-
PDF
- Split View
-
Views
-
Cite
Cite
Russell A. Poldrack, John D. E. Gabrieli, Characterizing the neural mechanisms of skill learning and repetition priming: Evidence from mirror reading, Brain, Volume 124, Issue 1, January 2001, Pages 67–82, https://doi.org/10.1093/brain/124.1.67
Close - Share Icon Share
Abstract
The changes in brain activity related to skill learning and repetition priming in a mirror-reading task were examined using functional MRI. Subjects exhibited significant learning across five training sessions and this learning generalized significantly to different spatial transformations (inverted-mirror reversed text and normal letters spelled backwards). Mirror reading, compared with reading normal text, was associated with extensive activation in occipital, temporal, parietal and frontal regions. Learning to read mirror-reversed (MR) text was associated with increased activation in left inferior temporal, striatal, left inferior prefrontal and right cerebellar regions and with decreased activity in the left hippocampus and left cerebellum. Short-term repetition priming was associated with reduced activity in many of the regions active during mirror reading and extensive item-specific practice (long-term repetition priming) resulted in a virtual elimination of activity in those regions. Short- and long-term repetition priming thus appeared to rely upon common neural mechanisms. Nearly all of the regions exhibiting significant learning-related changes also exhibited increased repetition priming effects, suggesting common neural substrates for priming and skill learning in this task. Comparison of MR items with other spatially transformed typographies showed that the learning-related changes were general to all of the spatial transformations. The results confirm the importance of striatofrontal neural networks for the acquisition of skills, and suggest that skill learning and repetition priming may have common substrates within a particular task.
Introduction
The ability of humans and other animals to learn from experience, once thought to be a unitary function, is now recognized to be supported by multiple memory systems with different functional characteristics and neural bases (Squire, 1992; Cohen and Eichenbaum, 1993). A fundamental distinction in memory is between the declarative and non-declarative (or procedural) memory systems. Declarative memory supports explicit recollection of events and facts and is thought to rely upon the medial temporal lobe and diencephalic structures; damage to these regions leads to amnesia, a global impairment of declarative memory. Non-declarative memory comprises a number of memory functions that are independent of the medial temporal lobe, as evidenced by their sparing in people with amnesia, and are thought to rely upon a number of cortical and subcortical structures. Non-declarative memory functions include skill learning (the acquisition of general task procedures with practice), repetition priming (item-specific learning) and classical conditioning.
Skill learning and repetition priming: common systems?
Some previous work has suggested that non-declarative memory may include independent systems specialized for skill learning and repetition priming. In particular, dissociations between skill learning and repetition priming have been demonstrated in studies of patient groups. For example, Heindel and colleagues examined priming (using a word-stem completion task) and skill learning (using a rotary pursuit task) in patients with Huntington's disease and Alzheimer's disease (Heindel et al., 1989). They found a double dissociation, in which Huntington's disease patients exhibited normal repetition priming on the stem completion task but impaired motor learning on the rotary pursuit task, whereas Alzheimer's disease patients showed the opposite pattern. However, because skill learning and repetition priming were measured using different tasks these results are equally consistent with a dissociation between different types of knowledge (e.g. lexical/semantic versus perceptual versus motor learning) than a dissociation between skill learning and repetition priming per se. Dissociations between skill learning and repetition priming have also been demonstrated within a single task in normal subjects. For example, Schwartz and Hashtroudi (1991) found that skill learning and repetition priming were uncorrelated in both word identification and inverted-reading tasks. Although these findings seem to suggest separate skill learning and repetition priming systems, others (Poldrack et al., 1999b) have argued on the basis of behavioural and computational evidence that these data can be accommodated by a single mechanism for both skill learning and repetition priming (in any particular task).
These two views of non-declarative memory make opposing claims concerning the neural bases of skill learning and repetition priming; the independence theory predicts that separate brain regions should exhibit neural changes related to skill learning and repetition priming, whereas the single-system theory predicts that similar areas should exhibit learning-related and priming-related changes within a particular task (although these areas would probably differ for different tasks). We examined this question in a preliminary fashion using functional MRI (fMRI) in a study of the mirror-reading task (Poldrack et al., 1998). In this task, subjects are presented with mirror-reversed (MR) text to read. With practice, subjects can become quite skilled at this task and learning is persistent, lasting at least 1 year (Kolers, 1976). Learning occurs normally in amnesic patients undertaking the mirror-reading task (Cohen and Squire, 1980; Martone et al., 1984), suggesting that it does not rely upon the medial temporal lobe. Such skill learning appears to be impaired in patients with Huntington's disease (Martone et al., 1984), suggesting that it may rely upon the striatum or striatofrontal networks.
In our previous study, subjects were presented with novel MR words and pseudowords, and asked to perform lexical decisions on these items; the lexical decision task was used instead of the usual reading-aloud procedure because of the constraints of fMRI on overt speech. Subjects participated in two scanning sessions, and in between these sessions were trained for three sessions on the mirror-reading task. In addition, they received extensive practice on a small set of repeated items during training. During scanning, lexical decisions on novel MR items were compared with those on items presented in plain text, as well as comparing them in the second session with items that had been practised during training. Only the posterior portion of the brain was imaged using fMRI. We found a network of occipital, superior parietal and inferior temporal regions involved in mirror reading compared with reading of plain text. Increased activity following learning was observed in the left inferior temporal cortex, while decreased activity was observed in the lateral occipital and superior parietal cortex. Repetition priming has generally been associated with decreased neural activity for repeated compared with novel stimuli (Squire et al., 1992; Demb et al., 1995; Gabrieli et al., 1996) and we found such priming-related decreases in a number of regions, including the left inferior temporal region that exhibited a learning-related increase in activity, i.e. as this region became more engaged in the task (evidenced by the learning-related increase) it began to exhibit a greater priming effect (evidenced by the priming-related decrease). These data were taken as preliminary evidence for common neural substrates in skill learning and repetition priming; however, the study did not include the conditions necessary to test this view strongly.
In the present study we examined short-term repetition priming, both before and after training on the mirror-reading task, by presenting sets of new MR items and then presenting the same items soon after (within 24 s). This design allowed us to examine directly how priming changed as a function of skill learning. We also included a set of repeated items during the training session in order to identify regions showing long-term repetition priming effects. Thus, we were able to determine whether short-term priming and long-term priming had common neural substrates.
What is learned in mirror reading?
In our previous study (Poldrack et al., 1998) we found decreasing activation with learning in regions of the right dorsal visual path (superior parietal cortex) and increasing activation in the left ventral visual path (inferior temporal cortex). These changes were interpreted as reflecting a shift from visuospatial transformation to direct recognition of the MR stimuli, but there were no manipulations in the previous study to test directly the nature of the processes underlying these changes. There are, for example, several possible explanations for the increasing inferior temporal activation that are equally consistent with the previous data. First, the increase in activation could reflect development of novel representations for individual MR letters [consistent with the results of Masson (1986)]. A second possibility is that increase in activation reflected processes that were letter-specific but not specific to the MR typography, such as skill in the recognition of transformed versions of particular letters. Another possibility is that the increase reflected general visual processing procedures that were specific to the MR typography (as proposed by Kolers, 1975), but not letter-specific as argued by Masson (Masson, 1986). A fourth possiblity is that the increase reflected processes that are neither letter-specific nor specific to the MR typography.
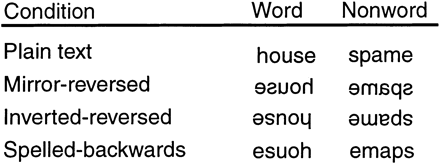
The present study included two control conditions with different transformed typographies [inverted-reversed (IR) and spelled-backwards (SB); see Fig. 1] to allow investigation of the specificity of learning in the mirror-reading task. Learning that is specific to the MR typography should result in changes in activation for only MR items, whereas non-specific changes in processing should result in equivalent learning-related changes for all typographies. Differences in activity between SB and IR items provide evidence about the degree to which learning in the task is specific to visuospatial transformation.
Material and methods
Participants
Sixteen volunteers from the Stanford community (seven males, nine females; mean age 20.3 years, SD 1.7) participated in the study. All subjects were right-handed [based on the Briggs and Nebes (1975) handedness inventory] and were native speakers of English. Informed consent was obtained for each participant prior to the experiment in a manner approved by the Stanford University Human Subjects Committee, which also approved the study, and participants were screened for any possible contraindications prior to entering the MR suite.
Materials
For the mirror-reading scans, a list of 1584 words was drawn from the Kucera and Francis database (Kucera and Francis, 1967), with word frequency ranging from 10 to 100 occurrences (mean 29.8, SD 20.9) and word length from five to eight letters (mean 6.5, SD 1.1). For each word, a matched pseudoword was created by changing one consonant in the word, with the constraint that the word remained pronounceable. Words from this list were randomly assigned to conditions in the experiment, with the constraint that word frequency and word length did not differ between conditions.
Experimental design
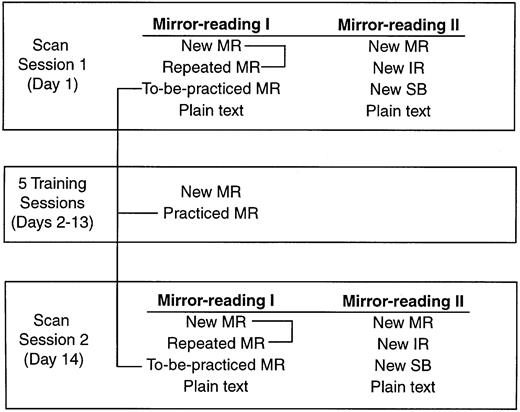
The study consisted of two fMRI scanning sessions with five training sessions in between. Figure 1 presents an overview of the stimuli used in the fMRI scans and training and Fig. 2 presents a schematic representation of the experimental design. In the first fMRI session, subjects participated in three functional scans. The first scan, performed as part of a separate study, is not reported here. In each of the two following scans, subjects made lexical decisions based on four different types of stimuli; the order of these scans and the order of conditions within the scans were counterbalanced across subjects. In one scan (Mirror Reading I), the stimuli consisted of MR (new items, first presentation), MR repeated (second presentation of MR items), MR practised (items to be practised during training sessions) and plain text. In the other scan (Mirror Reading II), the stimuli consisted of MR, inverted-reversed, spelled backwards and plain text (items in all conditions in Mirror Reading II were unique). A cue (`<' or `>') was presented laterally to the stimulus 200 ms before each stimulus in the Mirror Reading II scan to alert the subject to the direction from which they should read, so that items in the spelled-backward condition were not read as non-words. In both mirror reading scans, items were presented for 2000 ms with an 800 ms interstimulus interval and subjects were asked to press a response key as quickly as possible if the item was a real word. Each mirror reading scan consisted of four cycles through each of the four conditions and lasted a total of 460.8 s (during which 160 images were collected); an additional four images were collected at the beginning of each scan and discarded. The order of the two scans (Mirror Reading I and II) was counterbalanced across subjects.
