-
PDF
- Split View
-
Views
-
Cite
Cite
G. Deuschl, R. Wenzelburger, K. Löffler, J. Raethjen, H. Stolze, Essential tremor and cerebellar dysfunction Clinical and kinematic analysis of intention tremor, Brain, Volume 123, Issue 8, August 2000, Pages 1568–1580, https://doi.org/10.1093/brain/123.8.1568
Close - Share Icon Share
Abstract
The cerebellum is assumed to play a major role in the pathophysiology of essential tremor (ET). As intention tremor is considered one of the classical features of cerebellar disease, we have assessed a large group of patients with ET for the semiology of the tremor and have performed objective quantitative analysis of a grasping movement in patients with ET, cerebellar disease and a normal control group. We found 25% of the patients to have a moderate or severe kinetic tremor with clear-cut features of a classical intention tremor. Another 33% of the patients had a mild intentional component of their kinetic tremor. Patients with intention tremor (ETIT) did not differ from those with predominant postural tremor (ETPT) with respect to alcohol sensitivity of the tremor and the frequency of a family history. ETIT patients were older and more often showed head and trunk involvement. The onset of this intention tremor has been assessed retrospectively. It was found to begin at a randomly distributed time interval after the onset of the postural tremor, but older patients had a shorter time to development of intention tremor. Quantitative accelerometry of postural tremor showed similar tremor frequencies in both patient groups, but ETIT patients had a slightly larger tremor amplitude. Quantitative analysis of a grasping movement using an infrared-camera system was performed in two subgroups of the patients with ETPT and ETIT and control groups with cerebellar disease or normal subjects. The intention tremor could be quantified objectively as an increased amplitude of curvature during the deceleration and target phase of the movement. The amplitude measurements of intention tremor were clearly abnormal and of comparable magnitude for ETPT and cerebellar disease. Additionally, the patients with ETIT had a significantly slowed grasping movement during the deceleration and target period. Hypermetria was significantly increased for the patients with ETIT and cerebellar disease. We conclude that intention tremor is a feature of ET. ETIT patients have abnormalities of their upper limb function compatible with cerebellar disease. This suggests that patients with more advanced ET show abnormalities of cerebellar functions.
Introduction
Essential tremor (ET) is the most frequent movement disorder and can present with different clinical characteristics (Deuschl et al., 1998). Although the condition is widespread and has attracted much research interest, it is surprising that even a simple description of the clinical presentation of ET is not yet fully clear. The classical definition of the clinical symptoms of ET includes a `mainly postural tremor, not made strikingly worse during action' (Marsden, 1984; Findley and Koller, 1987). This description has been contested in the past (Biary and Koller, 1987; Louis et al., 1998a). Indeed, the intention tremor of some of these patients can be almost indistinguishable from cerebellar tremor and these cases have been classified as `severe essential tremor' (Marsden, 1984). There seems to be general agreement, firstly, that the dominant feature of ET is postural tremor and, secondly, that resting tremor is only rarely a feature of ET (Larssen and Sjögren, 1960; Marsden, 1984). The question is how to describe and label a movement disorder occurring during action of the extremities.
Traditionally, the semiology of action tremors in general has separated postural and intention tremor, the former defined as tremor occurring during active holding of the hands against gravity and the latter occurring during the finger–nose or finger–finger test (Oppenheim, 1894; Holmes, 1939; Adams and Victor, 1989). This distinction was lost during the past years in the description of ET (Deuschl et al., 1998). Instead the semiological characteristics of tremor during movement have been collectively labelled as kinetic tremor in ET (Findley and Koller, 1995; Louis et al., 1998a), lumping together tremor during purposeless active movements and goal-directed movements. This is advantageous as long as pure clinical descriptions of the tremor are sufficient. But whenever a patient suffered from intention tremor, clinical neurology has always interpreted this to indicate cerebellar malfunction or even cerebellar disease (Oppenheim, 1894; Holmes, 1939; Adams and Victor, 1989). This might have been one of the reasons for avoiding the label `intention tremor' in the setting of ET, as there are no other clear-cut signs of cerebellar functional abnormalities in ET. Thus, the present study was designed to assess the semiology of the tremor in a large cohort of patients with ET. In order to avoid observer bias, we decided to include a quantitative approach to analyse the tremor characteristics in the upper extremity. To this aim, we analysed the movement paths of hand movements with an optoelectronic camera system in 26 of those patients. Two control groups were assessed, one age-matched control group and one group of patients with cerebellar disease.
This approach allows further insights into the involvement of the cerebellum in the pathophysiology of ET. Assuming the cerebellum to be the source of the rhythmic activity leading to tremor in ET, it seems plausible to expect subtle abnormalities of those movements that need cerebellar control for their correct execution. The paradigm used in the present study shows not only specific patterns of terminal tremor, but also the whole kinematics of a reaching movement. In earlier studies, the following aspects have been considered to indicate cerebellar malfunction during a reaching movement.
The cerebellum is likely to be involved in the co-ordination of multi-joint movements. A decomposition of movements was described as the critical abnormality based on clinical observations in cerebellar disease (Holmes, 1939). The timing of the activation of the different muscles involved in the movement is considered the crucial parameter regulated by the cerebellum. Irregularity and hypermetria are common findings in cerebellar disease (Topka et al., 1998a). Dysmetria and overshooting has been related to EMG abnormalities such as a more gradual build-up and prolongation of the agonist muscle (Hore et al., 1991; Hallett and Massaquoi, 1993; Bastian and Thach, 1995) and a delayed onset of the phasic activity of the antagonist muscle (Flament and Hore, 1986; Hore et al., 1991). Experimental data demonstrating these abnormal movements are limited. Single and multi-joint movements have been studied in a patient with an infarction in the territory of the superior cerebellar artery, and preserved single but impaired compound movements with irregular trajectories and an increased curvature of the hand path have been found (Goodkin et al., 1993). In a kinematic study of unrestrained reaching movements in patients with various cerebellar disorders, Bastian and colleagues found abnormally curved trajectories of the wrist and a tendency to move only one joint at a time (Bastian et al., 1996). The terminal phase of multi-joint movements was influenced especially by visual feedback in patients with Friedreich's ataxia and cerebellar degeneration (Day et al., 1998): these movements showed excessive deviations or directional changes in the terminal path during visual feedback, in contrast to large errors at the end of the movement when carried out in darkness.
The velocity of movement is reduced in most studies in animal models of cerebellar disease and in cerebellar patients: slowness of movements have been studied during single-joint movements in animal models (Meyer-Lohmann et al., 1975; Flament and Hore, 1986, 1988) and in humans (Hallett et al., 1975b; Hore et al., 1991; Hallett and Massaquoi, 1993). Accleration time was prolonged (Hallett and Massaquoi, 1993), peak joint acceleration was decreased and peak deceleration relative to peak acceleration was increased (Hore et al., 1991). Several studies have investigated the influence of movement speed on movement kinematics in patients with cerebellar disorders. In the study by Massaquoi and Hallett the aberrations of the hand trajectory were more pronounced when subjects performed fast horizontal planar multi-joint movements (Massaquoi and Hallett, 1996). In contrast, others have found the curvature of movement trajectories slightly longer when subjects performed slow and accurate movements and shorter when subjects executed fast reaching movements during unrestrained vertical multi-joint movements (Bastian et al., 1996).
Clinical signs of cerebellar disease include intention tremor (Holmes, 1939; Vilis and Hore, 1977; Flament and Hore, 1986; Hore and Flament, 1986; Hore et al., 1991; Diener and Dichgans, 1992). It is difficult to measure the amount of intention tremor, because it typically occurs during a voluntary movement. Earlier attempts used triaxial accelerometry (Frost, 1978; Jankovic and Frost, 1981) or high-resolution infrared tracking of a movement path with active markers (Day et al., 1998). We investigated a simple reaching movement with a quantitative movement analysis device (MacReflex, Qualisys, Sweden).
Subjects and methods
Subjects
We studied 79 consecutive out-patients with ET (Table 1). All patients fulfilled the diagnostic criteria of classical ET as defined by the consensus statement of the Movement Disorders Society (Deuschl et al., 1998). No patient had any sensory loss or corticospinal tract signs. This group of 79 patients had a full neurological examination and was investigated with a standardized assessment of the clinical features of the tremor and accelerometric and electromyographic measurement of the hand tremor (see below). The data from quantitative measurement of accelerometry and EMG were compared with a large normal control group from our laboratory (Raethjen et al., 1998).
In a subgroup of 26 subjects selected from the above-mentioned ET patients, reaching movements were studied with kinematic methods (Table 1). The selection was performed primarily according to the presence of a definite intention tremor. This criterion was met by 18 patients with a clinical rating score of 2 or 3 bilaterally (see methods). A random sample of eight ET patients without definite intention tremor (rating 0 or 1 unilaterally only) was also subjected to kinematic analysis; 42.3% were female and 57.7% were male. The patients of this subgroup were aged between 16 and 84 years (mean 62.5 years). The mean age was not significantly different from the control group (Student's t-test).
