing movement representation at a central level and are consistent with the view that dystonia is not a purely motor disorder, but it also involves non-motor (sensory, cognitive) aspects related to movement processing and planning.
Introduction
Primary focal dystonia is a disorder of motor output consisting of sustained muscle contractions that cause uncontrollable postures and movements impeding the execution of voluntary finalistic movements in the affected body part(s) (Fahn et al., 1998). A subgroup of primary focal dystonias, previously known as ‘occupational cramps’ and now more frequently referred to as task-specific dystonias, affect and are brought on by the execution of skilled, mostly manual, activities, such as writing, practicing specific sports (e.g. golf) or playing a musical instrument (Rosenbaum et al., 1988; Jankovic et al., 2008).
Primary dystonia has classically been explained as a disorder affecting primarily the final organization and execution phases of movement (Berardelli et al., 1998). However, in recent years, substantive evidence has been produced that shows how other cognitive aspects of motor control, such as the processing of sensory input and imagination of movement, are also abnormal in this disorder, suggesting altered functioning in networks interconnecting frontal and parietal cortices to subcortical structures, primarily the basal ganglia (Abbruzzese et al., 2001; Quartarone et al., 2005; Fiorio et al., 2006, 2011). Cognitive abnormalities of motor control in patients with primary dystonia include impairment in the temporal discrimination between tactile stimuli (Tinazzi et al., 2002, 2004; Aglioti et al., 2003), in the vibration-induced illusion of movement (Grünewald et al., 1997; Frima et al., 2008) and in pure motor imagery tasks, such as mental rotation of corporeal and inanimate objects (Quartarone et al., 2005; Fiorio et al., 2006). Some of these abnormalities have also been demonstrated in subjects who do not manifest, but are genetically predisposed to, dystonia, suggesting they may represent endophenotypes of dystonia, rather than compensatory effects of the movement disorder (Bradley et al., 2010; Kimmich et al., 2011). Further, sensory processing abnormalities of vibrotactile stimulation with abnormal brain responses have been demonstrated on the affected and unaffected sides in people with writer’s cramp, also supporting the notion of an endophenotype (Tempel et al., 1990; Butterworth et al., 2003). On the other hand, only limited information is available on the ability of patients with dystonia to process the time-dependent features (e.g. speed and duration) of movement in real time (Ruiz et al., 2011; Strübing et al. 2012).
The processing of time-dependent features of movement has a crucial role in predicting whether the outcome of a complex motor sequence, such as handwriting or playing a musical passage, will be consistent with its ultimate goal, or results instead in an execution error. In a recent study, Ruiz et al. (2011) used an event-related potential paradigm to show abnormal EEG oscillatory patterns temporally preceding execution errors in musicians with dystonia, suggesting that error prediction, which is strongly related to both temporal and spatial features of movement, may be dysfunctional in patients with task-specific dystonia. This work provides evidence that forward control processes underlying motor prediction may be faulty in task-specific dystonia. However, it is still undefined whether this dysfunction consists uniquely of an abnormal integration between proprioceptive inputs and motor output during execution or whether it depends also on an abnormal way to process the time-dependent components of movement.
In this study, we were interested in comparing the ability to extrapolate temporal features of different movements (human body motion, inanimate object motion) between a group of patients with writer’s cramp, the most common form of primary task-specific focal dystonia, and a group of healthy control subjects of similar age and gender distribution. This goal was pursued by engaging subjects in an implicit time processing task. Implicit timing is engaged whenever sensorimotor information is temporally structured and can be used to predict the duration of future events (Coull and Nobre, 2008). For tasks in which implicit timing is indexed by the temporal predictability of perceptual input, timing is used to build an expectation of when the next stimulus will appear (Coull and Nobre, 2008).
Participants were asked to predict the end of visually perceived movements, either of a body segment (handwriting, i.e. the action performed by the human body segment specifically affected by writer’s cramp) or of an inanimate object (a moving circle reaching a spatial target). In line with evidence of other abnormalities of cognitive processing of movement in task-specific dystonia (Tinazzi et al., 2009; Fiorio et al., 2011), we hypothesized that patients with focal hand dystonia would be less accurate than healthy subjects in predicting the outcome of the movement of a given visual stimulus. We also hypothesized that this reduced accuracy would be more marked for human body motion (handwriting) compared with inanimate object motion, as a result of an altered process of embodiment of the perceived movement, which is known to produce distortions in the judgement of time (Nather et al., 2011). Given that our experimental tasks required subjects to perform a temporal prediction after short-term memory storage of a perceived movement, we added to our paradigm a task assessing visual working memory.
Materials and methods
Subjects
Fourteen patients [five males and nine females, mean ± standard deviation (SD) age 42.3 ± 12.3 years] affected by writer’s cramp in the right hand without any other neurological condition and 17 healthy subjects (6 males and 11 females, mean ± SD age 38.4 ± 15.6 years) were recruited for the study. In patients with writer’s cramp, mean ± SD disease duration was 9.6 ± 7.4 years, and the mean ± SD severity score on the Writer’s Cramp Rating Scale (Wissel et al., 1996) was 14.7 ± 4.9. Objective measurement of writing speed in patients with writer’s cramp was performed by recording the movement of the tip of a special pen by a digitizing tablet connected to a personal computer. Data were analysed using a commercially available software package for writing movement analysis providing spatial and temporal parameters (Writing Analyzer, eTT srl). Subjects were asked to write the sentence ‘il mattino ha l’oro in bocca’ using their normal everyday writing style in terms of stroke size and speed. As dystonic symptoms tend to increase with prolonged handwriting, subjects were required to write the same sentence 10 times without interruption. Writing speed was calculated on data collected from the 10th sentence by dividing the trajectory path (in cm) for the movement time (in s). Mean writing speed was 4.33 ± 0.69 cm/s.