Following the first fMRI session, subjects returned for five training sessions over 2 weeks. In each session, the subject was presented with four blocks of 96 MR items; half of the items were pseudowords and half were real words. In each block, 48 of the items were unique and were seen only once during training, whereas 48 were repeated from the MR practised condition in fMRI session 1; these repeated items appeared in every block for a total of 20 repetitions. Unlike the scanning session, in which subjects had only 2800 ms to perform the task on each item, the stimulus remained on the screen until a response was made during the training sessions. Subjects pressed one button if the item was a word and a different button if the item was a non-word. This differed from the go/no-go response that was peformed during fMRI scanning.
Two weeks after the first fMRI scan and following completion of all training sessions, subjects returned for a second fMRI session. The scans performed in this session were identical in design to those performed in session 1, with the exception that all items were new (except those in the MR practised condition, which were repeated across the first scan session and all training sessions). Order of task presentation within each scan was counterbalanced across subjects and each item presented during scanning appeared equally often in fMRI sessions 1 and 2 across subjects.
Stimuli were generated by a Macintosh computer and back-projected onto a screen located above the subject's neck via a magnet-compatible projector. The projected image appeared on a mirror mounted above the subject's head. Subjects responded by pressing a switch with the right index finger and responses were collected by a computer interfaced to the switch using the PsyScope button box.
MRI data acquisition
Imaging was performed on a 1.5 T GE Signa whole-body MRI system. A prototype birdcage receive-only head coil was used for signal reception. Head movement was minimized using a bite-bar formed with the subject's dental impression. The onset of scanning was controlled by the experimental presentation program via a pulse output for precise synchronization of scanning and stimulus presentation.
Sixteen contiguous axial images were imaged (6 mm thickness) parallel to the AC–PC (anterior–posterior commissure) line. A T2*-sensitive gradient echo spiral pulse sequence (Glover and Lai, 1998) was used for functional imaging with parameters of repetition time = 1440 ms, echo time = 40 ms, flip angle = 80°, field of view = 24 cm and two spiral interleaves (for a total time per image of 2880 ms and in-plane resolution of 2.9 mm). T1-weighted spin-echo images were collected for the same set of slices. A high-resolution three-dimensional SPGR (spoiled gradient-recalled) volume was also collected for use in spatial normalization.
MRI data analysis
Functional data were reconstructed by gridding to a Cartesian matrix before two-dimensional Fourier transform and a correction for blurring resulting from off-resonance (Irarrazabal et al., 1996) was applied. After reconstruction, functional images were motion-corrected using AIR 3.0 with interpolation to 3 mm cubic voxels. Images were then spatially normalized to the MNI305 space (which approximates the stereotactic space of Talairach and Tournoux, 1988) with SPM99 using a 12 parameter affine transformation followed by non-linear warping using 7×8×7 discrete cosine basis functions. Data were smoothed with a Gaussian filter (8 mm full-width at half-maximum).
Data were analysed in SPM99 using an initial analysis treating subjects as a fixed effect (i.e. including all images for each subject). One subject was excluded from the analysis of Mirror Reading I and two subjects were excluded from the analysis of Mirror Reading II because extensive artefacts were evident in the individual subject analyses. Global signal variation was removed using proportional scaling and low-frequency signal components were removed by including low-frequency covariates in the design matrix. Regions were identified in which activity was either significantly greater (i.e. activation) or significantly less (i.e. deactivation) during all of the transformed text conditions compared with the plain text condition across both scans in each scanning session, providing an estimate of active regions that is not biased towards any of the particular conditions. This analysis was performed separately across the two sessions to avoid exclusion of regions exhibiting learning-related changes. Regions of significant activation were first identified by thresholding the statistical parametric map at P = 0.001 (uncorrected for multiple comparisons) along with a cluster extent threshold of at least 10 voxels. The activations identified by this analysis were characterized further according to the theory of Gaussian random fields in order to provide a correction for multiple comparisons (P = 0.05). Multiple maxima within a cluster of activation were identified if they were at least 8 mm apart.
Because global signal normalization can result in spurious deactivations if the global signal level is correlated with the task, we performed a correlation analysis to rule this out so that deactivations and session×condition interactions could be confidently interpreted. For each session and subject, the correlation was measured between a delayed boxcar representing the task conditions and the global signal level for Mirror Reading I. Two such analyses were performed using delayed boxcar reference functions, one representing only new MR and plain text, and another representing all MR conditions compared with plain text. These correlations were compared with zero using a one-sample t test across subjects. There was no significant correlation between global signal and the task function (rs ranging from –0.09 to 0.06, Ps > 0.1). This suggests that the deactivations and session×condition interactions observed in this study reflect true signal changes rather than artefacts of global signal normalization.
The regions identified in this initial fixed-effect analysis were then used to perform region-of-interest (ROI) analyses treating subjects as a random effect [using repeated measures analysis of variance (ANOVA) with the Huyhn–Feldt correction for non-sphericity]. The ROI was determined using contrasts that were orthogonal to those examined in the ROI analysis to ensure unbiased comparisons. For each maximum voxel location identified in the unbiased comparison, the adjusted magnetic resonance signal was summed over that voxel and all voxels that were connected to that voxel (using an 18-connectivity scheme) in which there was significant activation in the unbiased analysis (P < 0.001). This resulted in a maximum of 19 voxels being included in each ROI. Specific comparisons between conditions were performed using linear contrasts.
Results
Behavioural results
Response times and accuracy for subjects during fMRI scanning are presented in Fig. 3. Behavioural data during scanning were unavailable for two of the 16 subjects due to malfunction of the button box. Correct responses for each scan were analysed using a 2 (session)×4 (condition) repeated measures ANOVA with the Huyhn–Feldt correction for non-sphericity.
Mirror Reading I
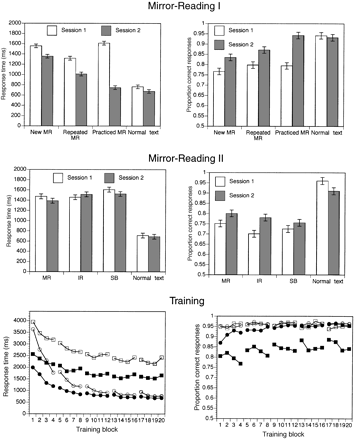
For response times, there were significant main effects of session and condition, along with a significant interaction (all Ps < 0.001). Paired comparisons (contrasts) were used to examine differences between conditions. In both sessions, MR items were read more slowly than plain text (Ps < 0.001) and new MR items were read more slowly than repeated MR items (Ps < 0.001). Performance on new MR items was faster in session 2 than session 1 (P < 0.001), whereas there was only a marginally significant difference between sessions 1 and 2 for plain text (P = 0.09). There was a larger absolute increase for MR compared with plain text, but the proportional change in response time was similar (13.0% speed-up for MR versus 11.7% speed-up for plain text). Performance on practised MR items (which were seen 20 times during training) was faster in session 2 than in session 1 (P < 0.001) and did not differ from plain text in session 2 (P = 0.16).
For accuracy, there were main effects of session (P = 0.015) and condition (P < 0.001) and a significant interaction (P < 0.001). Planned comparisons showed that accuracy was greater for plain than for new MR items in both sessions 1 and 2 (Ps < 0.001). There was no significant difference in accuracy between new and repeated MR items in either session 1 or session 2 (Ps > 0.1). Accuracy on MR items increased from session 1 to session 2 (P = 0.003), but there was no difference in accuracy between sessions for plain text (P = 0.69). Accuracy on practised items was greater in session 2 than session 1 (P < 0.001), and did not differ from accuracy on plain text items in session 2 (P = 0.63).
Mirror Reading II
For response times, there was a significant main effect of condition only (P < 0.001); the effect of session (P = 0.41) and the interaction (P = 0.35) were not significant. Responses to all classes of transformed items were slower than plain text in both sessions 1 and 2 (Ps < 0.001). There was no significant difference between IR and MR items (P = 0.80) in session 1, whereas responses to SB items were slower than MR items (P = 0.04). In session 2 the difference between MR and IR (P = 0.05) and the difference between MR and SB (P = 0.04) were both significant. None of the conditions exhibited significant changes in response time between sessions (Ps > 0.18), although inspection of the results suggests that responses to MR and SB items became faster with practice, whereas responses to IR items became slower with practice. The fact that there was no significant decrease from session 1 to session 2 in MR response times (whereas such a decrease did occur for the same condition in Mirror Reading 1) suggests that the inclusion of additional spatially transformed conditions or the use of the directional cue may have affected performance on MR items in Mirror Reading II.
For accuracy, there was a significant effect of session (P < 0.001) and a significant interaction (P = 0.003); the effect of session was not significant (P = 0.25). Performance on all transformed conditions was less accurate than plain items in both sessions (Ps < 0.001). Accuracy increased from session 1 to session 2 for MR (P = 0.05) and IR (P = 0.002), whereas there was no such change for SB items (P = 0.21). Accuracy on plain text items decreased significantly from session 1 to session 2 (P = 0.04).
Training
Training data for all 16 subjects were analysed separately for response time (correct responses only) and accuracy using a four condition (new word, new non-word, repeated word, repeated non-word), 20 (training block) repeated measures ANOVA; these data are presented in Fig. 3. For response times, there were significant main effects of condition and training block and a significant interaction (all Ps < 0.001). Response times decreased with training for all conditions, with repeated items exhibiting a greater speed-up. For accuracy, there were significant main effects of condition (P < 0.001) and training block (P < 0.001) and a significant interaction (P = 0.005). Performance on non-words was near ceiling, suggesting a speed–accuracy trade-off between words and non-words. However, the increase in accuracy over training rules out a speed–accuracy trade-off explanation for the decreased response times over training.
fMRI results
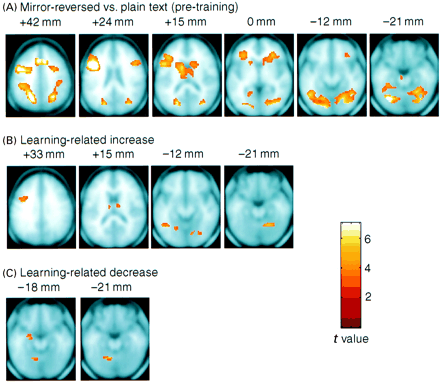
Results from the fixed-effect analysis are rendered onto a standard brain in Fig. 4 and are presented on averaged anatomical slices in Fig. 5. It should be noted that the slice prescription in some subjects resulted in a truncation of the image volume at approximately Z = 45. Thus, data were not available from the most superior aspects of the parietal and frontal cortices.