Twelve healthy subjects (three men and nine women) matched for age (27–82 years, mean 58 years) served as controls for the quantitative hand movement analysis. Twelve patients with cerebellar diseases of various origin (see Table 2) were included as a second control group. Their age was 30–69 years (mean 43 years). All the subjects with cerebellar disease exhibited upper limb ataxia and intention tremor or overshooting arm movements on clinical examination (finger–nose test). Our aim was to include patients with different stages of the disease. See Table 2 for tremor-related items of the Ataxia Rating Scale (Trouillas et al., 1997).
The protocol was approved by the ethical committee of the medical faculty of the University of Kiel and all the subjects signed an informed consent statement.
Clinical scores
Tremor rating was performed by trained neurologists on a four-point scale (0–3) according to a modification of the clinical tremor rating scale of Fahn and colleagues (Fahn et al., 1993), for each side of the upper limb. According to the criteria of the consensus statement of the Movement Disorder Society on tremor (Deuschl et al., 1998), intention tremor was defined as being present when the tremor amplitude increased during visually guided movements towards a target, if the possibility of a position-specific tremor or a postural tremor at the end of a movement was excluded and if amplitude fluctuated significantly as the target was approached. Mostly irregular jerks are present in the target phase of goal-directed movements. Intention tremor was rated in the terminal period of the finger–nose test: 0 = no intention tremor; 1 = probable intention component; 2 = definite intention component; 3 = functionally incapacitated due to intention tremor. The term `probable intention component' was included because in mild cases the distinction may be questionable if such tremor is present or not.
The disability due to tremor of the hands was scored at rest, during posture and during action. It was assessed with the following items: rest tremor was scored according to the social handicap (Zimmermann et al., 1994): 0 = no problem with tremor at rest; 1 = slight disturbance, slight handicaps in stressful situations or in social contacts; 2 = moderate disturbance, tremor is always obvious for others in everyday contacts; 3 = severe disturbance, patient avoids all social contacts. To assess the amount of postural tremor, the ability of pouring water from one test tube into another was examined: 0 = no tremor visible; 1 = slight tremor, but no water is spilled; 2 = spills some water, but <30%; 3 = spills >30% of the water. We will show later that this item does indeed correlate better with postural tremor than with intention tremor. Kinetic tremor was estimated according to difficulties with feeding and drinking: 0 = normal; 1 = slightly abnormal, soup is spilled occasionally; 2 = moderately abnormal, can drink from a cup or glass, but needs two hands; 3 = severely abnormal, must use a straw.
Additionally, the topographical distribution of the tremor was assessed. The presence of tremor was noted (rating 0 or 1) if the legs, trunk, head, face, tongue or voice were involved.
The patients were considered to suffer from hereditary tremor if at least two relatives in two generations were known to be affected. This is a very conservative estimation but it was our aim to exclude false-positive cases.
Accelerometry
Postural and rest tremor of the hands was recorded while the subjects were seated on a comfortable armchair with the forearm supported holding their hands outstretched in a pronated position or relaxing their hands, respectively. The movements were measured by two unidirectional piezoelectric accelerometers attached to the dorsum of the hands 9 cm distal to the processus styloideus ulnae on the distal part of the third metacarpal bone. The EMGs from the flexor carpi ulnaris and the extensor carpi ulnaris muscle were recorded with surface electrodes positioned 2 cm apart, close to the motor points of the respective muscle. The two accelerometer signals and the four EMG-channels were sampled over 32 s at a rate of 800 Hz and stored for off-line analysis. The power spectra of the time series of the accelerometer data and the rectified EMG-data were calculated with a software (Lauk et al., 1999) using a specifically designed stochastic time series analysis method (Timmer et al., 1996), but only the accelerometric data are reported here. Peak frequencies were determined and the total power of the spectra between 1 and 25 Hz were used as a measure of tremor amplitude because this has been found to be the better measure of tremor amplitude (Timmer et al., 1996).
Kinematic analysis
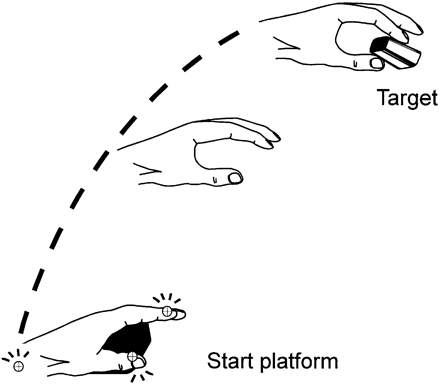
Subjects were seated on a comfortable chair with their back supported and the active hand lying on a table in front of them. The shoulder was in a neutral position with the upper arm in a vertical position, the forearm with an ~90° flexion at the elbow and the palm of the hand lying on a small platform (6 × 6 cm) at a height of 4 cm with the fingers lying relaxed on the table. This position was chosen because test trials have shown that the subjects could maximally relax in this position and in order to have a defined starting position while awaiting the next reaching movement. The target consisted of a plug with a diameter of 1 cm that was fixed to a heavy support and the subjects were instructed to reach out and precisely grasp the plug with their thumb and index finger (Fig. 1). The target was located 34 cm above the table in a parasagittal plane at a comfortable distance of ~50 cm from the body. The target distance was adjusted individually according to the height of the subject, in order to prevent any substantial movement of the trunk.
The subjects placed their hands on the resting platform, then grasped the target, released it and went back to the resting position. The movements were self-initiated and self-paced. The subjects were instructed to move at a comfortable velocity. There were no instructions concerning the duration of the grasping and resting periods. All subjects performed training trials until they were familiar with the task. At least 10 trials of the symptomatic arm were sampled.
The movements were recorded with a camera system. Reflective markers with a diameter of 5 mm were placed on the radial surface of the following landmarks: tip of the thumb, tip of the index finger and at the epicondylus of the wrist. A passive infrared movement analysis system (MacReflex version 3.2, Qualisys) was used to sample the 3D positions of the markers at a rate of 50 Hz. By using four cameras, the loss of data points could be minimized even when the hand performed irregular turning or flexion movements. About 10% of the trials had to be excluded because markers were obscured. In preceding test trials we could ascertain that our system met the manufacturer's specification of a spatial accuracy of at least 0.4 mm.
Movement paths of the reflective markers were sampled as Cartesian coordiantes and stored for off-line analysis. Movement speed was calculated as the change of the position over time (dp/dt).
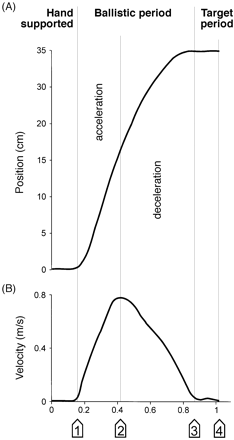
The aim of this study was to characterize the kinetic tremor in the various periods of the hand movement (see Fig. 2). Therefore, the hand transport was broken down into different periods (von Hofsten, 1991). During the first period the hand rested on the platform (support period). The support period was defined as beginning 500 ms before the start of the movement and ending when the velocity of the wrist exceeded 0.05 m/s. The following period was called the acceleration period and lasted until the maximum wrist velocity was reached. The subsequent period was called the deceleration phase and lasted until the velocity fell below 0.05 m/s. The final period of the movement was called the target period, which ended when the grasping movement of the first and second finger was terminated. During the target period additional accelerations and decelerations of the hand trajectory were observed even in most of the healthy control subjects. They are considered to represent submovements for terminal corrections, but intention tremor was typically also most prominent in the target period.
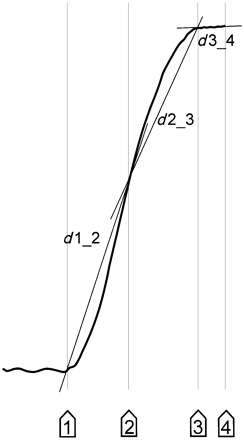
The sum of each successive change of the (3D) wrist position during the movement was calculated as a measure of the hand path. The path was evaluated for the acceleration, deceleration and target period separately to assign spatial abnormalities to the respective period of the movement (see Fig. 2). The comparison of the total movement path with a straight distance between starting and target positions had been proposed as an index of curvature in unrestrained arm movements (Atkeson and Hollerbach, 1985; Day et al., 1998). We adopted this measure to quantify the amplitude of kinetic tremor by an index of movement curvature that comprises also low-frequency irregularities of the movement and we computed this separately for each period of the movement. The straight-line distances between successive landmarks of the hand's trajectory (start of the movement, end of acceleration period, end of deceleration period and the end of target period, see Fig. 3, for definitions see above) were subtracted from the hand path during each of these periods of the movement (during acceleration, deceleration and in the target period), resulting in four indices of path curvature: we assessed the path curvature index during the total movement, during acceleration, deceleration and in the target period. Grip aperture was analysed by measuring the distance between the markers attached to the thumb and index finger.
