-
PDF
- Split View
-
Views
-
Cite
Cite
Shibley Rahman, Barbara J. Sahakian, John R. Hodges, Robert D. Rogers, Trevor W. Robbins, Specific cognitive deficits in mild frontal variant frontotemporal dementia, Brain, Volume 122, Issue 8, August 1999, Pages 1469–1493, https://doi.org/10.1093/brain/122.8.1469
Close - Share Icon Share
Abstract
Eight patients with relatively mild frontal variant frontotemporal dementia (fvFTD) were compared with age- and IQ-matched control volunteers on tests of executive and mnemonic function. Tests of pattern and spatial recognition memory, spatial span, spatial working memory, planning, visual discrimination learning/attentional set-shifting and decision-making were employed. Patients with fvFTD were found to have deficits in the visual discrimination learning paradigm specific to the reversal stages. Furthermore, in the decision-making paradigm, patients were found to show genuine risk-taking behaviour with increased deliberation times rather than merely impulsive behaviour. It was especially notable that these patients demonstrated virtually no deficits in other tests that have also been shown to be sensitive to frontal lobe dysfunction, such as the spatial working memory and planning tasks. These results are discussed in relation to the possible underlying neuropathology, the anatomical connectivity and the hypothesized heterogeneous functions of areas of the prefrontal cortex. In particular, given the nature of the cognitive deficits demonstrated by these patients, we postulate that, relatively early in the course of the disease, the ventromedial (or orbitofrontal) cortex is a major locus of dysfunction and that this may relate to the behavioural presentation of these patients clinically described in the individual case histories.
Introduction
The term `frontotemporal dementia' (FTD), coined in the consensus statement from the Lund and Manchester Groups (1994), represents a heterogeneous group of disorders with variable clinical and neuropathological manifestations. The term encompasses a number of conditions in which progressive dementia with bilateral degeneration of the frontal and/or temporal cortex occurs, previously referred to as Pick's disease (Pick, 1892), dementia of the frontal type (Neary et al., 1988), frontal lobe degeneration of the non-Alzheimer type (Brun, 1987, 1993; Gustafson, 1993), dementia lacking distinct histopathological features (Knopman et al., 1990), primary progressive aphasia (Mesulam, 1982; Hodges and Patterson, 1996; Snowden et al., 1996a, b), and semantic dementia (Snowden et al., 1989; Hodges et al., 1992, 1999). Neuropathological findings post-mortem may vary in such cases. Some have tau-positive intraneuronal inclusions (Pick bodies), whereas others have severe neuronal depletion with spongiosis but lack distinct inclusions; Alzheimer pathology is virtually never found (e.g. Mann, 1998). The precise mapping between clinical phenotype, distribution of pathology, type of pathology, and genotype needs further clarification. In a proportion of cases, however, the disorder is familial and linked to tau gene mutations on chromosome 17 (Hutton et al., 1998; Spillantini et al., 1998); it has been suggested that these familial chromosome-17-linked cases have a greater preponderance of parkinsonian features and lack Pick bodies, but share a distinctive abnormality of tau (Spillantini et al., 1998).
This study concerns aspects of cognition in patients with what we have termed frontal variant FTD (fvFTD) (Hodges et al., 1999), in which frontal involvement is the principal cause of the circumscribed presentation of a behavioural syndrome. This terminology parallels that used by Edwards-Lee and colleagues (Edwards-Lee et al., 1997) to describe patients with predominantly temporal lobe involvement, who typically have profound language deficits with the features of semantic dementia. There is little controversy over the fact that fvFTD affects younger patients, with the peak onset in the presenium (Neary and Snowden, 1996). Cases above 75 years of age appear relatively rarely (Miller et al., 1991; Gregory and Hodges, 1996). The exact prevalence of FTD is currently uncertain, but there is emerging evidence that FTD is the second commonest form of dementia in the presenium, and may represent up to a fifth of cases (Knopman et al., 1990). A biopsy study of patients with presenile dementia revealed a prevalence of 17% with non-Alzheimer pathology causing a syndrome of FTD (Neary et al., 1986). On clinical grounds alone, Neary et al. (1988) estimated that 19% of 130 cases seen in the neurological unit over a period of 5 years met criteria for frontal lobe dementia.
Ultimately, patients develop global cognitive impairment and severe disabilities in the functions of daily living. It is, however, of considerable importance for effective patient management that patients are identified in the earliest stages of the disease, particularly in the light of the psychological, social and financial consequences within families (e.g. Sperlinger and Furst, 1994). Clinically, the majority of patients with fvFTD are brought along to a clinic unaware of the major pervasive changes in their personality, behaviour and social conduct that have been observed by the informants. Patients may appear apathetic or withdrawn, or they may become socially disinhibited with facetiousness and inappropriate jocularity. Mental rigidity and an inability to appreciate the subtler aspects of language, such as irony, are common, as is stereotypical or ritualistic behaviour. Their ability to plan and organize complex activities (e.g. work, social engagements, etc.) is almost invariably impaired. This is traditionally considered to be due to deficits in goal-setting and attainment, as well as in mental flexibility and set-shifting (Gregory and Hodges, 1993, 1996).
There is often indifference to domestic and occupational responsibilities, a lack of empathy for family and friends, and gradual withdrawal from all social interactions. A form of severe self-neglect may occur as a direct consequence of frontal lobe dysfunction (Orrell et al., 1989). Many patients show a change in eating habits with an escalating desire for sweet food coupled with reduced satiety; the increasing gluttony may lead to enormous weight gain. The other components of the Klüver–Bucy syndrome (hypersexuality and oral exploratory behaviour) are less commonly observed (Cummings and Duchen, 1981). Language deficits include a mild anomia or dynamic aphasia (Snowden et al., 1996a, b) and repetitive speech acts, with muteness supervening in advanced cases. Visuospatial skills are typically remarkably preserved. Memory is typically intact, but patients may have difficulty with concentration and immediate working memory. Patients with fvFTD may present with prominent psychiatric symptoms (Neary et al., 1988; Miller et al., 1991; Gregory and Hodges, 1993, 1996). Depression may occur but is rarely severe (Gregory and Hodges, 1993, 1996). Compulsive disorders are more common and include, amongst others, cleaning, hygiene rituals, checking, and preoccupation with symmetry (Miller et al., 1995). Furthermore, Gustafson and Risberg (1992) report that hallucinations and delusions are prevalent in dementia (including FTD) and that psychotic traits may give the impression of a functional psychosis such as schizophrenia; however, in other series overt psychotic features in fvFTD have been found to be very rare and fleeting (e.g. Gregory et al., 1998; Gregory and Hodges, 1996).
In contrast to the clinical descriptions of profound `frontal' changes in patients with fvFTD, formal studies have often failed to show a specific profile, at least until late in the course of the disease (Miller et al., 1991). The failure to find clear neuropsychological differences between dementia of the Alzheimer type and FTD is likely to relate to variation in patient selection and to the particular choice of neuropsychological tests (Miller et al., 1991; Elfgren et al., 1994; Frisoni et al., 1995; Gregory et al., 1997). For example, Pachana and colleagues (Pachana et al., 1996) compared 15 patients with FTD (including both frontal and temporal cases) and 16 cases of dementia of the Alzheimer type, but did not match for severity or duration of dementia. They found a difference in the severity of non-verbal memory functioning in the predicted direction, but no differences in a range of language, attentional and executive tasks. More recently, Gregory and colleagues (Gregory et al., 1997) assessed the success of a brief battery of bedside cognitive tasks in distinguishing between groups of patients with dementia of the Alzheimer type and those with the purely frontal variant of FTD matched on the Mini-Mental State Examination (MMSE). Traditional tests of memory, attention, language comprehension and executive function failed to separate the groups.
Given the potential utility of cognitive neuropsychology in the accurate diagnosis of fvFTD, the performance of patients on various `frontal' tests is of special interest. One longitudinal study of patients demonstrated an unchanged cognitive profile across a series of assessments apart from on frontal tests, which showed progressive worsening (Venneri et al., 1996). Groups of patients with frontal lobe dementia have previously been shown to be impaired in verbal fluency tasks (Miller et al., 1991; Elfgren et al., 1993), but this finding is non-specific. The use of letter-initial verbal fluency performance in dementia of the frontal type, dementia of the Alzheimer type and control subjects matched for age, sex and level of education, was examined in a recent study (Pasquier et al., 1995). It was found that verbal fluency tests, whilst sensitive tools for detecting dementia, did not seem useful in distinguishing between patients with dementia of the Alzheimer type and those with dementia of the frontal type, as the two groups did not differ in the number of words generated, intrusions or perseverations. One other test that has been used most consistently in the assessment of frontal lobe dementia is the Wisconsin Card Sorting Test (WCST), which in a clinical setting has been applied to identify frontal lobe dysfunction (Milner, 1963). Although results across studies may be difficult to compare as different administration and sorting principles are used, many patients with fvFTD have been found to demonstrate deficits in the WCST, particularly those with lower MMSE scores, i.e. in the dementing range (Neary et al., 1988; Miller et al., 1991; Elfgren et al., 1993). Deficits on the WCST are difficult to attribute to specific neural damage, both within and outside prefrontal cortical regions (Robinson et al., 1990; Anderson et al., 1991; Corcoran and Upton, 1993). The lack of neural specificity of the WCST probably arises because of the complexity of the test, which has many cognitive components (e.g. including response inhibition and working memory). Indeed, decompositions of the test requirements in monkeys have shown that different forms of perseveration may result from damage to different sectors of the prefrontal cortex (Dias et al., 1996).
It is therefore clear that performance on the WCST cannot always be used to define with confidence the neural loci of cognitive deficits in patients with fvFTD. It also emerges from the literature that some patients, despite marked neurobehavioural changes and eventual clear-cut atrophy on MRI, may achieve all categories. As an example, Neary and colleagues (Neary et al., 1988) provide a graphic description of a male electrician who was suspended from work at the age of 55 years because of increasingly clownish behaviour and a reduction in his level of personal and social behaviour. His behaviour became progressively more puerile and disinhibited, resulting in his retirement and forcing his wife to assume responsibility for all household and financial affairs. Nonetheless, his performance on the modified form of the WCST remained intact, achieving six complete categories. Kertesz (1998) also recently noted that three out of 12 patients with a diagnosis of frontal lobe dementia had normal or nearly normal scores on frontal lobe function tests despite severe behavioural disturbances. A recent observation of Gregory and colleagues (Gregory et al., 1999) may also be of some relevance. They describe two patients who presented with a marked change in personality and behaviour, and who indeed progressed over a number of years in a way conforming to a clinical picture of fvFTD, but in whom there remained remarkably little abnormality on neuropsychological testing. Whilst they had normal HMPAO (99mTc-hexamethyl-propyleneamineoxide)-single photon emission computed tomography (SPECT) imaging when first investigated, both patients subsequently developed abnormalities on HMPAO-SPECT, frontal atrophy on MRI and a neuropsychological profile usually considered typical of fvFTD.
There may be a neuroanatomical basis for the discrepancy between the behaviour of patients and their neuropsychological performance. Specific architectonic differentiation and connectivity of the prefrontal cortex has been identified (Barbas and Pandya, 1991; Yeterian and Pandya, 1994). It is now widely accepted that a series of parallel subcortical circuits exist that link regions of the prefrontal cortex to the basal ganglia (Alexander et al., 1986; Alexander and Crutcher, 1990). Therefore, arguably, as the various subdivisions of the frontal lobes may subserve distinct and specific functions, traditional neurocognitive assessment may fail to test the functions of the areas of the prefrontal cortex which are predominantly affected by pathology. It has been recognized previously that successful identification of the neuroanatomical region involved may help ultimately in the direct selection of behavioural investigations and, conversely, to study which behaviours are correlated with particular brain regions (Stuss, 1993).
In the present study we examined the nature of cognition in patients with fvFTD with particular emphasis upon frontal lobe function. We have included only patients who are affected relatively mildly, defined (in parallel with patients with mild dementia of the Alzheimer type) for patients with a MMSE score of ≥20 (Folstein et al., 1975). In attempting to determine the major domains of neuropsychological deficit in patients with mild fvFTD, and thereby to develop suitable probes to examine deficits and the putative underlying loci of pathology, it is useful to note that the patients are similar to those with defined neurosurgical lesions of the ventromedial prefrontal cortex. In a classic paper, Eslinger and Damasio (1985) described a patient (EVR) who by the age of 35 years was successful in a professional occupation, happily married and the father of two children. After successful resection of an orbitofrontal meningioma involving a bilateral excision of the orbital and lower mesial cortices, he could not meet personal and professional responsibilities. He was unable to hold down his job, and his marital life eventually deteriorated until his wife left home with the children and filed for divorce after 17 years of marriage. Despite these problems, results of neurological examination and standard cognitive neuropsychological assessment were entirely normal (e.g. normal performance on the WCST and Word Fluency Test) and his intelligence was superior [97th percentile on the Wechsler Adult Intelligence Scale (WAIS)]. Clinically, lesions of the dorsolateral frontal lobe have traditionally been found to tend to affect planning and other higher cognitive functions, whereas lesions of the orbitofrontal cortex with its connections with the limbic system tend to have a greater effect on social behaviour and emotion (e.g. Sarazin et al., 1998).
A paradigm has been developed by Bechara and his co-workers to bring out the cognitive deficits in patients such as EVR. This paradigm requires subjects to sample repeatedly from four decks of cards. After each selection, they receive a given amount of reward consisting of facsimile money. Two of the four packs produce large payouts with larger penalties, but the other two packs produce small payouts with smaller penalties. The most productive strategy is therefore to sample from the two packs with small payouts and penalties, which indeed control subjects adopt. Patients with damage to the ventromedial, but not dorsolateral, sectors of prefrontal cortex have been found to persist in drawing cards from the high payout/penalty decks (Bechara et al., 1994). The task developed by Bechara and his colleagues is attractive because it models aspects of real-life decision-making that patients with ventromedial lesions find difficult. These decisions typically involve choices between actions associated with differing magnitudes of reward and punishment under conditions where the underlying contingencies relating actions to relevant outcomes remain hidden. To explain the nature of deficits in patients such as EVR, the `somatic marker hypothesis' has been posited (Damasio, 1995, 1996). The key idea in this hypothesis is that `somatic marker' signals influence the processes of response to stimuli at multiple levels of operation, some of which occur overtly (consciously `in mind') and some of which occur covertly (non-consciously, in a non-minded manner). Damasio speculates that the neural network required for such markers to operate includes the (i) ventromedial prefrontal cortices, (ii) central autonomic effectors such as the amygdala, which can activate somatic states in the viscera, vascular bed, endocrine system and non-specific neurotransmitter systems, and (iii) somatosensory cortices (SI, SII and insula) which can receive signals from the soma.
In two further hypotheses a relationship between the cognitive and behavioural presentation of patients is also proposed. Rolls and colleagues (Rolls et al., 1994) have observed that patients with ventral frontal lesions make more errors in the reversal (or similar extinction) tasks and complete fewer reversals than control patients with damage elsewhere in the frontal lobes or other brain regions. Subjects were required to learn to obtain points by touching one stimulus when it appeared on a video monitor but withholding a response when a different visual stimulus appeared. After the subjects had acquired the visual discrimination, the reinforcement contingencies unexpectedly reversed. Patients with ventral frontal (orbitofrontal) lesions often reported verbally that the contingencies had changed, but were unable to alter their behaviour appropriately. Some aspects of the performance of the patients were particularly intriguing. The perseverative touching of a previously rewarded stimulus observed in humans appeared to be consistent with work with non-human primates showing impaired reversal and extinction after orbitofrontal lesions. Furthermore, the performance on reversal and extinction tests in the group of patients with damage to the ventral part of the frontal lobes was found to correlate highly with responses to a questionnaire examining the degree of disinhibited and socially inappropriate behaviour. Rolls therefore argued that neuropsychological deficits in patients with ventral frontal lesions might be manifested as disorders of emotion, if various emotions are to be considered as states elicited by rewarding and punishing stimuli; for example, fear may be a state elicited by stimuli associated with punishment (after Mowrer, 1947), and joy may be a state which is associated with rewarding stimuli. Therefore, the difficulty in modifying stimulus–reward associations may contribute to the emotional changes shown in daily life. This may be consistent with the hypothesis that the orbitofrontal cortex is normally involved in executing behaviour when the behaviour is performed by evaluating the associations of environmental stimuli with reinforcement (Rolls, 1996b). Interestingly, the same patients with ventral frontal lesions that Rolls described performed normally on the Tower of London task.
In an alternative hypothesis, proposed by Plaisted and Sahakian (1997), the reversal learning deficits are also considered, and its starting-point is the well established finding that damage to the prefrontal cortex results in loss of inhibitory control over inappropriate responses to any current situation. Recently, in the primate literature, Dias and colleagues (Dias et al., 1996, 1997) have reported a double dissociation in the prefrontal cortex of affective and attentional shifts in the marmoset following excitotoxic lesions to the lateral and orbitofrontal cortex. Whereas damage to the lateral prefrontal cortex (Brodmann area 9) in monkeys causes a loss of inhibitory control in attentional selection (an extradimensional shift), damage to the orbitofrontal cortex in monkeys causes a loss of inhibitory control in `affective' processing (a reversal), thereby impairing the ability to alter behaviour in response to fluctuations in the emotional significance of stimuli. Plaisted and Sahakian, therefore, in explaining the marked deficits in social cognition observed in patients with fvFTD, put emphasis upon an inability of orbitofrontal inhibitory mechanisms to suppress inappropriate behaviours elicited by the immediate environment. They argue that such disinhibition would prevent the selection of alternative and more appropriate action plans which are dictated by long-term goals. Behaviour would hence be dominated by the immediate emotional evaluation of the stimuli, regardless of any available emotional or somatic information about the consequences of more distal action plans.
In the present study, we considered how the theories described above, which have tried to specify how dysfunction of the orbitofrontal cortex can lead to deficits in social cognition, may apply in this particular clinical disorder. We were therefore interested not only in identifying the cognitive deficits of patients with mild fvFTD, but also in examining whether these patients were unimpaired on certain tests of executive and mnemonic function. One obvious implication of our study concerns the future development of tests sensitive to the cognitive impairments demonstrated by patients in the mild stages of fvFTD.
In relation to Damasio's hypothesis, we attempted to examine the precise nature of decision-making deficits using a task in which subjects were required to make choices or decisions between contingencies that were presented in a readily comprehensible visual format. This task allowed us to assess the degree to which deficits in decision-making shown by mild fvFTD patients were sensitive to the quality of information available about the identity of the reinforced response, as well as differences in opportunities to earn reinforcement. Using this task, we were also able to model a further feature of real-life decision-making. Many taxing decisions involve the commitment of resources (e.g. money, time) to particular plans at the expense of others; it is therefore important to weigh up available opportunities against each other, judge the relative probabilities of a successful outcome and then choose how much of current resources or reward to commit to the chosen strategy. The decision-making paradigm used in this study enabled us to assess how much of their resources (in this instance, an accumulated points score) subjects were willing to commit to a given contingency in the hope of earning yet more reinforcement.
Secondly, in relation to the theories of Rolls (1994) and Plaisted and Sahakian (1997), we examined specifically whether patients with mild fvFTD performed differently from matched controls in the reversal stages of an attentional set-shifting paradigm. This particular paradigm of visual discrimination learning, which also included `intradimensional' and `extradimensional' shifts, has been described in greater detail elsewhere (Roberts et al., 1988; Downes et al., 1989). It allowed us to examine whether patients with fvFTD had specific deficits in the reversal stages, and, if so, what were the precise nature of these deficits.
Methods
Subjects
Patients
Eight male out-patients with a clinical diagnosis of fvFTD and with mean age 57.9 years (SD 9.60 years) took part in the study. Written informed consent was obtained from all participants in this study prior to subsequent assessment. The study was approved by the Cambridge Local Research Ethics Committee (reference number LREC96/113).
These patients attended the Memory and/or the Early Dementia Clinics at Addenbrooke's Hospital, Cambridge. These are multidisciplinary clinics where patients are seen by a neurologist, psychiatrist and neuropsychologist. All patients were examined by a senior neurologist (J.R.H.). The patients were diagnosed on the basis of criteria summarized in Table 1 (Gregory and Hodges, 1993, 1996), with some similarities to the Lund and Manchester criteria (Lund and Manchester Groups, 1994). Care was taken to include only patients with relatively mild FTD.
Assessment of the cases included an informant history, physical examination, neuropsychological tests, routine screening blood tests and structural (CT or MRI) and functional (HMPAO-SPECT) brain imaging. A battery of traditional neuropsychological tests was also administered at various intervals following diagnosis. Table 2 summarizes all the available results from these tests, which were administered at a similar time to the computerized neuropsychological assessment and provided references for detailed descriptions of the tests themselves. Patients with a past history of known or suspected transient or cerebral ischaemic event or stroke, alcoholism, head injury or other major medical illnesses (e.g. cancer or thyroid dysfunction) were excluded. One patient was actively receiving medication during the study. Case H was receiving nabumetane, meptazimide and amitriptyline. One other patient had also been receiving medication, but this was only previous to the time of testing. Case D had been prescribed fluvoxamine to control extreme eating habits, but this was discontinued after the development of a tremor. Case D had also been prescribed thioridazine to improve his sleep pattern, but this was also discontinued.
Control subjects
To compare the performance of the patients with that of controls, we selected eight subjects from the MRC Cognition and Brain Sciences Unit's control panel, matched for age, sex and premorbid predictions of IQ, as measured by the National Adult Reading Test (NART) (Nelson, 1982). Subjects with a history of alcoholism, drug abuse, learning disability or neurological or psychiatric illness were excluded, as were subjects with clinically apparent hearing and/or visual handicaps liable to affect their performance. All patients and controls were able to complete the full battery of tests.
Subject characteristics
One-way analysis of variance (ANOVA) revealed that the two groups did not differ in terms of age [F(1,14) = 0.0027, P = 0.959] or in estimates of WAIS full-scale, verbal and performance IQs as predicted from the number of errors made on the NART [full-scale IQ: F(1,14) = 0.224, P = 0.644; verbal IQ: F(1,14) = 0.267, P = 0.614; performance IQ: F(1,14) = 0.305, P = 0.589]. The fvFTD patients and the control group were both administered the MMSE (Folstein et al., 1975). One-way ANOVA revealed that the mean MMSE scores for two groups were significantly different [F(1,14) = 5.70, P = 0.0316]. Subject characteristics are detailed in Table 3.
Patient case histories
The clinical histories of each of the eight patients in the present study are described below. A summary of the clinical characteristics of these patients is provided in Table 4.
Case A
This patient is a married financial director (born in 1942) without past personal history of psychiatric illness but with a family history of suicide (maternal grandfather) and depression (maternal aunt). He was referred to a psychiatrist by his general practitioner in October 1991, after his wife had received a letter from his employers stating that his job was in jeopardy because of his extremely poor performance. His wife provided a 3-year history of concern regarding his deterioration in intellectual function and an insidious personality change characterized by increasing irritability, antisocial and inappropriate behaviour (e.g. retiring to bed during visits from friends). His wife also reported that he showed loss of judgement with his personal finances, running up considerable debts on his credit cards and buying unnecessary, expensive items. He lacked any interest in his wife and children, and his driving had also become erratic, such that his family felt unsafe with him at the wheel. The patient showed no insight into his problems and was mystified by the letter from his employers. An assessment by a psychiatrist found no evidence of depression or formal psychiatric disorder. He was referred to the Memory Clinic in 1991. Examination and all investigations including routine haematology, biochemistry, EEG, CT and MRI were normal. The SPECT scan was minimally abnormal, with a suggestion of diffuse patchy hypoperfusion. Neuropsychological evaluation, with the exception of verbal fluency, was normal. A tentative diagnosis of fvFTD was made on clinical grounds.
Since 1991 there has been a gradual decline in his personality and behaviour. By March 1995 he had become extremely apathetic and had begun to overeat, gaining several stones in weight. He currently spends his time at home watching TV. He does not engage in any spontaneous conversation. He attends to a few specified household chores, which he performs in a rigid and highly stereotyped manner, e.g. hoovering at exactly 4 p.m. daily. He has also become sexually disinhibited, increasingly antisocial and shows no interest in his family. He has occasional urinary incontinence. Subsequent MRI imaging in 1995 and 1998 showed progressive frontal lobe atrophy. Repeat HMPAO-SPECT scans in 1995 and 1998 have also revealed bifrontal hypoperfusion.
Case B
This patient (born in 1927) is now aged 70 years. He left school at 15 years and became an apprentice, but only a few years later, after joining the Burma Oil Company in India, he underwent rapid promotion so that within 10 years he was a manager and in charge of several hundred employees. At that time, he was under very considerable strain and began to have peculiar attacks of loss of consciousness which would last for a number of hours. These were subsequently diagnosed as strokes, although this seems dubious. He was unwell enough to be sent back from India and underwent extensive investigation in the early 1970s. In about 1975 he had a brain scan and was told that this showed brain atrophy due to a presenile dementia. In the 10 years after his retirement he underwent a progressive change in personality, behaviour and social interaction. The features of this included poor motivation and apathy, with a complete loss of interest in his wife and inability to organize any activity. He took up a couple of jobs but they lasted for only a few weeks. His current state is of complete inertia. Many days can pass in the house without him initiating conversation, although he still reads novels and apparently can be motivated if taken to museums, particularly if they relate to the Second World War. There have been some minor changes in eating and his manners. Approximately 15 years ago there was a period of sexual disinhibition but he subsequently appears to have lost his libido. His wife describes him as being `non-existent'.
On examination, there were a number of physical signs. Most striking was his restriction in eye movements. The horizontal range appeared limited, but he had virtually non-existent upgaze. There was also poor balance and a hint of some rigidity in the limbs. There was a positive pout, no clear grasping and no limb apraxia. On basic cognitive testing, he was fully orientated with good registration of information and rapid serial calculation. He had good visuospatial function and naming ability; he was also very good on current events. By contrast, his verbal fluency was markedly reduced. In March 1998 he underwent a number of investigations. An MRI scan confirmed marked frontal lobe atrophy, compatible with the diagnosis of FTD. A SPECT scan revealed reduced perfusion in the left frontal and temporal regions. All other routine investigations were normal.
Case C
This patient (born in 1939) presented in 1995. He admitted to no symptoms, although he did realize that his family had been finding it difficult to cope with him. His family reported that he was `letting things go' on the farm, whereas previously he was methodical and well organized. In addition, he had become excessively socially withdrawn and was initiating very little conversation at home. He needed reminding to change his clothes and to wash, and his dietary habits had changed in that he now liked to consume lots of sweet food.
He came from a very large family with 10 siblings, an elderly mother and a father who died of a heart attack at the age of 75 years. There was no family history of dementia. He had a surly, unsociable manner, but this impression was occasionally dispelled by a sudden smile. Examination revealed a bilateral palmomental reflex, pout reflex and glabellar tap, and difficulty with Luria three-step commands. He was well orientated with a normal digit span, but had reduced verbal fluency. His acquisition and recall of a name and address was surprisingly good. On formal neuropsychological assessment, language skills were well preserved, although his conversational ability was reduced. Traditional assessment of frontal lobe function revealed disorganized and somewhat erratic performance. In 1997, formal neuropsychological assessment revealed that he was unable to perform normally on formal tests of memory, but performance on tests of perceptual and visuospatial analysis was within normal limits. Unlike previously, he failed to sort the Weigl tokens according to shape and colour.
In 1995 a cerebral perfusion SPECT scan revealed dramatic extensive reductions in perfusion in the frontal and anterior temporal regions. Therefore, taken with the clinical findings, a tentative diagnosis of fvFTD was made. A cerebral perfusion SPECT scan was repeated in February 1997, which showed quite a marked progression in the changes in the frontal lobes. An MRI scan performed at that time demonstrated a gross degree of frontal atrophy.
Case D
This patient, a married music teacher born in 1942, was referred in November 1992 by his general practitioner following concern regarding his behaviour from both relatives and colleagues at work. It was reported that he had become increasingly vague and subdued, appeared slowed and lacked initiative. His driving had become rather hazardous, e.g. pulling out into the path of oncoming traffic. He was also noted to have lost his sense of humour. At work it was stated that he had difficulty in coping with the children during class, but he had no problems in completing his regular newspaper crossword. In February 1990 he had suffered a myocardial infarction from which he made a full recovery, and was found to have elevated cholesterol levels.
By March 1993 he was noted to have substantial difficulty in reading music and playing the piano simultaneously, and had problems playing and conducting, tasks at which he had previously excelled. He showed poor judgement and forethought. His affect was flat and his behaviour stereotyped, and he started to have difficulty controlling his food intake. He was referred to the Memory Clinic at Addenbrooke's Hospital and underwent full assessment in 1993. A psychiatric assessment found no evidence of functional illness, in particular no evidence of an affective disorder. There was no family history of psychiatric disorder, but the patient had suffered an episode of depression in his 30s. On physical examination he had positive pout and palmomental reflexes. Neuropsychological testing showed that his verbal and performance IQs were well maintained. His memory, naming and perceptual abilities were normal. On two simple frontal lobe tests (verbal fluency and a modified version of the WCST), he showed no deficit. Routine haematological and CSF examination were normal, as were SPECT, CT scan and MRI. A tentative diagnosis of fvFTD was made on the basis of the history. He has been assessed neuropsychologically on a number of occasions.
By July 1993 he was gaining weight excessively and would often eat two full meals at one sitting. At school, he began to behave in a disinhibited manner, making inappropriate sexual remarks. Because of this he was asked to leave his job. By December 1994 he had become markedly apathetic and, uncharacteristically, would sit watching TV for hours at a time. His conversational abilities were reduced and he became increasingly rigid and repetitive in his behaviour. He also began to eat inedible substances. In September 1997 a cerebral perfusion SPECT scan was undertaken, and this revealed a reduction in perfusion in both frontal cortices (in particular the inferior part, and especially on the right). The rest of the cerebral perfusion appeared within normal limits. At the present time (1998) he does little except keep to the routine of taking his dog for a daily walk, the route of which is never varied. He does not help spontaneously in the house, and his self-care and social manners have deteriorated greatly.
Case E
Patient E (born in 1944) was referred to the Memory Clinic from the Burton Hospitals NHS Trust in 1997 with the provisional clinical diagnosis of fvFTD. He himself denied any symptoms, but his wife and close friend reported a gradual change in personality over the last 2–3 years. He began to act in rather strange ways, for instance, grabbing someone else's steak at dinner because it looked larger than his own, and getting up and leaving half-way through a dinner party to watch TV. They noticed that he was becoming progressively withdrawn and at other times he would act in a rather disinhibited and blunt fashion. He also had difficulty with planning and organizing and dealing with more than one thing at once. There was no change in his self-care or eating habits, and his wife did not observe any obsessive or compulsive behaviour. He did express a slightly paranoid idea of conspiracy, but this may have been related to the fact that he felt that there was nothing wrong with him and therefore could not understand why he was being referred for investigations. Despite the changes in personality and behaviour, his memory remains very good and he is extremely knowledgeable about Manchester United Football Club and also the stock market. There is no past history of significant head injury. He has never abused alcohol. There is no family history of dementia. His mother is aged 78 years and still alive. His father died of a brain tumour. He has one sister, who is well, and a daughter aged 23 years.
On examination, he was found to have a positive pout response but no other release signs. He was well orientated in time and place, and his memory was good. There were no language or visuospatial deficits, but he did show a marked reduction in verbal fluency for both letters and categories. More formal neuropsychological testing showed borderline performance on tests of memory, with normal language and perceptual ability but poor performance on tests sensitive to frontal lobe function. A cerebral perfusion SPECT scan performed at Addenbrooke's Hospital in March 1997 showed a marked reduction in perfusion bilaterally to the frontal lobes, which concurred with the results of a CT scan performed at Burton NHS Trust in August 1996 that had revealed marked frontal lobe atrophy.
Case F
This patient was initially referred to a psychiatrist in May 1989, at the age of 35 years, with a short history of `odd ideas'. He believed that his workmates were smiling at him in suggestive ways and that he was a special and wonderful person. He also believed that his thoughts were being interfered with by an electronic gadget and that the TV and radio were making specific reference to him. Furthermore, he believed that his actions were being controlled by an external force. There was no past psychiatric history, and he was physically fit. There was a strong family psychiatric history; his paternal grandparents and uncle all died by suicide. His developmental, sexual and occupational histories were unremarkable, except that they were characterized by stability. On further questioning, his wife reported a gradual onset of mental inflexibility and social withdrawal, a decline in his sense of humour and a loss of interest in hobbies for ~1 year before this psychotic illness.
He made a good response to neuroleptic medication but never developed any insight into his illness. Over the next 2 years he was maintained on neuroleptic medication, and any attempt at withdrawal resulted in reappearance of his paranoid beliefs. Over this period, however, his behaviour changed substantially. He began to collect and hoard women's underwear and pornographic magazines, which he `hid' in the cupboard of his bedroom, but which were obvious to his wife whenever she opened the cupboard doors. He became sexually disinhibited, masturbating frequently. Over the next few months all his psychotic symptoms disappeared and he was left apathetic and disinhibited with poor foresight and planning. He appeared very flat all of the time, and even during a crisis at home he remained unperturbed. During all of this, he denied any problems at all and said that he was fine. He also denied any problems with his memory, thinking or organizational abilities. On physical examination he was found to have pout and grasp reflexes.
Bedside cognitive testing revealed him to be fully orientated, with good registration and recall. He showed reduced verbal fluency. Detailed neuropsychological tests (e.g. the WCST) confirmed the presence of frontal dysfunction. A CT scan revealed selective frontal atrophy, but a SPECT scan was unhelpful and showed only patchily reduced uptake of tracer. In August 1991 a diagnosis of fvFTD was made. During the period after diagnosis his clinical condition has deteriorated further. He has become gradually less able to organize himself or activities, is extremely impulsive and has occasional urinary incontinence. He requires constant supervision and is about to embark on respite admissions to his local psychiatric hospital.
Case G
This patient, a retired chartered accountant born in 1924, was referred to Addenbrooke's Hospital in March 1996 with a 3-year history of a gradual decline in motivation and interest. He had demonstrated a marked reduction in spontaneous conversation, often appeared apathetic and showed no empathy with family members. He had previously been a rather flamboyant Australian. His wife reported that his day-to-day episodic memory remained good. He denied any symptoms. Interestingly, his mother died in a home at the age of 81 years, possibly (in retrospect) having been suffering from dementia for the preceding 5 years. His father had a stroke in his 60s. He has seven siblings. He is an ex-smoker and drinks alcohol only occasionally.
On examination he was found to be fully orientated. His digit span was 7 forwards and 7 backwards. His verbal fluency was a little low for his background. Formal neuropsychological assessment showed quite marked anomia, which seemed to be in word-finding rather than semantic disorder. He performed quite well on visuospatial tests. His recognition memory for faces was at a good level but his recognition of memory for words was impaired. On tasks traditionally considered to be sensitive to frontal lobe function (verbal fluency, bimanual hand co-ordination, drawing to rule, Weigl, Stroop), the impression was of frontal inefficiency, although he did not show marked failure. A CT scan showed a mild degree of cerebral atrophy. An MRI scan demonstrated a degree of atrophy more prominent in the region of the anterior portion of the left temporal lobe, and the frontal lobe bilaterally. A clinical diagnosis was made of fvFTD.
Case H
This patient (born in 1940) was referred via the Orthopaedic and Psychiatric Departments to the Memory Clinic at Addenbrooke's Hospital, Cambridge. During a recent stay for investigation of sciatica and back pain, it was noted that his behaviour and affect on the ward were odd. For instance, he reported that his normal amount of exercise consisted of running at least 100 miles per week and he made inappropriately personal comments to the staff. He had been employed in a local security company, but had left his job 3 years previously, at the time of his marital break-up. He was unable to give a clear explanation of these events. He made no complaints related to his mental or intellectual state.
It was difficult to elicit a clear history because of his circumlocutory style, and his tendency to tell rather grandiose tales about his time in the Army. For example, he described an incident in Lebanon when he `awoke suddenly in the middle of the night, woke his 27 fellow soldiers and killed 900 enemy soldiers who were creeping up on them'. His son reported that he had always exaggerated about his Army career, but that he had started telling extremely unlikely tales only about a year previously. His ex-wife had also noted a change in personality in the last few years, with an exaggeration of his premorbid state. He has become disinhibited, with a tendency to tell inappropriate and rude jokes, with episodes of verbal aggression and emotional lability.
His father died of motor neuron disease, further details of which are pending. His mother is aged 79 years and is still alive and well, and he has five siblings who are all fit. He has been quite a heavy smoker for all of his life, and had consumed alcohol whilst in the Army but not more recently.
On examination there were no physical signs. He was well orientated. His acquisition of a name and address was poor. He was unable to name the Prime Minister, the last Prime Minister or the American President. His verbal fluency was poor; he was able to suggest the names of only nine animals in a minute. He scored within the normal range on the Cognitive Estimates Test. He had a striking impairment in abstract thinking, as he was quite unable to interpret proverbs and unable to answer even the simplest questions on similarities. Formal neuropsychological assessment showed him to have a rather low premorbid IQ but, even allowing for this, his performance on tests of naming and drawing was poor, and he was unable to complete the WCST. In February 1998, the clinical diagnosis was made of early fvFTD. Blood tests and CSF investigation revealed no abnormality. An MRI scan showed a degree of global atrophy, with accentuation in the frontal and anterior temporal lobes particularly on the left side. EMG was normal.
Computerized neuropsychological assessment
Procedure
The majority of tests were taken from the Cambridge Neuropsychological Test Automated Battery (CANTAB). These tests were administered using a portable Datalux 486 microcomputer fitted with a touch-sensitive screen. Subjects were seated comfortably ~0.5 m from the touchscreen, and it was explained to them that they would have to respond to stimuli presented on the monitor screen by touching them. In order to introduce subjects to the apparatus, they were initially given a `motor screening task' in which they were asked to respond to a series of 10 flashing crosses presented at varying locations on the monitor screen by placing the index finger of their preferred hand on the centre of the cross. This followed a short demonstration by the tester in which three consecutive crosses were touched. After completion of this task, subjects were given the following cognitive tasks in the order described below.
Pattern and spatial recognition (Sahakian et al., 1988)
Two tasks designed to assess recognition memory for both patterns and spatial locations were administered. In the pattern recognition task, subjects are presented with a series of 12 abstract patterns and their task is to remember them. Following a 5-s delay, each pattern is re-presented in reverse order, paired with a novel pattern, and subjects are asked to touch the pattern they have seen previously. This procedure is then repeated with a further 12 patterns.
In the spatial recognition task, five squares are presented sequentially in different locations around the screen. In the recognition phase, subjects are presented with a choice of two squares in different locations, one of which is novel; they must touch the location in which the square has previously appeared. The procedure is repeated a further three times.
Spatial span (Owen et al., 1990)
This is a computerized version of the Corsi Block Tapping task (Milner, 1971). Briefly, each trial begins with nine white boxes presented in fixed locations on the monitor screen. Initially, two of the boxes change colour, one after the other, in a predetermined sequence. The end of the sequence is indicated by a tone. Subjects are then asked to point to the boxes in the order in which they have changed colour. After successful completion of a sequence, the number of boxes changing colour increases by one, up to a maximum of nine. The test is terminated when three consecutive failures occur at any one sequence length. During each trial, the number in the bottom left corner of the screen indicated the length of the current sequence. The spatial span was calculated as the highest level at which the subject has successfully recalled at least one sequence of boxes. This measure was used to assess the ability of subjects to hold information on-line in order to plan a series of moves in tasks such as the spatial working memory task and the one-touch Tower of London task.
Spatial working memory (Owen et al., 1990)
This is a test of spatial working memory for humans which is formally analogous to the Olton radial arm maze (Olton and Samuelson, 1976), a spatial working memory task for the rat. Both tasks require memory for locations already visited. In this task, subjects are required to `search through' a number of coloured boxes presented on the monitor screen (by touching each one) in order to find blue `tokens', which are hidden inside. On any one trial, only a single token is hidden in one of the boxes. Once found, the next token is hidden. The key instruction is that once a token has been found within a particular box, then that box will not be used again to hide a token.
Two types of error are possible. First, a subject may return to open a box in which a token has already been found (a `between-search' error). Secondly, a subject may return to a box already opened and shown to be empty earlier in the same trial (a `within-search' error). There are four trials with each of four, six and eight boxes. The task is scored according to the number of between- and within-search errors at each level of difficulty and also for the use of an efficient search strategy (Owen et al., 1990). Both scores are a measure of spatial working memory, but the between-search error is a more stringent one as the subject is required to remember across searches which boxes had contained the blue tokens while conducting a new search (Joyce and Robbins, 1991).
A particularly efficient strategy for completing this task is to follow a predetermined search sequence, starting with a particular box and then returning to start each new sequence with that same box as soon as a token is found (editing the sequence when a token is found in that box). The extent to which such a strategy is used is estimated from the number of search sequences starting with a novel box for just the more difficult six- and eight-box problems. The total of these scores provides a measure of strategy for each subject, a high score (many sequences starting with a novel box) representing poor use of a strategy and vice versa.
Visual discrimination learning/attentional set shifting (Downes et al., 1989)
The task requires subjects to learn a series of two-alternative forced-choice visual discriminations using feedback provided automatically by the computer. The task is composed of nine stages presented in the same fixed order. For each stage, continuation to the next stage is dependent on a criterion of six successive correct discriminations being reached. If criterion is not reached at the 50th trial of a stage, the test is discontinued and the subject is not able to proceed to the following stage. The task starts with a simple discrimination and its reversal for stimuli varying in just one dimension (e.g. two different white-line configurations). A second, alternative dimension is then introduced (purple-filled shapes) and compound discrimination and its reversal are tested. To succeed, subjects must continue to respond to the previously relevant dimension whilst ignoring the presence of the new, irrelevant dimension. At the intradimensional shift stage, novel exemplars of each of the two dimensions are introduced and subjects must continue to respond to one of the two exemplars from the previously relevant dimension. Following another reversal, the extradimensional shift and its reversal are presented, again using novel exemplars of each stimulus dimension. In order to succeed at the extradimensional shift stage, the subject must shift `attentional' or `response' set to the previously irrelevant stimulus dimension, whilst ignoring the previously relevant dimension. The extradimensional shift stage is akin to a change in category in the Wisconsin Card Sorting Test.
One-touch Tower of London (Owen et al., 1995)
This task is a modified version of the CANTAB Tower of London task, itself ultimately derived from the Tower of London task developed by Shallice and McCarthy (Shallice, 1982). Subjects are first trained with a number of problems from the original Tower of London task and are then presented with the following modified task. Two sets of coloured balls appear on the screen, one in the upper half of the screen and one in the lower half. They are described to the subjects as `snooker' or `pool' balls as they appear to hang in vertical pockets. There are three pockets in each half of the screen, capable of holding three balls, two balls and one ball, respectively. The numbers 1, 2, 3, 4 and 5 are printed in large boxes across the bottom of the screen. At the start of each trial, the upper and lower pockets appear empty on the screen. After a delay of 1 s, a tone alerts the subject to the screen and a red ball, a blue ball and a green ball are placed in a predetermined arrangement in the pockets of the upper and lower displays. The subjects are instructed to examine the positions of the balls on the screen and then to imagine how they might rearrange the balls in the lower display to match those in the upper display without actually moving any of the balls. For any given arrangement, the subjects are asked to find the solution that requires the minimum number of moves, and then to press the corresponding number on the bottom of the screen. If a subject's first response is incorrect, he is required to try again until the correct number is selected. The importance of accuracy rather than speed of response is emphasized. After the practice problems, subjects are given test trials of varying problem difficulty arranged in a constant, pseudorandom order.
Decision-making (Rogers et al., 1999a)
Figure 1 shows a typical display from a decision-making task. The subject is told that the computer has hidden at random a yellow token inside one of the red or blue boxes arrayed at the top of the screen, and that he has to decide whether this token is hidden inside a red box or a blue box. The subject indicates his decision by touching either the response panel marked `red' or the response panel marked `blue'. After making this initial choice, the subject attempts to increase a total points score, shown in green towards the left-hand side of the display, by placing a bet on this choice being correct. The available bets appear in a sequence, one after another, centred in the box positioned towards the right-hand side of the display. Each bet is displayed for a period of 5 s before being replaced by its successor, and the subject is free to select any bet by touching the box in which the sequence appeared. Immediately following such a selection, one of the boxes opens to reveal the location of the token, accompanied by either a `You win!' message and a short rising musical scale, or a `You lose!' message and a low tone. If a subject chooses the correct colour the bet placed is added to the total points score; if the subject chooses the wrong colour the bet is subtracted. The subject is instructed to treat the points as being valuable and to accumulate as many as possible during the test. However, no real monetary significance is attached to the total points accumulated by the end of the task.
The subject performs the task in two separate conditions. In one condition (`ascending'), the first bet offered is small but is replaced by larger and larger bets until the subject makes a selection. In the other condition (`descending'), the first bet offered is large but is replaced by smaller and smaller bets. Each bet represents a fixed percentage of the current total points score, although this is never made clear to the subject. Five bets are offered on each trial, so that in the ascending condition the order of available bets is as follows: 5%, 25%, 50%, 75% and 95%. In the descending condition the order is reversed. In both conditions, each bet is presented with a short tone whose pitch corresponds to the size of the bet: higher tones accompany larger bets and lower tones accompany lower bets. If the subject fails to select a bet by the end of a sequence, the last bet is chosen automatically.
The ascending and descending conditions both consist of four sequences of nine displays. At the start of the sequence, the subject is given 100 points and is asked to increase this total by as much as possible. If a subject's score falls to just one point the current sequence ends and the next begins. The order of presentation of the two conditions is counterbalanced across the two subject groups. The same set of fixed, pseudorandom sequences is presented to both the subject groups.
Three features of this task are important. First, by manipulating the ratio of red and blue boxes from trial to trial it is possible to examine a subject's decision-making behaviour over a variety of differentially weighted contingencies. For example, some ratios (e.g. 9 red : 1 blue) represent two contingencies that are quite unequal in terms of the probabilities associated with the two possible outcomes. In contrast, other ratios (e.g. 4 red : 6 blue) represent contingencies that are more balanced. Therefore, a subject's choice of contingency, speed of choice and size of bet are expected to differ as a function of the ratio of red to blue boxes. Secondly, by allowing subjects to determine for themselves how much of their points score they wish to bet after each red/blue decision, we were able to assess individual efficiency in placing accumulated reinforcement at risk with the intention of acquiring more reward. For example, one might suppose that a ratio of 9 red : 1 blue represents an opportunity to bet more points on a red decision in order to gain more reward, while a ratio of 6 blue : 4 red represents a situation in which more conservative behaviour might be appropriate. In this way, the aspect of decision-making in real-life situations can be modelled, i.e. individual choices about the investment of resources (such as time and money, etc.) in available opportunities. Finally, offering the bets in both an ascending and a descending order enables the isolation of merely impulsive behaviour from genuine risk-taking behaviour (after Miller, 1992). One would expect a subject, if impulsive, to be unable to withhold manual responses to the sequence of bets as they are presented, and therefore to choose early bets in both the ascending and the descending sequence. In contrast, one would expect a subject, if actively risk-taking, to choose late bets in the ascending condition and early bets in the descending condition. A large difference between the mean percentage bet in these two conditions therefore indicates impulsivity whereas a low difference indicates risk-seeking.
The three principal measures in this task are: (i) the speed of decision-making, i.e. how long it takes the subject to decide which colour of box is hiding the token, as measured by the mean deliberation time; (ii) the quality of decisions—as an ineffective approach is to bet continuously on the least likely of the two possible outcomes, one measure is how much of the time the subject chooses the most likely outcome; and (iii) risk adjustment, i.e. the rate at which a subject increases the percentage of the available points bet in response to more favourable ratios of red to blue boxes (e.g. 9 red : 1 blue versus 4 red : 6 blue).
Data analysis
The majority of the experimental data were analysed using the Statistical Package for the Social Sciences [SPSS V6.1.1] (SPSS Inc., Chicago, Ill., USA) running on an Apple Power Macintosh 6100/66 computer. Data were transformed where appropriate (Tukey, 1977). Some data were not suitable for parametric analysis even after transformation, and were therefore analysed non-parametrically. The data shown in the figures always represent untransformed values.
For most of the dependent variables, ANOVA was used to compare the performance of patients with fvFTD with that of the controls. The ANOVA model was a two-factor design that included a between-subjects factor (group) and a within-subject factor (e.g. difficulty level).
In the analysis of the decision-making task, initially the principal measures were subjected to repeated measures ANOVA with the following between- and within-subject factors: group (fvFTD versus controls); order of condition (ascending/descending versus descending/ascending); condition (ascending versus descending); decision (red versus blue); ratio (6 : 4 versus 7 : 3 versus 8 : 2 versus 9 : 1). The mean deliberation times (i.e. the speed of decision-making) were log10 transformed to ensure homogeneity of variance, while the proportions of trials on which subjects chose the most likely outcome (i.e. the quality of decision-making) were arcsin-transformed, as is particularly appropriate whenever the variance is proportional to the mean (Howell, 1997). In those instances in which the additional assumption of homogeneity of covariance in repeated measures ANOVA was violated, as assessed using the Mauchly sphericity test, the number of degrees of freedom against which the F term was tested was reduced by the value of Greenhouse–Geisser epsilon (Howell, 1997).
Results
Computerized neuropsychological assessment
Pattern and spatial recognition memory
The mean percentage scores for both the pattern recognition and the spatial recognition memory test are presented in Table 5.
The mean percentage correct scores were compared, following arcsin transformation [f(x) = 2 arcsin ÷x. One-way ANOVAs revealed that there was no significant difference in the percentage of correct scores between the patients and controls on both the pattern recognition task [F(1,14) = 0.216, P = 0.650] and the spatial recognition task [F(1,14) = 1.64, P = 0.222]. The mean response latencies for both tasks were also compared, following logarithmic transformation [f(x) = log10 (x)] to reduce positive skew in the distribution. Further one-way ANOVAs revealed that the fvFTD patients were significantly slower on the pattern recognition task [F(1,14) = 5.57, P = 0.0333] and highly significantly slower on the spatial recognition task [F(1,14) = 9.81, P = 0.0073].
Spatial span
Mean spatial spans for the two groups are also presented in Table 5. There was no significant difference between the two groups in their spatial spans [F(1,14) = 1.52, P = 0.238]. The total numbers of errors for the fvFTD and control groups were 12.6 (SD = 2.77) and 12.6 (SD = 3.16), respectively. The total usage errors for the fvFTD and control groups were 3.50 (SD = 1.07) and 3.13 (SD = 1.25), respectively. There was no significant difference between the two groups in the total number of errors [F(1,14) = 0, P = 1] or in the total number of usage errors [F(1,14) = 0.417, P = 0.529].
Spatial working memory
Data for the spatial working memory task are presented in Table 6. The mean number of between-search errors made for each of the four-, six- and eight-box stages of the task was subjected to repeated measures ANOVA with group and level (four, six or eight boxes) as factors. There was no main effect of group [F(1,14) = 1.01, P = 0.333] but there was a significant main effect of difficulty [F(2,28) = 49.3, P < 0.0001]. There was no significant interaction between group and difficulty factors [F(2,28) = 0.220, P = 0.803]. Within-search errors were at a minimal level in both groups and hence no meaningful analysis was possible.
One-way ANOVA revealed no difference between the two groups in the use of an efficient search strategy [F(1,14) = 3.09, P = 0.101]. The relationship between the strategy score and the sum of between-search errors obtained in the four-, six- and eight-box problems was further examined using Pearson's product moment correlation coefficient, r. As expected, there was a significant correlation between the strategy score and the number of between-search errors for patients [r = 0.709, P = 0.049]. The correlation between the strategy score and the number of between-search errors tended to significance for controls [r = 0.692, P = 0.057].
Visual discrimination learning/attentional set shifting
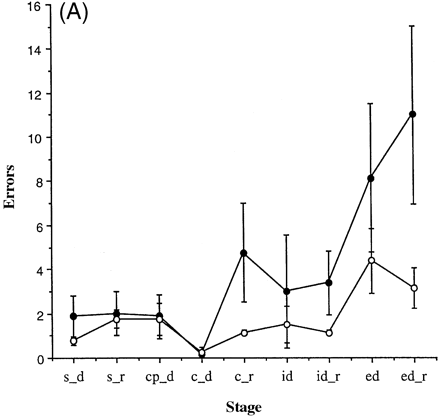
All the subjects and the controls attempted each of the nine stages of the attentional set-shifting paradigm. Only two subjects (patients E and H) failed to reach criterion at the final stage, the extradimensional shift reversal; their error scores for this particular stage, therefore, represent a conservative estimate of their deficit at this stage. Figure 2A shows the errors for each stage of the task for both the group of patients and for the controls. Two-way ANOVA of the errors to criterion at the intradimensional and extradimensional shift stages, following square-root transformation [f(x) = ÷(x + 0.5)], showed no significant effect of group [F(1,14) = 0.66, P = 0.431], a significant effect of stage [F(1,14) = 5.37, P = 0.036] and no significant group × stage interaction [F(1,14) = 0.22, P = 0.646].
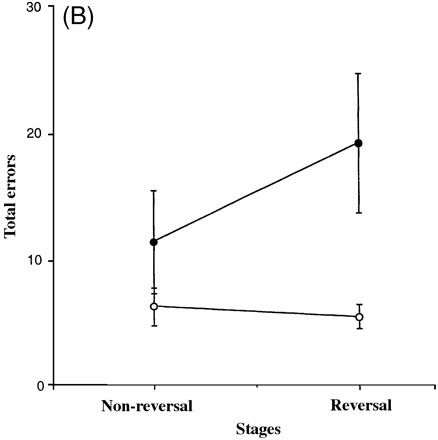
The total number of errors at the compound discrimination reversal, intradimensional shift reversal and extradimensional shift reversal stages, as well as the total number of errors for the corresponding non-reversal stages, was calculated for each subject. Figure 2B shows graphically the average total number of errors made by the two groups in the reversal and non-reversal stages. As the assumptions of ANOVA were violated even after transformation, these data were analysed non-parametrically. To examine specifically whether the effect of reversal differed between the two groups, the total number of errors made on the non-reversal stages was subtracted from the total number of errors made on the reversal stages for each subject. A two-tailed Mann–Whitney U test on this difference was then used, with a Bonferroni correction applied to the analysis such that the critical region was defined by P < 0.017. The Mann–Whitney U test revealed that the two groups differed significantly in the difference in the total number of errors made on the reversal and non-reversal stages [U = 8, exact two-tailed P = 0.0104]. Therefore, the patients with fvFTD did appear to diverge in performance from the controls specifically in the reversal stages of the task, the patients with fvFTD demonstrating a specific deficit on the reversal stages compared with controls. In addition, the number of trials before each subject changed his previously correct response (a perseverative error score) was noted for each of the three stages, compound discrimination reversal, intradimensional shift reversal and extradimensional shift reversal. The total number of perseverative errors made in these three stages was then calculated for each subject. Interestingly, one-way ANOVA revealed that there was no significant difference between the two groups in the total number of perseverative errors [F(1,14) = 2.01, P = 0.178].
One-touch Tower of London
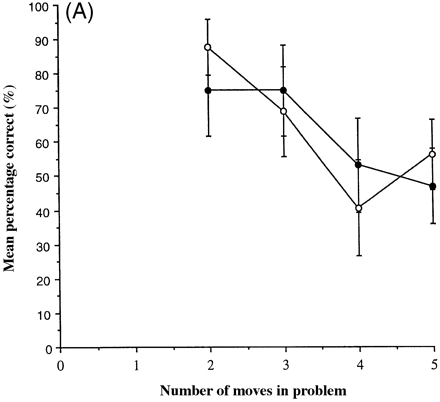
The main measure of planning accuracy was the percentage of problems solved correctly at each level of difficulty. A graph of this measure for both patients and controls is shown in Fig. 3A.
These data were subjected to repeated measures ANOVA with group and difficulty level as factors. There was a highly significant effect of difficulty [F(3,42) = 12.0, P < 0.0001] but no effect of group [F(1,14) < 0.001, P = 0.960] and no group×difficulty interaction [F(3,42) = 1.64, P = 0.195]. Another measure of planning accuracy was the mean number of attempts required before correct solution at each level of difficulty. These data were subjected to repeated measures ANOVA with group and difficulty level as factors, following square-root transformation [f(x) = ÷(x + 0.5)]. There was a highly significant effect of difficulty [F(3,42) = 16.2, P < 0.0001] but no effect of group [F(1,14) = 0.66, P = 0.430] and no group × difficulty interaction [F(3,42) = 1.91, P = 0.142].
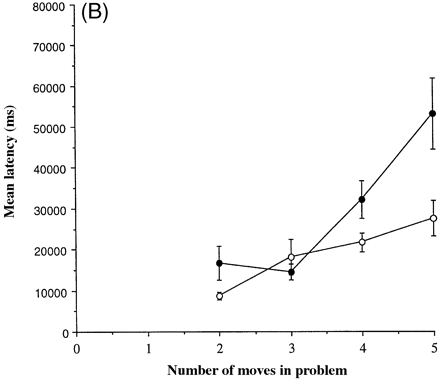
One further measure from this task was analysed following log10 transformation. This measure, the mean latency at each level of difficulty, is shown in Fig. 3B. For the mean latencies overall, there was a strong tendency towards a significant effect of group [F(1,14) = 4.26, P = 0.058], a highly significant effect of difficulty [F(3,42) = 36.2, P < 0.0001] and a significant group × difficulty interaction [F(3,42) = 3.41, P = 0.026].
Decision making
Speed of decision-making.
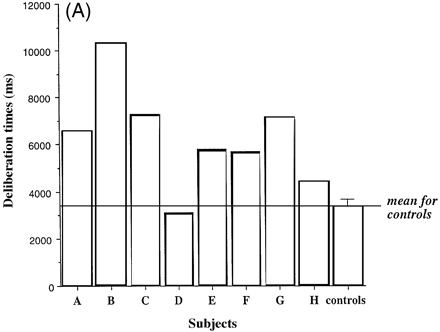
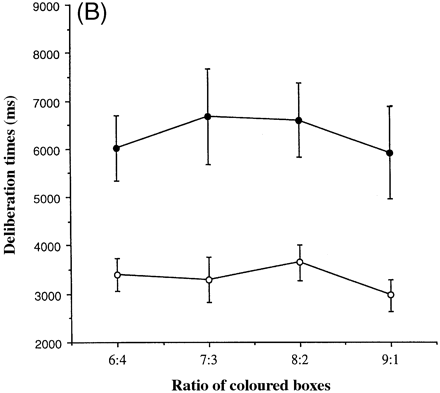
Figure 4A shows the mean deliberation times for the group of controls and for each of the eight patients associated with deciding which colour of box is hiding the yellow token, as a function of the ratio of red to blue boxes. Figure 4B shows the mean deliberation times for the groups of patients and controls.
Notably, the fvFTD group showed large and significant increases in the time needed to make their decisions, so that the mean deliberation time was 3325 ms for the controls but 6302 ms for the fvFTD patients [F(1,12) = 13.9, P = 0.003]. All subjects took more time to make their decisions on the first occasion that they completed the task compared with the second [average for the first occasion = 5624 ms versus 4363 ms for the second occasion]. In other words, the latency was slower in the ascending condition for those subjects whose order of conditions was ascending–descending and in the descending condition for those subjects whose order was descending–ascending, yielding a significant two-way interaction between order and condition [F(1,12) = 8.62, P = 0.012]. However, the three-way interaction between these two factors and that of group was not significant [F(1,12) = 0.01, P = 0.916]. The mean time needed to decide the colour of the box was not significantly increased at any particular ratio of red to blue boxes, suggesting that the patients did not have particular difficulties in the face of relatively poor information about the identity of the rewarded response. Analysis of the enhanced performance of the group of fvFTD patients and the controls revealed that neither age nor premorbid verbal IQ was a significant covariate of deliberation times (age: t = 1.51, P = 0.163; verbal IQ: t = –0.945, P = 0.367), and the results above were unaffected.
Quality of decisions.
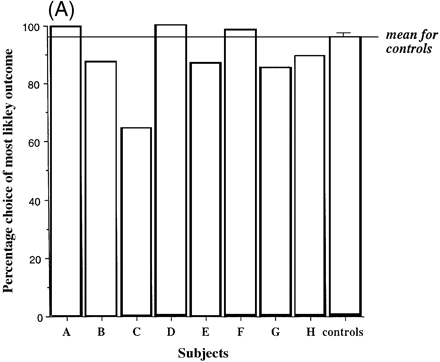
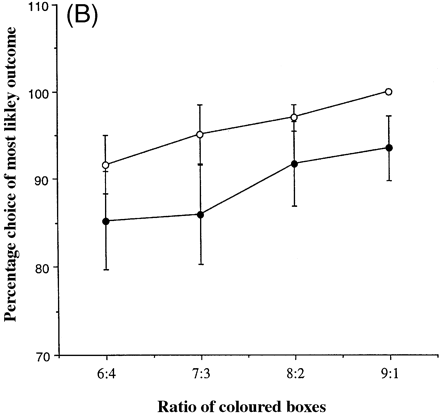
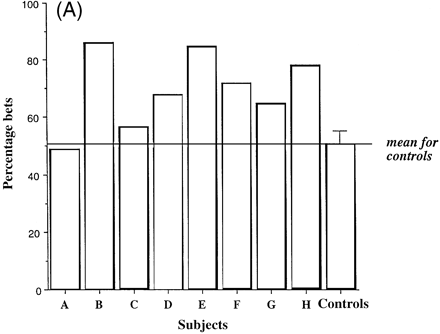
Figure 5A shows for the group of controls and for each of the eight patients the percentage of trials on which subjects chose the most likely of the two possible outcomes (i.e. the colour with the greatest number of boxes), as a function of the number of red and blue boxes. It is noteworthy that case C, with marked hypoperfusion of the frontal, frontoparietal and anterior temporal regions revealed by SPECT and with a MMSE score of 22, demonstrated marked impairment in the quality of decision-making. Figure 5B shows the same measure (the quality of decision-making) for the groups of patients and controls.
Averaging over group, the percentage of choices of the most likely outcome increased significantly at the more favourable compared with the less favourable ratios (ratio 6 : 4, 88.5%; ratio 7 : 3, 90.5%; ratio 8 : 2, 94.4%; ratio 9 : 1, 96.8%). Overall, there was no difference in the tendency to make optimal choices between the patients with fvFTD and controls [F(1,12) = 2.16, P = 0.167]. Analysis of the performance of the group of fvFTD patients and the controls revealed that neither age nor premorbid verbal IQ was a significant covariate of the probability that the most likely outcome was chosen (age: t = –0.770, P = 0.459; verbal IQ: t = 0.855, P = 0.412), and the results above were unaffected.
Risk adjustment.
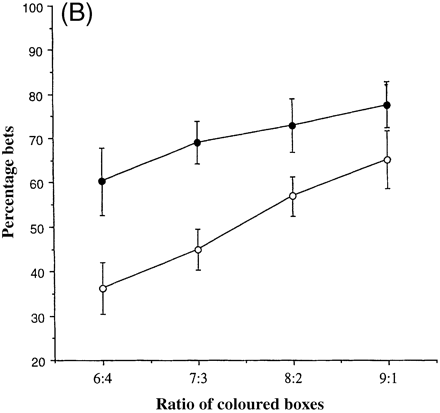
Figure 6A shows the percentage of their total points score that each of the eight subjects and the group of controls were prepared to risk in order to earn more points, as a function of the ratio of red to blue boxes. Most patients showed markedly increased bets compared with controls. Figure 6B shows the percentage of the points score for each of the groups of patients and controls. It is important to note that this analysis is restricted to those trials on which the subjects did in fact choose the most likely outcome, since it is only by comparing performance on such trials that we can assess subjects' sensitivity to the available opportunities to earn reward represented by different ratios.
Irrespective of group, subjects increased the percentage bet as a function of the ratio of red to blue boxes at a relatively constant rate (ratio 6 : 4, 48.3%; ratio 7 : 3, 56.9%; ratio 8 : 2, 65.0%; ratio 9 : 1, 71.4%). The patients with fvFTD bet more points at each ratio than the controls (patients: ratio 6 : 4, 60.3%; ratio 7 : 3, 69.0%; ratio 8 : 2, 73.0%; ratio 9 : 1, 77.6%; controls: ratio 6 : 4 36.3%; ratio 7 : 3, 44.9%; ratio 8 : 2, 56.9%; ratio 9 : 1, 65.2%). There was therefore a highly significant group difference [F(1,12) = 9.01, P = 0.011]. All subjects placed larger bets in the descending compared with the ascending condition [F(1,12) = 5.51, P = 0.037].
In order to determine if the order of ascending and descending conditions affected the outcome, for example as a consequence of impulsive choices in the fvFTD group, we examined the group × condition interaction. This was not, in fact, significant [F(1,12) < 0.001, P = 0.982]. There was also no significant group × probability interaction [F(2,36) = 1.09, P = 0.344]. Analysis of the performance of the group of fvFTD patients and the controls revealed that neither age nor premorbid verbal IQ was a significant covariate of the bets (age: t = –0.319, P = 0.756; verbal IQ: t = –1.366, P = 0.202), and the results above were unaffected.
Discussion
In this study we examined the cognitive performance of patients in the relatively mild stages of fvFTD on a broad range of computerized tests of executive and visuospatial function that have not previously been used in the assessment of fvFTD patients. As predicted, the patients with fvFTD were selectively impaired in the decision-making paradigm and there was some evidence that there was an impairment in the reversal stages of the attentional set-shifting paradigm. It is also of considerable interest that, in the present study, these patients were unimpaired in their performance in the spatial span and spatial working memory tasks. In addition, although an executive impairment was suggested by the difference in the groups on the mean latency measure of the one-touch Tower of London task, the accuracy of planning performance assessed using the same task was found to be relatively unimpaired.
Deficits of working memory have previously been implicated in disorders affecting the prefrontal cortex (Goldman-Rakic, 1987); furthermore, deficits of planning, demonstrated using the Tower of London task, have been found to be consequent upon disorders affecting the frontal lobes (Shallice, 1982). The performance of the fvFTD patients reported here differs specifically from that of unilateral or bilateral frontal excision patients (Owen et al., 1990Owen et al., 1995) in a number of key areas. The frontal-lesioned patients exhibited significant impairments in error scores on the spatial working memory task and in their use of strategy (Owen et al., 1990). These patients were also impaired on the one-touch Tower of London task in the accuracy of performance (Owen et al., 1995). The working memory and planning deficits were not considered to be due to an impairment in retaining a sequence of spatial moves in short-term memory for a sufficient length of time, as the patients had an unimpaired spatial span.
In considering the precise nature of cognitive deficits demonstrated by patients with mild fvFTD, it is certainly useful to note that dissociations of function within areas of the prefrontal cortex have been reported previously, and may shed some light on the specific deficits that were observed in the patients with fvFTD in this study. Recently, Bechara and colleagues (Bechara et al., 1998) have reported a dissociation in performance on neuropsychological assessment in patients with frontal lobe lesions. In their study, all subjects with ventromedial lesions were impaired on their gambling task, whereas only a subset of these subjects—those with the most anteriorly placed lesions—were normal on the working memory tasks; however, subjects with right dorsolateral/high mesial lesions were impaired on the working memory tasks but not on the gambling task.
An explanation for why the patients with fvFTD in this study, with presumed frontal lobe pathology, are relatively unimpaired in some traditional `frontal' tasks (spatial working memory and planning assessed using the one-touch Tower of London) may be derived from what is known about the precise neural substrates underlying successful performance in these tasks, together with consideration of the possible site of pathology in fvFTD. First, in recent PET studies Owen et al. (1996) have investigated components of working memory using a close analogue of the spatial working memory task described in the present study. These studies indicated two major areas of activation in the dorsolateral and ventrolateral prefrontal cortex. Further data suggest that the ventrolateral area (Brodmann area 47) may play a role in the `passive' receipt of items for memory (as in spatial span performance), whereas the dorsolateral prefrontal cortex (Brodmann areas 46 and 9) is important for the `monitoring' role by which candidate sequences are compared with the goal sequence. Secondly, in an investigation by Baker and colleagues (Baker et al., 1996) using the same Tower of London task as used in this study, regional increases in cerebral blood flow were produced in a distributed cortical network that included the superior occipitoparietal cortex and three main zones in the frontal cortex: the premotor area, a band of activation including the dorsolateral prefrontal cortex bilaterally and the frontopolar cortex on the right. In the present study, one possible reason, therefore, for the relative lack of impairment in patients with fvFTD in the spatial span, spatial working memory and the one-touch Tower of London tasks is that the site of pathology in fvFTD involves predominantly the orbitofrontal or ventromedial part of the frontal lobes, which is not associated with altered regional cerebral blood flow in these tasks. The future use of volumetric structural MRI or other forms of neuroimaging may be able to establish, with superior spatial resolution compared with SPECT, whether the primary site of damage in mild fvFTD is the orbitofrontal or the ventromedial part of the frontal lobe. However, in early disease it is far from clear whether structural changes will be evident, thus necessitating a neuropsychological approach, as adopted here.
Visual discrimination learning/attentional set-shifting
In the present study, we report evidence for an impairment in the attentional set-shifting (intradimensional/extradimensional) paradigm in patients with fvFTD that is relatively specific to the reversal stages demonstrated using only eight patients. Notably, the fvFTD patients were unimpaired compared with controls with respect to errors at the extradimensional shift stage, a stage which had previously been shown to pose particular difficulties for patients with frontal lobe excisions (Owen et al., 1991), medicated patients with severe Parkinson's disease (Owen et al., 1992) and patients in the preclinical and early clinical stages of Huntington's disease (Lawrence et al., 1998Lawrence et al., 1996). The extradimensional shift stage is akin to the category shift in the WCST, and the lack of impairment concurs with reports of normal performance on the WCST by patients with non-progressive frontal lobe damage associated with sparing of the dorsolateral cortex (Drewe, 1974; Stuss and Benson, 1984; Eslinger and Damasio, 1985). A recent PET imaging study using an adapted version of the attentional set-shifting paradigm showed that, compared with the intradimensional shift scans, the extradimensional shift activated the frontal pole on the left side and areas 9/46 on the right side, whereas reversal learning engaged neural circuitry associated with the ventromedial prefrontal cortex (Rogers et al., 1999b). In relation to this and the non-human primate work by Dias and colleagues (Dias et al., 1996, 1997), the specific reversal learning deficit of patients with fvFTD early in the course of the disease is consistent with the hypothesis that the pathology predominantly affects the orbitofrontal cortex or its connections rather than the dorsolateral, prefrontal cortex.
The orbitofrontal cortex is considered to be especially involved with stimulus–reward learning, and may participate in a distributed network comprising the temporal cortex, amygdala, ventral striatum and mediodorsal thalamus. Reasons for the impairment in the reversal stages may include a loss of inhibitory control in response to the previously rewarded response, a failure to learn new stimulus–reinforcement associations, or an interaction between the two. A classic study (Jones and Mishkin, 1972) showed that, for both object reversal and spatial reversal, lesions of the orbitofrontal cortex caused the animals to perseverate in the formerly relevant response. Once they had overcome this tendency they were able to learn the new stimulus–reward association. The study by Dias and colleagues (Dias et al., 1996) also emphasized the loss of inhibitory control following orbitofrontal lesions in affective shifting. The low number of perseverative errors made by the fvFTD patients in this study in the reversal stages suggests that the increased number of errors in the reversal stages made by the patients may arise from deficits in the formation of new stimulus–reinforcement associations. In relation to this, it is interesting to note that damage to the orbitofrontal cortex has been found to produce disturbances in learning stimulus–reinforcement associations; in a study by Iversen and Mishkin (Iversen and Mishkin, 1970), medial orbital-lesioned monkeys took many more trials to learn to respond to the previously unrewarded stimulus than controls, but were no slower than controls to inhibit their responding to the previously rewarded stimulus. This certainly concurs with more recent studies that demonstrate that lesions of the medial orbital region do disrupt the ability of monkeys to learn stimulus–reward associations (Baylis and Gaffan, 1991), and therefore impairments in learning about the affective properties of stimuli may be dissociable from deficits in inhibitory control, and may themselves contribute to some of the emotional impairments associated with damage to the orbitofrontal cortex.
This concept is supported by examination of the function of the orbitofrontal cortex at the neuronal level. There is major visual input to many neurons in the orbitofrontal cortex, and many neurons represent the reinforcement association of visual stimuli (Rolls et al., 1996a). In one study in which the primary reinforcer used was taste, many neurons were found to show visual–taste reversal in one trial or a very few trials (Thorpe et al., 1983). In addition to these neurons, which encode the reward association of visual stimuli, other neurons in the orbitofrontal cortex were found that detected non-reward. These responded, for example, when an expected reward was not obtained when a visual discrimination task was reversed. Therefore, it would seem that the orbitofrontal cortex does enable the rapid reversal of behaviour by stimulus–reinforcement association relearning when the association of stimuli with reinforcers is altered.
The results presented here appear consistent with the inhibition hypothesis proposed by Plaisted and Sahakian (Plaisted and Sahakian, 1997), as the inability to form stimulus–reinforcement associations when contingencies change would be sufficient to prevent these patients from responding to future social situations and to be able to form coherent action sequences appropriate to long-term goals. It might appear that the results are also broadly consistent with the position of Rolls and colleagues (Rolls et al., 1994) in that social–emotional changes may be due to a failure to alter behaviour as a consequence of difficulties in the remapping of stimulus–reinforcement associations. It is, however, very clear that patients with severe reversal deficits do not necessarily exhibit clinically disinhibited behaviour. At the single-case level, for example, case G scored only three errors in total in the reversal stages, but did demonstrate clinically marked behavioural disinhibition during cognitive testing.
There is converging evidence that the deficits in reversal learning may be related to abnormalities in the functioning of serotonergic neurotransmitter systems in the prefrontal cortex. Normal subjects with tryptophan depletion induced by a low-tryptophan drink, when required to perform a simple rule-reversal in the presence of an irrelevant dimension (the reversal of compound discrimination stage), needed more trials to learn this rule compared with subjects receiving placebo (Park et al., 1994). This is similar to the performance of the fvFTD patients in this study using the same paradigm. Low tryptophan intake is known to reduce the supply of tryptophan to the brain by lowering the plasma tryptophan level and by restricting the entry of plasma tryptophan into the brain through competition from other amino acids for transport across the blood–brain barrier (Young et al., 1985). Nomura (Nomura, 1992) has reported an analogous effect in rodents: rats given a low-tryptophan diet showed impaired learning in an operant-type discrimination learning paradigm. There is currently some neurochemical evidence, derived from post-mortem studies, to suggest that there exist profound postsynaptic serotonergic defects in patients with FTD in comparison with controls (Sparks and Markesbery, 1991). It is noteworthy that Sparks and Markesbery (Sparks and Markesbery, 1991) reported in their study reduced total serotonin receptor binding to samples of tissue from autopsy-proven cases of FTD from the frontal pole (Brodmann areas 10 and 11), temporal pole (Brodmann area 38) and the hypothalamus, but not in the nucleus basalis of Meynert, in comparison with controls.
It is nonetheless important to consider that the deficits in reversal learning may also reflect pathology affecting the caudate nucleus. Divac and colleagues (Divac et al., 1967) originally showed in primates that a lesion involving the ventrocaudal neostriatum impaired visual discrimination reversal. The hypothesis has therefore emerged that the ventrocaudal striatum is a critical link in the stimulus–response or habit-learning circuit (Mishkin et al., 1984). Neurones which reflect the responses of orbitofrontal neurons are found in the ventral head of the caudate nucleus and the ventral striatum, which receive input from the orbitofrontal cortex (Williams et al., 1993). The existence of severe degeneration of the caudate in frontotemporal degenerative dementia has long been recognized (e.g. Filley et al., 1994); the possibility of a minor degree of atrophy in the caudate nucleus in some of the patients who took part in this study cannot be completely excluded.
Decision-making
Previously, Damasio (Damasio, 1996) has argued that patients with ventromedial lesions in his paradigm perform badly because they may have difficulties in their ability `to sense', overtly or not, which decks are risky and which are profitable. That paradigm has not, however, been able to distinguish between the principal factors determining their risk-taking behaviour. Using a different decision-making paradigm, we have shown that the patients with fvFTD were different from age- and IQ-matched controls in that they were willing to bet a much higher proportion of their accumulated reward at all ratios. Furthermore, they showed no significant difference from controls in their tendency to choose the most likely outcome. Therefore, to summarize, although patients with fvFTD may make accurate probability judgements, they are unable to adjust the levels of their bets accordingly. As they are not merely impulsive in making their decisions, they appear to be true risk-takers.
There are broad similarities in the performance of the patients with fvFTD and the patients with ventromedial lesions studied by Damasio and colleagues (Bechara et al., 1994, 1998). Both groups of patients make abnormal decisions that are no longer personally advantageous, and in particular have difficulty planning their future over immediate, medium and long ranges. Furthermore, Bechara (Bechara et al., 1998) has argued that failure of inhibitory control is not a primary reason for the poor performance of subjects with ventromedial lesions on their gambling task. This argument was based partly on the observation that, in their task, subjects switch decks whenever they receive punishment, just as normal controls do, but they return more often to the decks that yield high immediate reward (the bad decks). The switch away from the bad deck immediately after punishment did not, arguably, indicate a lack of inhibition of the natural tendency to switch decks after a negative outcome (Bechara et al., 1994). There is little evidence that defective inhibitory control is the primary reason for the poor performance of the fvFTD patients in the decision-making task presented here, as the patients exhibited much longer deliberation times, did not consistently choose early bets in both the ascending and the descending sequence, and generally appeared able to adjust their bet successfully as a function of the ratio of coloured boxes (and hence the information encoded by them about the likelihood of a rewarded response), albeit at a rather inflated level.
In general, neither the group described by Damasio nor our patient group were able to adjust their behaviour successfully appropriately to the opportunities offered by the task. One explanation that has been proposed for the poor performance in the gambling task of Damasio's patients with ventromedial lesions is that there is a failure to anticipate future outcomes (Bechara et al., 1997). Indeed, there is some evidence from a recent electrophysiological study to support the hypothesis that the orbitofrontal cortex is involved in encoding information that is used to guide goal-directed behaviour (Schoenbaum et al., 1998). In that study, the activity of neurons in the rat orbitofrontal cortex and basolateral amygdala was recorded during instrumental learning in an olfactory discrimination task. Neurones in both these regions fired selectively during the anticipation of rewarding or aversive outcomes; this emerged early in training before the rats had learned reliably to avoid the aversive outcome. Schoenbaum and colleagues speculated that expectations about the occurrence of future events might be useful in forming associations that represent accurate predictive information, and that experience was required to determine whether anticipated outcomes were matched by actual outcomes. Within this conceptual framework, one possible reason for the poor performance of the patients with fvFTD in the decision-making task is that there exists a failure to anticipate expected outcomes, as may be the case for patients with ventromedial lesions, as a consequence of the pathology affecting the ventromedial (or orbitofrontal) cortex.
In their performance in the decision-making task, it is particularly noteworthy that the patients with fvFTD are strikingly similar to patients with orbitofrontal prefrontal cortex lesions, in that both groups demonstrate much higher deliberation times associated with their decisions (Rogers et al., 1999a). This behavioural difference is manifest in situations requiring choice between competing courses of action or response. It has been noted previously that similar increases in deliberation times in the decision-making task may be produced in those normal volunteers with acutely reduced tryptophan and reduced 5-hydroxytryptamine function who demonstrate the most severe deficits in decision-making. Therefore, in addition to the reversal learning deficits following a low-tryptophan drink reported by Park and colleagues (Park et al., 1994) and described above, as well as neuropathological post-mortem observations (e.g. Sparks and Markesbery, 1991), the results from the decision-making task provide some support for the hypothesis that abnormalities in the serotonergic system contribute to the neuropsychological profile of patients with fvFTD.
Putative neural substrates underlying the cognitive profile in fvFTD
One important consideration in concluding that patients with mild fvFTD demonstrate a relative lack of deficits on tests sensitive to lateral prefrontal function is that these tests may exhibit different sensitivity to those used to examine ventromedial or orbitofrontal function. Whilst this may be a concern, we feel that it is unlikely to be the case for a number of reasons. First, the one-touch Tower of London was shown to be very sensitive to the effects of frontal lobe lesions (Owen et al., 1995). Power analysis of those data suggests that it would have been possible to obtain significant deficits in that study using the same number of patients as used here. Secondly, the spatial working memory task and the Tower of London tasks have previously been found to be sensitive to cognitive deficits in patients with Huntington's disease (Lange et al., 1995; Lawrence et al., 1998 Lawrence et al., 1996), medicated patients with Parkinson's disease (Owen et al., 1992) and patients with multiple system atrophy and Steele–Richardson–Olsewski syndrome (Robbins et al., 1994b). Thirdly, recent results from our laboratory (L. H. A. Watkins, R. D. Rogers, B. J. Sahakian, A. E. Rosser and T. W. Robbins, unpublished observations) have demonstrated that patients with Huntington's disease, whilst impaired on the one-touch Tower of London task, are unimpaired on the decision-making task. When taken together with results from the present study, such a double dissociation arguably provides strong evidence against a difference in task sensitivity (Kinsbourne, 1971). Indeed, the deficits seen in different patient groups are consistent with what is known about the neural substrates underlying successful performance in these tasks in normal healthy volunteers discussed earlier (Baker et al., 1996; Owen et al., 1996). Finally, in relation to the performance of patients with fvFTD on the attentional set-shifting task, as the extradimensional shift is known to be more difficult than reversal shifting for normal subjects (Roberts et al., 1988), it is unlikely that the deficit in reversal shifting described here (or indeed the aforementioned deficit in extradimensional shifting in other patient groups) is simply due to differences in task sensitivity.
Overall, the finding that patients with mild fvFTD demonstrate deficits in tasks sensitive to orbitofrontal or ventromedial function, rather than function of more lateral areas, may be consistent with a number of other different converging lines of evidence. In their formulation of the neuropathological criteria for FTD, the Lund and Manchester groups (Lund and Manchester Groups, 1994) described the distribution of gross changes as typically being predominant in the frontal and anterior temporal lobes, adding that, in two of the three subtypes (frontal lobe degeneration and motor neuron disease type), there is usually no gross atrophy of the striatum, amygdala or hippocampus, in contrast to Pick disease type, where asymmetry and striatal atrophy appear to be common. At the microscopic level, they noted that changes in FTD were most notable in the frontal convexity cortex, sometimes within the orbitofrontal cortex. Various studies have recognized that the anterior and medial temporal areas and the orbitofrontal cortex show the most severe atrophic changes (Sanders et al., 1939; Cummings and Duchen, 1981; Frisoni et al., 1996); post-mortem studies of single cases have often revealed focal atrophy within the orbitofrontal region of the frontal lobe and medial temporal structures (see, for example, a case reported by Hodges and Gurd, 1994).
The neuropsychological findings described in this study are further supported by neuroimaging findings regarding the specificity of changes in cerebral blood flow in patients with frontal lobe dementia. In one HMPAO-SPECT study (Starkstein et al., 1994), blood flow was found to be significantly lower in the frontal lobes, the anterior temporal cortex and the basal ganglia in patients with frontal lobe dementia compared with patients with dementia of the Alzheimer type. Within the frontal lobe dementia group, blood flow was significantly lower in the orbital than in the dorsal frontal cortex, and in the anterior temporal than in the dorsal temporal cortex. These findings concur with those from other neuroimaging studies using PET. For example, Kumar and colleagues (Kumar et al., 1990) demonstrated metabolic deficits bilaterally in the orbitofrontal, the anterior cingulate and the temporal lobes with relative sparing of the parietal lobes in three patients with marked behavioural changes including sexual disinhibition, irritability, laughing bursts and loud speech. It is possible that a number of the various clinical symptoms of patients with fvFTD may be the consequence of a specific disruption of the frontostriatal circuitry involving the ventromedial (or orbitofrontal) prefrontal cortex (for a review, see Masterman and Cummings, 1997). An important aspect of future research will be to specify the relative contributions of areas other than the prefrontal cortex—with which the prefrontal cortex may indeed share connectivity—to the development of cognitive and behavioural symptomatologies as demonstrated in this study. It is known that in a few cases focal lesions to non-frontal areas may produce a `frontal-like' syndrome (e.g. Strub, 1989; Petty et al., 1996).
As expected for patients with pathology localized predominantly to the anterior cortical regions, the patients with fvFTD in this study were unimpaired in accuracy measures on tests of mnemonic function (pattern and spatial recognition memory). Performance on the pattern-recognition task has been shown to be impaired in patients with temporal lobe excisions and amygdalohippocampectomy, whilst the spatial recognition task is sensitive to frontal lobe excisions (Owen et al., 1995). The recognition tasks are also sensitive to the cognitive deficits seen in probable dementia of the Alzheimer type, mild and moderate patients being equivalently impaired on pattern and spatial recognition (Sahakian et al., 1988). The increased latencies on these tasks in our patient group may indicate some impaired ability to identify and retrieve the appropriate memory trace. These results are consistent with the general lack of mnemonic deficits in such background neuropsychological tests as the Logical Memory subtest used to detect posterior cortical involvement (Table 2). These patients with mild fvFTD were also found to have relatively preserved language and visuospatial function in background neuropsychological assessment, consistent with previous findings in the literature (e.g. Gustafson et al., 1992). Finally, it is interesting to note that, out of the eight patients included in this study, only one had an MMSE score (22) below the cut-off score for dementia (24). This supports the view that this tool is generally inadequate for the screening of fvFTD (e.g. Gregory and Hodges, 1996).
Conclusion
We have found that patients with fvFTD in the relatively mild stages of disease demonstrate cognitive deficits not usually identified using traditional neuropsychological testing. Specifically, these patients exhibited more marked impairments in the reversal stages of the attentional set-shifting paradigm and also in risk-taking behaviour in the decision-making paradigm. These neuropsychological deficits may be interpreted as a consequence of pathology affecting a distinct part of the prefrontal cortex (the orbitofrontal or ventromedial cortex), and appear to be consistent with current evidence from functional neuroimaging and post-mortem neuropathological studies. The understanding of this condition is of particular relevance to the development of better methods of early diagnosis and assessment of efficacious therapeutic interventions. It may also, in the longer term, be helpful for the development of a strategy for the rehabilitation of patients with fvFTD. Crucial to this, there is a clear clinical need for the development of further neuropsychological tasks to provide sensitive measures of orbitofrontal or ventromedial prefrontal lobe dysfunction.
Criteria for the diagnosis of fvFTD
| I. Presentation with an insidious and progressive disorder of personality and behaviour of at least 6 months' duration characterized by at least five of the following features: loss of insight, disinhibition, restlessness, distractibility, emotional lability, reduced empathy or unconcern for others, lack of foresight and planning, impulsivity, social withdrawal, apathy or lack of spontaneity, poor self-care, reduced verbal output, verbal stereotypes or echolalia, perseveration (verbal or motor),features of the Klüver–Bucy syndrome (gluttony, sexual hyperactivity). |
| II. Historical evidence of preserved memory for day-to-day events. |
| III. Neuropsychological evidence of disproportionate frontal dysfunction. |
| IV. Absence of history of significant head injury, stroke, score >4 on the Hachinski scale, alcohol abuse, diagnosis of Parkinsonism or other movement disorder. |
| V. Psychiatric phenomena may be present. |
| I. Presentation with an insidious and progressive disorder of personality and behaviour of at least 6 months' duration characterized by at least five of the following features: loss of insight, disinhibition, restlessness, distractibility, emotional lability, reduced empathy or unconcern for others, lack of foresight and planning, impulsivity, social withdrawal, apathy or lack of spontaneity, poor self-care, reduced verbal output, verbal stereotypes or echolalia, perseveration (verbal or motor),features of the Klüver–Bucy syndrome (gluttony, sexual hyperactivity). |
| II. Historical evidence of preserved memory for day-to-day events. |
| III. Neuropsychological evidence of disproportionate frontal dysfunction. |
| IV. Absence of history of significant head injury, stroke, score >4 on the Hachinski scale, alcohol abuse, diagnosis of Parkinsonism or other movement disorder. |
| V. Psychiatric phenomena may be present. |
Criteria for the diagnosis of fvFTD
| I. Presentation with an insidious and progressive disorder of personality and behaviour of at least 6 months' duration characterized by at least five of the following features: loss of insight, disinhibition, restlessness, distractibility, emotional lability, reduced empathy or unconcern for others, lack of foresight and planning, impulsivity, social withdrawal, apathy or lack of spontaneity, poor self-care, reduced verbal output, verbal stereotypes or echolalia, perseveration (verbal or motor),features of the Klüver–Bucy syndrome (gluttony, sexual hyperactivity). |
| II. Historical evidence of preserved memory for day-to-day events. |
| III. Neuropsychological evidence of disproportionate frontal dysfunction. |
| IV. Absence of history of significant head injury, stroke, score >4 on the Hachinski scale, alcohol abuse, diagnosis of Parkinsonism or other movement disorder. |
| V. Psychiatric phenomena may be present. |
| I. Presentation with an insidious and progressive disorder of personality and behaviour of at least 6 months' duration characterized by at least five of the following features: loss of insight, disinhibition, restlessness, distractibility, emotional lability, reduced empathy or unconcern for others, lack of foresight and planning, impulsivity, social withdrawal, apathy or lack of spontaneity, poor self-care, reduced verbal output, verbal stereotypes or echolalia, perseveration (verbal or motor),features of the Klüver–Bucy syndrome (gluttony, sexual hyperactivity). |
| II. Historical evidence of preserved memory for day-to-day events. |
| III. Neuropsychological evidence of disproportionate frontal dysfunction. |
| IV. Absence of history of significant head injury, stroke, score >4 on the Hachinski scale, alcohol abuse, diagnosis of Parkinsonism or other movement disorder. |
| V. Psychiatric phenomena may be present. |
Performance of the patients in the study on a battery of background neuropsychological tests
| . | FTD patients . | Controls . | Comparison . |
|---|---|---|---|
| Data shown are mean (standard deviation) values. Controls were taken from the MRC Cognition and Brain Sciences Unit control panel, and were matched on the basis of age and verbal IQ estimated by the NART. Controls = control subjects. Forward and reverse memory span from the Wechsler Memory Scale (WMS-R) (Wechsler, 1987); logical memory subtest from the WMS-R (Wechsler, 1987);TROG = test for the reception of grammar (Bishop, 1983); VOSP = Visual Object and Space Perception Battery (Warrington and James, 1991).*5% cut-off scores for subjects on the VOSP incomplete letters, silhouettes, object decision, position discrimination, number location and cube analysis tests are 17, 16, 15, 18, 7 and 6 (age <50 years) and 16, 15, 14, 18, 7 and 6 (age ≥50 years), respectively. | |||
| NART errors | 16.3 (10.3) | 13.1 (7.18) | P > 0.05 |
| Memory span (forwards) | 6.38 (1.30) | 6.63 (1.19) | P > 0.05 |
| Memory span (backwards) | 5.13 (1.36) | 4.88 (1.13) | P > 0.05 |
| Logical memory (immediate/24) | 11.9 (3.66) | 13.0 (4.57) | P > 0.05 |
| Logical memory (delayed/24) | 7.90 (2.72) | 9.55 (2.43) | P > 0.05 |
| TROG (number correct/80) | 76.9 (2.61) | 78.7 (2.00) | P > 0.05 |
| TROG (blocks correct/20) | 18.0 (1.91) | 19.4 (0.79) | P > 0.05 |
| VOSP: object (incomplete letters/20) | 18.8 (1.33) | (all patients above cut-off*) | |
| VOSP: object (silhouettes/30) | 19.7 (5.13) | (all patients above cut-off*) | |
| VOSP: object (object decision/20) | 18.5 (1.64) | (all patients above cut-off*) | |
| VOSP: space (position discrimination /20) | 18.7 (1.03) | (all patients above cut-off, except one*) | |
| VOSP: space (number location/10) | 8.17 (1.94) | (all patients above cut-off, except one*) | |
| VOSP: space (cube analysis/10) | 9.33 (0.82) | (all patients above cut-off*) | |
| . | FTD patients . | Controls . | Comparison . |
|---|---|---|---|
| Data shown are mean (standard deviation) values. Controls were taken from the MRC Cognition and Brain Sciences Unit control panel, and were matched on the basis of age and verbal IQ estimated by the NART. Controls = control subjects. Forward and reverse memory span from the Wechsler Memory Scale (WMS-R) (Wechsler, 1987); logical memory subtest from the WMS-R (Wechsler, 1987);TROG = test for the reception of grammar (Bishop, 1983); VOSP = Visual Object and Space Perception Battery (Warrington and James, 1991).*5% cut-off scores for subjects on the VOSP incomplete letters, silhouettes, object decision, position discrimination, number location and cube analysis tests are 17, 16, 15, 18, 7 and 6 (age <50 years) and 16, 15, 14, 18, 7 and 6 (age ≥50 years), respectively. | |||
| NART errors | 16.3 (10.3) | 13.1 (7.18) | P > 0.05 |
| Memory span (forwards) | 6.38 (1.30) | 6.63 (1.19) | P > 0.05 |
| Memory span (backwards) | 5.13 (1.36) | 4.88 (1.13) | P > 0.05 |
| Logical memory (immediate/24) | 11.9 (3.66) | 13.0 (4.57) | P > 0.05 |
| Logical memory (delayed/24) | 7.90 (2.72) | 9.55 (2.43) | P > 0.05 |
| TROG (number correct/80) | 76.9 (2.61) | 78.7 (2.00) | P > 0.05 |
| TROG (blocks correct/20) | 18.0 (1.91) | 19.4 (0.79) | P > 0.05 |
| VOSP: object (incomplete letters/20) | 18.8 (1.33) | (all patients above cut-off*) | |
| VOSP: object (silhouettes/30) | 19.7 (5.13) | (all patients above cut-off*) | |
| VOSP: object (object decision/20) | 18.5 (1.64) | (all patients above cut-off*) | |
| VOSP: space (position discrimination /20) | 18.7 (1.03) | (all patients above cut-off, except one*) | |
| VOSP: space (number location/10) | 8.17 (1.94) | (all patients above cut-off, except one*) | |
| VOSP: space (cube analysis/10) | 9.33 (0.82) | (all patients above cut-off*) | |
Performance of the patients in the study on a battery of background neuropsychological tests
| . | FTD patients . | Controls . | Comparison . |
|---|---|---|---|
| Data shown are mean (standard deviation) values. Controls were taken from the MRC Cognition and Brain Sciences Unit control panel, and were matched on the basis of age and verbal IQ estimated by the NART. Controls = control subjects. Forward and reverse memory span from the Wechsler Memory Scale (WMS-R) (Wechsler, 1987); logical memory subtest from the WMS-R (Wechsler, 1987);TROG = test for the reception of grammar (Bishop, 1983); VOSP = Visual Object and Space Perception Battery (Warrington and James, 1991).*5% cut-off scores for subjects on the VOSP incomplete letters, silhouettes, object decision, position discrimination, number location and cube analysis tests are 17, 16, 15, 18, 7 and 6 (age <50 years) and 16, 15, 14, 18, 7 and 6 (age ≥50 years), respectively. | |||
| NART errors | 16.3 (10.3) | 13.1 (7.18) | P > 0.05 |
| Memory span (forwards) | 6.38 (1.30) | 6.63 (1.19) | P > 0.05 |
| Memory span (backwards) | 5.13 (1.36) | 4.88 (1.13) | P > 0.05 |
| Logical memory (immediate/24) | 11.9 (3.66) | 13.0 (4.57) | P > 0.05 |
| Logical memory (delayed/24) | 7.90 (2.72) | 9.55 (2.43) | P > 0.05 |
| TROG (number correct/80) | 76.9 (2.61) | 78.7 (2.00) | P > 0.05 |
| TROG (blocks correct/20) | 18.0 (1.91) | 19.4 (0.79) | P > 0.05 |
| VOSP: object (incomplete letters/20) | 18.8 (1.33) | (all patients above cut-off*) | |
| VOSP: object (silhouettes/30) | 19.7 (5.13) | (all patients above cut-off*) | |
| VOSP: object (object decision/20) | 18.5 (1.64) | (all patients above cut-off*) | |
| VOSP: space (position discrimination /20) | 18.7 (1.03) | (all patients above cut-off, except one*) | |
| VOSP: space (number location/10) | 8.17 (1.94) | (all patients above cut-off, except one*) | |
| VOSP: space (cube analysis/10) | 9.33 (0.82) | (all patients above cut-off*) | |
| . | FTD patients . | Controls . | Comparison . |
|---|---|---|---|
| Data shown are mean (standard deviation) values. Controls were taken from the MRC Cognition and Brain Sciences Unit control panel, and were matched on the basis of age and verbal IQ estimated by the NART. Controls = control subjects. Forward and reverse memory span from the Wechsler Memory Scale (WMS-R) (Wechsler, 1987); logical memory subtest from the WMS-R (Wechsler, 1987);TROG = test for the reception of grammar (Bishop, 1983); VOSP = Visual Object and Space Perception Battery (Warrington and James, 1991).*5% cut-off scores for subjects on the VOSP incomplete letters, silhouettes, object decision, position discrimination, number location and cube analysis tests are 17, 16, 15, 18, 7 and 6 (age <50 years) and 16, 15, 14, 18, 7 and 6 (age ≥50 years), respectively. | |||
| NART errors | 16.3 (10.3) | 13.1 (7.18) | P > 0.05 |
| Memory span (forwards) | 6.38 (1.30) | 6.63 (1.19) | P > 0.05 |
| Memory span (backwards) | 5.13 (1.36) | 4.88 (1.13) | P > 0.05 |
| Logical memory (immediate/24) | 11.9 (3.66) | 13.0 (4.57) | P > 0.05 |
| Logical memory (delayed/24) | 7.90 (2.72) | 9.55 (2.43) | P > 0.05 |
| TROG (number correct/80) | 76.9 (2.61) | 78.7 (2.00) | P > 0.05 |
| TROG (blocks correct/20) | 18.0 (1.91) | 19.4 (0.79) | P > 0.05 |
| VOSP: object (incomplete letters/20) | 18.8 (1.33) | (all patients above cut-off*) | |
| VOSP: object (silhouettes/30) | 19.7 (5.13) | (all patients above cut-off*) | |
| VOSP: object (object decision/20) | 18.5 (1.64) | (all patients above cut-off*) | |
| VOSP: space (position discrimination /20) | 18.7 (1.03) | (all patients above cut-off, except one*) | |
| VOSP: space (number location/10) | 8.17 (1.94) | (all patients above cut-off, except one*) | |
| VOSP: space (cube analysis/10) | 9.33 (0.82) | (all patients above cut-off*) | |
Subject characteristics
| . | FvFTD patients . | Control subjects . |
|---|---|---|
| Data shown are mean (standard deviation) values. | ||
| FSIQ = predicted full-scale IQ; VIQ = predicted verbal IQ; PIQ = predicted performance IQ. | ||
| Age (years) | ||
| Mean | 57.9 (9.60) | 58.1 (9.60) |
| Range | 43–73 | 44–73 |
| Sex (M/F) | 8/0 | 8/0 |
| FSIQ | 114 (8.38) | 116 (6.28) |
| VIQ | 114 (9.33) | 116 (6.97) |
| PIQ | 113 (6.64) | 115 (5.01) |
| MMSE score | ||
| Mean | 27.8 (2.49) | 29.9 (0.35) |
| Range | 22–30 | 29–30 |
| . | FvFTD patients . | Control subjects . |
|---|---|---|
| Data shown are mean (standard deviation) values. | ||
| FSIQ = predicted full-scale IQ; VIQ = predicted verbal IQ; PIQ = predicted performance IQ. | ||
| Age (years) | ||
| Mean | 57.9 (9.60) | 58.1 (9.60) |
| Range | 43–73 | 44–73 |
| Sex (M/F) | 8/0 | 8/0 |
| FSIQ | 114 (8.38) | 116 (6.28) |
| VIQ | 114 (9.33) | 116 (6.97) |
| PIQ | 113 (6.64) | 115 (5.01) |
| MMSE score | ||
| Mean | 27.8 (2.49) | 29.9 (0.35) |
| Range | 22–30 | 29–30 |
Subject characteristics
| . | FvFTD patients . | Control subjects . |
|---|---|---|
| Data shown are mean (standard deviation) values. | ||
| FSIQ = predicted full-scale IQ; VIQ = predicted verbal IQ; PIQ = predicted performance IQ. | ||
| Age (years) | ||
| Mean | 57.9 (9.60) | 58.1 (9.60) |
| Range | 43–73 | 44–73 |
| Sex (M/F) | 8/0 | 8/0 |
| FSIQ | 114 (8.38) | 116 (6.28) |
| VIQ | 114 (9.33) | 116 (6.97) |
| PIQ | 113 (6.64) | 115 (5.01) |
| MMSE score | ||
| Mean | 27.8 (2.49) | 29.9 (0.35) |
| Range | 22–30 | 29–30 |
| . | FvFTD patients . | Control subjects . |
|---|---|---|
| Data shown are mean (standard deviation) values. | ||
| FSIQ = predicted full-scale IQ; VIQ = predicted verbal IQ; PIQ = predicted performance IQ. | ||
| Age (years) | ||
| Mean | 57.9 (9.60) | 58.1 (9.60) |
| Range | 43–73 | 44–73 |
| Sex (M/F) | 8/0 | 8/0 |
| FSIQ | 114 (8.38) | 116 (6.28) |
| VIQ | 114 (9.33) | 116 (6.97) |
| PIQ | 113 (6.64) | 115 (5.01) |
| MMSE score | ||
| Mean | 27.8 (2.49) | 29.9 (0.35) |
| Range | 22–30 | 29–30 |
Summary of clinical characteristics of the patients in the study
| Initial . | Age, sex . | Length of time since clinical diagnosis (years) . | Relevant aspects of family history . | MMSE . | Neuroimaging . |
|---|---|---|---|---|---|
| MMSE = Mini-Mental State Examination, M = male. | |||||
| A | 54, M | 7 | Maternal grandfather died by suicide; depression (maternal aunt) | 29 | SPECT (1991): minimally abnormal with a suggestion of diffuse patchy hypoperfusion. |
| SPECT (1995, 1998): bifrontal hypoperfusion. | |||||
| MRI (1995, 1998): progressive frontal lobe atrophy. | |||||
| B | 70, M | 1 | — | 28 | SPECT (1998): reduced perfusion in left frontal and temporal regions. |
| MRI (1998): marked frontal lobe atrophy. | |||||
| C | 58, M | 3 | — | 22 | SPECT (1995): dramatic extensive reductions in perfusion in the frontal and anterior temporal regions. |
| SPECT (1997): marked progression in the changes in the frontal lobes. | |||||
| MRI (1997): gross degree of frontal atrophy. | |||||
| D | 55, M | 5 | — | 29 | SPECT, CT, MRI (1993): normal. |
| SPECT (1997): reduced perfusion in both frontal cortices (in particular the inferior part and especially on the right). | |||||
| E | 53, M | 1 | — | 28 | CT (1996): marked frontal lobe atrophy. |
| SPECT (1997): marked reduction in perfusion bilaterally to the frontal lobes. | |||||
| F | 43, M | 7 | Paternal grandparents and uncle all died by suicide | 30 | CT (1991): selective frontal atrophy. |
| SPECT (1991): patchy reduced uptake of tracer. | |||||
| G | 73, M | 2 | Mother died at age 81 years, having possibly been suffering from dementia for the preceding 5 years | 27 | CT (1996): mild degree of cerebral atrophy. |
| MRI (1996): a degree of atrophy more prominent in the region of the anterior portion of the left temporal lobe and the frontal lobe bilaterally. | |||||
| H | 57, M | 1 | Father died of motor neuron disease (mother still alive and well) | 29 | MRI (1998): degree of global atrophy, with accentuation in the frontal and anterior temporal lobes, particularly on the left side. |
| Initial . | Age, sex . | Length of time since clinical diagnosis (years) . | Relevant aspects of family history . | MMSE . | Neuroimaging . |
|---|---|---|---|---|---|
| MMSE = Mini-Mental State Examination, M = male. | |||||
| A | 54, M | 7 | Maternal grandfather died by suicide; depression (maternal aunt) | 29 | SPECT (1991): minimally abnormal with a suggestion of diffuse patchy hypoperfusion. |
| SPECT (1995, 1998): bifrontal hypoperfusion. | |||||
| MRI (1995, 1998): progressive frontal lobe atrophy. | |||||
| B | 70, M | 1 | — | 28 | SPECT (1998): reduced perfusion in left frontal and temporal regions. |
| MRI (1998): marked frontal lobe atrophy. | |||||
| C | 58, M | 3 | — | 22 | SPECT (1995): dramatic extensive reductions in perfusion in the frontal and anterior temporal regions. |
| SPECT (1997): marked progression in the changes in the frontal lobes. | |||||
| MRI (1997): gross degree of frontal atrophy. | |||||
| D | 55, M | 5 | — | 29 | SPECT, CT, MRI (1993): normal. |
| SPECT (1997): reduced perfusion in both frontal cortices (in particular the inferior part and especially on the right). | |||||
| E | 53, M | 1 | — | 28 | CT (1996): marked frontal lobe atrophy. |
| SPECT (1997): marked reduction in perfusion bilaterally to the frontal lobes. | |||||
| F | 43, M | 7 | Paternal grandparents and uncle all died by suicide | 30 | CT (1991): selective frontal atrophy. |
| SPECT (1991): patchy reduced uptake of tracer. | |||||
| G | 73, M | 2 | Mother died at age 81 years, having possibly been suffering from dementia for the preceding 5 years | 27 | CT (1996): mild degree of cerebral atrophy. |
| MRI (1996): a degree of atrophy more prominent in the region of the anterior portion of the left temporal lobe and the frontal lobe bilaterally. | |||||
| H | 57, M | 1 | Father died of motor neuron disease (mother still alive and well) | 29 | MRI (1998): degree of global atrophy, with accentuation in the frontal and anterior temporal lobes, particularly on the left side. |
Summary of clinical characteristics of the patients in the study
| Initial . | Age, sex . | Length of time since clinical diagnosis (years) . | Relevant aspects of family history . | MMSE . | Neuroimaging . |
|---|---|---|---|---|---|
| MMSE = Mini-Mental State Examination, M = male. | |||||
| A | 54, M | 7 | Maternal grandfather died by suicide; depression (maternal aunt) | 29 | SPECT (1991): minimally abnormal with a suggestion of diffuse patchy hypoperfusion. |
| SPECT (1995, 1998): bifrontal hypoperfusion. | |||||
| MRI (1995, 1998): progressive frontal lobe atrophy. | |||||
| B | 70, M | 1 | — | 28 | SPECT (1998): reduced perfusion in left frontal and temporal regions. |
| MRI (1998): marked frontal lobe atrophy. | |||||
| C | 58, M | 3 | — | 22 | SPECT (1995): dramatic extensive reductions in perfusion in the frontal and anterior temporal regions. |
| SPECT (1997): marked progression in the changes in the frontal lobes. | |||||
| MRI (1997): gross degree of frontal atrophy. | |||||
| D | 55, M | 5 | — | 29 | SPECT, CT, MRI (1993): normal. |
| SPECT (1997): reduced perfusion in both frontal cortices (in particular the inferior part and especially on the right). | |||||
| E | 53, M | 1 | — | 28 | CT (1996): marked frontal lobe atrophy. |
| SPECT (1997): marked reduction in perfusion bilaterally to the frontal lobes. | |||||
| F | 43, M | 7 | Paternal grandparents and uncle all died by suicide | 30 | CT (1991): selective frontal atrophy. |
| SPECT (1991): patchy reduced uptake of tracer. | |||||
| G | 73, M | 2 | Mother died at age 81 years, having possibly been suffering from dementia for the preceding 5 years | 27 | CT (1996): mild degree of cerebral atrophy. |
| MRI (1996): a degree of atrophy more prominent in the region of the anterior portion of the left temporal lobe and the frontal lobe bilaterally. | |||||
| H | 57, M | 1 | Father died of motor neuron disease (mother still alive and well) | 29 | MRI (1998): degree of global atrophy, with accentuation in the frontal and anterior temporal lobes, particularly on the left side. |
| Initial . | Age, sex . | Length of time since clinical diagnosis (years) . | Relevant aspects of family history . | MMSE . | Neuroimaging . |
|---|---|---|---|---|---|
| MMSE = Mini-Mental State Examination, M = male. | |||||
| A | 54, M | 7 | Maternal grandfather died by suicide; depression (maternal aunt) | 29 | SPECT (1991): minimally abnormal with a suggestion of diffuse patchy hypoperfusion. |
| SPECT (1995, 1998): bifrontal hypoperfusion. | |||||
| MRI (1995, 1998): progressive frontal lobe atrophy. | |||||
| B | 70, M | 1 | — | 28 | SPECT (1998): reduced perfusion in left frontal and temporal regions. |
| MRI (1998): marked frontal lobe atrophy. | |||||
| C | 58, M | 3 | — | 22 | SPECT (1995): dramatic extensive reductions in perfusion in the frontal and anterior temporal regions. |
| SPECT (1997): marked progression in the changes in the frontal lobes. | |||||
| MRI (1997): gross degree of frontal atrophy. | |||||
| D | 55, M | 5 | — | 29 | SPECT, CT, MRI (1993): normal. |
| SPECT (1997): reduced perfusion in both frontal cortices (in particular the inferior part and especially on the right). | |||||
| E | 53, M | 1 | — | 28 | CT (1996): marked frontal lobe atrophy. |
| SPECT (1997): marked reduction in perfusion bilaterally to the frontal lobes. | |||||
| F | 43, M | 7 | Paternal grandparents and uncle all died by suicide | 30 | CT (1991): selective frontal atrophy. |
| SPECT (1991): patchy reduced uptake of tracer. | |||||
| G | 73, M | 2 | Mother died at age 81 years, having possibly been suffering from dementia for the preceding 5 years | 27 | CT (1996): mild degree of cerebral atrophy. |
| MRI (1996): a degree of atrophy more prominent in the region of the anterior portion of the left temporal lobe and the frontal lobe bilaterally. | |||||
| H | 57, M | 1 | Father died of motor neuron disease (mother still alive and well) | 29 | MRI (1998): degree of global atrophy, with accentuation in the frontal and anterior temporal lobes, particularly on the left side. |
Group comparisons of visuospatial memory tasks
| Group . | Pattern recognition . | Spatial recognition . | Spatial span . |
|---|---|---|---|
| Data shown are mean (standard deviation) values. | |||
| fvFTD patients | 77.6 (15.7) | 73.1 (16.2) | 4.88 (1.46) |
| Control subjects | 81.8 (7.70) | 81.9 (8.84) | 5.63 (0.92) |
| Group . | Pattern recognition . | Spatial recognition . | Spatial span . |
|---|---|---|---|
| Data shown are mean (standard deviation) values. | |||
| fvFTD patients | 77.6 (15.7) | 73.1 (16.2) | 4.88 (1.46) |
| Control subjects | 81.8 (7.70) | 81.9 (8.84) | 5.63 (0.92) |
Group comparisons of visuospatial memory tasks
| Group . | Pattern recognition . | Spatial recognition . | Spatial span . |
|---|---|---|---|
| Data shown are mean (standard deviation) values. | |||
| fvFTD patients | 77.6 (15.7) | 73.1 (16.2) | 4.88 (1.46) |
| Control subjects | 81.8 (7.70) | 81.9 (8.84) | 5.63 (0.92) |
| Group . | Pattern recognition . | Spatial recognition . | Spatial span . |
|---|---|---|---|
| Data shown are mean (standard deviation) values. | |||
| fvFTD patients | 77.6 (15.7) | 73.1 (16.2) | 4.88 (1.46) |
| Control subjects | 81.8 (7.70) | 81.9 (8.84) | 5.63 (0.92) |
Spatial working memory data
| Group . | BSE (four boxes) . | BSE (six boxes) . | BSE (eight boxes) . | Strategy score . |
|---|---|---|---|---|
| Data shown are mean (standard deviation) values. BSE = number of between-search errors. | ||||
| fvFTD | 2.75 (3.41) | 12.9 (9.89) | 26.6 (10.6) | 36.6 (4.78) |
| Control subjects | 1.13 (1.89) | 9.50 (9.17) | 22.0 (10.0) | 31.8 (6.23) |
| Group . | BSE (four boxes) . | BSE (six boxes) . | BSE (eight boxes) . | Strategy score . |
|---|---|---|---|---|
| Data shown are mean (standard deviation) values. BSE = number of between-search errors. | ||||
| fvFTD | 2.75 (3.41) | 12.9 (9.89) | 26.6 (10.6) | 36.6 (4.78) |
| Control subjects | 1.13 (1.89) | 9.50 (9.17) | 22.0 (10.0) | 31.8 (6.23) |
Spatial working memory data
| Group . | BSE (four boxes) . | BSE (six boxes) . | BSE (eight boxes) . | Strategy score . |
|---|---|---|---|---|
| Data shown are mean (standard deviation) values. BSE = number of between-search errors. | ||||
| fvFTD | 2.75 (3.41) | 12.9 (9.89) | 26.6 (10.6) | 36.6 (4.78) |
| Control subjects | 1.13 (1.89) | 9.50 (9.17) | 22.0 (10.0) | 31.8 (6.23) |
| Group . | BSE (four boxes) . | BSE (six boxes) . | BSE (eight boxes) . | Strategy score . |
|---|---|---|---|---|
| Data shown are mean (standard deviation) values. BSE = number of between-search errors. | ||||
| fvFTD | 2.75 (3.41) | 12.9 (9.89) | 26.6 (10.6) | 36.6 (4.78) |
| Control subjects | 1.13 (1.89) | 9.50 (9.17) | 22.0 (10.0) | 31.8 (6.23) |
Decision-making task. This figure shows a typical display from the decision-making task (see text for details). Note that the ratio of red to blue boxes (i.e. the relative probabilities of the two outcomes) changed from trial to trial.
Attentional set-shifting task: (A) errors; (B) total number of errors at reversal stages of compound discrimination and intradimensional and extradimensional shift (CD_R + ID_R + ED_R) and non-reversal stages (CD + ID + ED). Error bars represent 1 SEM. Solid circles = fvFTD; open circles = controls.
One-touch Tower of London task: (A) mean percentage correct; (B) mean latency. Error bars represent 1 SEM. Solid circles = fvFTD; open circles = controls.
Decision-making (speed) task. Deliberation times associated with deciding which colour of box is hiding the yellow token, as a function of the red and blue boxes (see text for details). (A) Individual characteristics in the form of a bar chart showing data for each of the patients and the average performance of the controls. (B) Group characteristics. Error bars represent 1 SEM. Solid circles = fvFTD; open circles = controls.
Decision-making (quality) task. Percentage choice of the most likely outcome as a function of red and blue boxes (see text for details). (A) Individual characteristics in the form of a bar chart showing data for each of the patients and the average performance of the controls. (B) Group characteristics. Error bars represent 1 SEM. Solid circles = fvFTD; open circles = controls.
Decision-making (risk adjustment) task. Percentage points risked in order to earn more points as a function of red and blue boxes (see text for details). (A) Individual characteristics in the form of a bar chart showing data for each of the patients and the average performance of the controls. (B) Group characteristics. Error bars represent 1 SEM. Solid circles = fvFTD; open circles = controls.
The authors wish to thank two anonymous referees for their helpful comments on a previous version of the manuscript, M. A. Mehta for statistical advice, S. Davies and N. Graham for assistance in compiling Table 2, and all those who took part in this study. This work was supported by a Programme Grant from the Wellcome Trust to T.W.R., B.J.S., Professor B. J. Everitt and Dr A. C. Roberts, a MRC Programme Grant to J.R.H., a MRC LINK grant to B.J.S., J.R.H., Dr J. Semple and T.W.R. and an MRC studentship to S.R.
References
Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing [see comments]. [Review].
Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. [Review].
Anderson SW, Damasio H, Jones RD, Tranel D. Wisconsin Card Sorting Test performance as a measure of frontal lobe damage.
Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, et al. Neural systems engaged by planning: a PET study of the Tower of London task.
Barbas H, Pandya DN. Pattern of connections in the prefrontal cortex in the rhesus monkey associated with cortical architecture. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. p. 35–58.
Baylis LL, Gaffan D. Amygdalectomy and ventromedial prefrontal ablation produce similar deficits on food choice and in simple object discrimination learning for an unseen reward.
Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex.
Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy [see comments].
Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex.
Brun A. Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology.
Corcoran, R, Upton, D. A role for the hippocampus in card sorting? [see comments].
Cummings JL, Duchen LW. The Klüver-Bucy syndrome in Pick's disease: clinical and pathologic correlations.
Damasio, AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. [Review].
Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and emotional shifts.
Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from `on-line' processing.
Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus.
Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Robbins RG, Robbins TW. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson's disease: evidence for a specific attentional dysfunction.
Drewe EA. The effect of type and area of brain lesion on the Wisconsin Card Sorting Test performance.
Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia.
Elfgren C, Passant U, Risberg J. Neuropsychological findings in frontal lobe dementia.
Elfgren C, Brun A, Gustafson L, Johansen A, Minthon L, Passant U, et al. Neuropsychological tests as discriminators between dementia of Alzheimer type and frontotemporal dementia.
Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR.
Filley CM, Kleinschmidt-De Masters BK, Gross KF. Non-Alzheimer fronto-temporal degenerative dementia. A neurobehavioral and pathologic study.
Folstein MF, Folstein SE, McHugh PR. `Mini-mental state': a practical method for grading the cognitive state of patients for the clinician.
Frisoni GB, Pizzolato G, Geroldi C, Rossato A, Bianchetti A, Trabucchi M. Dementia of the frontal type: neuropsychological and [99Tc]-HM-PAO SPECT features.
Frisoni GB, Beltramello A, Geroldi C, Weiss C, Bianchetti A, Trabucchi M. Brain atrophy in frontotemporal dementia.
Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behaviour by representational memory. In: Plum F, editor. Handbook of physiology, Sect 1, Vol. 5, Pt. 1. Bethesda (MD): American Physiological Society; 1987. p. 373–417.
Gregory CA, Hodges JR. Dementia of frontal type and the focal lobar atrophies.
Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer's disease. [Review].
Gregory CA, Orrell M, Sahakian BJ, Hodges JR. Can frontotemporal dementia and Alzheimer's disease be differentiated using a brief battery of tests?
Gregory CA, McKenna PJ, Hodges JR. Dementia of frontal type and simple schizophrenia: two sides of the same coin?
Gregory CA, Serra-Mestres J, Hodges JR. The early diagnosis of the frontal variant of frontotemporal dementia: how sensitive are standard neuroimaging and neuropsychological tests?
Gustafson L. Clinical picture of frontal lobe degeneration of non-Alzheimer type.
Gustafson L, Risberg J. Deceptions and delusions in Alzheimer's disease and frontal lobe dementia. In: Katona C, Levy R, editors. Delusions and hallucinations of old age. London: Gaskell; 1992.
Gustafson L, Brun A, Passant U. Frontal lobe degeneration of non-Alzheimer type. [Review].
Hodges JR, Gurd JM. Remote memory and lexical retrieval in a case of frontal Pick's disease [see comments].
Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study.
Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy.
Hodges JR, Patterson K, Ward R, Garrard P, Bak T, Perry R, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer's disease: a comparative neuropsychological study.
Hutton M, Lendon LC, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17.
Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity.
Jones B, Mishkin M. Limbic lesions and the problem of stimulus–reinforcement associations.
Joyce EM, Robbins TW. Frontal lobe function in Korsakoff and non-Korsakoff alcoholics: planning and spatial working memory.
Kertesz A. The quantification of behavior in frontotemporal dementia. In: Kertesz A, Munoz DG, editors. Pick's disease and Pick complex. New York: Wiley-Liss; 1998. p. 47–68.
Kinsbourne M. Cognitive deficit: experimental analysis. In: McGaugh JL, editor. Psychobiology. New York: Academic Press; 1971. p. 285–348.
Knopman DS, Mastri AR, Frey WH 2d, Sung JH, Rustan T. Dementia lacking in distinctive histologic features: a common non-Alzheimer degenerative dementia.
Kumar A, Schapiro MB, Haxby JV, Grady CL, Friedland RP. Cerebral metabolic and cognitive studies in dementia with frontal lobe behavioral features.
Lange KW, Sahakian BJ, Quinn NP, Marsden CD, Robbins TW. Comparison of executive and visuospatial memory function in Huntington's disease and dementia of Alzheimer type matched for degree of dementia.
Lawrence AD, Sahakian BJ, Hodges JR, Rosser AE, Lange KW, Robbins TW. Executive and mnemonic functions in early Huntington's disease.
Lawrence AD, Hodges JR, Rosser AE, Kershaw A, ffrench-Constant C, Rubinsztein DC, et al. Evidence for specific cognitive deficits in preclinical Huntington's disease.
Mann DM. Dementia of frontal type and dementias with subcortical gliosis. [Review].
Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. [Review].
Masterman DL, Cummings JL. Frontal–subcortical circuits: the anatomic basis of executive, social and motivated behaviors. [Review].
Miller LA. Impulsivity, risk-taking, and the ability to synthesize fragmented information after frontal lobectomy.
Miller BL, Cummings JL, Villanueva-Meyer J, Boone K, Mehringer CM, Lesser IM, et al. Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics [see comments].
Miller BL, Darby AL, Swartz JR, Yener GG, Mena I. Dietary changes, compulsions and sexual behaviour in frontotemporal degeneration.
Milner, B. Interhemispheric differences in the localization of psychological processes in man. [Review].
Mishkin M, Malamut B, Bachevalier J. Memories and habits: two neural systems. In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of learning and memory. New York: Guilford Press; 1984. p. 65–7.
Mowrer OH. On the dual nature of learning—a re-interpretation of `conditioning' and `problem solving'.
Neary D, Snowden J. Fronto-temporal dementia: nosology, neuropsychology, and neuropathology. [Review].
Neary D, Snowden JS, Bowen DM, Sims NR, Mann DM, Benton JS, et al. Neuropsychological syndromes in presenile dementia due to cerebral atrophy.
Neary D, Snowden JS, Northen B, Goulding P. Dementia of frontal lobe type.
Nomura M. Effects of bifemelane on discrimination learning of serotonergic-dysfunction rats.
Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats.
Orrell MW, Sahakian BJ, Bergmann K. Self-neglect and frontal lobe dysfunction.
Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man.
Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man.
Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, et al. Fronto-striatal cognitive deficits at different stages of Parkinson's disease.
Owen AM, Sahakian BJ, Hodges JR, Summers BA, Polkey CE, Robbins TW. Dopamine-dependent frontostriatal planning deficits in early Parkinson's disease.
Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man.
Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study.
Pachana NA, Boone KB, Miller BL, Cummings JL, Berman N. Comparison of neuropsychological functioning in Alzheimer's disease and frontotemporal dementia.
Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, et al. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory.
Pasquier F, Lebert F, Grymonprez L, Petit H. Verbal fluency in dementia of frontal lobe type and dementia of Alzheimer type.
Petty RG, Bonner D, Mouratoglou V, Silverman M. Acute frontal lobe syndrome and dyscontrol associated with bilateral caudate nucleus infarctions.
Plaisted KC, Sahakian BJ. Dementia of frontal type—living in the here and now.
Robbins TW, James M, Owen AM, Lange KW, Lees AJ, Leigh PN, et al. Cognitive deficits in progressive supranuclear palsy, Parkinson's disease, and multiple system atrophy in tests sensitive to frontal lobe dysfunction.
Roberts AC, Robbins TW, Everitt BJ. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates.
Robinson AL, Heaton RK, Lehman RA, Stilson DW. The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions.
Rogers RD, Everitt BJ, Baldacchino A, Johnson AJ, Swainson R, London M, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms.
Rogers RD, Andrews TC, Grasby PM, Brooks D, Robbins TW. Contrasting cortical and sub-cortical PET activations produced by reversal learning and attentional set-shifting in humans. J Cogn Neurosci. In press 1999b.
Rolls ET. A neurophysiological and computational approach to the functions of the temporal lobe cortical visual areas in invariant object recognition. In: Harris L, Jenkin M, editors. Computational and biological mechanisms of visual coding. Cambridge: Cambridge University Press; 1996a. p. 184–220.
Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage.
Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, et al. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson's disease.
Sanders J, Schenk VWD, Van Venn P. A family with Pick's disease. In: Uitgave NV van de, editor. Amsterdam: Noord-Hollandsche; 1939.
Sarazin M, Pillon B, Giannakopoulos P, Rancurel G, Samson Y, Dubois B. Clinicometabolic dissociation of cognitive functions and social behavior in frontal lobe lesions.
Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning.
Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy.
Snowden JS, Griffiths HL, Neary D. Progressive language disorder associated with frontal lobe degeneration.
Snowden JS, Neary D, Mann DMA. Frontotemporal lobar degeneration: frontotemporal dementia, progressive aphasia, semantic dementia. New York: Churchill Livingstone; 1996b.
Sparks DL, Markesbery WR. Altered serotonergic and cholinergic synaptic markers in Pick's disease.
Sperlinger D, Furst M. Service experience of people with pre-senile dementia: a study of carers in one London borough.
Spillantini MG, Bird TD, Ghetti B. Frontotemporal dementia and parkinsonism linked to chromosome 17: a new group of tauopathies. [Review].
Starkstein SE, Migliorelli R, Tesón A, Sabe L, Vázquez S, Turjanski M, et al. Specificity of changes in cerebral blood flow in patients with frontal lobe dementia.
Strub RL. Frontal lobe syndrome in a patient with bilateral globus pallidus lesions.
Stuss DT. Assessment of neuropsychological dysfunction in frontal lobe degeneration. [Review].
Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey.
Venneri A, Grassi F, Caffarra P. Dementia of the frontal lobe type: report of the neuroimaging and neuropsychological results of a case study.
Warrington EK, James MJ. The Visual Object and Space Perception Battery (VOSP). Bury St Edmunds, UK: The Thames Valley Test Company; 1991.
Williams GV, Rolls ET, Leonard CM, Stern C. Neuronal responses in the ventral striatum of the behaving macaque.
Yeterian EH, Pandya DN. Laminar origin of striatal and thalamic projections of the prefrontal cortex in rhesus monkeys.