Mirror reading versus plain text
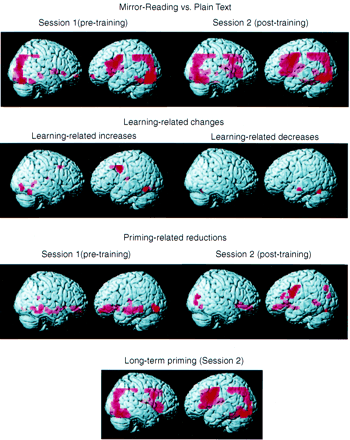
Stereotactic locations for activations during reading of novel MR items compared with plain text in each session are presented in Table 1. Mirror reading in session 1 (pre-training) was associated with extensive activation along both the dorsal and ventral visual streams, including the occipital, parietal and inferior temporal regions. In addition, there was activation throughout the posterior left prefrontal cortex, including the premotor and inferior frontal cortices, and less extensive activation in the right prefrontal cortex. Activation was also found in the right cerebellum and right basal ganglia (putamen/globus pallidus). Mirror reading in session 2 (post-training) engaged a similar set of regions.
Learning-related changes
In order to examine directly changes related to learning, activation for mirror reading compared with plain text was compared across the pre-training and post-training scanning sessions (by testing for a condition×session interaction). These results are presented in Table 2 and are illustrated in Fig. 4. Learning-related increases were found in the right cerebellum, left inferior frontal/premotor cortex, left inferior temporal/fusiform regions, anterior cingulate and in the tail of the caudate nucleus.
Only two regions showed significant decreases in activation in the fixed-effects analysis related to skill learning in the mirror-reading task: the left cerebellum and the left hippocampus. However, the superior parietal region that exhibited a learning-related decrease in the previous study (Poldrack et al., 1998) was not fully included in the imaging window for this analysis (because of missing data for some subjects due to the slice prescription). An additional fixed-effect group analysis was run on the eight subjects for whom the slice prescription covered the superior parietal cortex (i.e. with data extending to at least Z = 56), in order to determine whether a decrease occurred for those subjects. However, no significant decrease was found in the superior parietal region in this analysis.
Short-term repetition priming
Regions exhibiting significant priming-related reductions in activation (novel MR > repeated MR) are presented in Fig. 4 and their stereotactic locations are presented in Table 3. In session 1 (pre-training), priming-related reductions were evident bilaterally along the ventral visual pathway, including the inferior occipital, fusiform and parahippocampal cortices, with activation extending into the hippocampus proper in the left hemisphere. Following training, reductions in ventral path activation were still evident. In addition, extensive priming-related reductions became evident in the left inferior frontal and premotor regions.
Long-term repetition priming
Long-term priming (measured as the difference between novel and practised MR items in session 2) was associated with extensive reductions in activation compared with new MR items. These regions (shown in Fig. 4 and listed in Table 3) included bilateral inferior frontal and premotor cortex, bilateral intraparietal and occipital cortex, bilateral basal ganglia (caudate/putamen) and the anterior cingulate.
Mirror reading of practised items was compared with reading of plain text, in order to determine whether differences in neural processing persisted even when response times and accuracy did not differ from plain text. This analysis found regions of activation in the precuneus, orbitofrontal cortex and right inferior parietal cortex. Thus, the occipital, parietal and frontal activations apparent during reading of new MR items were not evident during reading of highly trained MR items compared with plain text.
ROI analysis of priming effects
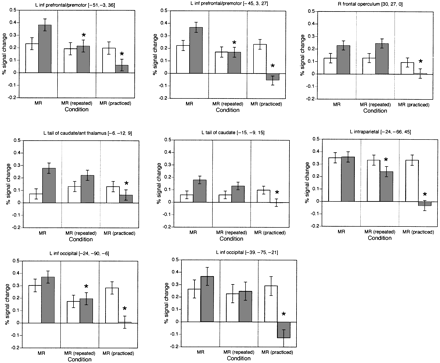
In order to examine more directly priming effects in the context of skill learning, magnetic resonance signal was examined in each of the ROIs based upon all transformed conditions compared with plain text; these data were analysed using a 2 (session)×4 (condition: novel MR, repeated MR, practised MR and plain text) repeated measures ANOVA. A significant session×condition interaction (P < 0.05) was found in eight regions. The data from these ROIs are presented (as percentage signal change compared with plain text) in Fig. 6.
Long-term priming effects (i.e. less activity for practised compared with new MR items) occurred in all ROIs. Short-term priming effects (i.e. reduced signal change for repeated compared with novel MR stimuli) were not significant in any of the ROIs prior to training (although trends were visible in the left occipital cortex), whereas short-term priming effects were significant following training in the left inferior frontal, left intraparietal and posterior left inferior occipital regions, with visible trends toward such priming in the caudate nucleus and anterior left inferior occipital cortex. The right frontal operculum exhibited long-term priming but no evidence of either skill learning or short-term priming in either session.
Effects of different spatial transformations
The brain regions engaged by different visuospatial transformations (in Mirror Reading II) were examined using contrasts to compare brain activity arising from items with those transformations. When compared with reading of plain text in session 1, reading of MR, IR and SB items all engaged a set of brain regions similar to those engaged by mirror reading in Mirror Reading I (see Fig. 4). For this reason, we focused on those regions that exhibited differential response between the different transformations. These regions are shown in Fig. 4 and their stereotactic locations are listed in Tables 4 and 5.
Compared with MR items, there was little additional activation for items spelled backwards; in session 1 there was activation in the right inferior frontal cortex and in session 2 there was activation in the left cerebellum. There were a number of regions that exhibited greater activation for MR compared with SB items. In both sessions, there was activation in the posterior parahippocampal/lingual gyri bilaterally. In session 1, there was additional activation in the right hippocampus, whereas a number of regions were activated in session 2, including the left dorsolateral prefrontal cortex, left anterior fusiform gyrus, left cerebellar nuclei, cingulate cortex and left inferior parietal cortex (additional regions listed in Table 4).
In the comparison of IR items to MR items, the bilateral inferior occipital/temporal cortices and right cerebellum were active in both sessions (stereotactic locations are listed in Table 5). Additionally, in session 1 there was activation for IR items in the right frontal operculum, whereas in session 2 there was activation in the intraparietal cortex bilaterally, right superior parietal cortex and left cerebellum. For MR compared with IR items, the right lingual/parahippocampal cortex was active in both sessions; additionally, the left occipital and left inferior frontal cortex was active in session 1 and the left cerebellar nuclei and precuneus were active in session 2.
ROI analysis of spatial transformation effects
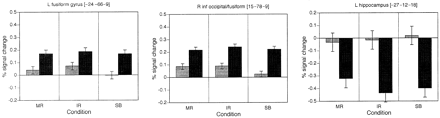
In order to determine the degree to which learning-related effects were specific to the MR font, magnetic resonance signal for each condition in Mirror Reading II was examined in each of the regions independently identified in the analysis of learning-related effects (listed in Table 2). These data were analysed using a 2 (session)×4 (condition: MR, IR, SB and plain text) repeated measures ANOVA. Two regions in this analysis exhibited significant condition×session interactions (P < 0.05): the right inferior occipital/fusiform cortex (which exhibited a learning-related increase) and the left hippocampus (which showed a learning-related decrease). Signal from these regions is displayed in Fig. 7. The left fusiform cortex was also included because of a priori interest based upon a previous study (Poldrack et al., 1998), although the session×condition was only marginally significant in this region (P = 0.13). In each of these regions, learning resulted in similar increases in activity for each of the spatial transformations. There was no evidence of changes that were specific to MR text.
Discussion
We examined neural changes related to skill learning and repetition priming in a mirror-reading task using fMRI. The results indicated that learning was associated with increased activation in left inferior temporal, left inferior prefrontal, striatal and right cerebellar regions and with decreased activity in the left hippocampus and left cerebellum. These results partially replicate the findings of our previous study (Poldrack et al., 1998), and extend those results by demonstrating the involvement of striatofrontal circuits in skill learning on this task. Short-term repetition priming was associated with reduced activity in many of the regions active during mirror reading and extensive item-specific practice resulted in a virtual elimination of activity in those regions. Nearly all of the regions exhibiting significant learning-related changes also exhibited increased priming-related reductions, suggesting common neural substrates for priming and skill learning in this task. Comparison of MR items with other transformed typographies showed that the learning-related changes associated with skill learning are not specific to the MR typeface, extending equally to IR and SB items.
Neural mechanisms of skill learning
Learning in the mirror-reading task was associated with increased activation in a number of regions. Increasing activity in the left prefrontal and right cerebellar regions may reflect increased engagement of lexical search and/or retrieval systems necessary to perform the lexical decision task. It is unlikely that these changes reflect duty cycle or total time-on-task (Poldrack, 2000) because response time decreased from session 1 to session 2. Rather, the increase in the frontal-cerebellar system may reflect increased lexical processing that is a consequence of increasingly accurate decoding of the transformed stimuli. Early in training, most of the time on task is spent on visually decoding the transformed stimuli (because the amount of time available to perform the task is limited), whereas later in training a greater proportion of that time on task can be spent on lexical processing.