Twelve patients had received treatment with botulinum toxin not <6 months before the study; the remaining two patients had never received treatment for dystonia before the study. Demographic and clinical information for the patient group is provided in Table 1.
Demographic and clinical information for patients with writer’s cramp
| Patient/gender . | Age, years . | Disease duration . | Severity scorea . | Therapy . |
|---|---|---|---|---|
| 1/F | 47 | 7 | 24 | BTX |
| 2/M | 49 | 10 | 16 | BTX |
| 3/F | 56 | 18 | 20 | BTX |
| 4/F | 33 | 9 | 16 | BTX |
| 5/F | 43 | 23 | 14 | BTX |
| 6/F | 22 | 1 | 8 | |
| 7/M | 39 | 3 | 6 | |
| 8/F | 26 | 3 | 12 | BTX |
| 9/M | 55 | 5 | 12 | BTX |
| 10/F | 52 | 8 | 18 | BTX |
| 11/F | 42 | 8 | 12 | BTX |
| 12/M | 45 | 10 | 16 | BTX |
| 13/M | 60 | 25 | 20 | BTX |
| 14/F | 23 | 4 | 12 | BTX |
| Patient/gender . | Age, years . | Disease duration . | Severity scorea . | Therapy . |
|---|---|---|---|---|
| 1/F | 47 | 7 | 24 | BTX |
| 2/M | 49 | 10 | 16 | BTX |
| 3/F | 56 | 18 | 20 | BTX |
| 4/F | 33 | 9 | 16 | BTX |
| 5/F | 43 | 23 | 14 | BTX |
| 6/F | 22 | 1 | 8 | |
| 7/M | 39 | 3 | 6 | |
| 8/F | 26 | 3 | 12 | BTX |
| 9/M | 55 | 5 | 12 | BTX |
| 10/F | 52 | 8 | 18 | BTX |
| 11/F | 42 | 8 | 12 | BTX |
| 12/M | 45 | 10 | 16 | BTX |
| 13/M | 60 | 25 | 20 | BTX |
| 14/F | 23 | 4 | 12 | BTX |
a Writer’s Cramp Rating Scale (Wissel et al., 1996).
BTX = botulinum toxin.
Demographic and clinical information for patients with writer’s cramp
| Patient/gender . | Age, years . | Disease duration . | Severity scorea . | Therapy . |
|---|---|---|---|---|
| 1/F | 47 | 7 | 24 | BTX |
| 2/M | 49 | 10 | 16 | BTX |
| 3/F | 56 | 18 | 20 | BTX |
| 4/F | 33 | 9 | 16 | BTX |
| 5/F | 43 | 23 | 14 | BTX |
| 6/F | 22 | 1 | 8 | |
| 7/M | 39 | 3 | 6 | |
| 8/F | 26 | 3 | 12 | BTX |
| 9/M | 55 | 5 | 12 | BTX |
| 10/F | 52 | 8 | 18 | BTX |
| 11/F | 42 | 8 | 12 | BTX |
| 12/M | 45 | 10 | 16 | BTX |
| 13/M | 60 | 25 | 20 | BTX |
| 14/F | 23 | 4 | 12 | BTX |
| Patient/gender . | Age, years . | Disease duration . | Severity scorea . | Therapy . |
|---|---|---|---|---|
| 1/F | 47 | 7 | 24 | BTX |
| 2/M | 49 | 10 | 16 | BTX |
| 3/F | 56 | 18 | 20 | BTX |
| 4/F | 33 | 9 | 16 | BTX |
| 5/F | 43 | 23 | 14 | BTX |
| 6/F | 22 | 1 | 8 | |
| 7/M | 39 | 3 | 6 | |
| 8/F | 26 | 3 | 12 | BTX |
| 9/M | 55 | 5 | 12 | BTX |
| 10/F | 52 | 8 | 18 | BTX |
| 11/F | 42 | 8 | 12 | BTX |
| 12/M | 45 | 10 | 16 | BTX |
| 13/M | 60 | 25 | 20 | BTX |
| 14/F | 23 | 4 | 12 | BTX |
a Writer’s Cramp Rating Scale (Wissel et al., 1996).
BTX = botulinum toxin.
The experimental paradigm consisted of (i) one session assessing temporal expectation of human body segment versus inanimate object motion and (ii) one session assessing visual working memory. All subjects were right-handed and gave informed consent for participation in the study. Experimental procedures were approved by the local ethics committee and were conducted in accordance with the Declaration of Helsinki.
Temporal expectation task
This experimental session consisted of two different perceptual tasks: a target task, involving the perception of a common movement of a human body segment (a right hand writing a sentence), and a control task, involving the perception of a movement of an inanimate object (a ball reaching a target). These movements were presented as video files on a computer screen at a fixed speed. The human body motion video displayed the movement of a hand writing a proverb sentence in Italian (‘il mattino ha l’oro in bocca’) with a black pencil on an A4-size white paper sheet positioned on the table; the inanimate object motion video consisted of a linear movement, directed from left to right, of a black ball on a white screen heading towards a black vertical bar. The two videos displayed movements under the same visual angle, had the same total duration (20 s), and the order of administration was counterbalanced across the two groups. The tasks have been programmed using dedicated software (E-Prime 2.0, SciencePlus).
