-
PDF
- Split View
-
Views
-
Cite
Cite
Noam Sobel, Vivek Prabhakaran, Catherine A. Hartley, John E. Desmond, Gary H. Glover, Edith V. Sullivan, John D. E. Gabrieli, Blind smell: brain activation induced by an undetected air-borne chemical, Brain, Volume 122, Issue 2, February 1999, Pages 209–217, https://doi.org/10.1093/brain/122.2.209
Close - Share Icon Share
Abstract
EEG and behavioural evidence suggests that air-borne chemicals can affect the nervous system without being consciously detected. EEG and behaviour, however, do not specify which brain structures are involved in chemical sensing that occurs below a threshold of conscious detection. Here we used functional MRI to localize brain activation induced by high and low concentrations of the air-borne compound oestra-1,3,5(10),16-tetraen-3yl acetate. Following presentations of both concentrations, eight of eight subjects reported verbally that they could not detect any odour (P = 0.004). Forced choice detection performed during the presentations revealed above-chance detection of the high concentration, but no better than chance detection of the low concentration compound. Both concentrations induced significant brain activation, primarily in the anterior medial thalamus and inferior frontal gyrus. Activation in the inferior frontal gyrus during the high concentration condition was significantly greater in the right than in the left hemisphere (P = 0.03). A trend towards greater thalamic activation was observed for the high concentration than the low concentration compound (P = 0.08). These findings localize human brain activation that was induced by an undetectable air-borne chemical (the low concentration compound).
Introduction
Sensory stimuli can affect behaviour without being consciously perceived. The classic example for this effect is that of blindsight (Weiskrantz et al., 1974; Hécaen and Albert, 1978; Weiskrantz, 1986) in which patients with cortical damage in primary visual cortex, that led to post-chiasmatic homonymous field loss, demonstrated above-chance detection of visual stimuli despite denying having seen the stimulus. There is evidence that an analogous phenomenon, that may be termed blind smell, not only exists in the chemical sensing of healthy humans, but also affects their behaviour. Stern and McClintock (1998) demonstrated that undetectable secretions collected from the axillae of donor women can alter the timing of ovulation and menstruation in other women who had the secretions wiped on to their upper lip. Conditioning with undetected odours can induce negative mood (Kirk-Smith et al., 1983; but see Black and Smith, 1994), and undetected odours can affect mood and patterns of EEG activity (Lorig et al., 1990; Schwartz et al., 1994). Whereas behavioural reports offer an indirect measure of the possible effect of air-borne chemicals on the nervous system, and EEG measures offer an unlocalized measure of brain activity, functional imaging offers the means to localize the brain regions that mediate chemical sensing without awareness.
Undetected quantities of the air-borne compound oestra-1,3,5(10),16-tetraen-3yl acetate have been reported to induce changes in body temperature, skin conductance, respiration and heart rate, and surface electrical potential recorded from the epithelium of the vomeronasal organ in men but less in women (Monti-Bloch et al., 1994; Moran et al., 1995; this compound was called PH15 in these previous reports). These findings led the latter authors to propose that this compound may function as a human pheromone. Here we use functional MRI (fMRI) to localize brain activation induced by this air-borne chemical.
Material and methods
Stimuli
The compounds used were high (10–2 M) and low (10–8 M) concentrations of oestra-1,3,5(10),16-tetraen-3yl acetate (supplied by EROX corporation, Fremont, Calif., USA) in the diluent mineral oil. As the surface potential recorded at the vomeronasal organ is larger following higher concentration compounds (Monti-Bloch et al., 1994), we expected a similar dose-dependence in the fMRI signal.
Subjects
Eight healthy right-handed men, mean age 29 years, participated in the study. The study was approved by the Stanford IRB committee in accordance with the declaration of Helsinki. All subjects signed an agreement of informed consent.
Stimuli generation
Methods of air dilution olfactometry were modified to accommodate the MRI environment (for methods in detail, see Sobel et al., 1997). The system enabled switching from stimulus to no-stimulus conditions in less than 500 ms. The alternation from stimulus to no-stimulus conditions produced no auditory, visual, tactile or thermal cues regarding the alteration between conditions.