The left inferior temporal and fusiform cortex also exhibited increased activation with training on the mirror-reading task. These increases were previously attributed to the development of novel representations of the MR letters (Poldrack et al., 1998). However, in the present study there was a similar learning-related increase in activation for MR, IR and SB stimuli (Fig. 7), which suggests that learning is specific neither to individual letters nor to particular typographies. This finding is consistent with several behavioural studies, which have found (contrary to Masson, 1986) that learning in the mirror-reading task is not specific to the particular typography encountered during training (Horton, 1985; Tardif and Craik, 1989; Horton and McKenzie, 1995). These studies have suggested instead that facilitation on the mirror-reading task is based upon lexical and/or semantic representations instead of orthographic (letter form) representations. Based upon these results and other relevant findings, we hypothesize that the learning-related increase in left inferior temporal and fusiform regions found in the present study and in that of Poldrack and colleagues (Poldrack et al., 1998) may reflect increased engagement of lexical/phonological processes as learning progresses. There is evidence that the inferior temporal region is engaged in service of phonological processing in reading, particularly for unfamiliar word-like stimuli; e.g. comparisons of visual presentation of pseudowords compared with letterstrings have demonstrated activation in this area (e.g. Frith et al., 1995; Price et al., 1996). In addition, this region is activated during the performance of phonological monitoring tasks with auditory stimuli (Demonet et al., 1992, 1994; Zatorre et al., 1996), suggesting that the region is involved in phonological processing more generally. The increase in inferior temporal activation in the present study may reflect increased access to phonological representations by spatially transformed stimuli. It is not, however, currently clear how such increased access could generalize across the different stimulus types. Alternatively, increased activation in this region may reflect increased neural synchronization of lexical/phonological processing with spatial transformation processing occurring in the parietal cortex. Similar changes in effective connectivity have been observed during associative object-spatial learning (Buchel et al., 1999). Further work is necessary to determine which of these interpretations is correct.
Role of the striatum in non-declarative memory
Learning in the mirror-reading task was associated with increased activation in the caudate nucleus. This finding extends previous findings of basal ganglia activity associated with motor skill learning (Seitz et al., 1990; Grafton et al., 1995) and cognitive skill learning (Poldrack et al., 1999a). These results are consistent with neuropsychological findings in Huntington's disease and Parkinson's disease, demonstrating impaired learning of motor skills (Heindel et al., 1988, 1989), perceptual skills (Martone et al., 1984) and cognitive skills (Saint-Cyr et al., 1988; Knowlton et al., 1996a). Together these findings suggest that the striatum is a critical structure for skill learning, but its particular role remains to be fully specified. A number of authors (Mishkin et al., 1984; Knowlton et al., 1996a) have suggested that the striatum may be involved in the formation of stimulus–response associations (also called habit learning), whereas we have previously suggested (Poldrack et al., 1999a) that the striatum may play a critical role in mediating dynamic shifts in the involvement of different processing systems as learning progresses. The results of the present study, although not conclusive, are more consistent with the process-switching account than the habit-learning account. Learning-related changes in the striatum were not specific to the MR typography, as would be expected if striatal activation reflected specific stimulus–response associations. However, further studies are necessary to determine conclusively the particular role of the striatum.
The role of the striatum may be segregated further on the basis of its cortical connectivity. Cognitive skill learning has been associated with activation in the head of the caudate (Poldrack et al., 1999a), which is strongly connected to the dorsolateral prefrontal regions that are also active during cognitive skill learning (e.g. Yeterian and Pandya, 1991). In the present study, perceptual skill learning was associated with activation in the tail of the caudate nucleus, which is reciprocally connected to visual processing regions including the inferior temporal cortex (Saint-Cyr et al., 1990). Motor skill learning has been primarily associated with activation in the putamen (Seitz et al., 1990; Grafton et al., 1995), which is extensively connected with the motor cortex. Thus, the different territories of the striatum may support different classes of skill learning, either through their different corticostriatal inputs or their different outputs to frontal cortex.
Relationship between skill learning and repetition priming
The results of the present study provide neural evidence in favour of the proposal that skill learning and repetition priming may rely upon common cognitive and neural processes within a particular task (Logan, 1990; Poldrack et al., 1999b; Gupta and Cohen, 2001). All of the regions identified in our ROI analysis as exhibiting skill-related changes in activation also exhibited long-term repetition priming effects, and a number of them also exhibited short-term priming effects as well (with all but one region exhibiting at least a trend for short-term priming). These data are plainly inconsistent with the notion that skill learning and repetition priming are independent.
This study represents, to our knowledge, the first finding of significant repetition priming effects in the striatum. On the face of it, this finding is inconsistent with previous neuropsychological results, which had demonstrated normal repetition priming effects in Huntington's disease patients on a mirror-reading task (Martone et al., 1984). However, the design of that study may have encouraged subjects to use declarative memory (which is relatively spared in early Huntington's disease). Mirror-reversed words were presented in triplets and the order of the words was not varied when the triplets were repeated. Thus, upon decoding the first word the subject could explicitly remember the other two words in the triplet, rather than decoding all three words. This design feature also explains the impairment of repetition priming in amnesic patients found by Cohen and Squire (1980), who used the same triplet procedure. Further work is necessary, however, to confirm this hypothesis and the lexical decision procedure developed in the present task may be a useful tool for studying mirror reading in a single-word paradigm.
The medial temporal lobe and skill learning
The independence of skill learning from the medial temporal lobe (hippocampus and related cortices) is well established on the basis of neuropsychological studies of amnesic patients (Cohen and Eichenbaum, 1993). Although activation was observed in the right hippocampus and bilateral parahippocampal cortices during mirror reading, it is unlikely that these regions were crucially involved in acquisition of the mirror-reading skill. Instead, they may reflect responses to the novelty of the spatially transformed stimuli (Stern et al., 1996; Gabrieli et al., 1997). Consistent with this is the finding that right hippocampal and bilateral parahippocampal activation were greater for MR compared with spelled-backward stimuli, which have novel word forms but familiar letter forms. These responses would probably support later explicit remembering of the stimuli (Brewer et al., 1998; Wagner et al., 1998), but are unlikely to support acquisition of the mirror-reading skill.
There is some evidence from lesion studies in animals that the medial temporal lobe and striatum may act competitively during learning. For example, Packard and colleagues found that rats with fornix lesions (which disconnect the hippocampus) were better than control rats at acquiring a win–stay maze skill, whereas animals with caudate lesions were impaired at acquiring this skill (Packard et al., 1989). These data and others (e.g. Eichenbaum et al., 1988) suggest that the medial temporal lobe and striatum may compete for control of behaviour during learning. There is also evidence for such competition in humans as they learned to perform a probabilistic classification task during fMRI scanning (Poldrack et al., 1999a). The caudate nucleus was active throughout learning, consistent with the deficient learning observed on the task in patients with Huntington's disease (Knowlton et al., 1996b). The hippocampus was initially deactivated compared with the baseline task, and that deactivation increased during early learning but then decreased later in learning (consistent with the timecourse of learning on the task in amnesic patients; Knowlton et al., 1994). This finding suggested that activity in the hippocampal system was suppressed during skill learning, perhaps via striatal or ventral tegmental projections to the hippocampus (e.g. Gasbarri et al., 1994).
In the present study we found a similar learning-related increase in deactivation of the left hippocampus, which was similar for each of the stimulus transformations (Fig. 7). Because performance was only imaged at two points in time, we are unable to outline the complete timecourse of this deactivation, but the finding converges with the results of Poldrack and colleagues to suggest that the hippocampus may be generally deactivated as a skill is acquired (Poldrack et al., 1999a). This deactivation may reflect a dynamic relationship between the declarative and non-declarative memory systems, in which the two systems compete with each other to control behaviour. In both the present study and that of Poldrack et al. (1999a), the hippocampal deactivation occurred in the anterior hippocampus, suggesting that the deactivation may reflect a specific suppression of memory retrieval processes (Gabrieli et al., 1997), whereas the posterior parahippocampal regions involved in memory encoding remain active. Thus, the acquisition of memory traces may continue (explaining the ability of normal subjects to explicitly remember the stimuli) while the retrieval of these traces is suppressed during performanace. Further work is necessary to confirm the generality of hippocampal suppression during skill learning and to characterize its functional importance.