Another important movement parameter is the overshooting of the movement, known to be a common feature of cerebellar disease (Hallett et al., 1975a). We defined the vertical overshoot as the difference between the highest position of the wrist marker during the grasping movement minus the vertical marker position at the end of the target period. Mean values were calculated for all trials. The overshoot was measured in the vertical, parasagittal plane, because it reflects most of the deviations of the hand in cerebellar disease according to previous kinematic studies on the movement disorder in cerebellar disease (Topka et al., 1998a).
Statistical analysis
Groups were compared with the one-way analysis of variance (ANOVA) including age as a co-factor (SPSS 9.0). In case of a significant influence of the group factor on the dependent variables, post hoc comparisons were computed using Kirk's t-test (Kirk, 1982). In case of ordinal values (rating scales) the Kruskal–Wallis one-way ANOVA and the Mann–Whitney U-test were computed, respectively. The results were corrected for the influence of multiple comparisons according to Bonferroni. In case of nominal variables the χ2 test was used and correlation analysis were computed by using Spearman's rho. The level of significance was set to 0.05.
If there was a difference between the arms of at least one point on the clinical rating scale of postural or action tremor, the more affected side was taken as symptomatic side. The dominant hand was analysed when clinical side differences were absent.
Results
Clinical data
The age of the 79 patients with ET (Table 1) was 16–88 years (mean 57.8 years); 52.6% of the patients were women and 47.4% men. The duration of the disease was between 2 and 64 years (mean 18.4 years). The mean duration of head tremor was 12.5 ± 8.8 years. The tremor of the voice, trunk or leg had a mean duration of 2.5 ± 1.7 years; 63.3% of the patients reported a family history of ET involving at least two generations; 60.3% reported a reduction of tremor following ingestion of alcoholic beverage.
The clinical examination of hand tremor showed rest tremor in seven (9%), postural tremor in 94.7% and kinetic tremor in 80.8% of the patients. Intention tremor was found in 59% of the patients at least for one hand; 33.3% had a probable intention tremor; 24.4% had definite intention tremor; and two patients had functionally useless hands due to intention tremor.
For further analysis those patients with definite intention tremor at least for one hand or with probable intention tremor in both hands (n = 41) were labelled as `essential tremor with intention tremor' (ETIT) and the remaining patients (n = 38) as `essential tremor with postural tremor' (ETPT). The two groups differed significantly in several aspects (Table 3): patients with ETIT were older, and showed some special features of the topographic distribution; they had head tremor and tremor of the trunk more often. Voice tremor was also more common in the patients with ETIT, but the difference just failed to be significant. The degree of disability due to kinetic tremor and the overall degree of disability was greater for ETIT. No difference was found for the duration of the disease, sex predominance or the number of patients with hereditary tremor.
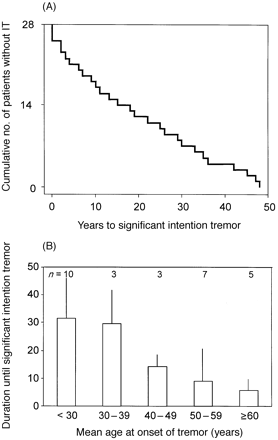
It is not known at what time intention tremor develops during the course of the disease. If ETIT represents a separate entity among the patients with ET, one would assume that the intention tremor component develops either from the very beginning of symptomatic tremor in these patients or after a more or less constant time period. Thus, we asked patients retrospectively for the onset time of symptoms of postural tremor and the onset time of intention tremor. The latter was estimated from the time when the patients first remembered the serious disabilities (e.g. must drink with a straw) that could be seen during the neurological investigation in our department. Care was taken to get data as reliable as possible. Physician and patient discussed the medical history and tried to link retrospectively the beginning of the intention tremor to life events. According to this procedure, the data from 28 of the 41 patients were considered to be reliable enough to be included in the present analysis. Figure 4A shows the time interval between the first symptoms of postural tremor and the beginning of intention tremor as a survival function with the endpoint defined as the onset of serious intention tremor. It is evident that intention tremor is not present at the very start of ET symptoms. It is a slowly developing condition in patients suffering from pure postural tremor. It neither develops early after the onset of postural tremor, nor is there a preferred time interval between the onset of postural and intention tremor. In Figure 4B the time interval between the onset of postural tremor and the development of intention tremor is displayed as a function of the age at onset of postural tremor. According to these data, intention tremor develops after a longer time period in younger onset ETIT patients than in those with tremor onset after 40 years of age. But some younger patients developed intention tremor after a very short time period, i.e. 2–3 years. The mean disease duration in the ETPT and ETIT patients is the same (Table 3), but some patients develop intention tremor while others have a longstanding history of uncomplicated postural tremor. This could reflect either a different rate of progression of the disease in the two groups or that additional factors come into action which have not yet been identified.
Accelerometry
Peak spectral frequency and total power of the postural hand tremor were measured in all but five ET patients. The frequency of postural tremor in ETPT did not significantly differ from ETIT (6.5 versus 6.4 Hz). Frequency was negatively correlated with age (r = –0.45, P < 0.01) and with the age at onset of the disease (r = –0.45, P < 0.05), but there was no correlation to disease duration.
As the total power values were distributed according to a log(10) function, median values are reported here. Total power of postural tremor was significantly higher in ETIT than in ETPT (median 0.51 versus 0.2 mg2, P < 0.05) and a positive correlation to the clinical rating of postural tremor could be observed (r = 0.48, P < 0.01). There was a weak, but significant correlation between age and postural tremor amplitude (r = 0.32, P < 0.01), but no correlation with age at onset or disease duration. A positive correlation between the total power of postural tremor and the postural tremor rating was found (r = 0.48, P < 0.01). Among the clinical tests, pouring water from one test tube into the other was highly correlated with postural tremor as measured with accelerometry (r = 0.48, P < 0.01), but it did not correlate with intention tremor. The correlation between power and the ratings of action as well as intention tremor was only weak and not significant (r = 0.26 and 0.33, repectively).
Quantitative measurement of intention tremor
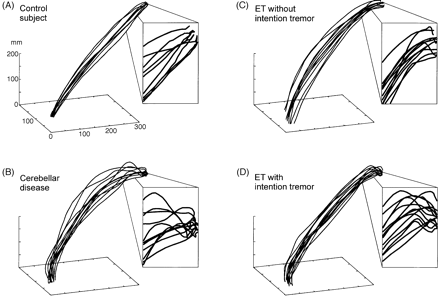
The individual movement paths of the wrist and finger markers showed reproducible and subject-specific curvatures in all patients and healthy controls (see Fig. 5). The most prominent feature of hand movement was a significant increase of the curvature index when approaching the target in patients with cerebellar disease and in many patients with ET. For the following analysis we separated normal controls from patients with ETPT, ETIT and cerebellar disease. If not otherwise stated, group comparisons are reported versus healthy controls. All ANOVAs were calculated with age as a co-factor in order to account for the slight difference of the mean age in the ETPT and ETIT subgroups.
The difference between the real hand path and the straight line was determined as a quantitative approach to measure the amplitude of intention tremor. This difference was calculated separately for the deceleration and for the terminal period and was labelled as curvature index (see Subjects and methods). The underlying idea was to match the neurophysiological test with the clinical definition of intention tremor, which is maximal in the terminal period just before the target is reached. It was therefore predicted that the amplitudes of the curvature are larger in the terminal than in the deceleration period.
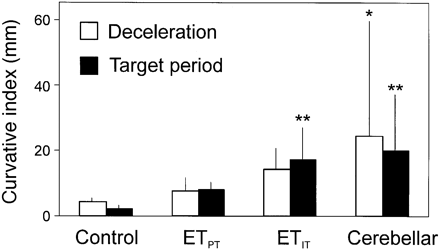
During the acceleration and deceleration periods there was a trend to higher values of the curvature index at the wrist in both subgroups of ET. Cerebellar disease patients exhibited significantly increased tremor amplitudes (P < 0.05) in the deceleration period but not during acceleration (see Fig. 6 and Table 4). In the target period the group differences were much clearer. In both ETIT and in cerebellar patients, the curvature index was significantly larger (P = 0.001) than in the controls, but there were no differences between these patient groups, or between the ETIT and ETPT subgroups.
The curvature index of the target period was correlated with the clinical rating of kinetic tremor (r = 0.43, P < 0.05), but not all indices of wrist curvature were correlated with the postural tremor rating (r < 0.2).
Movement overshoot
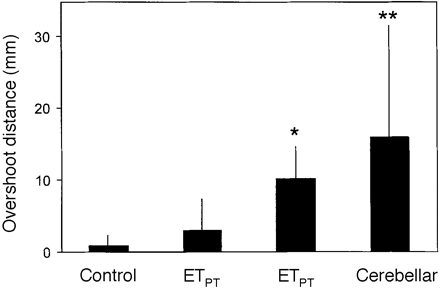
The relevant parameter for dysmetria during a grasping movement was the vertical overshoot of the hand. The mean overshoot of the wrist was 1.0 ± 0.8 mm in the healthy controls, 3.3 ± 4.1 mm in ETPT , 10.1 ± 4.8 mm in ETIT and 16.5 ± 16.0 mm in cerebellar disease (mean values ± standard deviation). Group comparison revealed a significant increase of overshoot in ETIT (P < 0.05) as well as in cerebellar disease (P < 0.001) compared with controls (Fig. 7). Although the ETPT group was clinically selected according to the absence of intention tremor in the finger–nose test, overshooting was slightly more pronounced in these patients. Overshooting was significantly lower in ETPT than in cerebellar disease (P < 0.01), but the comparison between ETIT and cerebellar disease revealed only a trend towards higher values in the latter group. We observed more overshoot in ETIT compared with the ETPT subgroup, but the difference failed to reach significance (Fig. 7). In the ETIT group no correlation could be observed between the overshoot of wrist and the amplitude of kinetic tremor during the deceleration or target periods (highest r = 0.29). This supports the notion that overshooting was not necessarily due to tremor activity in ET patients.