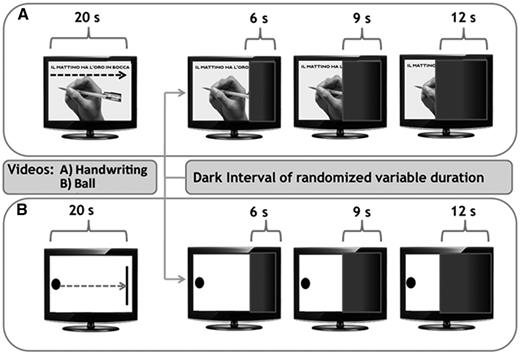
For each of the two tasks, subjects were shown the full video for a single time without receiving any instruction. The actual task consisted of watching the same video, but it was interrupted after a variable interval from its onset (pre-dark interval) by a dark interval of variable duration (dark interval). During the dark interval, subjects were asked to indicate when the movement represented in the video reached its end by clicking on the space bar of the keyboard. Thus, participants used their knowledge of velocity of motion to extrapolate duration of the movement of the body segment (or object) represented in the video. For the sake of the analysis, we refer to the period spanning from the beginning of the dark interval to the subject’s response as ‘reproduced interval’, whereas we refer to the real duration of the dark interval as ‘target interval’. Participants did not receive any feedback on interval duration or their performance throughout the whole session. Three different dark intervals (6, 9 and 12 s) were used, which were randomized across trials. Each condition was administered six times in a randomized order, for 36 trials per experimental session. The experimental paradigm is summarized in Fig. 1.
A schematic view of the experimental design. Two videos were shown to the subjects: the human body motion video showed a common movement of a human body segment (a right hand writing a sentence), whereas the inanimate object motion video showed a movement of an inanimate object (a ball reaching a target). The task consisted of watching the same video, but it was interrupted, after a variable interval from its onset, by a dark interval of variable duration (6, 9 or 12 s). During the dark interval, subjects were required to extrapolate the temporal duration of the movement displaced by clicking on the space bar when they thought that the movement had reached its end.
Visual working memory task
During this task, subjects were serially shown 7 × 7 visual bidimensional matrices containing six black target squares. Each matrix appeared at the centre of the computer screen for 5 s, and subsequently disappeared. During the 5 s, subjects had to look at the matrix and memorize the exact position of the six black target squares. After a variable time interval (500 ms or 5 s), the next matrix appeared, and subjects had to express, by pressing one of two keyboard pads, whether the position of the six black target squares of the new matrix was identical to or different from that of the previous matrix. Therefore, subjects had to simultaneously compare each matrix with the preceding one, and memorize it for comparison with the following one. The whole task comprises two series of 16 consecutive matrices each; the interstimulus interval was 500 ms in one series and 5 s in the other. The two series were presented in counterbalanced order across participants.
Data analysis
Performance on the temporal expectation task was analysed measuring the absolute value of error, the coefficient of variability and the percentage of anticipation responses. These parameters allowed us to describe the ability in time processing determining the magnitude (absolute value of error) and the variability (coefficient of variability) of the timing error, as well as the strategy adopted by subjects during time prediction (percentage of anticipation responses). The timing error was calculated as (reproduced interval − target interval). The absolute value of this error was then normalized with respect to the corresponding target interval and was expressed as a percentage; only normalized absolute timing errors were analysed. This parameter provides a direct measure of the accuracy of subjects in estimating the corresponding target interval: the greater the absolute error, the greater the error in temporal judgement regardless of its direction.
The coefficient of variability was calculated as SD/mean of the reproduced intervals, representing an index of performance variability in temporal judgement.
Finally, the percentage of anticipation responses (calculated on the total number of responses) represents the number of responses in which subjects pressed the space button before the real end of the movement (negative error). This parameter gives information on the tendency to under- or overestimate the target interval.
The performance on visual working memory was measured calculating accuracy (percentage of correct responses) and reaction time (in ms).
Statistical analysis
In Session 1, timing parameters (absolute error, coefficient of variability and percentage of responses in anticipation) were analysed by means of a repeated measures ANOVA with group (patients with writer’s cramp, healthy control subjects) as between subject factor and task (target, control) and target interval (6, 9 and 12 s) as within-subjects factors. Accuracy and reaction time data obtained in the visual working memory session were analysed between groups by means of an unpaired t-test, separately for each interstimulus interval (500 or 5000 ms). Post hoc analysis of significant interactions was performed by means of t-tests applying the Bonferroni correction for multiple comparisons where necessary. P-values of <0.05 were considered as threshold for statistical significance. Finally, the Spearman’s correlation coefficient was applied to assess any correlation between performance on perceptual timing of motion and severity and duration of the disease and writing speed. Statistical analysis was performed with SPSS 13.0.