Activations for new MR items compared with plain text items in Mirror Reading I scans
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| R = right; L = left. P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. ‡Activation in the left and right occipitoparietal regions comprised one larger cluster; we report the right hemisphere maximum, although it did not comprise a separate cluster. | |||||
| Session 1 (pre-training) | |||||
| L intraparietal sulcus/occipital (BA 7/18/19) | –24 | –69 | 45 | 1113* | 9.43† |
| L inferior frontal (BA 44/45) | –45 | 6 | 24 | 695* | 7.97† |
| R superior cerebellum | 39 | –72 | –21 | 494* | 7.72† |
| L inferior temporal (BA 37) | –45 | –54 | –18 | 65* | 6.26† |
| R intraparietal/inferior parietal (BA 7/40) | 36 | –42 | 42 | 40 | 5.36† |
| L orbitofrontal cortex | –12 | 60 | –12 | 12 | 4.58† |
| R parahippocampal cortex | 21 | –30 | –6 | 32 | 4.45† |
| R cerebellum | 30 | –51 | –21 | 41 | 4.39 |
| Brainstem | 3 | –27 | –18 | 76* | 4.38 |
| R orbitofrontal cortex | 12 | 60 | –9 | 13 | 4.16 |
| R putamen/GP | 24 | 9 | 3 | 28 | 3.70 |
| R anterior thalamus | 12 | 0 | 3 | 11 | 3.67 |
| R frontal operculum (BA 47) | 30 | 30 | –3 | 13 | 3.39 |
| Session 2 (post-training) | |||||
| L inferior frontal (BA 44/45) (cluster also included L caudate) | –48 | 0 | 36 | 3037* | 12.62† |
| L intraparietal sulcus/occipital/inferior temporal (BA 7/18/19/37) | –24 | –63 | 45 | 2875* | 9.05† |
| R inferior occipital/intraparietal sulcus/superior cerebellum (BA 18/19/7) | 42 | –78 | –15 | ‡ | 8.87† |
| Brainstem | –6 | –27 | –21 | 277* | 6.46† |
| R inferior frontal (BA 44/45) | 48 | 9 | 27 | 147* | 6.35† |
| L parahippocampal | –15 | –51 | 3 | 85 | 5.27† |
| R hippocampus | 24 | –27 | –3 | 18 | 4.19 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| R = right; L = left. P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. ‡Activation in the left and right occipitoparietal regions comprised one larger cluster; we report the right hemisphere maximum, although it did not comprise a separate cluster. | |||||
| Session 1 (pre-training) | |||||
| L intraparietal sulcus/occipital (BA 7/18/19) | –24 | –69 | 45 | 1113* | 9.43† |
| L inferior frontal (BA 44/45) | –45 | 6 | 24 | 695* | 7.97† |
| R superior cerebellum | 39 | –72 | –21 | 494* | 7.72† |
| L inferior temporal (BA 37) | –45 | –54 | –18 | 65* | 6.26† |
| R intraparietal/inferior parietal (BA 7/40) | 36 | –42 | 42 | 40 | 5.36† |
| L orbitofrontal cortex | –12 | 60 | –12 | 12 | 4.58† |
| R parahippocampal cortex | 21 | –30 | –6 | 32 | 4.45† |
| R cerebellum | 30 | –51 | –21 | 41 | 4.39 |
| Brainstem | 3 | –27 | –18 | 76* | 4.38 |
| R orbitofrontal cortex | 12 | 60 | –9 | 13 | 4.16 |
| R putamen/GP | 24 | 9 | 3 | 28 | 3.70 |
| R anterior thalamus | 12 | 0 | 3 | 11 | 3.67 |
| R frontal operculum (BA 47) | 30 | 30 | –3 | 13 | 3.39 |
| Session 2 (post-training) | |||||
| L inferior frontal (BA 44/45) (cluster also included L caudate) | –48 | 0 | 36 | 3037* | 12.62† |
| L intraparietal sulcus/occipital/inferior temporal (BA 7/18/19/37) | –24 | –63 | 45 | 2875* | 9.05† |
| R inferior occipital/intraparietal sulcus/superior cerebellum (BA 18/19/7) | 42 | –78 | –15 | ‡ | 8.87† |
| Brainstem | –6 | –27 | –21 | 277* | 6.46† |
| R inferior frontal (BA 44/45) | 48 | 9 | 27 | 147* | 6.35† |
| L parahippocampal | –15 | –51 | 3 | 85 | 5.27† |
| R hippocampus | 24 | –27 | –3 | 18 | 4.19 |
Activations for new MR items compared with plain text items in Mirror Reading I scans
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| R = right; L = left. P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. ‡Activation in the left and right occipitoparietal regions comprised one larger cluster; we report the right hemisphere maximum, although it did not comprise a separate cluster. | |||||
| Session 1 (pre-training) | |||||
| L intraparietal sulcus/occipital (BA 7/18/19) | –24 | –69 | 45 | 1113* | 9.43† |
| L inferior frontal (BA 44/45) | –45 | 6 | 24 | 695* | 7.97† |
| R superior cerebellum | 39 | –72 | –21 | 494* | 7.72† |
| L inferior temporal (BA 37) | –45 | –54 | –18 | 65* | 6.26† |
| R intraparietal/inferior parietal (BA 7/40) | 36 | –42 | 42 | 40 | 5.36† |
| L orbitofrontal cortex | –12 | 60 | –12 | 12 | 4.58† |
| R parahippocampal cortex | 21 | –30 | –6 | 32 | 4.45† |
| R cerebellum | 30 | –51 | –21 | 41 | 4.39 |
| Brainstem | 3 | –27 | –18 | 76* | 4.38 |
| R orbitofrontal cortex | 12 | 60 | –9 | 13 | 4.16 |
| R putamen/GP | 24 | 9 | 3 | 28 | 3.70 |
| R anterior thalamus | 12 | 0 | 3 | 11 | 3.67 |
| R frontal operculum (BA 47) | 30 | 30 | –3 | 13 | 3.39 |
| Session 2 (post-training) | |||||
| L inferior frontal (BA 44/45) (cluster also included L caudate) | –48 | 0 | 36 | 3037* | 12.62† |
| L intraparietal sulcus/occipital/inferior temporal (BA 7/18/19/37) | –24 | –63 | 45 | 2875* | 9.05† |
| R inferior occipital/intraparietal sulcus/superior cerebellum (BA 18/19/7) | 42 | –78 | –15 | ‡ | 8.87† |
| Brainstem | –6 | –27 | –21 | 277* | 6.46† |
| R inferior frontal (BA 44/45) | 48 | 9 | 27 | 147* | 6.35† |
| L parahippocampal | –15 | –51 | 3 | 85 | 5.27† |
| R hippocampus | 24 | –27 | –3 | 18 | 4.19 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| R = right; L = left. P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. ‡Activation in the left and right occipitoparietal regions comprised one larger cluster; we report the right hemisphere maximum, although it did not comprise a separate cluster. | |||||
| Session 1 (pre-training) | |||||
| L intraparietal sulcus/occipital (BA 7/18/19) | –24 | –69 | 45 | 1113* | 9.43† |
| L inferior frontal (BA 44/45) | –45 | 6 | 24 | 695* | 7.97† |
| R superior cerebellum | 39 | –72 | –21 | 494* | 7.72† |
| L inferior temporal (BA 37) | –45 | –54 | –18 | 65* | 6.26† |
| R intraparietal/inferior parietal (BA 7/40) | 36 | –42 | 42 | 40 | 5.36† |
| L orbitofrontal cortex | –12 | 60 | –12 | 12 | 4.58† |
| R parahippocampal cortex | 21 | –30 | –6 | 32 | 4.45† |
| R cerebellum | 30 | –51 | –21 | 41 | 4.39 |
| Brainstem | 3 | –27 | –18 | 76* | 4.38 |
| R orbitofrontal cortex | 12 | 60 | –9 | 13 | 4.16 |
| R putamen/GP | 24 | 9 | 3 | 28 | 3.70 |
| R anterior thalamus | 12 | 0 | 3 | 11 | 3.67 |
| R frontal operculum (BA 47) | 30 | 30 | –3 | 13 | 3.39 |
| Session 2 (post-training) | |||||
| L inferior frontal (BA 44/45) (cluster also included L caudate) | –48 | 0 | 36 | 3037* | 12.62† |
| L intraparietal sulcus/occipital/inferior temporal (BA 7/18/19/37) | –24 | –63 | 45 | 2875* | 9.05† |
| R inferior occipital/intraparietal sulcus/superior cerebellum (BA 18/19/7) | 42 | –78 | –15 | ‡ | 8.87† |
| Brainstem | –6 | –27 | –21 | 277* | 6.46† |
| R inferior frontal (BA 44/45) | 48 | 9 | 27 | 147* | 6.35† |
| L parahippocampal | –15 | –51 | 3 | 85 | 5.27† |
| R hippocampus | 24 | –27 | –3 | 18 | 4.19 |
Regions exhibiting significant learning-related changes in activity
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. | |||||
| Learning-related increase | |||||
| R superior cerebellum | 24 | –60 | –24 | 70* | 6.15† |
| L inferior frontal/premotor (BA 44/6) | –42 | 3 | 39 | 137* | 5.33† |
| L inferior temporal (BA 37) | –54 | –60 | –15 | 35 | 4.41† |
| L fusiform (BA 19/37) | –24 | –66 | –9 | 17 | 4.33 |
| L anterior cingulate | –9 | 18 | 42 | 18 | 4.18 |
| R inferior occipital/fusiform (BA 19/37) | 15 | –78 | –9 | 59 | 4.12 |
| R tail of caudate | 9 | –15 | 15 | 48 | 3.96 |
| R anterior cingulate | 15 | 12 | 42 | 18 | 3.86 |
| L cingulate sulcus | –18 | 0 | 45 | 20 | 3.77 |
| R lingual gyrus (BA 19) | 12 | –51 | –3 | 30 | 3.75 |
| Learning-related decrease | |||||
| L cerebellum | –15 | –63 | –21 | 35 | 4.94† |
| L hippocampus | –27 | –12 | –18 | 17 | 4.06 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. | |||||
| Learning-related increase | |||||
| R superior cerebellum | 24 | –60 | –24 | 70* | 6.15† |
| L inferior frontal/premotor (BA 44/6) | –42 | 3 | 39 | 137* | 5.33† |
| L inferior temporal (BA 37) | –54 | –60 | –15 | 35 | 4.41† |
| L fusiform (BA 19/37) | –24 | –66 | –9 | 17 | 4.33 |
| L anterior cingulate | –9 | 18 | 42 | 18 | 4.18 |
| R inferior occipital/fusiform (BA 19/37) | 15 | –78 | –9 | 59 | 4.12 |
| R tail of caudate | 9 | –15 | 15 | 48 | 3.96 |
| R anterior cingulate | 15 | 12 | 42 | 18 | 3.86 |
| L cingulate sulcus | –18 | 0 | 45 | 20 | 3.77 |
| R lingual gyrus (BA 19) | 12 | –51 | –3 | 30 | 3.75 |
| Learning-related decrease | |||||
| L cerebellum | –15 | –63 | –21 | 35 | 4.94† |
| L hippocampus | –27 | –12 | –18 | 17 | 4.06 |
Regions exhibiting significant learning-related changes in activity
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. | |||||