In summary, overshooting of the wrist movement was more frequent in ETIT and in cerebellar disease, whereas the ETPT group exhibited only slightly increased values.
Slowing of movement
Slowing of movements is a feature common to many different movement disorders, such as chorea (Agostino et al., 1992), spasticity (Knutsson and Martensson, 1980), Parkinson's disease (Agostino et al., 1992) and cerebellar diseases (Wild et al., 1996), but the different periods of movement are differently affected in these diseases.
A significant slowing of the total reach-to-grasp movement could be observed in the ETPT (P < 0.05), ETIT (P = 0.001) and the cerebellar disease patients (P < 0.0001) (Table 5). As this finding is non-specific, we analysed the hypothesis that the slowness could be confined to the terminal periods of the movement.
Group comparisons of the time spent during the acceleration period revealed no significant differences, but the duration of the deceleration period was significantly increased in ETIT (P < 0.05; increase in all but one patient). Deceleration was also prolonged in six of eight ETPT patients and in eight of 12 cerebellar disease patients, but the group comparison failed to demonstrate statistically significant differences. The analysis of the target period, which comprised all terminal correction movements after the ballistic period, revealed a nearly uniform prolongation in all the patient groups (all with P < 0.001).
Intention tremor might be the cause of the prolongation of movement in the target period in patients with cerebellar disease and also in ETIT. The ETPT patients, however, who were selected according to the absence of intention tremor in the finger–nose test, also performed more terminal corrections than controls, accounting for an increased duration of the target period. We speculate that this uniform finding common to ET and cerebellar disease could be due to a tremor-related inaccuracy of the ballistic movement period, leading to an increased need for terminal corrections.
Discussion
The present study has demonstrated that intention tremor is part of the clinical spectrum of ET. We have shown that ET patients with intention tremor have some specific associated clinical features, and we would like to put forward the interpretation that the presence of intention tremor represents a more advanced stage of the condition, rather than a separate entity among the clinical syndromes of ET. The second aim of the study was to emphasize the similarity of the movement abnormality of ET with the one of cerebellar disease. We will put forward the hypothesis that this reflects an abnormality of cerebellar function in ET.
The clinical syndrome of ET
Marsden proposed ET to be a heterogeneous syndrome rather than a single clinical entity (Marsden, 1984). Although the dominant clinical feature of ET was always considered to be a `postural tremor not made strikingly worse during action' (Fahn, 1984; Marsden, 1984; Findley, 1986; Findley and Koller, 1987). Marsden always mentioned that some patients suffer from severe action and even intention tremor. In his classification of four subtypes of ET he labelled this condition as `severe essential tremor' (Marsden, 1984). This `severe essential tremor' is the extreme variant of what has been called ETIT in the present study.
The first question to be discussed is whether this tremor should be called intention or kinetic tremor. According to a recent nomenclature, `action tremor' is divided into postural, kinetic and isometric tremor (Deuschl et al., 1998). `Kinetic tremor' is subdivided into `simple kinetic tremor', tested during non-goal-directed movements, and `intention tremor', which was defined as tremor with increasing amplitude during movements towards a target (Deuschl et al., 1998). Therefore, kinetic tremor is a neutral term that does not specify whether intention tremor is meant or not. Most of the recent reports on ET introduce the term `kinetic tremor' (Lou and Jankovic, 1991; Bain et al., 1994; Findley and Koller, 1995b; Hubble et al., 1997; Louis et al., 1998b), probably to circumvent the difficulty that intention tremor is pathogenetically linked to a functional disturbance of the cerebellum. As this hypothesis is still not proven, it is probably wise to use the more neutral term. For this discussion, the present observations may be important because the clinical findings favour the existence of intention tremor in ET, and they have been confirmed by results of quantitative movement analysis for the first time. We have analysed a paradigm which is equivalent to the clinical task used to test for intention tremor. First, compared with normal subjects we found that the tremor in ETIT increases more during the target period than during the acceleration and deceleration period. Therefore, this tremor fulfils the criteria for intention tremor because it increases when the hand approaches an object. Secondly, we have asked patients with clear-cut cerebellar disease to perform the same paradigm. Their test results were the same as the ones of the patients with ETIT. Hence, the classical intention tremor of cerebellar disease is indistinguishable from the tremor seen in ETIT during the target period. We conclude that intention tremor indeed is a feature of ET at least in our subgroup labelled as ETIT. Kinetic tremor is a correct label for this tremor as long as intention tremor is included in the definition.
We could show that the involvement of more proximal topographic regions like the head, voice or trunk accompanies the occurrence of intention tremor. This is a feature which is often seen in cerebellar tremor, and may indicate that other cerebellar mechanisms are activated in ETIT than in ETPT. Greater age seems to be a risk factor for the development of this more severe form of ET, but we could not confirm in our patient group that head and voice tremor is significantly more common in females (Hubble et al., 1997). The present data give ample evidence that the condition develops in older patients after a shorter time period of postural tremor than in younger ones. This cannot be interpreted as a trivial selection bias (i.e. older patients have less time to express this symptom compared with young-onset patients), because we have two young patients who developed intention tremor within 3 years and the disease duration does not differ significantly between ETPT and ETIT. But we failed to find an unequivocal predictor indicating the development of intention tremor in a single patient. Neither a family history of ET, a specific clinical course running in some families nor the responsiveness to alcohol could be shown to be predicting factors. The clinical finding of intention tremor may be of great clinical interest, because this symptom may cause confusion about the diagnosis of ET even among neurologists. It is the most important reason for disability in ET (Busenbark et al., 1991) and in our experience, the symptom is the least favourable to treat with conventional medication (Biary and Koller, 1987; Koller et al., 1987). Future studies will have to focus more on this feature in ET.
The pathophysiology of ET
The present quantitative data of the movement analysis give some new hints at a possible involvement of the cerebellum in the generation of ET. We have used a simple reach-to-grasp paradigm to analyse some features of the central organization of movement in ET and cerebellar disease. The basic idea was to compare ET patients with and without intention tremor, and patients with cerebellar disease. We have assessed whether they share similar components of their movement abnormalities.
The first abnormality was the slowness of goal-directed movements found to be similar in ET and cerebellar disease. Slowness of the acceleration phase was found for the bradykinesia of Parkinson's disease (Gentilucci and Negrotti, 1999), spasticity (Knutsson and Martensson, 1980) and Huntington's disease (Berardelli et al., 1999), but the different movement periods were almost equally affected in these conditions, whereas the slowness was confined to the deceleration and target period, but not during the acceleration phase in our patients with cerebellar disease. Therefore, we consider this to be a finding which is relatively specific for cerebellar malfunction, but it cannot be excluded that bradykinesia during the target phase is due to time for additional terminal corrections. Anyway, the same type of slowness was found for ETIT and cerebellar disease.
The second abnormality was the overshoot of hand movements when reaching a target, which was present not only in cerebellar disease and in many ET patients, but also in normal subjects. Hypermetria of fast pointing movements mainly results from excessive motion about the shoulder and elbow joint in cerebellar disease (Topka et al., 1998a). Hypermetria may be caused by abnormalities in the control of dynamic movement variables, especially of interaction torques, which is believed to be governed by the cerebellum (Bastian et al., 1996; Topka et al., 1998b). Our findings of a vertical overshooting might additionally be a side effect of the decomposition of movements, which is a well known phenomenon in cerebellar disease (Bastian et al., 1996). It has been shown that ballistic movements show a delayed deceleration phase in ET (Britton et al., 1994), which may lead to a similar overshoot as in the present paradigm. Interestingly, we found the hypermetria of the present investigation to be more pronounced in the ETIT than in the ETPT patients. We will show in an accompanying paper that the disturbance of ballistic movements is also more pronounced in ETIT than in the ETPT patients (B. Köster, G. Deuschl, M. Lank, J. Timmer, B. Guschlbauer, C. H. Lücking). Thus, we conclude that hypermetria found in patients with ET might indicate a disturbance of cerebellar function that is more pronounced in patients with ETIT than those with ETPT.
The third abnormality of both cerebellar disease and ET was intention tremor. This is typically found in cerebellar disease and has been confirmed for ETIT with the present experiments. Intention tremor is a clinically defined feature, but it can be picked up with the method applied in the present study. The method to extract intention tremor from the present raw data is not trivial. Instead of using theoretical trajectories as the ideal path (Jeannerod, 1986), we divided the movement into several segments and expressed the amount of intention tremor as the difference of the hand path and the straight-line connections between these segments. This procedure was a refinement of the measurements used by Day and colleagues, who compared the total movement path with the shortest distance between the start and end points of the movement (Day et al., 1998). This measurement certainly combines irregular with regular curvature but it matches closely the clinical observation of intention tremor. As abnormal tremor amplitudes were only found for the target period and to some extent, already in the deceleration period, it is likely that we have, in fact, depicted the intention tremor according to the clinical definition.
How can our finding of cerebellar abnormalities be interpreted in the context of the present knowledge concerning ET? There is ample evidence from different sources that the cerebellar function is affected in ET. The animal model of harmalin tremor shares some features with ET. In this condition, the cerebellar cortex and cerebellar nuclei show timelocked discharges with the tremor and the inferior olive is considered to be the underlying oscillator (Wilms et al., 1999). Although there are some features that are at variance between harmaline tremor in humans (Lewin, 1928; Pennes and Hoch, 1957) and ET, there are no clinical features definitely contradicting this hypothesis. Furthermore, clinical observations in patients with localized strokes in the cerebellum or its connections demonstrate a disappearance of tremor ipsilateral to a cerebellar or pontine stroke (Dupuis et al., 1989; Kim and Lee, 1994; Nagaratnam and Kalasabail, 1997). PET investigations found the cerebellum to be hyperactive not only in ET, but also in other forms of tremor (Hallett and Dubinsky, 1993; Jenkins and Frackowiak, 1993; Wills et al., 1994; Boecker and Brooks, 1998). Many patients with ET have a reduction of their tremor intensity during alcohol consumption and PET activation shows a similar reduction of cerebellar hyperactivity following alcohol use (Boecker et al., 1996). Thus, the severity of cerebellar activation seems to correlate with the severity of ET. Finally, the analysis of ballistic movements demonstrated a disturbance of the triphasic pattern of ballistic arm movements that is similar (Britton et al., 1994), but not identical, to the pattern seen in cerebellar diseases (Hallett et al., 1975b). In an accompanying paper, we will show that this abnormality is mainly seen in patients with intention tremor in ET (B. Köster, G. Deuschl, M. Lank, J. Timmer, B. Guschlbauer, C. H. Lücking).
The present demonstration of movement abnormalities in ET compatible with cerebellar disease might indicate a cerebellar malfunction in advanced ET and fit well into the above mentioned findings. As not only intention tremor but also hypermetria and slowness of movements were found, we suggest that cerebellar functions are affected and no longer able to support accurate movement performance. The alternative hypothesis would be that the movement abnormalities observed are all secondary to the more vigorous tremor in ETIT which causes the cerebellar feed-forward and feed-back regulation to decompensate. However, this hypothesis would require that the amplitudes of postural tremor in ETPT should differ markedly from ETIT, which was not found. An additional argument against this `decompensation hypothesis' is that abnormalities of gait typical for cerebellar disease can be found in patients with ET, even when leg tremor is absent (Stolze et al., 1999).
Altogether, the present findings converge into the hypothesis that cerebellar functions are affected in ET, and we suggest that this is due to the generation of the abnormal tremor rhythms in those parts of the cerebellum which are normally necessary to perform the functions being defective in ETIT.
Conclusion
The semiology of ET has been imprecise concerning the clinical manifestations of tremor. According to our results, intention tremor can be found in ET and, thus, should be included in the clinical spectrum of the condition. Whenever intention tremor is present in ET head and trunk tremor are more likely to occur and the patients with intention tremor have a higher degree of disability. The occurrence of intention tremor suggests abnormalities of cerebellar functions. The present quantitative movement analysis showed that further aspects like prolongation of the movement time and overshooting further strengthen this view. We will show in accompanying papers that ballistic movements are similarly abnormal in ETIT as in cerebellar disease and that there is a gait abnormality in ETIT which is similar to the one of cerebellar disease.
Clinical characteristics of the patients with ET
| . | Total group . | Subgroup for movement analysis . |
|---|---|---|
| *mg = milli-gravities. | ||
| Number | 79 | 26 |
| Age (years) (mean ± SD) | 57.8 ± 17.2 | 62.5 ± 14.6 |
| Sex (%) (male/female) | 47.4/52.6 | 57.7/42.3 |
| Duration of the disease (years) | 18.4 ± 15.5 | 18.5 ± 14.1 |
| Age at onset (years) | 39.7 ± 19.6 | 44 ± 19.8 |
| Severity of postural tremor (score) | 1.38 ± 0.91 | 1.96 ± 0.77 |
| Amplitude of postural tremor at the more affected side (mg2*) (median) | 0.4 ± 1.5 | 1.2 ± 6.3 |
| . | Total group . | Subgroup for movement analysis . |
|---|---|---|
| *mg = milli-gravities. | ||
| Number | 79 | 26 |
| Age (years) (mean ± SD) | 57.8 ± 17.2 | 62.5 ± 14.6 |
| Sex (%) (male/female) | 47.4/52.6 | 57.7/42.3 |
| Duration of the disease (years) | 18.4 ± 15.5 | 18.5 ± 14.1 |
| Age at onset (years) | 39.7 ± 19.6 | 44 ± 19.8 |
| Severity of postural tremor (score) | 1.38 ± 0.91 | 1.96 ± 0.77 |
| Amplitude of postural tremor at the more affected side (mg2*) (median) | 0.4 ± 1.5 | 1.2 ± 6.3 |
Clinical characteristics of the patients with ET
| . | Total group . | Subgroup for movement analysis . |
|---|---|---|
| *mg = milli-gravities. | ||
| Number | 79 | 26 |
| Age (years) (mean ± SD) | 57.8 ± 17.2 | 62.5 ± 14.6 |
| Sex (%) (male/female) | 47.4/52.6 | 57.7/42.3 |
| Duration of the disease (years) | 18.4 ± 15.5 | 18.5 ± 14.1 |
| Age at onset (years) | 39.7 ± 19.6 | 44 ± 19.8 |
| Severity of postural tremor (score) | 1.38 ± 0.91 | 1.96 ± 0.77 |
| Amplitude of postural tremor at the more affected side (mg2*) (median) | 0.4 ± 1.5 | 1.2 ± 6.3 |
| . | Total group . | Subgroup for movement analysis . |
|---|---|---|
| *mg = milli-gravities. | ||
| Number | 79 | 26 |
| Age (years) (mean ± SD) | 57.8 ± 17.2 | 62.5 ± 14.6 |
| Sex (%) (male/female) | 47.4/52.6 | 57.7/42.3 |
| Duration of the disease (years) | 18.4 ± 15.5 | 18.5 ± 14.1 |
| Age at onset (years) | 39.7 ± 19.6 | 44 ± 19.8 |
| Severity of postural tremor (score) | 1.38 ± 0.91 | 1.96 ± 0.77 |
| Amplitude of postural tremor at the more affected side (mg2*) (median) | 0.4 ± 1.5 | 1.2 ± 6.3 |
Patients with cerebellar disease
| Patient no. . | Aetiology . | Age (years) . | Sex . | Duration of disease (years) . | Diadocho-kinesis* . | Finger– finger* . | Finger–nose* . | Tremor rating . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | posture . | kinetic . |
| *The items finger–nose test, finger–finger test and diadochokinesis refer to a subscore of the Ataxia Rating Scale (Trouillas et al., 1997), items 11–13 (finger–finger = instability; finger–nose = intention tremor). †SCA 2 = spinocerebellar atrophy, type 2. | |||||||||
| 1 | MS | 32 | M | 0.2 | 1 | 1 | 1 | 2 | 0 |
| 2 | SCA 2† | 37 | M | 30 | 3 | 2 | 2 | 3 | 2 |
| 3 | MS | 38 | M | 9 | 3 | 2 | 3 | 2 | 2 |
| 4 | MS | 30 | F | 1 | 2 | 2 | 1 | 1 | 1 |
| 5 | Stroke | 69 | M | 1 | 1 | 1 | 1 | 2 | 2 |
| 6 | MS | 40 | M | 1 | 1 | 0 | 1 | 0 | 1 |
| 7 | MS | 33 | F | 10 | 2 | 1 | 2 | 1 | 2 |
| 8 | MS | 38 | M | 5 | 0 | 1 | 1 | 0 | 1 |
| 9 | MS | 67 | M | 30 | 3 | 4 | 4 | 3 | 3 |
| 10 | MS | 37 | F | 18 | 2 | 1 | 1 | 0 | 2 |
| 11 | MS | 32 | F | 7 | 3 | 2 | 2 | 1 | 1 |
| 12 | Cerebellitis | 61 | M | 0.2 | 1 | 2 | 3 | 3 | 3 |
| Patient no. . | Aetiology . | Age (years) . | Sex . | Duration of disease (years) . | Diadocho-kinesis* . | Finger– finger* . | Finger–nose* . | Tremor rating . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | posture . | kinetic . |
| *The items finger–nose test, finger–finger test and diadochokinesis refer to a subscore of the Ataxia Rating Scale (Trouillas et al., 1997), items 11–13 (finger–finger = instability; finger–nose = intention tremor). †SCA 2 = spinocerebellar atrophy, type 2. | |||||||||
| 1 | MS | 32 | M | 0.2 | 1 | 1 | 1 | 2 | 0 |
| 2 | SCA 2† | 37 | M | 30 | 3 | 2 | 2 | 3 | 2 |
| 3 | MS | 38 | M | 9 | 3 | 2 | 3 | 2 | 2 |
| 4 | MS | 30 | F | 1 | 2 | 2 | 1 | 1 | 1 |
| 5 | Stroke | 69 | M | 1 | 1 | 1 | 1 | 2 | 2 |
| 6 | MS | 40 | M | 1 | 1 | 0 | 1 | 0 | 1 |
| 7 | MS | 33 | F | 10 | 2 | 1 | 2 | 1 | 2 |
| 8 | MS | 38 | M | 5 | 0 | 1 | 1 | 0 | 1 |
| 9 | MS | 67 | M | 30 | 3 | 4 | 4 | 3 | 3 |
| 10 | MS | 37 | F | 18 | 2 | 1 | 1 | 0 | 2 |
| 11 | MS | 32 | F | 7 | 3 | 2 | 2 | 1 | 1 |
| 12 | Cerebellitis | 61 | M | 0.2 | 1 | 2 | 3 | 3 | 3 |
Patients with cerebellar disease
| Patient no. . | Aetiology . | Age (years) . | Sex . | Duration of disease (years) . | Diadocho-kinesis* . | Finger– finger* . | Finger–nose* . | Tremor rating . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | posture . | kinetic . |
| *The items finger–nose test, finger–finger test and diadochokinesis refer to a subscore of the Ataxia Rating Scale (Trouillas et al., 1997), items 11–13 (finger–finger = instability; finger–nose = intention tremor). †SCA 2 = spinocerebellar atrophy, type 2. | |||||||||
| 1 | MS | 32 | M | 0.2 | 1 | 1 | 1 | 2 | 0 |
| 2 | SCA 2† | 37 | M | 30 | 3 | 2 | 2 | 3 | 2 |
| 3 | MS | 38 | M | 9 | 3 | 2 | 3 | 2 | 2 |
| 4 | MS | 30 | F | 1 | 2 | 2 | 1 | 1 | 1 |
| 5 | Stroke | 69 | M | 1 | 1 | 1 | 1 | 2 | 2 |
| 6 | MS | 40 | M | 1 | 1 | 0 | 1 | 0 | 1 |
| 7 | MS | 33 | F | 10 | 2 | 1 | 2 | 1 | 2 |
| 8 | MS | 38 | M | 5 | 0 | 1 | 1 | 0 | 1 |
| 9 | MS | 67 | M | 30 | 3 | 4 | 4 | 3 | 3 |
| 10 | MS | 37 | F | 18 | 2 | 1 | 1 | 0 | 2 |
| 11 | MS | 32 | F | 7 | 3 | 2 | 2 | 1 | 1 |
| 12 | Cerebellitis | 61 | M | 0.2 | 1 | 2 | 3 | 3 | 3 |
| Patient no. . | Aetiology . | Age (years) . | Sex . | Duration of disease (years) . | Diadocho-kinesis* . | Finger– finger* . | Finger–nose* . | Tremor rating . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | posture . | kinetic . |
| *The items finger–nose test, finger–finger test and diadochokinesis refer to a subscore of the Ataxia Rating Scale (Trouillas et al., 1997), items 11–13 (finger–finger = instability; finger–nose = intention tremor). †SCA 2 = spinocerebellar atrophy, type 2. | |||||||||
| 1 | MS | 32 | M | 0.2 | 1 | 1 | 1 | 2 | 0 |
| 2 | SCA 2† | 37 | M | 30 | 3 | 2 | 2 | 3 | 2 |
| 3 | MS | 38 | M | 9 | 3 | 2 | 3 | 2 | 2 |
| 4 | MS | 30 | F | 1 | 2 | 2 | 1 | 1 | 1 |
| 5 | Stroke | 69 | M | 1 | 1 | 1 | 1 | 2 | 2 |
| 6 | MS | 40 | M | 1 | 1 | 0 | 1 | 0 | 1 |
| 7 | MS | 33 | F | 10 | 2 | 1 | 2 | 1 | 2 |
| 8 | MS | 38 | M | 5 | 0 | 1 | 1 | 0 | 1 |
| 9 | MS | 67 | M | 30 | 3 | 4 | 4 | 3 | 3 |
| 10 | MS | 37 | F | 18 | 2 | 1 | 1 | 0 | 2 |
| 11 | MS | 32 | F | 7 | 3 | 2 | 2 | 1 | 1 |
| 12 | Cerebellitis | 61 | M | 0.2 | 1 | 2 | 3 | 3 | 3 |
Differences between patients with ETPTand ETIT
| . | ETPT . | ETIT . | Significance . |
|---|---|---|---|
| No. of patients (%) | 38 (48) | 41 (52) | n.s. |
| Age (years) (mean ± SD) | 51.8 ± 17.3 | 63.4 ± 15.2 | P < 0.05 |
| Sex (%) (male/female) | 50/50 | 53.7/46.3 | n.s. |
| Duration of symptomatic tremor (mean years ± SD) | 19 ± 16.3 | 17.8 ± 15 | n.s. |
| Hereditary tremor (%) | 57.9 | 68.3 | n.s. |
| Upper extremity (%) | 94.7 | 100 | n.s. |
| Head tremor (%) | 42.1 | 68.3 | P < 0.05 |
| Voice tremor (%) | 18.4 | 34.1 | n.s. |
| Tongue (%) | 0 | 12.2 | P < 0.05 |
| Tremor of the trunk (%) | 5.4 | 22 | P < 0.05 |
| Lower extremities (%) | 21.1 | 26.8 | n.s. |
| Disability due to kinetic tremor (mean rank ± SD) | 0.9 ± 0.8 | 1.85 ± 0.7 | P < 0.01 |
| Amplitude of postural tremor (median ± quartile range in mg2) | 0.2 ± 0.6 | 0.5 ± 2.7 | P < 0.05 |
| Frequency of postural tremor (mean ± SD in Hz) | 6.4 ± 1.2 | 6.5 ± 1.5 | n.s. |
| . | ETPT . | ETIT . | Significance . |
|---|---|---|---|
| No. of patients (%) | 38 (48) | 41 (52) | n.s. |
| Age (years) (mean ± SD) | 51.8 ± 17.3 | 63.4 ± 15.2 | P < 0.05 |
| Sex (%) (male/female) | 50/50 | 53.7/46.3 | n.s. |
| Duration of symptomatic tremor (mean years ± SD) | 19 ± 16.3 | 17.8 ± 15 | n.s. |
| Hereditary tremor (%) | 57.9 | 68.3 | n.s. |
| Upper extremity (%) | 94.7 | 100 | n.s. |
| Head tremor (%) | 42.1 | 68.3 | P < 0.05 |
| Voice tremor (%) | 18.4 | 34.1 | n.s. |
| Tongue (%) | 0 | 12.2 | P < 0.05 |
| Tremor of the trunk (%) | 5.4 | 22 | P < 0.05 |
| Lower extremities (%) | 21.1 | 26.8 | n.s. |
| Disability due to kinetic tremor (mean rank ± SD) | 0.9 ± 0.8 | 1.85 ± 0.7 | P < 0.01 |
| Amplitude of postural tremor (median ± quartile range in mg2) | 0.2 ± 0.6 | 0.5 ± 2.7 | P < 0.05 |
| Frequency of postural tremor (mean ± SD in Hz) | 6.4 ± 1.2 | 6.5 ± 1.5 | n.s. |
Differences between patients with ETPTand ETIT
| . | ETPT . | ETIT . | Significance . |
|---|---|---|---|
| No. of patients (%) | 38 (48) | 41 (52) | n.s. |
| Age (years) (mean ± SD) | 51.8 ± 17.3 | 63.4 ± 15.2 | P < 0.05 |
| Sex (%) (male/female) | 50/50 | 53.7/46.3 | n.s. |
| Duration of symptomatic tremor (mean years ± SD) | 19 ± 16.3 | 17.8 ± 15 | n.s. |
| Hereditary tremor (%) | 57.9 | 68.3 | n.s. |
| Upper extremity (%) | 94.7 | 100 | n.s. |
| Head tremor (%) | 42.1 | 68.3 | P < 0.05 |
| Voice tremor (%) | 18.4 | 34.1 | n.s. |
| Tongue (%) | 0 | 12.2 | P < 0.05 |
| Tremor of the trunk (%) | 5.4 | 22 | P < 0.05 |
| Lower extremities (%) | 21.1 | 26.8 | n.s. |
| Disability due to kinetic tremor (mean rank ± SD) | 0.9 ± 0.8 | 1.85 ± 0.7 | P < 0.01 |
| Amplitude of postural tremor (median ± quartile range in mg2) | 0.2 ± 0.6 | 0.5 ± 2.7 | P < 0.05 |
| Frequency of postural tremor (mean ± SD in Hz) | 6.4 ± 1.2 | 6.5 ± 1.5 | n.s. |
| . | ETPT . | ETIT . | Significance . |
|---|---|---|---|
| No. of patients (%) | 38 (48) | 41 (52) | n.s. |
| Age (years) (mean ± SD) | 51.8 ± 17.3 | 63.4 ± 15.2 | P < 0.05 |
| Sex (%) (male/female) | 50/50 | 53.7/46.3 | n.s. |
| Duration of symptomatic tremor (mean years ± SD) | 19 ± 16.3 | 17.8 ± 15 | n.s. |
| Hereditary tremor (%) | 57.9 | 68.3 | n.s. |
| Upper extremity (%) | 94.7 | 100 | n.s. |
| Head tremor (%) | 42.1 | 68.3 | P < 0.05 |
| Voice tremor (%) | 18.4 | 34.1 | n.s. |
| Tongue (%) | 0 | 12.2 | P < 0.05 |
| Tremor of the trunk (%) | 5.4 | 22 | P < 0.05 |
| Lower extremities (%) | 21.1 | 26.8 | n.s. |
| Disability due to kinetic tremor (mean rank ± SD) | 0.9 ± 0.8 | 1.85 ± 0.7 | P < 0.01 |
| Amplitude of postural tremor (median ± quartile range in mg2) | 0.2 ± 0.6 | 0.5 ± 2.7 | P < 0.05 |
| Frequency of postural tremor (mean ± SD in Hz) | 6.4 ± 1.2 | 6.5 ± 1.5 | n.s. |
Curvature index in the different periods of the reaching movement
| Kinetic tremor . | Controls . | ETPT . | ETIT . | Cerebellar disease . |
|---|---|---|---|---|
| The curvature index measurement was calculated by subtracting the straight line between the positions of the wrist marker at the beginning and end of the respective periods of the movements from the length of the real hand paths (in mm). It represents kinetic and intention tremor as well as non-rhythmic movement curvature. *P < 0.05; **P < 0.01 when compared with controls. Values are displayed as mean (± standard deviation). | ||||
| In the total movement | 44.0 (18.9) | 55.2 (12.1) | 79.0 (14.1) | 118.6 (93.7) |
| During acceleration | 21.5 (5.3) | 22.6 (5.1) | 24.9 (9.2) | 28.6 (19.9) |
| During deceleration | 5.4 (1.3) | 8.5 (5.0) | 15.2 (4.3) | 26.5 (33.9)* |
| During target period | 2.5 (1.3) | 8.8 (3.2) | 18.5 (8.7)** | 20.5 (18.1)** |
| Kinetic tremor . | Controls . | ETPT . | ETIT . | Cerebellar disease . |
|---|---|---|---|---|
| The curvature index measurement was calculated by subtracting the straight line between the positions of the wrist marker at the beginning and end of the respective periods of the movements from the length of the real hand paths (in mm). It represents kinetic and intention tremor as well as non-rhythmic movement curvature. *P < 0.05; **P < 0.01 when compared with controls. Values are displayed as mean (± standard deviation). | ||||
| In the total movement | 44.0 (18.9) | 55.2 (12.1) | 79.0 (14.1) | 118.6 (93.7) |
| During acceleration | 21.5 (5.3) | 22.6 (5.1) | 24.9 (9.2) | 28.6 (19.9) |
| During deceleration | 5.4 (1.3) | 8.5 (5.0) | 15.2 (4.3) | 26.5 (33.9)* |
| During target period | 2.5 (1.3) | 8.8 (3.2) | 18.5 (8.7)** | 20.5 (18.1)** |
Curvature index in the different periods of the reaching movement
| Kinetic tremor . | Controls . | ETPT . | ETIT . | Cerebellar disease . |
|---|---|---|---|---|
| The curvature index measurement was calculated by subtracting the straight line between the positions of the wrist marker at the beginning and end of the respective periods of the movements from the length of the real hand paths (in mm). It represents kinetic and intention tremor as well as non-rhythmic movement curvature. *P < 0.05; **P < 0.01 when compared with controls. Values are displayed as mean (± standard deviation). | ||||
| In the total movement | 44.0 (18.9) | 55.2 (12.1) | 79.0 (14.1) | 118.6 (93.7) |
| During acceleration | 21.5 (5.3) | 22.6 (5.1) | 24.9 (9.2) | 28.6 (19.9) |
| During deceleration | 5.4 (1.3) | 8.5 (5.0) | 15.2 (4.3) | 26.5 (33.9)* |
| During target period | 2.5 (1.3) | 8.8 (3.2) | 18.5 (8.7)** | 20.5 (18.1)** |
| Kinetic tremor . | Controls . | ETPT . | ETIT . | Cerebellar disease . |
|---|---|---|---|---|
| The curvature index measurement was calculated by subtracting the straight line between the positions of the wrist marker at the beginning and end of the respective periods of the movements from the length of the real hand paths (in mm). It represents kinetic and intention tremor as well as non-rhythmic movement curvature. *P < 0.05; **P < 0.01 when compared with controls. Values are displayed as mean (± standard deviation). | ||||
| In the total movement | 44.0 (18.9) | 55.2 (12.1) | 79.0 (14.1) | 118.6 (93.7) |
| During acceleration | 21.5 (5.3) | 22.6 (5.1) | 24.9 (9.2) | 28.6 (19.9) |
| During deceleration | 5.4 (1.3) | 8.5 (5.0) | 15.2 (4.3) | 26.5 (33.9)* |
| During target period | 2.5 (1.3) | 8.8 (3.2) | 18.5 (8.7)** | 20.5 (18.1)** |
Timing of movement periods
| Variables . | Controls . | ETPT . | ETIT . | Cerebellar disease . |
|---|---|---|---|---|
| The duration of the different movement periods are shown (in seconds). Values are displayed as mean (± standard deviation). *P < 0.05; **P < 0.01 when compared with controls. | ||||
| Total movement duration | 0.88 (0.11) | 1.1 (0.2)* | 1.14 (0.16)** | 1.21 (0.22)** |
| Duration of ballistic period | 0.78 (0.11) | 0.87 (0.16) | 0.92 (0.13)* | 0.96 (0.16) |
| Duration of acceleration | 0.3 (0.07) | 0.3 (0.08) | 0.3 (0.09) | 0.43 (0.07) |
| Duration of deceleration | 0.48 (0.1) | 0.57 (0.11) | 0.62 (0.11)* | 0.61 (0.17) |
| Duration of target period | 0.1 (0.02) | 0.25 (0.07)** | 0.21 (0.08)* | 0.26 (0.12)** |
| Maximal wrist speed (m/s) | 0.65 (0.27) | 0.62 (0.22) | 0.57 (0.2) | 0.6 (0.19) |
| Variables . | Controls . | ETPT . | ETIT . | Cerebellar disease . |
|---|---|---|---|---|
| The duration of the different movement periods are shown (in seconds). Values are displayed as mean (± standard deviation). *P < 0.05; **P < 0.01 when compared with controls. | ||||
| Total movement duration | 0.88 (0.11) | 1.1 (0.2)* | 1.14 (0.16)** | 1.21 (0.22)** |
| Duration of ballistic period | 0.78 (0.11) | 0.87 (0.16) | 0.92 (0.13)* | 0.96 (0.16) |
| Duration of acceleration | 0.3 (0.07) | 0.3 (0.08) | 0.3 (0.09) | 0.43 (0.07) |
| Duration of deceleration | 0.48 (0.1) | 0.57 (0.11) | 0.62 (0.11)* | 0.61 (0.17) |
| Duration of target period | 0.1 (0.02) | 0.25 (0.07)** | 0.21 (0.08)* | 0.26 (0.12)** |
| Maximal wrist speed (m/s) | 0.65 (0.27) | 0.62 (0.22) | 0.57 (0.2) | 0.6 (0.19) |
Timing of movement periods
| Variables . | Controls . | ETPT . | ETIT . | Cerebellar disease . |
|---|---|---|---|---|
| The duration of the different movement periods are shown (in seconds). Values are displayed as mean (± standard deviation). *P < 0.05; **P < 0.01 when compared with controls. | ||||
| Total movement duration | 0.88 (0.11) | 1.1 (0.2)* | 1.14 (0.16)** | 1.21 (0.22)** |
| Duration of ballistic period | 0.78 (0.11) | 0.87 (0.16) | 0.92 (0.13)* | 0.96 (0.16) |
| Duration of acceleration | 0.3 (0.07) | 0.3 (0.08) | 0.3 (0.09) | 0.43 (0.07) |
| Duration of deceleration | 0.48 (0.1) | 0.57 (0.11) | 0.62 (0.11)* | 0.61 (0.17) |
| Duration of target period | 0.1 (0.02) | 0.25 (0.07)** | 0.21 (0.08)* | 0.26 (0.12)** |
| Maximal wrist speed (m/s) | 0.65 (0.27) | 0.62 (0.22) | 0.57 (0.2) | 0.6 (0.19) |
| Variables . | Controls . | ETPT . | ETIT . | Cerebellar disease . |
|---|---|---|---|---|
| The duration of the different movement periods are shown (in seconds). Values are displayed as mean (± standard deviation). *P < 0.05; **P < 0.01 when compared with controls. | ||||
| Total movement duration | 0.88 (0.11) | 1.1 (0.2)* | 1.14 (0.16)** | 1.21 (0.22)** |
| Duration of ballistic period | 0.78 (0.11) | 0.87 (0.16) | 0.92 (0.13)* | 0.96 (0.16) |
| Duration of acceleration | 0.3 (0.07) | 0.3 (0.08) | 0.3 (0.09) | 0.43 (0.07) |
| Duration of deceleration | 0.48 (0.1) | 0.57 (0.11) | 0.62 (0.11)* | 0.61 (0.17) |
| Duration of target period | 0.1 (0.02) | 0.25 (0.07)** | 0.21 (0.08)* | 0.26 (0.12)** |
| Maximal wrist speed (m/s) | 0.65 (0.27) | 0.62 (0.22) | 0.57 (0.2) | 0.6 (0.19) |
Prehension paradigm. The hand is resting on a platform. A reaching movement to the target is performed.
Subdivision of the movement. In (A) the trajectory of the wrist is shown measured in the vertical axis and in (B) the corresponding velocity profile is plotted. During the support period the hand lies on the start platform. The following ballistic period ends when the speed drops below 0.05 m/s for the first time, marking the beginning of the non-ballistic target period.
Calculation of the curvature index. The straight-line distances (d1_2, d2_3, d3_4) were determined between the successive landmarks of the movement (markers 1–4, see Fig. 2 for details). The straight distance between the wrist positions at the start and end of the total movement (d1_4) was also computed. These distances were subtracted from the real hand path, separately in each period of the movement to characterize the hand path curvature.
Time interval between the onset of postural tremor and the onset of intention tremor in 28 patients with ETIT. (A) Kaplan–Meier curve of the time between the onset of postural and intention tremor. The number of patients without intention tremor is plotted on the y-axis. The time intervals vary considerably and no preferred time interval is discernible. (B) The time to intention tremor is displayed for patient groups with similar onset intervals (groups of 10 years).
3D diagrams of the hand path of individual subjects. Overshooting and ataxia are most prominent in the patients with cerebellar disease and in the ETIT patient. Note that these diagrams of the hand path purely rely on positional data, so there is no information in the frequency domain.
The curvature index (see Fig. 3) was used as a measure of intention tremor. The amplitude of tremor was calculated by subtracting the length of the straight line between the positions of the wrist marker at the beginning and end of the respective periods of the movements from the length of the real hand paths (in mm). *P < 0.05; **P < 0.01 when compared with controls.
Overshooting of the wrist when approaching the target. *P < 0.05; **P < 0.01 when compared with controls.
The authors thank Mareike Witt for the preparation of data and Johannes Pohl for statistical support. This study was supported by a grant of the University of Kiel, the German Research Council (Deutsche Forschungsgemeinschaft) and by the German Ministry of Research (BMBF).
References
Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson's disease, Huntington's disease and dystonia.
Atkeson CG, Hollerbach JM. Kinematic features of unrestrained vertical arm movements.
Bain PG, Findley LJ, Thompson PD, Gresty MA, Rothwell JC, Harding AE, et al. A study of hereditary essential tremor.
Bastian AJ, Thach WT. Cerebellar outflow lesions: a comparison of movement deficits resulting from lesions at the levels of the cerebellum and thalamus.
Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints.
Berardelli A, Noth J, Thompson PD, Bollen EL, Curra A, Deuschl G, et al. Pathophysiology of chorea and bradykinesia in Huntington's disease. [Review].
Biary N, Koller W. Kinetic predominant essential tremor: successful treatment with clonazepam.
Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thompson PD, Findley LJ, et al. The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study.
Britton TC, Thompson PD, Day BL, Rothwell JC, Findley LJ, Marsden CD. Rapid wrist movements in patients with essential tremor: the critical role of the second agonist burst.
Busenbark KL, Nash J, Nash S, Hubble JP, Koller WC. Is essential tremor benign?
Day BL, Thompson PD, Harding AE, Marsden CD. Influence of vision on upper limb reaching movements in patients with cerebellar ataxia.
Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. [Review].
Dupuis MJ, Delwaide PJ, Boucquey D, Gonsette RE. Homolateral disappearance of essential tremor after cerebellar stroke.
Fahn S. Atypical, rare and unclassified tremors. In: Findley LJ, Capildeo R, editors. Movement disorders: tremor. London: Macmillan; 1984. p. 85–93.
Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson's disease and movement disorders. 2nd ed. Baltimore: Williams and Wilkins; 1993. p. 271–80.
Findley LJ, Koller WC. Definitions and behavioral classifications. In: Findley LJ, Koller WC, editors. Handbook of tremor disorders. New York: Marcel Dekker; 1995b. p. 1–6.
Flament D, Hore J. Movement and electromyographic disorders associated with cerebellar dysmetria.
Flament D, Hore J. Comparison of cerebellar intention tremor under isotonic and isometric conditions.
Frost JD Jr. Triaxial vector accelerometry: a method for quantifying tremor and ataxia.
Gentilucci M, Negrotti A. Planning and executing an action in Parkinson's disease.
Goodkin HP, Keating JG, Martin TA, Thach WT. Preserved simple and impaired compound movement after infarction in the territory of the superior cerebellar artery.
Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor.
Hallett M, Massaquoi SG. Physiologic studies of dysmetria in patients with cerebellar deficits. [Review].
Hallett M, Shahani BT, Young RR. EMG analysis of patients with cerebellar deficits.
Hallett M, Shahani BT, Young RR. EMG analysis of stereotyped voluntary movements in man.
Hore J, Flament D. Evidence that a disordered servo-like mechanism contributes to tremor in movements during cerebellar dysfunction.
Hore J, Wild B, Diener HC. Cerebellar dysmetria at the elbow, wrist, and fingers.
Hubble JP, Busenbark KL, Pahwa R, Lyons K, Koller WC. Clinical expression of essential tremor: effects of gender and age.
Jankovic J, Frost JD Jr. Quantitative assessment of parkinsonian and essential tremor: clinical application of triaxial accelerometry.
Jeannerod M. The formation of finger grip during prehension. A cortically mediated visuomotor pattern.
Jenkins IH, Frackowiak RS. Functional studies of the human cerebellum with positron emission tomography.
Knutsson E, Martensson A. Dynamic motor capacity in spastic paresis and its relation to prime mover dysfunction, spastic reflexes and antagonist co-activation.
Koller WC, Glatt S, Biary N, Rubino FA. Essential tremor variants: effect of treatment.
Larsson T, Sjögren T. Essential tremor: a clinical and genetic population study.
Lauk M, Timmer J, Lucking CH, Honerkamp J, Deuschl G. A software for recording and analysis of human tremor.
Lewin L. Untersuchungen über Banisteria Caapi Spr. (Ein südamerikanisches Rauschmittel).
Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients.
Louis ED, Ford B, Lee H, Andrews H, Cameron G. Diagnostic criteria for essential tremor: a population perspective.
Louis ED, Ford B, Wendt KJ, Cameron G. Clinical characteristics of essential tremor: data from a community-based study.
Marsden CD. Origins of normal and pathologic tremor. In: Findley LJ, Capildeo R, editors. Movement disorders: Tremor. London: Macmillan Press; 1984. p. 37–84.
Massaquoi S, Hallett M. Kinematics of initiating a two-joint arm movement in patients with cerebellar ataxia.
Meyer-Lohmann J, Conrad B, Matsunami K, Brooks VB. Effects of dentate cooling on precentral unit activity following torque pulse injections into elbow movements.
Nagaratnam N, Kalasabail G. Contralateral abolition of essential tremor following a pontine stroke.
Pennes HH, Hoch PH. Psychotomimetics, clinical and theoretical considerations: armine, Win-2299 and alline.
Raethjen J, Pawlas F, Wenzelburger R, Gerstmann F, Deuschl G. A normative study of physiological hand and finger tremor.
Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. Gait analysis in essential tremor – further evidence for a cerebellar dysfunction.
Timmer J, Lauk M, Deuschl G. Quantitative analysis of tremor time series.
Topka H, Konczak J, Dichgans J. Coordination of multi-joint arm movements in cerebellar ataxia: analysis of hand and angular kinematics.
Topka H, Konczak J, Schneider K, Boose A, Dichgans J. Multijoint arm movements in cerebellar ataxia: abnormal control of movement dynamics.
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology.
Vilis T, Hore J. Effects of changes in mechanical state of limb on cerebellar intention tremor.
von Hofsten C. Structuring of early reaching movements: a longitudinal study.
Wild B, Klockgether T, Dichgans J. Acceleration deficit in patients with cerebellar lesions. A study of kinematic and EMG-parameters in fast wrist movements.
Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study.