Task design
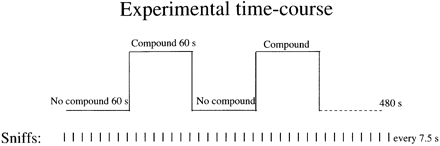
Alternating half blocks of the compound diluted in mineral oil versus pure mineral oil without the compound were generated. Eight such 60 s half blocks for a total duration of 480 s constituted a single scan (Fig. 1). During a scan, a line of script reading: `SNIFF AND RESPOND, IS THERE AN ODOR? PRESS THE RIGHT BUTTON FOR YES OR THE LEFT BUTTON FOR NO' was projected to the subject once every 7.5 s. Subjects sniffed and then responded by using the right index finger only to press one of two buttons. The number of sniffs and button presses was thus balanced over the stimulus and the no-stimulus conditions, and constituted a constant base-line. Response accuracy was recorded on a computer that controlled the solenoid valve determining stimulus presence and triggered the scanner, thus maintaining synchronization between the task, stimulus presentation and data acquisition.
The following instructions were given to subjects before the experiment: `During the scan you will receive constant sniff instructions. Occasionally there may be a substance imbedded in the air stream. Although this substance may be odorless, please try as hard as you can to detect its presence. If you sense any change in the air content, press the right button, if not, press the left'. Each subject was scanned once with the low concentration and once with the high concentration of the compound, in a randomized order across subjects. Subjects were not informed whether a given scan contained a stimulus or not.
The absence of a reliable subjective behavioural response to the compound led to a dilemma in the construction of the paradigm. In general, it is preferable to use short half-block durations in fMRI experiments. Long half-blocks determine a low frequency task, and low frequency analysis is highly susceptible to signal-noise in the scanning environment (Aguirre et al., 1997; Zarahn et al., 1997). There is, however, a high degree of habituation in the chemical senses, and a relatively long half-block of no-stimulus is necessary for these systems to return to baseline responsiveness. In previous studies using fMRI to study the main olfactory system (Sobel et al., 1997, 1998a, Sobel et al., b) we adjusted half-block duration to the shortest possible without compromising behavioural performance in detection that was above 90%. In this study, however, there was no similarly dependable measure of detection. Therefore, a long total experiment and half-block duration were arbitrarily set to assure response-recovery to the stimulus. Thus, every subject was also administered a sham scan, identical in length and mineral oil content to the other scans, but without any compound, in order to assess and compensate for individual regional noise levels at this low frequency.
Imaging parameters
Each subject was accommodated with a custom-built bite-bar to prevent head-motion. Imaging was performed using a 1.5 T whole-body MRI scanner (GE Signa, Rev. 5.5 Echospeed). For functional imaging, two 5-inch diameter local receive coils were used for signal reception. A T2* sensitive gradient echo spiral sequence (Glover and Lai, 1998), which is relatively insensitive to cardiac pulsatility motion artefacts, was employed with parameters of TR (repetition time) = 720 ms, TE (echo time) = 40 ms, flip angle = 65°. Spatial resolution was set by a 153 × 153 voxel matrix covering a 42 × 42 cm field of view resulting in an in-plane resolution of 2.75 × 2.75 mm. Four interleaves were collected for each frame, with total acquisition time of 2.88 s per frame. One hundred and seventy frames were acquired for a total scan duration of 490 s.
Eight 4 mm thick slices with a 2 mm inter-slice gap were acquired at an oblique plane traversing from the frontal pole to the temporal pole [typically 30° clockwise to the AC–PC (anterior–posterior commissure) plane, top right of Fig. 2]. This slice orientation was chosen so as to maximize the volume of olfactory cortex within the acquisition (Sobel et al., 1997).
The experimental sequence automatically initiated 12 s following scanning onset, allowing the first four frames to be discarded from the analysis. This eliminated transients arising before the achievement of dynamic equilibrium. T1-weighted flow compensated spin-warp anatomy images (TR = 500 ms, minimum TE) were acquired as a substrate on which to overlay functional data. Location of specific regions within the oblique slices was later cross-referenced to standard coronal plane images (90° to AC–PC plane) using a cross-referencing algorithm (Desmond and Lim, 1997) so as to validate anatomical localization in standardized coordinates.
Analysis of functional data
Image reconstruction was performed off-line on a Sun SparcStation. A gridding algorithm was employed to resample the raw data into a Cartesian matrix prior to processing with 2D FFT (fast Fourier transform). Motion artefacts were assessed and corrected (Woods et al., 1992; Friston et al., 1996). Once individual images were reconstructed, the time series of each pixel was obtained and correlation methods that take advantage of periodically oscillating events were used to analyse functional activation (Friston et al., 1994). A reference function was computed by convolving a square-wave at the task-frequency with a data-derived estimate of the haemodynamic response function. The frequency of the square-wave was computed by dividing the number of task cycles by the duration of the experiment, i.e. 4 cycles/480 s = 0.0083 Hz. Correlations between the reference function and each pixel response time-series were computed and normalized to create statistical parametric maps (SPM{Z}).