| Learning-related increase | |||||
| R superior cerebellum | 24 | –60 | –24 | 70* | 6.15† |
| L inferior frontal/premotor (BA 44/6) | –42 | 3 | 39 | 137* | 5.33† |
| L inferior temporal (BA 37) | –54 | –60 | –15 | 35 | 4.41† |
| L fusiform (BA 19/37) | –24 | –66 | –9 | 17 | 4.33 |
| L anterior cingulate | –9 | 18 | 42 | 18 | 4.18 |
| R inferior occipital/fusiform (BA 19/37) | 15 | –78 | –9 | 59 | 4.12 |
| R tail of caudate | 9 | –15 | 15 | 48 | 3.96 |
| R anterior cingulate | 15 | 12 | 42 | 18 | 3.86 |
| L cingulate sulcus | –18 | 0 | 45 | 20 | 3.77 |
| R lingual gyrus (BA 19) | 12 | –51 | –3 | 30 | 3.75 |
| Learning-related decrease | |||||
| L cerebellum | –15 | –63 | –21 | 35 | 4.94† |
| L hippocampus | –27 | –12 | –18 | 17 | 4.06 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. | |||||
| Learning-related increase | |||||
| R superior cerebellum | 24 | –60 | –24 | 70* | 6.15† |
| L inferior frontal/premotor (BA 44/6) | –42 | 3 | 39 | 137* | 5.33† |
| L inferior temporal (BA 37) | –54 | –60 | –15 | 35 | 4.41† |
| L fusiform (BA 19/37) | –24 | –66 | –9 | 17 | 4.33 |
| L anterior cingulate | –9 | 18 | 42 | 18 | 4.18 |
| R inferior occipital/fusiform (BA 19/37) | 15 | –78 | –9 | 59 | 4.12 |
| R tail of caudate | 9 | –15 | 15 | 48 | 3.96 |
| R anterior cingulate | 15 | 12 | 42 | 18 | 3.86 |
| L cingulate sulcus | –18 | 0 | 45 | 20 | 3.77 |
| R lingual gyrus (BA 19) | 12 | –51 | –3 | 30 | 3.75 |
| Learning-related decrease | |||||
| L cerebellum | –15 | –63 | –21 | 35 | 4.94† |
| L hippocampus | –27 | –12 | –18 | 17 | 4.06 |
Regions exhibiting significant priming-related reductions in activity
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *This activation occurred in a region of signal dropout due to magnetic susceptibility artefact; †significant at cluster-level corrected P < 0.05; ‡significant at voxel-level corrected P < 0.05. | |||||
| Short-term priming—pre-training | |||||
| Orbital frontal* | –21 | 36 | –9 | 305† | 6.12‡ |
| L hippocampus/parahippocampal cortex/fusiform gyrus | –39 | –42 | –9 | 687† | 4.65‡ |
| R parahippocampal cortex/fusiform gyrus | 33 | –42 | –6 | 146† | 4.39 |
| L inferior occipital (BA 18/19) | –21 | –78 | –9 | 168† | 4.05 |
| White matter | 24 | –48 | 18 | 10 | 3.37 |
| Short-term priming—post-training | |||||
| L inferior frontal/premotor (BA 44/6) | –45 | 3 | 24 | 234† | 5.47‡ |
| L parahippocampal | –12 | –48 | 6 | 97† | 5.26‡ |
| Orbital frontal* | 3 | 48 | –15 | 413† | 4.76‡ |
| R inferior occipital | 24 | –84 | 0 | 74† | 4.26 |
| L premotor (BA 6) | –30 | –6 | 45 | 19 | 4.00 |
| R inferior occipital | 15 | –72 | –9 | 19 | 3.73 |
| L cerebellum | –21 | –75 | –18 | 20 | 3.70 |
| L intraparietal sulcus (BA 7) | –18 | –75 | 42 | 24 | 3.62 |
| L lateral occipital (BA 18/19) | –24 | –81 | 18 | 10 | 3.49 |
| L inferior frontal (BA 45/47) | –54 | 30 | 18 | 11 | 3.28 |
| Long-term priming (post-training) | |||||
| L inferior frontal/premotor (BA 6/44/45/47)/caudate/putamen | –42 | 3 | 27 | 1536† | 12.37‡ |
| L intraparietal sulcus (BA 7)/occipital (BA 18/19) | –24 | –63 | 45 | 1177† | 9.55‡ |
| Anterior cingulate | –6 | 15 | 42 | 282† | 7.73‡ |
| R intraparietal sulcus (BA 7)/occipital (BA 18/19) | 27 | –66 | 42 | 964† | 7.27‡ |
| R frontal operculum (BA 44/47)/caudate/putamen | 30 | 27 | 0 | 409† | 5.84‡ |
| L inferior frontal/premotor (BA 6/44) | 48 | 9 | 27 | 122† | 5.48‡ |
| Brainstem | –3 | –27 | –18 | 16 | 3.70 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *This activation occurred in a region of signal dropout due to magnetic susceptibility artefact; †significant at cluster-level corrected P < 0.05; ‡significant at voxel-level corrected P < 0.05. | |||||
| Short-term priming—pre-training | |||||
| Orbital frontal* | –21 | 36 | –9 | 305† | 6.12‡ |
| L hippocampus/parahippocampal cortex/fusiform gyrus | –39 | –42 | –9 | 687† | 4.65‡ |
| R parahippocampal cortex/fusiform gyrus | 33 | –42 | –6 | 146† | 4.39 |
| L inferior occipital (BA 18/19) | –21 | –78 | –9 | 168† | 4.05 |
| White matter | 24 | –48 | 18 | 10 | 3.37 |
| Short-term priming—post-training | |||||
| L inferior frontal/premotor (BA 44/6) | –45 | 3 | 24 | 234† | 5.47‡ |
| L parahippocampal | –12 | –48 | 6 | 97† | 5.26‡ |
| Orbital frontal* | 3 | 48 | –15 | 413† | 4.76‡ |
| R inferior occipital | 24 | –84 | 0 | 74† | 4.26 |
| L premotor (BA 6) | –30 | –6 | 45 | 19 | 4.00 |
| R inferior occipital | 15 | –72 | –9 | 19 | 3.73 |
| L cerebellum | –21 | –75 | –18 | 20 | 3.70 |
| L intraparietal sulcus (BA 7) | –18 | –75 | 42 | 24 | 3.62 |
| L lateral occipital (BA 18/19) | –24 | –81 | 18 | 10 | 3.49 |
| L inferior frontal (BA 45/47) | –54 | 30 | 18 | 11 | 3.28 |
| Long-term priming (post-training) | |||||
| L inferior frontal/premotor (BA 6/44/45/47)/caudate/putamen | –42 | 3 | 27 | 1536† | 12.37‡ |
| L intraparietal sulcus (BA 7)/occipital (BA 18/19) | –24 | –63 | 45 | 1177† | 9.55‡ |
| Anterior cingulate | –6 | 15 | 42 | 282† | 7.73‡ |
| R intraparietal sulcus (BA 7)/occipital (BA 18/19) | 27 | –66 | 42 | 964† | 7.27‡ |
| R frontal operculum (BA 44/47)/caudate/putamen | 30 | 27 | 0 | 409† | 5.84‡ |
| L inferior frontal/premotor (BA 6/44) | 48 | 9 | 27 | 122† | 5.48‡ |
| Brainstem | –3 | –27 | –18 | 16 | 3.70 |
Regions exhibiting significant priming-related reductions in activity
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *This activation occurred in a region of signal dropout due to magnetic susceptibility artefact; †significant at cluster-level corrected P < 0.05; ‡significant at voxel-level corrected P < 0.05. | |||||
| Short-term priming—pre-training | |||||
| Orbital frontal* | –21 | 36 | –9 | 305† | 6.12‡ |
| L hippocampus/parahippocampal cortex/fusiform gyrus | –39 | –42 | –9 | 687† | 4.65‡ |
| R parahippocampal cortex/fusiform gyrus | 33 | –42 | –6 | 146† | 4.39 |
| L inferior occipital (BA 18/19) | –21 | –78 | –9 | 168† | 4.05 |
| White matter | 24 | –48 | 18 | 10 | 3.37 |
| Short-term priming—post-training | |||||
| L inferior frontal/premotor (BA 44/6) | –45 | 3 | 24 | 234† | 5.47‡ |
| L parahippocampal | –12 | –48 | 6 | 97† | 5.26‡ |
| Orbital frontal* | 3 | 48 | –15 | 413† | 4.76‡ |
| R inferior occipital | 24 | –84 | 0 | 74† | 4.26 |
| L premotor (BA 6) | –30 | –6 | 45 | 19 | 4.00 |
| R inferior occipital | 15 | –72 | –9 | 19 | 3.73 |
| L cerebellum | –21 | –75 | –18 | 20 | 3.70 |
| L intraparietal sulcus (BA 7) | –18 | –75 | 42 | 24 | 3.62 |
| L lateral occipital (BA 18/19) | –24 | –81 | 18 | 10 | 3.49 |
| L inferior frontal (BA 45/47) | –54 | 30 | 18 | 11 | 3.28 |
| Long-term priming (post-training) | |||||
| L inferior frontal/premotor (BA 6/44/45/47)/caudate/putamen | –42 | 3 | 27 | 1536† | 12.37‡ |
| L intraparietal sulcus (BA 7)/occipital (BA 18/19) | –24 | –63 | 45 | 1177† | 9.55‡ |
| Anterior cingulate | –6 | 15 | 42 | 282† | 7.73‡ |
| R intraparietal sulcus (BA 7)/occipital (BA 18/19) | 27 | –66 | 42 | 964† | 7.27‡ |
| R frontal operculum (BA 44/47)/caudate/putamen | 30 | 27 | 0 | 409† | 5.84‡ |
| L inferior frontal/premotor (BA 6/44) | 48 | 9 | 27 | 122† | 5.48‡ |
| Brainstem | –3 | –27 | –18 | 16 | 3.70 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *This activation occurred in a region of signal dropout due to magnetic susceptibility artefact; †significant at cluster-level corrected P < 0.05; ‡significant at voxel-level corrected P < 0.05. | |||||
| Short-term priming—pre-training | |||||
| Orbital frontal* | –21 | 36 | –9 | 305† | 6.12‡ |
| L hippocampus/parahippocampal cortex/fusiform gyrus | –39 | –42 | –9 | 687† | 4.65‡ |
| R parahippocampal cortex/fusiform gyrus | 33 | –42 | –6 | 146† | 4.39 |
| L inferior occipital (BA 18/19) | –21 | –78 | –9 | 168† | 4.05 |
| White matter | 24 | –48 | 18 | 10 | 3.37 |
| Short-term priming—post-training | |||||
| L inferior frontal/premotor (BA 44/6) | –45 | 3 | 24 | 234† | 5.47‡ |
| L parahippocampal | –12 | –48 | 6 | 97† | 5.26‡ |
| Orbital frontal* | 3 | 48 | –15 | 413† | 4.76‡ |
| R inferior occipital | 24 | –84 | 0 | 74† | 4.26 |
| L premotor (BA 6) | –30 | –6 | 45 | 19 | 4.00 |
| R inferior occipital | 15 | –72 | –9 | 19 | 3.73 |
| L cerebellum | –21 | –75 | –18 | 20 | 3.70 |
| L intraparietal sulcus (BA 7) | –18 | –75 | 42 | 24 | 3.62 |
| L lateral occipital (BA 18/19) | –24 | –81 | 18 | 10 | 3.49 |
| L inferior frontal (BA 45/47) | –54 | 30 | 18 | 11 | 3.28 |
| Long-term priming (post-training) | |||||
| L inferior frontal/premotor (BA 6/44/45/47)/caudate/putamen | –42 | 3 | 27 | 1536† | 12.37‡ |
| L intraparietal sulcus (BA 7)/occipital (BA 18/19) | –24 | –63 | 45 | 1177† | 9.55‡ |
| Anterior cingulate | –6 | 15 | 42 | 282† | 7.73‡ |
| R intraparietal sulcus (BA 7)/occipital (BA 18/19) | 27 | –66 | 42 | 964† | 7.27‡ |