Results
Temporal expectation task
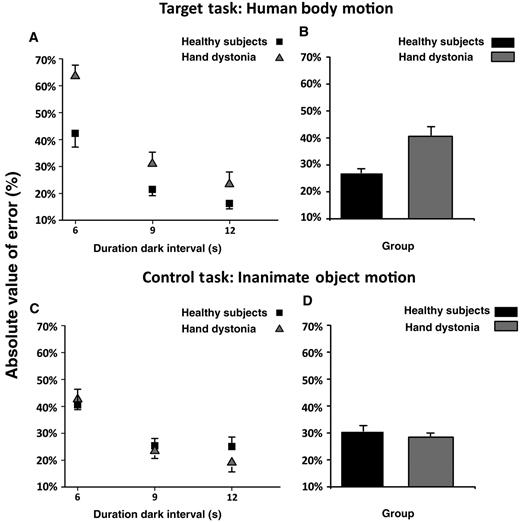
Absolute timing errors for both human body motion and inanimate object motion tasks are presented in Fig. 2. Patients with writer’s cramp exhibited a greater absolute timing error compared with control subjects at all target intervals in the human body motion task (handwriting). Accordingly, repeated measures ANOVA showed a significant group × task interaction [F(1,29) = 8.34; P = 0.007]; post hoc analysis showed that absolute timing error in the human body motion task was greater at all target intervals in the writer’s cramp group compared with the control subjects (P = 0.009), whereas there was no difference between groups on the absolute timing error in the inanimate object motion task (P = 0.54). Moreover, although control subjects exhibited a similar absolute timing error in both tasks (P = 0.22), patients with writer’s cramp exhibited a greater absolute timing error in the human body motion task compared with the inanimate object motion task (P = 0.010).
Absolute value of error expressed as per cent of the duration of target interval. Data of both patients with writer’s cramp and healthy control subjects are shown. (A and B) The results of the human body motion perceptual task, and (C and D) the results of the inanimate object motion task. On the x-axis, we show the duration of the dark interval in seconds (6, 9 or 12 s). On the y-axis, we show the duration of the absolute value of error expressed as per cent of the duration the target interval. Columns in B and D represent mean values across dark intervals. Mean data + standard error mean (SEM) are shown.
A significant main effect for target interval [F(2,58) = 35.99; P < 0.001] was also observed, and post hoc analysis showed that the absolute timing error was greater for the shorter dark intervals compared with the longer ones in both patients and control subjects (6 versus 9 s and 12 s: P < 0.001; 9 versus 12 s: P = 0.15).
No major effect of task or group × target interval or group × task × target interval interaction was observed (P > 0.05). Absolute timing error data (mean ± SD) are reported in Table 2.
Mean ± SD values of absolute timing error (%), coefficient of variability and percentage of responses in anticipation
| . | Dark interval (s) . | Absolute timing error (%) . | Coefficient of variability . | Percentage of responses in anticipation . |
|---|---|---|---|---|
| Healthy control subjects | ||||
| Human body motion | 6 | 42.32 ± 21.04 | 0.26 ± 0.10 | 23.56 ± 29.48 |
| 9 | 21.49 ± 9.51 | 0.18 ± 0.09 | 42.50 ± 28.86 | |
| 12 | 16.29 ± 8.29 | 0.14 ± 0.06 | 62.78 ± 30.35 | |
| Inanimate object motion | 6 | 40.58 ± 23.91 | 0.31 ± 0.12 | 44.15 ± 28.22 |
| 9 | 25.39 ± 11.20 | 0.19 ± 0.05 | 62.80 ± 31.44 | |
| 12 | 25.09 ± 14.48 | 0.16 ± 0.11 | 83.33 ± 28.89 | |
| Hand dystonia | ||||
| Human body motion | 6 | 63.5 ± 18.04 | 0.29 ± 0.07 | 26.14 ± 32.3 |
| 9 | 31.2 ± 16.10 | 0.15 ± 0.06 | 54.9 ± 37.3 | |
| 12 | 23.44 ± 16.99 | 0.11 ± 0.07 | 58.58 ± 36.80 | |
| Inanimate object motion | 6 | 42.66 ± 14.48 | 0.34 ± 0.08 | 35.74 ± 24.13 |
| 9 | 23.45 ± 10.39 | 0.23 ± 0.07 | 64.31 ± 28.2 | |
| 12 | 19.16 ± 13.08 | 0.12 ± 0.07 | 82.16 ± 30.2 | |
| . | Dark interval (s) . | Absolute timing error (%) . | Coefficient of variability . | Percentage of responses in anticipation . |
|---|---|---|---|---|
| Healthy control subjects | ||||
| Human body motion | 6 | 42.32 ± 21.04 | 0.26 ± 0.10 | 23.56 ± 29.48 |
| 9 | 21.49 ± 9.51 | 0.18 ± 0.09 | 42.50 ± 28.86 | |
| 12 | 16.29 ± 8.29 | 0.14 ± 0.06 | 62.78 ± 30.35 | |
| Inanimate object motion | 6 | 40.58 ± 23.91 | 0.31 ± 0.12 | 44.15 ± 28.22 |
| 9 | 25.39 ± 11.20 | 0.19 ± 0.05 | 62.80 ± 31.44 | |
| 12 | 25.09 ± 14.48 | 0.16 ± 0.11 | 83.33 ± 28.89 | |
| Hand dystonia | ||||
| Human body motion | 6 | 63.5 ± 18.04 | 0.29 ± 0.07 | 26.14 ± 32.3 |
| 9 | 31.2 ± 16.10 | 0.15 ± 0.06 | 54.9 ± 37.3 | |
| 12 | 23.44 ± 16.99 | 0.11 ± 0.07 | 58.58 ± 36.80 | |
| Inanimate object motion | 6 | 42.66 ± 14.48 | 0.34 ± 0.08 | 35.74 ± 24.13 |
| 9 | 23.45 ± 10.39 | 0.23 ± 0.07 | 64.31 ± 28.2 | |
| 12 | 19.16 ± 13.08 | 0.12 ± 0.07 | 82.16 ± 30.2 | |
Data of both patients with writer’s cramp and healthy control subjects on the human body motion and inanimate object motion perceptual tasks are reported.
Mean ± SD values of absolute timing error (%), coefficient of variability and percentage of responses in anticipation
| . | Dark interval (s) . | Absolute timing error (%) . | Coefficient of variability . | Percentage of responses in anticipation . |
|---|---|---|---|---|
| Healthy control subjects | ||||
| Human body motion | 6 | 42.32 ± 21.04 | 0.26 ± 0.10 | 23.56 ± 29.48 |
| 9 | 21.49 ± 9.51 | 0.18 ± 0.09 | 42.50 ± 28.86 | |
| 12 | 16.29 ± 8.29 | 0.14 ± 0.06 | 62.78 ± 30.35 | |
| Inanimate object motion | 6 | 40.58 ± 23.91 | 0.31 ± 0.12 | 44.15 ± 28.22 |
| 9 | 25.39 ± 11.20 | 0.19 ± 0.05 | 62.80 ± 31.44 | |
| 12 | 25.09 ± 14.48 | 0.16 ± 0.11 | 83.33 ± 28.89 | |
| Hand dystonia | ||||
| Human body motion | 6 | 63.5 ± 18.04 | 0.29 ± 0.07 | 26.14 ± 32.3 |
| 9 | 31.2 ± 16.10 | 0.15 ± 0.06 | 54.9 ± 37.3 | |
| 12 | 23.44 ± 16.99 | 0.11 ± 0.07 | 58.58 ± 36.80 | |
| Inanimate object motion | 6 | 42.66 ± 14.48 | 0.34 ± 0.08 | 35.74 ± 24.13 |
| 9 | 23.45 ± 10.39 | 0.23 ± 0.07 | 64.31 ± 28.2 | |
| 12 | 19.16 ± 13.08 | 0.12 ± 0.07 | 82.16 ± 30.2 | |
| . | Dark interval (s) . | Absolute timing error (%) . | Coefficient of variability . | Percentage of responses in anticipation . |
|---|---|---|---|---|
| Healthy control subjects | ||||
| Human body motion | 6 | 42.32 ± 21.04 | 0.26 ± 0.10 | 23.56 ± 29.48 |
| 9 | 21.49 ± 9.51 | 0.18 ± 0.09 | 42.50 ± 28.86 | |
| 12 | 16.29 ± 8.29 | 0.14 ± 0.06 | 62.78 ± 30.35 | |
| Inanimate object motion | 6 | 40.58 ± 23.91 | 0.31 ± 0.12 | 44.15 ± 28.22 |
| 9 | 25.39 ± 11.20 | 0.19 ± 0.05 | 62.80 ± 31.44 | |
| 12 | 25.09 ± 14.48 | 0.16 ± 0.11 | 83.33 ± 28.89 | |
| Hand dystonia | ||||
| Human body motion | 6 | 63.5 ± 18.04 | 0.29 ± 0.07 | 26.14 ± 32.3 |
| 9 | 31.2 ± 16.10 | 0.15 ± 0.06 | 54.9 ± 37.3 | |
| 12 | 23.44 ± 16.99 | 0.11 ± 0.07 | 58.58 ± 36.80 | |
| Inanimate object motion | 6 | 42.66 ± 14.48 | 0.34 ± 0.08 | 35.74 ± 24.13 |
| 9 | 23.45 ± 10.39 | 0.23 ± 0.07 | 64.31 ± 28.2 | |
| 12 | 19.16 ± 13.08 | 0.12 ± 0.07 | 82.16 ± 30.2 | |
Data of both patients with writer’s cramp and healthy control subjects on the human body motion and inanimate object motion perceptual tasks are reported.
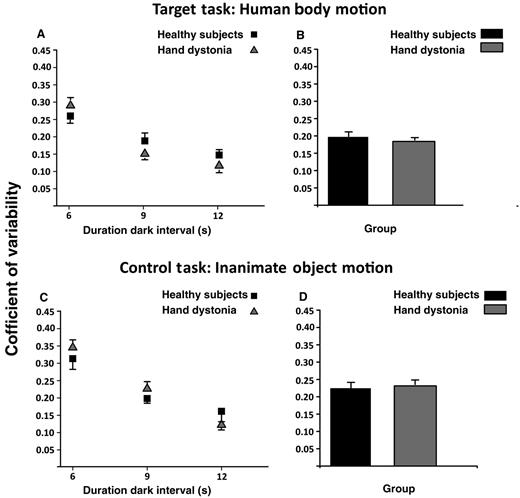
Variability data are reported in Fig. 3 and Table 2. Repeated measures ANOVA on coefficient of variability data showed a significant main effect for task [F(1,29) = 7.91, P = 0.009] and target interval [F(2,58) = 72.38, P < 0.001]. Performance variability was smaller in the human body motion task than in the inanimate object motion task (P = 0.009). Timing performance was proportionally more variable when target intervals were shorter (6 versus 9 s and 12 s: P < 0.001; 9 versus 12 s: P < 0.001). Conversely, there was no difference between groups, as we did not observe any significant main effect of either group or group × task, group × target interval and group × task × target interval interaction terms (all P > 0.05).
Coefficient of variability calculated as standard deviation/mean of the reproduced intervals. Data of both patients with writer’s cramp and healthy control subjects are shown. (A and B) Results of the human body motion perceptual task, and (C and D) results of the inanimate object motion task. On the x-axis, we show the duration of the dark interval in seconds (6, 9 or 12 s). On the y-axis, we show the value of the coefficient of variability. Columns in B and D represent mean values across dark intervals. Mean data + SEM are shown.
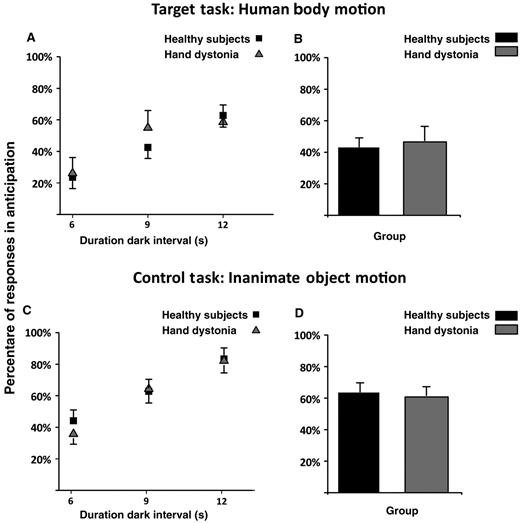
The percentages of response in anticipation for both target and control tasks are reported in Fig. 4 and Table 2. Repeated measures ANOVA showed a significant main effect for task [F(1,29) = 9.49, P = 0.004] and target interval [F(2,58) = 56.25, P < 0.001]. Both patients with writer’s cramp and healthy control subjects significantly underestimated more target intervals in the inanimate object motion task than in the human body motion task (P = 0.004). Further, both groups tended to underestimate more the duration of the target interval when it was longer (9 and 12 s) (6 versus 9 s and 12 s: P < 0.001; 9 versus 12 s: P < 0.001). No difference between groups was found (main effects of group and interaction group × task, group × target interval and group × task × target interval always not significant, P > 0.05).
Percentage of responses in anticipation. Data of both patients with writer’s cramp and healthy control subjects are shown. (A and B) Results of the human body motion perceptual task, and (C and D) results of the inanimate object motion task. On the x-axis, we show the duration of the dark interval in seconds (6, 9 or 12 s). On the y-axis, we show the percentage of the response in anticipation with respect to the total number of trials. Columns in B and D represent mean values across dark intervals. Mean data + SEM are shown.
The main analysis described earlier in text showed that patients with writer’s cramp exhibited a greater absolute timing error compared with control subjects in predicting the outcome of the human body motion task (handwriting). However, the magnitude of error (mean value across dark intervals) did not correlate either with disease severity (Spearman’s rho = 0.12; P = 0.69), disease duration (Spearman’s rho = 0.46; P = 0.10) or writing speed (Spearman’s rho = −0.18; P = 0.54), suggesting that it is independent from the severity and duration of motor symptoms.
Working memory task
No difference was found between the two groups of subjects on the visual working memory task at either 500 or 5000 ms interstimulus intervals. Data on the percentage of correct responses and reaction time, and respective P-values, are reported in Table 3.
Mean reaction time values and percentage of correct responses obtained on the working memory test
| Parameter . | ISI (ms) . | Groups . | P-value . | |
|---|---|---|---|---|
| . | . | Hand dystonia . | Healthy control subjects . | . |
| Reaction time (ms) | 500 | 1566.1 ± 527.9 | 1569.1 ± 484.3 | 0.98 |
| 5000 | 1644 ± 645.6 | 1794.6 ± 533.3 | 0.55 | |
| Percentage of correct responses | 500 | 78.8 ± 24.4 | 83.8 ± 14.2 | 0.16 |
| 5000 | 89.8 ± 28.2 | 91.6 ± 9.4 | 0.67 | |
| Parameter . | ISI (ms) . | Groups . | P-value . | |
|---|---|---|---|---|
| . | . | Hand dystonia . | Healthy control subjects . | . |
| Reaction time (ms) | 500 | 1566.1 ± 527.9 | 1569.1 ± 484.3 | 0.98 |
| 5000 | 1644 ± 645.6 | 1794.6 ± 533.3 | 0.55 | |
| Percentage of correct responses | 500 | 78.8 ± 24.4 | 83.8 ± 14.2 | 0.16 |
| 5000 | 89.8 ± 28.2 | 91.6 ± 9.4 | 0.67 | |
P indicates the results of unpaired t-test between groups.
ISI = interstimulus interval.
Mean reaction time values and percentage of correct responses obtained on the working memory test
| Parameter . | ISI (ms) . | Groups . | P-value . | |
|---|---|---|---|---|
| . | . | Hand dystonia . | Healthy control subjects . | . |
| Reaction time (ms) | 500 | 1566.1 ± 527.9 | 1569.1 ± 484.3 | 0.98 |
| 5000 | 1644 ± 645.6 | 1794.6 ± 533.3 | 0.55 | |
| Percentage of correct responses | 500 | 78.8 ± 24.4 | 83.8 ± 14.2 | 0.16 |
| 5000 | 89.8 ± 28.2 | 91.6 ± 9.4 | 0.67 | |
| Parameter . | ISI (ms) . | Groups . | P-value . | |
|---|---|---|---|---|
| . | . | Hand dystonia . | Healthy control subjects . | . |
| Reaction time (ms) | 500 | 1566.1 ± 527.9 | 1569.1 ± 484.3 | 0.98 |
| 5000 | 1644 ± 645.6 | 1794.6 ± 533.3 | 0.55 | |
| Percentage of correct responses | 500 | 78.8 ± 24.4 | 83.8 ± 14.2 | 0.16 |
| 5000 | 89.8 ± 28.2 | 91.6 ± 9.4 | 0.67 | |
P indicates the results of unpaired t-test between groups.
ISI = interstimulus interval.
Discussion
The main session of our experimental paradigm (temporal expectation task) was focused on a task evaluating the ability of subjects to visually perceive two different types of movement, one engaging the human body segment and action specifically related to dystonia in our patient group, and one involving movement of an inanimate object. Unlike other tasks assessing motor imagery or the mental rotation of movement, in which subjects are asked to provide a response on the spatial dimension of movement (Farah, 1989; Zacks, 2008), in our tasks, subjects were asked to estimate the time needed for a perceived movement to reach its completion relying uniquely on their mental representation of that movement. Hence, the performance on the temporal expectation task can be subdivided in the following phases: (i) visual perception and working memory storage of movement; (ii) extrapolation of the temporal features (velocity) of the perceived movement (implicit time processing); and (iii) estimation, through a simple motor response, of the end-point of the perceived movement in the absence of visual scanning of the movement itself.
The main finding of our study is that the accuracy of the performance on predicting the end of a visually perceived human body movement is lower in patients with writer’s cramp than in control subjects, whereas no difference was detected with inanimate object motion. This suggests that patients with writer’s cramp process the temporal components of movement less effectively than healthy control subjects, and that this dysfunction is likely to be stimulus selective, as shown in other cognitive processing abnormalities in dystonia, such as in mental rotation tasks (Fiorio et al., 2006).
Regardless of the presence of dystonia, the ability to estimate the time point at which the visually perceived movement ended was, first of all, influenced by the duration of the time interval in which subjects had to rely only on their mental representation of movement (i.e. the dark interval). In particular, shorter dark intervals were associated with higher tendency to shift the end of the perceived movement ahead in time, with greater variability of prediction response and greater normalized absolute timing error. Indeed, when humans are asked to reproduce various temporal intervals, longer durations are perceived as being shorter than the reference, and the opposite is true for short durations. This phenomenon of regression to the mean is known as Vierordt’s law (Vierordt, 1868), and it has also been found in studies of ‘pure’ temporal reproduction (Bangert et al., 2011). The normalized absolute timing error [(reproduced interval − target interval)/target interval] and the coefficient of variability (SD/mean of the reproduced intervals) are both approximate ratios of the target interval duration, thus allowing for comparison of performance at each interval independent of duration (Gooch et al., 2010). The effect of the target interval on these two parameters is, however, not surprising given that both tend to be larger for shorter target intervals compared with longer ones (Gooch et al., 2010; Broadway et al., 2011).
The implicit timing performance depended also, in a similar way in both groups of subjects, on the type of motion perceived, with lower accuracy (larger variability and higher frequency of anticipated responses) observed on the inanimate object motion task compared with the human body motion task. This result may depend on the differences between the two tasks. First, the tasks differed in the nature of the moving object—body segment as opposed to an inanimate object—and of the movement itself, a voluntary action as opposed to a purposeless movement. Second, the two videos presented motions at a different degree of complexity, with the handwriting movement exhibiting more complex spatial features than the linear movement of the inanimate graphic object. If the complexity of motion had been the main factor influencing implicit timing performance, one would have expected performance consistency and accuracy to decrease at the increase of task complexity also in the healthy control group; conversely, in healthy control subjects, the variability and percentage of responses in anticipation were larger on the inanimate object motion task than on the human body motion task, whereas absolute timing error did not differ between the two tasks. We favour a different interpretation for this result, which seems consistent with a previous study showing that the ability to predict the temporal outcome of a perceived action changes depending on whether the action is performed by a body segment (e.g. a limb) or by an inanimate object, such as a robotic arm; normal subjects tend to anticipate the outcome of the action performed by a robotic arm compared with that of a human arm (Craighero et al., 2008). This result may also be explained within the theoretical framework of embodiment, according to which the performance on timing tasks is influenced by the quality of the dynamic stimulus, with a tendency to overestimate the duration of movements that the perceiving subject is able to ‘embody’ (e.g. the handwriting movement), whereas this may not be applicable to the movement of an inanimate object (Nather et al., 2011).
Which of the phases that shape the performance on this temporal expectation task is most likely to be affected in our patients with writer’s cramp? First, it seems unlikely that the general visual perception and working memory storage of movements was abnormal, as patients with writer’s cramp performed similarly to control subjects on both the inanimate object motion and the visual working memory tasks. Moreover, a recent study failed to show abnormalities in recruitment of cerebral areas during passive movement observation in patients with focal hand dystonia (Castrop et al., 2012), further suggesting that it is unlikely that these patients present alterations in the visual processing of movement. Second, it seems unlikely that our patients were defective in the general ability to perceive time intervals, as the temporal expectation for inanimate object motion was unaffected in both patients and control subjects. Our findings point instead to an abnormal extrapolation of temporal features when they concern body motion in patients with writer’s cramp, suggestive of an alteration of the writing movement representation at a central level. Further, the lack of correlation between the magnitude of error in temporal prediction of body motion and clinical variables directly related to the illness, such as severity scores and disease duration, may suggest that this abnormal feature is not a direct expression of the process that causes the motor symptoms, but is likely to occur independently of it.
This finding is also supported by the lack of correlation between writing speed and absolute timing error of the human body motion task in patients with writer’s cramp. The implicit timing task adopted in the present study, in addition to the information extrapolated from the video about the velocity of the perceived movement, might require the use of the subject’s internal model of handwriting to predict the end of the movement. As the subjective internal model is based on previous experience of the movement (handwriting), and writing speed is reduced in patients with writer’s cramp (Wissel et al., 1996), the absence of correlation between timing performance on handwriting and writing speed strengthens our interpretation that abnormalities on the temporal expectation task are not a direct consequence of the motor abnormality.
An alternative explanation for this selective implicit timing abnormality exhibited by our patients is that this is not primarily determined by the object and nature of the movement, but rather by the higher degree of complexity of the handwriting motion compared with the inanimate object motion. As previously stated, we believe that this interpretation is less likely given that healthy control subjects showed a more accurate and consistent temporal expectation performance on the handwriting movement than on the inanimate object movement. In addition, the influence of complexity on cognitive aspects of motion, such as motor imagery and movement observation, has not been demonstrated consistently in recent studies addressing this issue (Kranczioch et al., 2010; Neely and Heath, 2010; Roosink and Zijdewind, 2010). In any event, we acknowledge that our experimental paradigm does not allow us to fully discard the relevance of complexity of motion to the interpretation of the abnormal implicit timing performance of our patients with writer’s cramp.
Different cerebral networks are likely to be responsible for the extrapolation of the different kinematic features of motion. Particularly, spatial and temporal parameters of motion, extrapolated from direction and velocity of perceived movements, may differ in their representation at a central level. In visuospatial perception, a dominant role of a parietal-premotor network has consistently been described in both human and monkey brain studies (Mountcastle et al., 1975; Grafton et al., 1996; de Jong et al., 2001; Beudel et al., 2009). At difference, the representation of temporal parameters of motion remains vaguely defined. Cerebral regions that have been implicated in timing perception include cerebellum, basal ganglia, supplementary motor cortex as well as prefrontal and inferior parietal cortices (Rao et al., 1997; Harrington et al., 1998; Ivry and Spencer, 2004; Vicario et al., 2010; for a review Koch et al., 2009). When subjects use temporal information inherent in the spatiotemporal trajectory of a dynamic visual stimulus to predict its final position, activations are reported in the lateral portion of the left inferior parietal cortex (Assmus et al., 2005), in sensorimotor regions of premotor and parietal cortices (Field and Wann, 2005) and in the cerebellum (O’Reilly et al., 2008). Anatomical and functional abnormalities corresponding to some of these areas were also reported in patients with focal dystonia. Indeed, primary dystonia is conceived today as a network disorder, in which several crucial nodes located in the cerebral cortex, for example, sensorimotor regions in frontal and parietal areas, and subcortical structures, for example, basal ganglia and cerebellum, might be involved (Neychev et al., 2011). Dysfunctional connectivity within this network of brain regions might lead to the increasing array of non-motor features observed in primary dystonia (Stamelou et al., 2012), which includes abnormalities of spatial and temporal discrimination of sensory stimuli (Bara-Jimenez et al., 2000; Tinazzi et al., 2002) and kinaesthesia (Grünewald et al.,1997; Frima et al., 2008), abnormalities in sensorimotor integration processes (Kaji et al., 1995; Abbruzzese et al., 2001) and dysfunctional cognitive processing of movement (Quartarone et al., 2005; Fiorio et al., 2006, 2011).
A possible interpretation of the abnormal performance on the temporal expectation of visually perceived handwriting movements in our patients with writer’s cramp implies an abnormality in the integrative role of the cerebellum over sensory and motor cortical areas while the subject is structuring a mental representation of the handwriting motor sequence (Wolpert et al., 1995). Indeed, the cerebellum plays a crucial role in processing visual motion (Baumann and Mattingley, 2010), with specific activation when temporal information has to be incorporated into perceptual predictions to make velocity judgements (O’Reilly et al., 2008). Recent research into animal models of dystonia, along with functional neuroimaging, electrophysiological and behavioural studies in hereditary and late-onset focal dystonia, has highlighted the contribution of the cerebellum to the pathophysiology of dystonia (Avanzino and Abbruzzese, 2012; Sadnicka et al., 2012). However, it needs to be pointed out that the absence of a task using another body motion stimulus different from an action specifically affected in focal hand dystonia does not allow us to conclude whether the temporal processing of body motion involves any type of corporeal movement or selectively a movement strictly related to the manifestation of dystonia.
Conclusion
This study shows, for the first time, that the ability to correctly predict the temporal characteristics of a writing movement is impaired in patients with writer’s cramp. This dysfunction cannot be explained as a consequence of an abnormal integration between sensory inputs and motor output, as subjects were not asked to execute the movement, but it rather depends on an abnormal way to process the time-dependent components of movement. Our results fit in with the idea that primary dystonia is not a pure motor problem, but involves sensory and cognitive aspects related to movement processing and planning. Assessing temporal expectation also in other focal dystonias is necessary to understand whether this cognitive abnormality is related to the core pathophysiological mechanisms shared by all forms of primary dystonia.
Expanding the investigation of timing abilities of patients with primary dystonia to other tasks might also be useful to interpret our findings. Future studies devoted to investigate implicit timing in focal dystonia should use ‘emergent timing’ tasks, in which implicit timing is indexed by the temporal regularity of a motor output and timing is said to emerge as a by-product of the dynamics of motor control (Coull and Nobre, 2008). Moreover, it would be interesting to evaluate, in the domain of temporal expectation, the performance of patients with dystonia in endogenous temporal expectations tasks, that is, when participants are asked to make explicit and deliberate use of temporal information to optimize performance, in contrast with exogenous temporal expectation tasks that imply an unintentional use of temporal information, as in our task. A better understanding of these mechanisms, and of their possible impact on the execution of fine complex movements, might ultimately prove useful for the development of novel rehabilitation strategies for dystonia.
References
Author notes
*These authors have equally contributed to this work.