To ensure that activations were not due to high levels of noise at our task frequency (0.0083 Hz), the individual sham scans were used to set an individual threshold for each subject. To construct individual functional activation maps of the high and low concentration conditions, pixels that satisfied the criterion of Z > Zmax of activation in the sham condition, and always higher than |Z| of 1.96, representing a significance level of P < 0.05, were selected. This map was then processed with a median filter with a spatial width of 2 pixels. The resulting activation map was overlaid on a T1-weighted structural image.
In order to quantify effects, the relevant regions were first individually outlined based on the anatomy image of each subject. Two measures were then used to quantify activations. The first was extent of activation. This measure is a count of the pixels that satisfied the criterion of |Z| > 1.96 (P < 0.05) within the region of interest, multiplied by the voxel volume. The second measure used was mean Z score in the entire region of interest. The advantage of the latter measure is that it does not employ a threshold that may arbitrarily exclude important information. The Z values obtained using this measure are typically low, as they are diluted over a large anatomical area. Mean Z values are also highly positively correlated with raw percentage fMRI-signal change (Sobel et al., 1998a).
Results
Behaviour
Following the scans, all eight of the eight subjects verbally reported that they could not detect any odour, and that they were guessing throughout the experiment (binomial, P = 0.004). Subjects were cued for response 64 times per scan. The number of overall positive responses (i.e. the subject thought there was an odour) did not significantly differ among the sham (mean = 17, SD = 12), low (mean = 16, SD = 13) and high (mean = 21, SD = 18) concentrations [repeated measures analysis of variance: F(2,7) = 1.68, P = 0.22]. Detection accuracy for the high and low concentration scans was computed by: [(hits + correct rejections)/64] × 100. Detection accuracy during the low concentration scan was at chance [48.8% accuracy, one-sample two-tailed t-test: t(7) = 0.78, P = 0.46] and above chance during the high concentration scan [56.4% accuracy, one-sample two-tailed t-test: t(7) = 2.48, p = 0.04]. A d′ analysis revealed that the difference in discriminability of the high and low concentrations approached significance [high versus low d′, two-tailed paired t-test: t(7) = 2.24, P = 0.06], that was not related to subjects' changes in strategy of response between the high concentration and low concentration scans [high versus low β, two-tailed paired t-test: t(7) = 0.151, P = 0.88].
Brain activation
Both high and low concentrations of the compound induced significant brain activation in all subjects (e.g. Fig. 2). Activations were most prominent in slices 3–8, with little activation in the most ventral portion of the acquisition in slices 1 and 2. The anterior medial thalamus near the fornix was significantly activated in seven of the eight subjects. The inferior frontal gyrus, bordering the premotor area, was significantly activated in six of the eight subjects. The region of the amygdala, the region of the hypothalamus and the hippocampus were significantly activated in five of the eight subjects. Additional regions were significantly activated in four or fewer of the subjects (Table 1). Only activation in the inferior frontal gyrus was significantly lateralized, with greater activation in the right than in the left hemisphere [two-tailed paired t-test on mean Z in region: t(7) = 2.811, P < 0.03] (Fig. 3).
A dose-dependent response was seen in the thalamus in six of the seven subjects with thalamic activation (binomial, P = 0.06) (Fig. 4) that was greater following stimulation with the high concentration of the compound in comparison with the low concentration (one-tailed paired t-test on extent activation in the region in all eight subjects: [t(7) = 1.56, P = 0.08] (Fig. 5). A non-significant trend towards dose-dependence was also evident in the cingulate gyrus and inferior frontal gyrus. Dose-dependence was not quantified in the region of the hypothalamus because there are no common landmarks enabling demarcation of the hypothalamus as a region of interest within the oblique plane of acquisition used.
Discussion
Awareness for the compound was poor in both conditions, but differed for the high and low concentrations. After the scans, all subjects were unaware that any compound had been presented. During the scans, while performing a forced choice detection paradigm, subjects were slightly, but significantly above chance in detecting the high concentration compound, but no better than chance in detecting the low concentration compound.
Determining whether a process occurs without awareness is a topic of some controversy (Eriksen, 1960; Cheesman and Merikle, 1986; Holender, 1986; Greenwald, 1992; Merikle, 1992). Measures of awareness range from the more strict, such as objective measures of performance that are obtained during the inter-stimulus intervals of an experiment, to more lenient measures, such as subjective reports obtained at the end of an experiment. In the present study, post-experimental responses revealed no subjective awareness of either high or low concentrations of the compound, but responses obtained during the experiment revealed objective awareness of the high concentration only. In the latter regard, the demonstration we have provided here in the chemical senses differs from the demonstration of blindsight. Whereas in blindsight patients deny a perceptual experience, although it is simultaneously affecting recorded behaviour (e.g. detection of motion), here, normal subjects only later deny a perceptual experience that previously affected behaviour (the detection in the high concentration condition). Thus, it is unclear whether post-experimental lack of awareness reflected perceptual unawareness during stimulus presentation, or subsequent lack of memory for perceptual experiences.
A question is raised regarding which intra-nasal nerve mediated the observed activations. In mammals, chemical signals may be transduced via a number of distinct neural subsystems, including the nervus terminalis, septal organ, trigeminal nerve (CNV 5), gustatory system, main olfactory system (CNV 1) and accessory olfactory (vomeronasal) system (Graziadei, 1977). In previous work performed with this compound it was shown that the compound induces a surface response in the human vomeronasal organ, and that the compound alters a host of physiological measures. The combination of the above led to the suggestion that this compound may be functioning as a human pheromone (Monti-Bloch et al., 1994; Moran et al., 1995). Support for the latter can be seen in that the regions activated in this study are homologous with the neuroanatomy of the vomeronasal system in mammals (Winans and Scalia, 1970; Kevetter and Winans, 1981; Risold and Swanson, 1995; reviewed by Wysocki, 1979; Johns, 1980; Halpern, 1987). The regions activated, however, are also a subset of the regions activated by above-threshold standard odorants (Zatorre et al., 1992; Levy et al., 1997; Sobel et al., 1997, 1998b; Yousem et al., 1997; Zald and Pardo, 1997), and are also regions in which one might expect activation following change in affect that is not only chemically induced. In the current study we had no way of restricting the compound to the vomeronasal organ while preventing it from reaching the olfactory epithelium, and therefore cannot determine here whether the activations witnessed were in fact the result of human vomeronasal organ activation, or the result of sub-detection threshold activation of any of the other nerves inervating the nasal passage, or a combination of both. This question will be addressed in further experimentation.
The difference in activation levels between the high and the low concentrations only approached significance, and one may claim that this is not in agreement with the expected response profile of a sensory system. Although similar concentration increases do induce an increase in activity at the peripheral level (measured electrically at the vomeronasal organ), it remains possible that a saturation effect is present at the central level as measured with fMRI. Furthermore, if in fact the activation reflects a human pheromonal-type response, it is possible that the system would not behave in a linear fashion characteristic to senses such as vision. The language of the chemical senses in general, and pheromones in particular, is unknown, and an increase in pheromone concentration may confer a different message rather than an increase in intensity of the same message.
Whereas the behavioural reports of unconscious odour perception described in the introduction indirectly measure the effect of air-borne chemicals on the nervous system, and EEG offers an unlocalized measure of brain activity, here we pinpoint brain regions activated by an air-borne chemical that is not consciously perceived (the low concentration). This finding joins other functional imaging reports that reveal brain activation induced without awareness (Berns et al., 1997; Sahraie et al., 1997; Whalen et al., 1998). Some overlap in the regions activated across these studies is evident. Whalen et al. (1998) demonstrated that masked presentation of negative compared with positive facial expressions induces an increase in activation in the amygdala. Amygdaloid activation was evident in our study as well; however, it did not show a tendency towards dose-dependence. Berns et al. (1997) demonstrated that an element of novelty in an unconsciously perceived stimulus induces activation in the premotor area, anterior cingulate and ventral striatum. Similar activation was seen in our study in the anterior cingulate and in the inferior frontal gyrus bordering the premotor area, and it therefore may be that these regions are involved in the attentional response to any stimuli (see also Menon et al., 1997) rather than in the chemosensory perception of the compound per se. Sahraie et al. (1997) studied activation induced by moving visual stimuli presented in the blind hemifield of a brain damaged patient. The cingulate gyrus, thalamus, inferior frontal gyrus and hippocampus, which were all activated by the undetected compound in this study, were also activated by moving visual stimuli of which the patient was unaware.
There are two opposing approaches as to how the nervous system gives rise to awareness. One approach holds that awareness is an emergent property, the result of activity in complex neuronal networks (e.g. Kinsbourne, 1988). The other approach holds that there may be a neural gate, i.e. a region or cluster of regions that is primarily responsible for transferring information to a level of conscious awareness (e.g. Crick, 1984; Bogen, 1995a). It is therefore tempting to target a region that is not active during the low concentration condition (no awareness) but active during the high concentration condition (low awareness) as such a candidate gate.
Whereas no region was dichotically activated by the high but not low concentration, the high concentration did induce a relative increase in activation in comparison with the low concentration in the thalamus in six of the seven subjects that displayed thalamic activation. These findings are intriguing in light of the role attributed to the thalamus in recent theories on the neuroanatomy of awareness (Crick, 1984; Bogen, 1995a, b; discussed in Baars, 1995; Kinsbourne, 1995; Koch, 1995; Newman, 1995). In these theories, the thalamus, specifically the intralaminar nuclei (Bogen, 1995a) and reticular complex (Crick, 1984), have been described as regions central to selective attention and awareness. These thalamic regions are within the anterior portion of the thalamus which had the most robust activation in this study. Whereas the thalamic relay of the chemical senses was thought to play a role only in complex chemical-sensing tasks such as odour discrimination (Price and Slotnick, 1983; Slotnick and Schoonover, 1992), here we see thalamic activation during a low-level task of detection. Smythies (1997) argued against theories of awareness that stress the role of the anterior regions of the thalamus using olfaction as a case in point. Smythies's rationale was that whereas selective olfactory attention and awareness to olfactory stimuli are both achievable, the role of the thalamus in these tasks is negligible (according to data from animals), and therefore the thalamus may not be central to the process of awareness. Our finding of anterior thalamic activation induced by an undetected air-borne chemical (the low concentration) in humans may merit reconsideration of the arguments suggested by Smythies, as it is apparent that the thalamus is activated by low-level chemical sensing in humans. A final word of caution, however, may be merited regarding the specific localization within the thalamus, as the centre of thalamic activation was near the expected location of the internal cerebral vein which may give rise to a drainage effect.
Regions activated in more than one subject
| Region . | No. of subjects showing significant activation . | x . | y . | z . |
|---|---|---|---|---|
| Coordinates in standard stereotactic space (Talairach and Tournoux, 1988) referring to centre of significant activation. Activations were also seen in the cerebellum. We were, however, cautious in considering these activations due to the increased probability of false positives in the most posterior portion of the acquisition that is out of the signal range of the centrally placed surface coils. | ||||
| Anterior medial thalamus | 7 | –3 | –4 | 13 |
| Inferior frontal gyrus | 6 | 51 | 20 | 23 |
| Amygdala | 5 | –23 | –8 | –9 |
| Hypothalamus | 5 | –5 | 0 | –8 |
| Hippocampus | 5 | –32 | –20 | –8 |
| Cingulate gyrus | 4 | –3 | 32 | 25 |
| Lateral orbitofrontal gyrus | 3 | 27 | 50 | –11 |
| Superior frontal gyrus | 3 | 5 | 60 | 18 |
| Piriform cortex | 3 | –30 | 4 | –14 |
| Peri-insular region | 2 | 42 | 8 | –5 |
| Superior temporal gyrus | 2 | 51 | 4 | –2 |
| Region . | No. of subjects showing significant activation . | x . | y . | z . |
|---|---|---|---|---|
| Coordinates in standard stereotactic space (Talairach and Tournoux, 1988) referring to centre of significant activation. Activations were also seen in the cerebellum. We were, however, cautious in considering these activations due to the increased probability of false positives in the most posterior portion of the acquisition that is out of the signal range of the centrally placed surface coils. | ||||
| Anterior medial thalamus | 7 | –3 | –4 | 13 |
| Inferior frontal gyrus | 6 | 51 | 20 | 23 |
| Amygdala | 5 | –23 | –8 | –9 |
| Hypothalamus | 5 | –5 | 0 | –8 |
| Hippocampus | 5 | –32 | –20 | –8 |
| Cingulate gyrus | 4 | –3 | 32 | 25 |
| Lateral orbitofrontal gyrus | 3 | 27 | 50 | –11 |
| Superior frontal gyrus | 3 | 5 | 60 | 18 |
| Piriform cortex | 3 | –30 | 4 | –14 |
| Peri-insular region | 2 | 42 | 8 | –5 |
| Superior temporal gyrus | 2 | 51 | 4 | –2 |
Regions activated in more than one subject
| Region . | No. of subjects showing significant activation . | x . | y . | z . |
|---|---|---|---|---|
| Coordinates in standard stereotactic space (Talairach and Tournoux, 1988) referring to centre of significant activation. Activations were also seen in the cerebellum. We were, however, cautious in considering these activations due to the increased probability of false positives in the most posterior portion of the acquisition that is out of the signal range of the centrally placed surface coils. | ||||
| Anterior medial thalamus | 7 | –3 | –4 | 13 |
| Inferior frontal gyrus | 6 | 51 | 20 | 23 |
| Amygdala | 5 | –23 | –8 | –9 |
| Hypothalamus | 5 | –5 | 0 | –8 |
| Hippocampus | 5 | –32 | –20 | –8 |
| Cingulate gyrus | 4 | –3 | 32 | 25 |
| Lateral orbitofrontal gyrus | 3 | 27 | 50 | –11 |
| Superior frontal gyrus | 3 | 5 | 60 | 18 |
| Piriform cortex | 3 | –30 | 4 | –14 |
| Peri-insular region | 2 | 42 | 8 | –5 |
| Superior temporal gyrus | 2 | 51 | 4 | –2 |
| Region . | No. of subjects showing significant activation . | x . | y . | z . |
|---|---|---|---|---|
| Coordinates in standard stereotactic space (Talairach and Tournoux, 1988) referring to centre of significant activation. Activations were also seen in the cerebellum. We were, however, cautious in considering these activations due to the increased probability of false positives in the most posterior portion of the acquisition that is out of the signal range of the centrally placed surface coils. | ||||
| Anterior medial thalamus | 7 | –3 | –4 | 13 |
| Inferior frontal gyrus | 6 | 51 | 20 | 23 |
| Amygdala | 5 | –23 | –8 | –9 |
| Hypothalamus | 5 | –5 | 0 | –8 |
| Hippocampus | 5 | –32 | –20 | –8 |
| Cingulate gyrus | 4 | –3 | 32 | 25 |
| Lateral orbitofrontal gyrus | 3 | 27 | 50 | –11 |
| Superior frontal gyrus | 3 | 5 | 60 | 18 |
| Piriform cortex | 3 | –30 | 4 | –14 |
| Peri-insular region | 2 | 42 | 8 | –5 |
| Superior temporal gyrus | 2 | 51 | 4 | –2 |
Task design. Alternating blocks of air passed over the compound diluted in mineral oil versus air passed over mineral oil only were generated. Subjects sniffed and responded once every 7.5 s continuously throughout the scan, thus constituting a constant baseline.
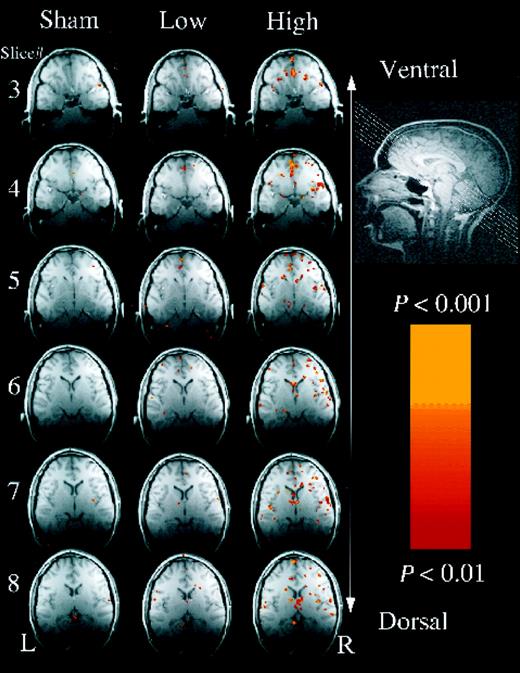
Example of results from a single subject. Slice orientation is shown at top right. Slices 1 and 2 had no activation and are not shown. Slices 3–8 in the three conditions (sham, low concentration, high concentration) are shown from top to bottom, respectively. A robust increase in activation from the low concentration to the high concentration conditions is evident. Regarding regions of interest, activation is evident: in the cingulate gyrus in slices 3–8, in the inferior frontal gyrus in slices 4–8, in the hypothalamus in slice 5, in the amygdala in slice 6, in the thalamus in slices 7 and 8.
Lateralization in the inferior frontal gyrus. Mean Z and extent of significant activation (|Z| > 1.96, P < 0.05) from all eight subjects in the left and right inferior frontal gyrus during the high and low concentration conditions. Mean Z was significantly greater in the right than in the left hemisphere in the high concentration condition [two-tailed paired t-test: t(7) = 2.81,P < 0 0.03], but not in the low concentration condition. The extent of activation tended to be greater in the right hemisphere in the high concentration [t(7) = 1.866, P = 0.1] but not low concentration. Bars are standard error of the mean.
Dose-response in the thalamus. Mean Z and extent of significant activation (|Z| > 1.96, P <0.05) in the thalamus of all eight subjects during the high and low concentration conditions. A one-tailed paired t-test revealed a trend towards dose-dependence with greater activation in the high versus the low concentration condition in both mean Z [t(7) = 1.4, P = 0.1] and extent activation [t(7) = 1.56, P = 0.08]. Bars are standard error of the mean.
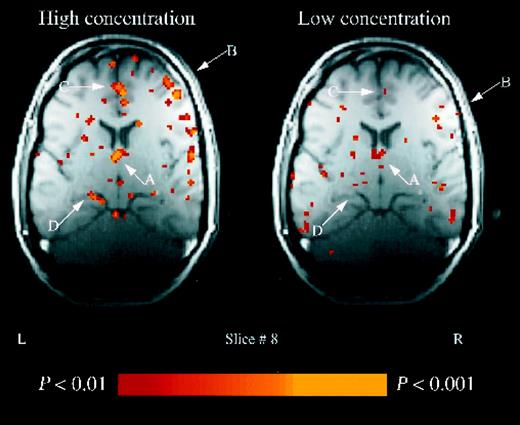
Thalamic activation. Example of slice 8 in a single subject. An increase in activation associated with the high concentration condition is evident in the: thalamus (A); inferior frontal gyrus (B); cingulate gyrus (C) and hippocampus (D).
We are most thankful to Lubert Stryer whose generous help made this work possible. N.S. was supported by SGF Smith Fellowship, E.V.S. by NIH-AA10723, AA05965, G.H.G. by NIH RR 09784. Additional support was received from Utah University Department of Psychiatry. We thank R. Khan, L. Monti-Bloch, C. Jennings-White and D. Berliner for their advice.
References
Aguirre GK, Zarahn E, D'Esposito M. Empirical analyses of BOLD fMRI statistics. II. Spatially smoothed data collected under null-hypothesis and experimental conditions.
Baars BJ. Tutorial commentary: surprisingly small subcortical structures are needed for the state of waking consciousness, while cortical projection areas seem to provide perceptual contents of consciousness [comment].
Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness.
Black SL, Smith DG. Has odor conditioning been demonstrated?: A critique of `unconscious odour conditioning in human subjects'.
Bogen JE. On the neurophysiology of consciousness. I. An overview. [Review].
Bogen JE. On the neurophysiology of consciousness. II. Constraining the semantic problem [see comments]. [Review].
Cheesman J, Merikle PM. Distinguishing conscious from unconscious perceptual processes.
Crick F. Function of the thalamic reticular complex: the searchlight hypothesis.
Desmond JE, Lim KO. On- and offline Talairach registration for structural and functional MRI studies.
Eriksen CW. Discrimination and learning without awareness: a methodological survey and evaluation.
Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series.
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series.
Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot.
Graziadei PPC. Functional anatomy of the mammalian chemoreceptor system. In: Müller-Schwarze D, Mozell MM, editors. Chemical signals in vertebrates. New York: Plenum Press; 1977. p. 435–54.
Greenwald AG. New look 3: unconscious cognition reclaimed [see comments].
Halpern M. The organization and function of the vomeronasal system. [Review].
Holender D. Semantic activation without conscious identification in dichotic listening, parafoveal vision, and visual masking: a survey and appraisal.
Johns MA. The role of the vomeronasal system in mammalian reproductive physiology. In: Müller-Schwarze D, Silverstein RM, editors. Chemical signals: vertebrates and aquatic invertebrates. New York: Plenum Press; 1980. p. 341–64.
Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the `vomeronasal amygdala'.
Kinsbourne M. Integrated field theory of consciousness. In: Marcel AJ, Bisiach E, editors. Consciousness in contemporary science. Oxford: Clarendon Press; 1988. p. 239–56.
Kinsbourne M. The intralaminar thalamic nuclei: subjectivity pumps or attention-action coordinators? [comment].
Kirk-Smith MD, Van Toller C, Dodd GH. Unconscious odour conditioning in human subjects.
Koch C. Visual awareness and the thalamic intralaminar nuclei [comment].
Levy LM, Henkin RI, Hutter A, Lin CS, Martins D, Schellinger D. Functional MRI of human olfaction.
Lorig TS, Herman KB, Schwartz GE. EEG activity during administration of low-concentration odors.
Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A. Combined event-related fMRI and EEG evidence for temporal-parietal cortex activation during target detection.
Merikle PM. Perception without awareness. Critical issues [comment].
Monti-Bloch L, Jennings-White C, Dolberg DS, Berliner DL. The human vomeronasal system.
Moran DT, Monti-Bloch L, Stensaas LJ, Berliner DL. Structure and function of the human vomeronasal organ. In: Doty RL, editor. Handbook of gustation and olfaction. New York: Marcel Dekker; 1995. p. 793–821.
Newman J. Thalamic contributions to attention and consciousness [comment]. [Review].
Price JL, Slotnick BM. Dual olfactory representation in the rat thalamus: an anatomical and electrophysiological study.
Risold PY, Swanson LW. Evidence for a hypothalamothalamocortical circuit mediating pheromonal influences on eye and head movements.
Sahraie A, Weiskrantz L, Barbur JL, Simmons A, Williams SC, Brammer MJ. Pattern of neuronal activity associated with conscious and unconscious processing of visual signals.
Schwartz GE, Bell IR, Dikman ZV, Fernandez M, Kline JP, Peterson JM, et al. EEG responses to low-level chemicals in normals and cacosmics.
Slotnick BM, Schoonover FW. Olfactory pathways and the sense of smell.
Smythies J. The functional neuroanatomy of awareness: with a focus on the role of various anatomical systems in the control of intermodal attention. [Review].
Sobel N, Prabhakaran V, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. A method for functional magnetic resonance imaging of olfaction.
Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, et al. Odorant-induced and sniff-induced activation in the cerebellum of the human.
Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, et al. Sniffing and smelling: separate subsystems in the human olfactory cortex.
Stern K, McClintock MK. Regulation of ovulation by human pheromones [see comments].
Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation.
Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge.
Winans SS, Scalia F. Amygdaloid nucleus: new afferent input from the vomeronasal organ.
Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images.
Wysocki CJ. Neurobehavioral evidence for the involvement of the vomeronasal system in mammalian reproduction. [Review].
Yousem DM, Williams SC, Howard RO, Andrew C, Simmons A, Allin M, et al. Functional MR imaging during odor stimulation: preliminary data.
Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation.
Zarahn E, Aguirre GK, D'Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions.


![Lateralization in the inferior frontal gyrus. Mean Z and extent of significant activation (|Z| > 1.96, P < 0.05) from all eight subjects in the left and right inferior frontal gyrus during the high and low concentration conditions. Mean Z was significantly greater in the right than in the left hemisphere in the high concentration condition [two-tailed paired t-test: t(7) = 2.81,P < 0 0.03], but not in the low concentration condition. The extent of activation tended to be greater in the right hemisphere in the high concentration [t(7) = 1.866, P = 0.1] but not low concentration. Bars are standard error of the mean.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/122/2/10.1093_brain_122.2.209/2/m_b020703.gif?Expires=1716511421&Signature=NoDGdtprheo9Twaqgw1tLpuBHgce1dBZLI5fLJK1mmq5262KTZfmKlPw9zaSwnxaQIz6iPsHGsPY1fDFd3HPP8rdvwvCHnGj8bCAr88TjNWb5xZ2cJLdBFDSx~PEy1MBuOGiHoS-KlEUn7gd2SakEJFOiTvbduP0z1PsUVsysxj~8g8JWfn31vMFTINYjw6RGpHRZdDrL-nsHbglZu-xhT7MZGycvQ1F0yryYSnld54~GAMpXqhHJysyz8RG4C5fKD9XY-Lx7O-w3Ost~5v-92gdCXwlN~PZO59iiqmalNEC2k6BMx4EFxUEatueHlcG9CPjHMfyzcmJZ9CgyqaB9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Dose-response in the thalamus. Mean Z and extent of significant activation (|Z| > 1.96, P <0.05) in the thalamus of all eight subjects during the high and low concentration conditions. A one-tailed paired t-test revealed a trend towards dose-dependence with greater activation in the high versus the low concentration condition in both mean Z [t(7) = 1.4, P = 0.1] and extent activation [t(7) = 1.56, P = 0.08]. Bars are standard error of the mean.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/122/2/10.1093_brain_122.2.209/2/m_b020704.gif?Expires=1716511421&Signature=1QevNXWGgtuHi-x2uN7OpQQdKnq-djvso1wnLTe9OXEe5MGaDsGhpaRIAqZWrToipRrKSIB-ROCgS7p9BCPDcmqby1YetuJRKfXT7wIUEcXO2~BgW6oY~i8fTd7kQwgdY5ouLERZTFb-wUKtY7xeUOeYHHgez8n61L9WiHdqWYymkbaLorxXbL0KVIZfD93rwWRwC6VLq-EKSgIecnn-jwCzaONNmVfblHqzvzmR8iXXw77hPPO~picvF8ME5n8NZnlDCYBwjd~oDNOjwhPoNf3EUt3Ctk~-rzRGSHf5zMhrcr4jc-D4CRp4ojdZVbrevaP8NUsCCquzyzuRiXiRTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)