| R frontal operculum (BA 44/47)/caudate/putamen | 30 | 27 | 0 | 409† | 5.84‡ |
| L inferior frontal/premotor (BA 6/44) | 48 | 9 | 27 | 122† | 5.48‡ |
| Brainstem | –3 | –27 | –18 | 16 | 3.70 |
Regions exhibiting significant activity for SB compared with MR stimuli
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at voxel-level corrected P < 0.05; †significant at cluster-level corrected P < 0.05. ‡This activation occurred in the region of signal dropout due to magnetic susceptibility artefact. | |||||
| Session 1: SB > MR | |||||
| R inferior frontal (BA 45/47) | 48 | 27 | –3 | 29 | 4.79* |
| Session 2: SB > MR | |||||
| L cerebellum | –6 | –81 | –18 | 17 | 4.29 |
| Session 1: MR > SB | |||||
| R lingual/parahippocampal cortex | 9 | –48 | –6 | 13 | 3.76 |
| L lingual/parahippocampal cortex | –9 | –45 | –6 | 29 | 3.69 |
| White matter | 3 | 21 | 9 | 18 | 3.63 |
| R hippocampus | 27 | –24 | –3 | 19 | 3.51 |
| Session 2: MR > SB | |||||
| L dorsolateral prefrontal cortex (BA 9/46) | –21 | 27 | 42 | 45† | 4.59 |
| L fusiform | –42 | –30 | –21 | 29 | 4.30 |
| L cerebellar nuclei | –12 | –48 | –27 | 68† | 4.27 |
| Anterior cingulate | –15 | 45 | 6 | 72† | 4.23 |
| L inferior parietal (BA 39/40) | –45 | –24 | 45 | 60† | 4.22 |
| R lingual/parahippocampal cortex | 12 | –45 | 0 | 119† | 4.18 |
| Posterior cingulate | –12 | –39 | 45 | 218† | 4.10 |
| L orbitofrontal‡ | –30 | 36 | –9 | 16 | 3.72 |
| R inferior parietal (BA 39/40) | 45 | –30 | 24 | 13 | 3.70 |
| R orbitofrontal | 12 | 48 | 0 | 11 | 3.69 |
| R fusiform | 33 | –54 | –18 | 15 | 3.56 |
| L lingual/parahippocampal cortex | –12 | –51 | 3 | 14 | 3.39 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at voxel-level corrected P < 0.05; †significant at cluster-level corrected P < 0.05. ‡This activation occurred in the region of signal dropout due to magnetic susceptibility artefact. | |||||
| Session 1: SB > MR | |||||
| R inferior frontal (BA 45/47) | 48 | 27 | –3 | 29 | 4.79* |
| Session 2: SB > MR | |||||
| L cerebellum | –6 | –81 | –18 | 17 | 4.29 |
| Session 1: MR > SB | |||||
| R lingual/parahippocampal cortex | 9 | –48 | –6 | 13 | 3.76 |
| L lingual/parahippocampal cortex | –9 | –45 | –6 | 29 | 3.69 |
| White matter | 3 | 21 | 9 | 18 | 3.63 |
| R hippocampus | 27 | –24 | –3 | 19 | 3.51 |
| Session 2: MR > SB | |||||
| L dorsolateral prefrontal cortex (BA 9/46) | –21 | 27 | 42 | 45† | 4.59 |
| L fusiform | –42 | –30 | –21 | 29 | 4.30 |
| L cerebellar nuclei | –12 | –48 | –27 | 68† | 4.27 |
| Anterior cingulate | –15 | 45 | 6 | 72† | 4.23 |
| L inferior parietal (BA 39/40) | –45 | –24 | 45 | 60† | 4.22 |
| R lingual/parahippocampal cortex | 12 | –45 | 0 | 119† | 4.18 |
| Posterior cingulate | –12 | –39 | 45 | 218† | 4.10 |
| L orbitofrontal‡ | –30 | 36 | –9 | 16 | 3.72 |
| R inferior parietal (BA 39/40) | 45 | –30 | 24 | 13 | 3.70 |
| R orbitofrontal | 12 | 48 | 0 | 11 | 3.69 |
| R fusiform | 33 | –54 | –18 | 15 | 3.56 |
| L lingual/parahippocampal cortex | –12 | –51 | 3 | 14 | 3.39 |
Regions exhibiting significant activity for SB compared with MR stimuli
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at voxel-level corrected P < 0.05; †significant at cluster-level corrected P < 0.05. ‡This activation occurred in the region of signal dropout due to magnetic susceptibility artefact. | |||||
| Session 1: SB > MR | |||||
| R inferior frontal (BA 45/47) | 48 | 27 | –3 | 29 | 4.79* |
| Session 2: SB > MR | |||||
| L cerebellum | –6 | –81 | –18 | 17 | 4.29 |
| Session 1: MR > SB | |||||
| R lingual/parahippocampal cortex | 9 | –48 | –6 | 13 | 3.76 |
| L lingual/parahippocampal cortex | –9 | –45 | –6 | 29 | 3.69 |
| White matter | 3 | 21 | 9 | 18 | 3.63 |
| R hippocampus | 27 | –24 | –3 | 19 | 3.51 |
| Session 2: MR > SB | |||||
| L dorsolateral prefrontal cortex (BA 9/46) | –21 | 27 | 42 | 45† | 4.59 |
| L fusiform | –42 | –30 | –21 | 29 | 4.30 |
| L cerebellar nuclei | –12 | –48 | –27 | 68† | 4.27 |
| Anterior cingulate | –15 | 45 | 6 | 72† | 4.23 |
| L inferior parietal (BA 39/40) | –45 | –24 | 45 | 60† | 4.22 |
| R lingual/parahippocampal cortex | 12 | –45 | 0 | 119† | 4.18 |
| Posterior cingulate | –12 | –39 | 45 | 218† | 4.10 |
| L orbitofrontal‡ | –30 | 36 | –9 | 16 | 3.72 |
| R inferior parietal (BA 39/40) | 45 | –30 | 24 | 13 | 3.70 |
| R orbitofrontal | 12 | 48 | 0 | 11 | 3.69 |
| R fusiform | 33 | –54 | –18 | 15 | 3.56 |
| L lingual/parahippocampal cortex | –12 | –51 | 3 | 14 | 3.39 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at voxel-level corrected P < 0.05; †significant at cluster-level corrected P < 0.05. ‡This activation occurred in the region of signal dropout due to magnetic susceptibility artefact. | |||||
| Session 1: SB > MR | |||||
| R inferior frontal (BA 45/47) | 48 | 27 | –3 | 29 | 4.79* |
| Session 2: SB > MR | |||||
| L cerebellum | –6 | –81 | –18 | 17 | 4.29 |
| Session 1: MR > SB | |||||
| R lingual/parahippocampal cortex | 9 | –48 | –6 | 13 | 3.76 |
| L lingual/parahippocampal cortex | –9 | –45 | –6 | 29 | 3.69 |
| White matter | 3 | 21 | 9 | 18 | 3.63 |
| R hippocampus | 27 | –24 | –3 | 19 | 3.51 |
| Session 2: MR > SB | |||||
| L dorsolateral prefrontal cortex (BA 9/46) | –21 | 27 | 42 | 45† | 4.59 |
| L fusiform | –42 | –30 | –21 | 29 | 4.30 |
| L cerebellar nuclei | –12 | –48 | –27 | 68† | 4.27 |
| Anterior cingulate | –15 | 45 | 6 | 72† | 4.23 |
| L inferior parietal (BA 39/40) | –45 | –24 | 45 | 60† | 4.22 |
| R lingual/parahippocampal cortex | 12 | –45 | 0 | 119† | 4.18 |
| Posterior cingulate | –12 | –39 | 45 | 218† | 4.10 |
| L orbitofrontal‡ | –30 | 36 | –9 | 16 | 3.72 |
| R inferior parietal (BA 39/40) | 45 | –30 | 24 | 13 | 3.70 |
| R orbitofrontal | 12 | 48 | 0 | 11 | 3.69 |
| R fusiform | 33 | –54 | –18 | 15 | 3.56 |
| L lingual/parahippocampal cortex | –12 | –51 | 3 | 14 | 3.39 |
Regions exhibiting significant activity for IR compared with MR stimuli
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. ‡This activation occurred in the region of signal dropout due to magnetic susceptibility artefact. | |||||
| Session 1: IR > MR | |||||
| R inferior occipital/temporal (BA 37/19) | 42 | –66 | –21 | 106* | 5.63† |
| R frontal operculum (BA 45/47) | 48 | 24 | 0 | 60* | 4.26 |
| R cerebellum | 30 | –45 | –21 | 13 | 3.93 |
| L inferior occipital/temporal (BA 37/19) | –33 | –72 | –18 | 53* | 3.90 |
| Session 2: IR > MR | |||||
| R inferior occipital/temporal (BA 37/19) | 42 | –66 | –21 | 101* | 5.52† |
| R intraparietal/inferior parietal | 39 | –36 | 42 | 87* | 5.14† |
| L inferior temporal (BA 37) | –39 | –57 | –21 | 190* | 4.97† |
| R superior parietal (BA 7) | 24 | –78 | 42 | 84* | 4.95† |
| L cerebellum | –9 | –81 | –21 | 25 | 4.34 |
| R cerebellum | 18 | –78 | –21 | 15 | 4.30 |
| Brainstem | 0 | –21 | –15 | 16 | 3.86 |
| L intraparietal cortex | –15 | –78 | 42 | 10 | 3.80 |
| Session 1: MR > IR | |||||
| R lingual/parahippocampal | 9 | –45 | –9 | 28 | 3.93 |
| L occipital (BA 17/18) | –12 | –90 | –3 | 13 | 3.67 |
| L inferior frontal (BA 44/45) | –51 | 24 | 24 | 26 | 3.57 |
| Session 2: MR > IR | |||||
| Orbitofrontal cortex‡ | –6 | 48 | –12 | 37 | 4.05 |
| R lingual/parahippocampal | 9 | –45 | –3 | 16 | 4.00 |
| L cerebellar nuclei | –15 | –48 | –27 | 26 | 3.99 |
| Precuneus | 0 | –57 | 33 | 57* | 3.84 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. ‡This activation occurred in the region of signal dropout due to magnetic susceptibility artefact. | |||||
| Session 1: IR > MR | |||||
| R inferior occipital/temporal (BA 37/19) | 42 | –66 | –21 | 106* | 5.63† |
| R frontal operculum (BA 45/47) | 48 | 24 | 0 | 60* | 4.26 |
| R cerebellum | 30 | –45 | –21 | 13 | 3.93 |
| L inferior occipital/temporal (BA 37/19) | –33 | –72 | –18 | 53* | 3.90 |
| Session 2: IR > MR | |||||
| R inferior occipital/temporal (BA 37/19) | 42 | –66 | –21 | 101* | 5.52† |
| R intraparietal/inferior parietal | 39 | –36 | 42 | 87* | 5.14† |
| L inferior temporal (BA 37) | –39 | –57 | –21 | 190* | 4.97† |
| R superior parietal (BA 7) | 24 | –78 | 42 | 84* | 4.95† |
| L cerebellum | –9 | –81 | –21 | 25 | 4.34 |
| R cerebellum | 18 | –78 | –21 | 15 | 4.30 |
| Brainstem | 0 | –21 | –15 | 16 | 3.86 |
| L intraparietal cortex | –15 | –78 | 42 | 10 | 3.80 |
| Session 1: MR > IR | |||||
| R lingual/parahippocampal | 9 | –45 | –9 | 28 | 3.93 |
| L occipital (BA 17/18) | –12 | –90 | –3 | 13 | 3.67 |
| L inferior frontal (BA 44/45) | –51 | 24 | 24 | 26 | 3.57 |
| Session 2: MR > IR | |||||
| Orbitofrontal cortex‡ | –6 | 48 | –12 | 37 | 4.05 |
| R lingual/parahippocampal | 9 | –45 | –3 | 16 | 4.00 |
| L cerebellar nuclei | –15 | –48 | –27 | 26 | 3.99 |
| Precuneus | 0 | –57 | 33 | 57* | 3.84 |
Regions exhibiting significant activity for IR compared with MR stimuli
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. ‡This activation occurred in the region of signal dropout due to magnetic susceptibility artefact. | |||||
| Session 1: IR > MR | |||||
| R inferior occipital/temporal (BA 37/19) | 42 | –66 | –21 | 106* | 5.63† |
| R frontal operculum (BA 45/47) | 48 | 24 | 0 | 60* | 4.26 |
| R cerebellum | 30 | –45 | –21 | 13 | 3.93 |
| L inferior occipital/temporal (BA 37/19) | –33 | –72 | –18 | 53* | 3.90 |
| Session 2: IR > MR | |||||
| R inferior occipital/temporal (BA 37/19) | 42 | –66 | –21 | 101* | 5.52† |
| R intraparietal/inferior parietal | 39 | –36 | 42 | 87* | 5.14† |
| L inferior temporal (BA 37) | –39 | –57 | –21 | 190* | 4.97† |
| R superior parietal (BA 7) | 24 | –78 | 42 | 84* | 4.95† |
| L cerebellum | –9 | –81 | –21 | 25 | 4.34 |
| R cerebellum | 18 | –78 | –21 | 15 | 4.30 |
| Brainstem | 0 | –21 | –15 | 16 | 3.86 |
| L intraparietal cortex | –15 | –78 | 42 | 10 | 3.80 |
| Session 1: MR > IR | |||||
| R lingual/parahippocampal | 9 | –45 | –9 | 28 | 3.93 |
| L occipital (BA 17/18) | –12 | –90 | –3 | 13 | 3.67 |
| L inferior frontal (BA 44/45) | –51 | 24 | 24 | 26 | 3.57 |
| Session 2: MR > IR | |||||
| Orbitofrontal cortex‡ | –6 | 48 | –12 | 37 | 4.05 |
| R lingual/parahippocampal | 9 | –45 | –3 | 16 | 4.00 |
| L cerebellar nuclei | –15 | –48 | –27 | 26 | 3.99 |
| Precuneus | 0 | –57 | 33 | 57* | 3.84 |
| Location . | x . | y . | z . | Cluster extent . | Max. t value . |
|---|---|---|---|---|---|
| P < 0.001 uncorrected, extent of at least 10 voxels. *Significant at cluster-level corrected P < 0.05; †significant at voxel-level corrected P < 0.05. ‡This activation occurred in the region of signal dropout due to magnetic susceptibility artefact. | |||||
| Session 1: IR > MR | |||||
| R inferior occipital/temporal (BA 37/19) | 42 | –66 | –21 | 106* | 5.63† |
| R frontal operculum (BA 45/47) | 48 | 24 | 0 | 60* | 4.26 |
| R cerebellum | 30 | –45 | –21 | 13 | 3.93 |
| L inferior occipital/temporal (BA 37/19) | –33 | –72 | –18 | 53* | 3.90 |
| Session 2: IR > MR | |||||
| R inferior occipital/temporal (BA 37/19) | 42 | –66 | –21 | 101* | 5.52† |
| R intraparietal/inferior parietal | 39 | –36 | 42 | 87* | 5.14† |
| L inferior temporal (BA 37) | –39 | –57 | –21 | 190* | 4.97† |
| R superior parietal (BA 7) | 24 | –78 | 42 | 84* | 4.95† |
| L cerebellum | –9 | –81 | –21 | 25 | 4.34 |
| R cerebellum | 18 | –78 | –21 | 15 | 4.30 |
| Brainstem | 0 | –21 | –15 | 16 | 3.86 |
| L intraparietal cortex | –15 | –78 | 42 | 10 | 3.80 |
| Session 1: MR > IR | |||||
| R lingual/parahippocampal | 9 | –45 | –9 | 28 | 3.93 |
| L occipital (BA 17/18) | –12 | –90 | –3 | 13 | 3.67 |
| L inferior frontal (BA 44/45) | –51 | 24 | 24 | 26 | 3.57 |
| Session 2: MR > IR | |||||
| Orbitofrontal cortex‡ | –6 | 48 | –12 | 37 | 4.05 |
| R lingual/parahippocampal | 9 | –45 | –3 | 16 | 4.00 |
| L cerebellar nuclei | –15 | –48 | –27 | 26 | 3.99 |
| Precuneus | 0 | –57 | 33 | 57* | 3.84 |
Behavioural data during scanning and training. In the bottom row open boxes = new non-words, open circles = practised non-words, filled boxes = new words, filled circles = practised words. Error bars reflect within-subject standard error.
Rendering of activation maps from fixed-effects analysis for mirror-reading, learning-related changes, short-term priming and long-term priming.
Projection of activiation maps from fixed-effects analysis for mirror-reading and learning-related changes on averaged anatomical slices.
ROI analysis of repetition priming effects. Open columns = session 1; stippled columns = session 2; inf = inferior; ant = anterior; L = left; R = right. Error bars reflect within-subject standard error. Asterisks denote significant difference from MR items (P < 0.05).
ROI analysis of spatial transformation effects. Open columns = session 1; stippled columns = session 2; inf = inferior. Error bars reflect within-subject standard error.
We wish to thank Joseph Hsieh and Jennifer Burrows for assistance in data collection and Skip Stebbins for helpful comments on a draft of this paper. This research was supported by the McDonnell-Pew Program in Cognitive Neuroscience and by the National Institutes of Health (P50NS26985 and AG12995).
References
Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered.
Buchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning.
Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge (MA): MIT Press; 1993.
Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that.
Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity.
Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, et al. The anatomy of phonological and semantic processing in normal subjects.
Demonet JF, Price C, Wise R, Frackowiak RS. A PET study of cognitive strategies in normal subjects during language tasks. Influence of phonetic ambiguity and sequence processing on phoneme monitoring.
Eichenbaum H, Fagan A, Mathews P, Cohen NJ. Hippocampal system dysfunction and odor discrimination learning in rats: impairment of facilitation depending on representational demands.
Frith CD, Kapur N, Friston KJ, Liddle PF, Frackowiak RSJ. Regional cerebral activity associated with the incidental processing of pseudo-words.
Gabrieli JDE, Desmond JE, Demb JB, Wagner AD. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes.
Gabrieli JDE, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe.
Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat.
Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot.
Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans.
Gupta P, Cohen NJ. Skill learning, repetition priming, and procedural memory: theoretical and computational analysis. Psychol Rev. In press 2001.
Heindel WC, Butters N, Salmon DP. Impaired learning of a motor skill in patients with Huntington's disease.
Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer's, Huntington's and Parkinson's disease patients.
Horton KD. The role of semantic information in reading spatially transformed text.
Horton KD, McKenzie BD. Specificity of perceptual processing in rereading spatially transformed materials.
Irarrazabal P, Meyer CH, Nishimura DG, Macovski A. Inhomogeneity correction using an estimated linear field map.
Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans.
Knowlton BJ, Squire LR, Paulsen JS, Swerdlow NR, Swenson M, Butters N. Dissociations within nondeclarative memory in Huntington's disease.
Kolers PA. Memorial consequences of automatized encoding.
Kucera H, Francis WN. Computational analysis of present-day American English. Providence (RI): Brown University Press; 1967.
Logan GD. Repetition priming and automaticity: common underlying mechanisms?
Martone M, Butters N, Payne M, Becker JT, Sax DS. Dissociations between skill learning and verbal recognition in amnesia and dementia.
Masson ME. Identification of typographically transformed words: instance-based skill acquisition.
Mishkin M, Petri HL. Memory and habits: some implications for the analysis of learning and retention. In: Squire LR, Butters N, editors. Neuropsychology of memory. New York: Guilford Press; 1984. p. 287–96.
Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems.
Poldrack RA. Imaging brain plasticity: conceptual and methodological issues—a theoretical review.
Poldrack RA, Desmond JE, Glover GH, Gabrieli JD. The neural basis of visual skill learning: an fMRI study of mirror reading.
Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill.
Poldrack RA, Selco SL, Field JE, Cohen NJ. The relationship between skill learning and repetition priming: experimental and computational analyses.
Price CJ, Wise RJ, Frackowiak RS. Demonstrating the implicit processing of visually presented words and pseudowords.
Saint-Cyr JA, Taylor AE, Lang AE. Procedural learning and neostriatal dysfunction in man.
Saint-Cyr JA, Ungerleider LG, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey.
Schwartz BL, Hashtroudi S. Priming is independent of skill learning.
Seitz RJ, Roland E, Bohm C, Greitz T, Stone-Elander S. Motor learning in man: a positron emission tomographic study.
Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. [Review].
Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: a functional anatomical study of memory.
Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, et al. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging.
Tardif T, Craik FIM. Reading a week later: perceptual and conceptual factors.
Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity.
Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys.