-
PDF
- Split View
-
Views
-
Cite
Cite
Josef Parvizi, Steven W. Anderson, Coleman O. Martin, Hanna Damasio, Antonio R. Damasio, Pathological laughter and crying: A link to the cerebellum, Brain, Volume 124, Issue 9, September 2001, Pages 1708–1719, https://doi.org/10.1093/brain/124.9.1708
Close - Share Icon Share
Abstract
Patients with pathological laughter and crying (PLC) are subject to relatively uncontrollable episodes of laughter, crying or both. The episodes occur either without an apparent triggering stimulus or following a stimulus that would not have led the subject to laugh or cry prior to the onset of the condition. PLC is a disorder of emotional expression rather than a primary disturbance of feelings, and is thus distinct from mood disorders in which laughter and crying are associated with feelings of happiness or sadness. The traditional and currently accepted view is that PLC is due to the damage of pathways that arise in the motor areas of the cerebral cortex and descend to the brainstem to inhibit a putative centre for laughter and crying. In that view, the lesions `disinhibit' or `release' the laughter and crying centre. The neuroanatomical findings in a recently studied patient with PLC, along with new knowledge on the neurobiology of emotion and feeling, gave us an opportunity to revisit the traditional view and propose an alternative. Here we suggest that the critical PLC lesions occur in the cerebro-ponto-cerebellar pathways and that, as a consequence, the cerebellar structures that automatically adjust the execution of laughter or crying to the cognitive and situational context of a potential stimulus, operate on the basis of incomplete information about that context, resulting in inadequate and even chaotic behaviour.
Introduction
Pathological laughter and crying (PLC) is a condition defined by relatively uncontrollable episodes of laughter, crying or both. The episodes either do not have an apparent motivating stimulus or are triggered by a stimulus that would not have led the subject to laugh or cry prior to the onset of the condition. In some instances, the stimulus may have an emotional valence contrary to the emotional expression. For example, patients can laugh in response to sad news or cry in response to a moving hand in the visual field, and the expression of laughter can abruptly change to crying (Poeck, 1985). PLC is a disorder of emotional expression rather than a primary disturbance of feelings. It is distinguishable from the mood disorders in which laughter and crying are associated with feelings of happiness or sadness, and from regular laughter or crying in which the emotional expression is consonant with the triggering stimulus. However, the essence of the actual laughter or crying behaviours (e.g. the facial expressions, the tears, etc.) is identical in PLC, in mood disorders and in the normal condition.
PLC has been noted in gelastic epilepsy (Arroyo et al., 1993), multiple sclerosis (Feinstein et al., 1997), pseudobulbar palsy (Black, 1982) and tumours in the cerebellopontine region (Achari and Colover, 1976) [including trigeminal neurinoma (Bhatjiwale et al., 2000), petroclival meningeoma (Shafqat et al., 1998), clival chordoma (Matsuoka et al., 1993) and pontine glioma (Lal and Chandy, 1992)]. PLC has also been noted in association with cerebrovascular lesions involving the descending corticobulbar pathways, most notably at the level of internal capsule, the cerebral peduncles and the basis pontis (Black, 1982; Poeck, 1985; Bassetti et al., 1996; Kim, 1997; Kim and Choi-Kwon, 2000). In a review of autopsy findings in 30 patients, PLC was never correlated with a single cortical lesion, but the internal capsule was damaged in all patients (Poeck, 1985). In a related condition, the so-called `fou rire prodromique' (Féré, 1903), pathological laughing is a transient manifestation that heralds a brainstem stroke involving the basis pontis or the cerebral peduncles (Wali, 1993).
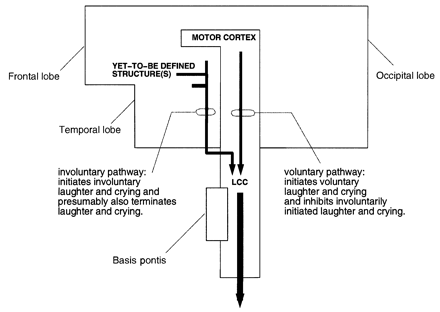
It has been hypothesized that PLC is caused by a loss of voluntary inhibition of a presumed centre for laughter and crying located in the upper brainstem (Wilson, 1924). This centre would be capable of coordinating the faciorespiratory functions associated with laughter and crying. Accordingly, laughter and crying would be triggered normally via two separate neuroanatomical pathways (Fig. 1). One pathway would run from yet-to-be-identified brain regions to the brainstem laughter and crying centre, and be concerned with involuntarily triggering (and presumably also involuntarily terminating) the faciorespiratory patterns associated with laughter and crying; the other pathway would run from the motor cortices to the centre, and be concerned with voluntarily controlling faciorespiratory actions. According to Wilson, `PLC is caused by lesions of the voluntary paths from the motor areas of the cerebral cortex or by any state in which these exercise imperfect control' over the laughter and crying centre (Wilson, 1924). This interpretation is identified in the literature as the `disinhibition' or `release phenomenon' hypothesis.
This view of PLC appeared in the beginning of last century when the knowledge about the function and anatomy of neural systems was limited, and the operation of the nervous system tended to be conceptualized in terms of serial processing and hierarchical control. Given that the motor acts of laughing and crying require the integration of facial and respiratory functions which are mediated by brainstem nuclei, it was presumed that the so-called laughter and crying centre must be located above the facial and respiratory nuclei, somewhere in the upper pons or midbrain. Since the presumed laughter and crying centre was a subcortical structure, it was then believed that its operation should be under the control of a descending pathway from the cerebral cortex, the pinnacle of the hierarchy.
This view provides an unsatisfactory explanation for PLC and prompts a number of questions for which there are no proper answers. For example, if the sole reason why a patient exhibits PLC is because the laughter and crying centre is `disinhibited' from voluntary cortical control, why do some patients change from laughing to crying in response to the same stimulus, and why can they exhibit responses that are entirely incongruent with the triggering stimulus? Why is it that some patients laugh in response to sad news, or cry in response to stimuli as banal as moving a hand in their visual field? Or, as Wilson himself wondered, why does PLC often occur in patients in whom the voluntary control of faciorespiratory functions is intact, and why are patients with bilateral and central facial palsy not especially prone to developing PLC? Also, if PLC is due to lesions of `voluntary' pathways from the motor cortex to a laughter and crying centre, how can patients with PLC voluntarily mimic laughter or crying?
A recently studied patient who developed PLC after an ischaemic stroke gave us an opportunity to revisit the traditional view of PLC and discuss it in light of new neuropathological findings and new knowledge of the biology of emotion and feeling. We suggest that PLC is not caused by a loss of direct motor cortical inhibition of a laughter and crying centre, but rather by dysfunction in circuits that involve the cerebellum and exert influence over brainstem nuclei as well as the cerebral cortex itself.
Case study
C.B. is a 51-year-old right-handed man with a high school education who was employed as a landscaper. C.B. suffered a stroke in March 1998. He had no personal or family history of neurological or psychiatric disease. Past medical history was significant for hypertension and hyperlipidaemia, for which he had been taking bisoprolol 5 mg and hydrochlorothiazide 6.25 mg daily as a combination tablet.
C.B.'s primary manifestation following the stroke was pathological laughter and crying. He would begin laughing and/or crying in response to stimuli that normally did not trigger such a response, and this would happen several times a day. According to both the patient and relatives, the pathological crying was present immediately after the stroke, and the pathological laughter was present `a short time later'. His impression was that laughter and crying attacks occurred with similar frequencies. Clinical examination in March 1998 also revealed gaze-evoked nystagmus with rightward gaze, and right rotatory nystagmus of both eyes at rest. A coordination examination showed ataxia with right finger-to-nose and heel-to-shin tests. Motor strength and stretch reflexes were normal. Testing of all sensory modalities did not reveal any abnormality. The status of all cranial nerve nuclei (including the facial nucleus) was also normal. Initial imaging with CT without contrast showed a right cerebellar infarct. One week later, the patient was transferred to rehabilitative care, and although his coordination and ambulation improved significantly, his PLC persisted unchanged.
Clinical observations
When we first examined C.B. ~14 months after his stroke (May 1999), PLC remained the dramatic feature of his clinical presentation. The laughter or crying attacks were sudden and of moderate intensity. He felt deeply embarrassed by the attacks and would struggle to stop them. He reported that he could exert little or no control over the onset of attacks, but he would usually gain some control within 30 s to 2 min of onset. We were able to observe transitions of laughter into crying in response to the same stimulus, but never of crying into laughter, and the patient did not recall transitions of crying into laughing. We also observed that PLC could be incongruent with the triggering stimulus. For instance, the patient would cry in response to a joke and laugh in response to a frustrating test failure.
We observed what appeared to be `priming' effects in determining whether he would laugh or cry in response to a given stimulus. If he had laughed recently, he was more likely to laugh in response to the next effective stimulus, independent of its actual emotional valence; and if he had recently laughed or cried, the threshold for responding with laughter or crying was lowered for subsequent stimuli. Because of these effects, there would be long periods, 30 min or even more, during which he would repeatedly burst into laughter or repeatedly cry in response to seemingly neutral stimuli. Except for the outbursts of laughter and crying, his personal and social behaviour was entirely appropriate. C.B. was acutely aware of this abnormal behaviour and was embarrassed by it. Moreover, he noted that, in spite of the lack of an appropriate laughter—or crying—inducing stimulus, he would eventually feel jolly or sad after a long episode of laughter or crying. A feeling was in fact being produced, consonant with the emotional expression, and in the absence of an appropriate stimulus for that emotional expression.
At the time we began studying C.B., his baseline rating on the PLC scale was 20 out of 54, a value indicative of moderate impairment [this scale is rated by the interviewer and quantifies different aspects of laughter and crying, including duration, relationship to external events, degree of voluntary control, inappropriateness and degree of resultant distress; for each of the criteria, the examiner makes a judgement about the severity of the symptoms on a scale of 0–3 points; the inter-rater reliability of the scale is 93% (Robinson et al., 1993)].
Responses on the Beck Depression Inventory and the Beck Anxiety Inventory did not reveal any significant depressive or anxiety-related symptoms. He performed within normal expectations on a broad range of cognitive tests. He performed primarily in the average range on measures of verbal and nonverbal intellectual abilities, consistent with expectations based on his background. Likewise, performances were within normal limits on measures of language, visuospatial and visuomotor abilities, and abstract problem solving. However, anterograde memory and sustained attention were mildly impaired, and he had a significant impairment in aspects of executive functions as measured by the Tower of Hanoi task (TOH) (Table 1).
As a treatment for PLC, the patient was given citalopram (Celexa®) 20 mg/day. Citalopram is a selective serotonin reuptake inhibitor (SSRI), which according to previous observations has been shown to eliminate the condition of PLC (Andersen et al., 1993). C.B.'s condition improved and his rating in the PLC-scale dropped to 2 at 2 weeks and to 0 at 6 weeks. He is still medicated with Celexa and the attacks have not recurred.
Neuroanatomical findings
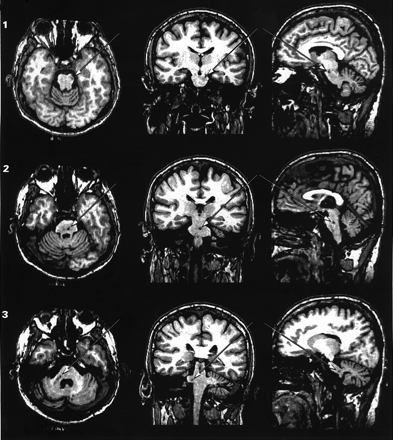
MRIs were obtained from the entire brain and reconstructed in three dimensions using the Brainvox program (Damasio and Frank, 1992; Frank et al., 1997), which permits neuroanatomical analysis on any arbitrary plane of section. C.B. underwent MRI scanning in a General Electric Signa scanner operating at 1.5 T, using the following protocol: SPGR 30, TR 24, TE 7, NEX 1, FOV 24 cm, matrix 256 × 192; 124 contiguous coronal slices were obtained with a thickness of 1.5–1.7 mm and an interpixel distance 0.94 mm. The slice thickness was adjusted to the size of the head in order to sample the entire brain, while avoiding wrap artefacts. Three individual 1 NEX SPGR data sets were co-registered post hoc with automated image registration (AIR 3.03) to produce a single data set of enhanced quality with pixel dimensions of 0.7 mm in plane and 1.5 mm between planes (Holmes et al., 1998). The axial and parasagittal sections shown in Fig. 2 were obtained from the reconstructed 3D image using the Brainvox program.
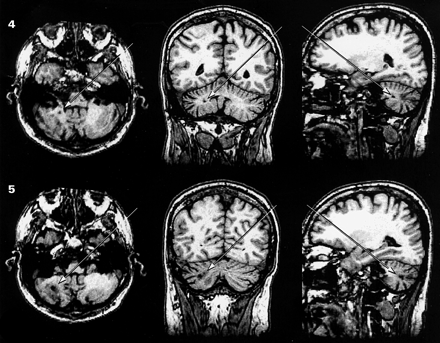
The MRIs revealed three lesions in the brainstem and two lesions in the cerebellum (Fig. 2). The first lesion was 2 mm × 1 mm × 1 mm in size located at the pontomesencephalic junction and affecting the lateral segment of the left cerebral peduncle (Fig. 2, lesion 1). The second lesion was 4 mm × 3 mm × 3 mm in size located at the midline basis pontis and at the level of mid-to-upper pons (Fig. 2, lesion 2). The third and fourth lesions were almost contiguous and both affected the right middle cerebellar peduncle from its brainstem root to the region below the superior semilunar lobule (Fig. 2, lesions 3–4). Together, these two lesions measured 12 mm in the axial plane and varied between 2 and 5 mm in the coronal and parasagittal planes. Finally, the fifth lesion measured 6 mm × 2 mm × 2 mm affecting, also on the right side, the middle cerebellar peduncle and the white matter immediately beneath the inferior semilunar lobule (Fig. 2, lesion 5). No lesions were detected elsewhere in the brain. In conclusion, all five lesions of Patient C.B. are located in the white matter of the brainstem or the cerebellum. These lesions are placed along pathways from telencephalic structures to the nuclei of the basis pontis, and from these nuclei to the cerebellum, thus disrupting the cerebro-ponto-cerebellar projections by means of which telencephalic structures communicate with the cerebellum (Fig. 3).
Based on the anatomical findings, the following can be concluded: C.B.'s cerebellum is partially deafferented from topographically organized descending telencephalic inputs, and the deafferentation is most severe with respect to connections from the left telencephalic structures to the right cerebellar hemisphere (although lesion 2 in the basis pontis will have a bilateral effect since it affects the pontine nuclei of one side and the crossing fibres from the pontine nuclei of the other). Considering that no other lesions were detected in any other brain region, and given that PLC only began after the patient acquired the lesions described here, it is justifiable to conclude that C.B.'s condition was caused by a partial deafferentation of the cerebellum.
Discussion
An alternative hypothesis
By what mechanism might a partial deafferentation of the cerebellum, as described above, lead to pathological laughing and crying? In answer to this question, we begin by placing the phenomena of laughter and crying in the perspective of the neuroanatomical and functional framework that has guided our research on emotion and feeling (Damasio, 1999; Damasio et al., 2000). Laughter and crying are complex patterns of movement that are usually integral parts of the enactment of specific emotions. Laughter is normally a part of the enactment of happiness (true laughter tends not to occur in a context of sadness), and crying is normally a part of the enactment of sadness (although it is possible to cry in a context of happiness, the pattern of crying is distinctive in that it does not include sobbing and it tends to appear as a hybrid of laughter and crying).
Laughter and crying are thus triggered by the sort of stimuli that are competent to trigger happiness or sadness. The triggering depends on the presence of such stimuli being perceived or recalled within a particular cognitive/social context, and on the detection of such stimuli/contexts by induction sites located in the telencephalon (examples of emotion induction sites include the ventromedial prefrontal cortex, the anterior cingulate cortex, the extended amygdala and the ventral striatum). In turn, the activated induction sites operate on effector sites (examples of which include the motor cortices as well as the hypothalamus, the periaqueductal grey matter, the cranial nerve nuclei and the premotor regions that interlock the operation of these structures).
In normal circumstances, a perceived external stimulus (e.g. being told a funny joke) or a recalled one (e.g. remembering the death of a friend) will trigger an emotional response, but only if the cognitive/social context is appropriate. Although the laughter and crying components of the emotional responses are largely pre-programmed and stereotyped, their intensity, duration and certain aspects of the overall pattern depend on the cognitive/social context in which the triggering stimulus appeared (Provine, 1996). For instance, a given stimulus might lead to a burst of laughter when the subject is in a conducive cognitive state and in a casual social context, while the same stimulus may lead to only a gentle smile or to no emotional expression at all in a tense social context.
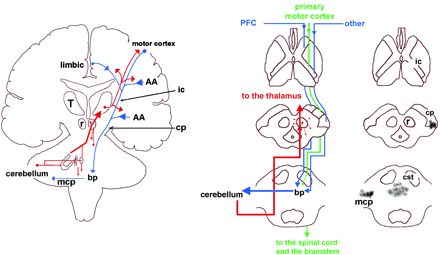
We believe that the cerebellum has an important role to play in the modulatory control described above. Specifically, we believe that the cerebellum adjusts laughter and crying behaviours according to specific contexts, e.g. social contexts in which such behaviours should be scaled down or even inhibited, and that the cerebellum might even set the threshold at which the induction–effector apparatus responds to a stimulus thus producing (or not producing) laughter or crying. These modulatory cerebellar actions would occur automatically as a result of learning (i.e. pairing certain social contexts to certain profiles and levels of emotional response).
We believe the cerebellum is prepared to perform these modulatory actions for two reasons. First, because the cerebellum receives projections from telencephalic structures that can convey to it the cognitive/social context of a stimulus, thus allowing the computations performed by the cerebellum to take into account such contexts. Secondly, because the cerebellar projections to the brainstem and telencephalic inductor and effector sites would allow the cerebellum to coordinate the varied responses whose ensemble constitute laughter or crying (which involve the coordination of a complex set of facial, laryngopharyngeal and rhythmic clonic diaphragmatic movements) altering the overall profile of these behaviours as well as their intensity and duration (Fig. 4).
Neurophysiological and neuroanatomical evidence relevant to the alternative hypothesis
It is known that the cerebellum plays an important role in the automatic execution of innate or previously learned movements (Thach, 1997, 1998; Hikosaka et al., 1999), and it has been shown that the contribution of the cerebellum pertains to coordinating and modulating movements rather than to generating these movements (Schmahmann, 1997b). In brief, the cerebellum modulates the profile, the intensity and the duration of movements according to the circumstances in which movements are to occur, as signalled by visual, auditory, somatosensory and vestibular inputs to the cerebellum. It is also reasonable to assume that a structure that is involved in coordinating and modulating movements according to sensory settings is equally capable of coordinating and modulating movements on the basis of cognitive contexts, since they too are implemented in the form of sensory-related processes in sensory-related cortical areas of varied orders.
There is evidence that, in addition to well-known projections from the spinal cord, proprioceptive dorsal column nuclei, vestibular nuclei and sensory and motor cortices, the cerebellum receives, via the nuclei in the basis pontis, neuroanatomical projections from a number of cortical areas related to cognitive processes. These nuclei receive afferents from cortical association areas in the prefrontal, parietal and temporal regions and from paralimbic structures in the parahippocampal and cingulate cortices (references in Brodal and Bjaalie, 1997; Schmahmann and Pandya, 1997b). It is important to note that projections from different cortical regions are relayed to the cerebellum by different sectors of the basis pontis. For example, while the motor cortices project to the cerebellum via the lateral pontine nuclei, projections from the non-motor prefrontal areas (Schmahmann and Pandya, 1997a) and anterior cingulate cortices are relayed by the medial pontine nuclei (Vilensky and Van Hoesen, 1981). Therefore, lesions in different regions of the basis pontis or at different sites along the cerebro-ponto-cerebellar pathways may give rise to different kinds of dysfunction. Gavrilescu and Kase report that PLC is often associated with infarcts limited to the medial, rather than the lateral territory of the basilar artery (Gavrilescu and Kase, 1995). In this regard, it is interesting to note that one of the lesions in Patient C.B. (lesion 2) was in the medial sector of the basis pontis. Thus it is possible that, in Patient C.B., the deafferentation of the cerebellum from prefrontal and cingulate inputs might have played an important role in PLC. This possibility is supported by the neuropsychological findings. As shown in Table 1, C.B.'s most significant impairment was on the Tower of Hanoi (TOH). The TOH is a spatiomotor problem-solving task, whose solution requires the execution of an ordered series of movements. There is evidence that damage to the prefrontal cortex and anterior cingulate can result in impaired performance on the TOH (Goel and Grafman, 1995). In the present study, we administered the TOH to C.B. over four successive trials to allow measurement of his ability to learn how to solve the task on the basis of experience. Instead of the expected improvement in performance over repeated trials, C.B.'s scores worsened suggesting that he was not able to bring his experience with the task to bear on subsequent response selection. Feinstein and colleagues compared multiple sclerosis patients with and without PLC and found that multiple sclerosis patients with PLC did poorly in the Stroop task (Feinstein et al., 1999). Like the TOH, the Stroop task has been linked to prefrontal and anterior cingulate function (MacLeod and MacDonald, 2000). According to our hypothesis, the deficits on the TOH and Stroop tasks are consonant with a dysfunction of the reciprocal communication between the prefrontal/anterior cingulate cortices and the cerebellum (Fig. 4).
In addition to the above-mentioned neuroanatomical projections from cortical areas involved in cognitive processing, the cerebellum receives projections from subcortical structures that are related to the processing of emotions. For example, hypothalamic (Dietrichs, 1984) and brainstem monoaminergic nuclei, such as the serotonergic raphe nuclei (Bishop and Ho, 1985; Kerr and Bishop, 1991) have direct projections to the cerebellum.
It is relevant to note that in a recent PET study we found alteration of blood flow in the nuclei of basis pontis and the cerebellum when the subjects re-enacted previously experienced emotional events (Damasio et al., 2000). In a group of 14 subjects who re-enacted a previous sad event, eight of whom cried during the acquisition of images, the left basis pontis and the right cerebellum showed increased activity (for reviews of other functional studies, see Fiez and Raichle, 1997; Schmahmann, 1997a, b; Schmahmann and Sherman, 1998).
According to our hypothesis, either deafferentation of the cerebellum from cortical and subcortical inputs related to cognitive/affective processing, or a specific lesion in the cerebellum itself, will alter the communication of the cerebellum to cortical and subcortical areas involved in generating emotional responses (Fig. 4A). The cerebellar outputs target the cortical and subcortical motor structures via the red nucleus and the ventral lateral nucleus of the thalamus (Afifi and Bergman, 1998). However, there is evidence that the cerebellum may also target a wide array of structures beyond the motor territories of the cerebral cortex. For instance, some projections from the fastigial nucleus of the cerebellum have been traced to the mediodorsal nucleus of the thalamus, which has strong projections to the prefrontal and cingulate cortices (references in Schmahmann and Pandya, 1997b). There are also direct projections from the cerebellum to the hypothalamus (Dietrichs, 1984). Moreover, Strick and colleagues have reported the presence of projections (via the thalamus) from the dentate nucleus to regions other than motor cortices (Middleton and Strick, 2001). A transneuronal retrograde tracer injected in the prefrontal areas 46d, 9m and 9l was found in the dentate nucleus. Interestingly, the dentate neurones labelled after virus injections into prefrontal areas were located in regions spatially separate from those labelled after virus injections into motor areas of the cerebral cortex.
In brief, given the anatomical evidence alone, it is apparent that the telencephalic structures in which both an emotionally competent stimulus as well as the relevant cognitive/social context are processed can convey important signals to the cerebellum. Likewise, it appears that the cerebellum conveys important signals to the emotion–effector sites (and perhaps also to the emotion–induction sites) and can influence their operation, thus contributing to the overall regulation of emotional expression (Fig. 4A).
Evidence from past neuropathological studies are compatible with the alternative hypothesis
Since the cerebro-ponto-cerebellar projections descend in the internal capsule towards the basis pontis (Fig. 3), the hypothesis, that PLC is caused by dysfunction in the loops interconnecting the telencephalic structures and the cerebellum, is compatible with the previous findings that PLC is often associated with lesions in the internal capsule. This also includes the PLC cases in multiple sclerosis. We believe PLC in patients with multiple sclerosis can be explained by our hypothesis because multiple sclerosis so often involves the descending corticobulbar pathways, some of which communicate with the cerebellum via the pontine nuclei. The hypothesis is also in accordance with the findings that patients with resected tumours in the cerebellar vermis often exhibit what is described as transient emotional lability (Pollack et al., 1995). Also of note is that children with posterior fossa syndrome show an emotional fluctuation ranging from giggling almost uncontrollably to crying irritably and inconsolably (Levisohn et al., 2000). Schmahmann and Sherman also report a patient (a 22-year-old woman) with the resection of a midline cerebellar tumour who made a sound similar to such a cry and developed `emotional lability' and `disinhibition' (Schmahmann and Sherman, 1998).
Furthermore, patients with Angelman syndrome, also known as the `happy puppet' syndrome, exhibit severe mental retardation and abnormal outbursts of laughter. The major phenotypic feature of this disease correlates with the loss of expression of Ube3a gene. In the mouse model for this disease, it was recently found that one of the regions in which the expression of this gene is reduced is the cerebellum (Albrecht et al., 1997) (the other brain regions are the hippocampus and the olfactory bulb, neither of which appear to be linked to the abnormal fits of laughter in patients with this syndrome).
Although we think that these observations support a link between the cerebellum and PLC, we are not proposing that PLC is the automatic consequence of any cerebellar lesion (i.e. no matter where in the cerebellum or in the corticopontine pathways). The critical cerebellar lesion must affect circuitry relevant for the adjustment of laughter and crying responses to cognitive/affective contexts. Although laughter and crying responses are motor responses, we presume that their related circuitry involves a cerebellar region different from the one engaged by `non-emotional' motor responses. Thus there is no reason to expect that any or even most cerebellar lesions would cause PLC. This qualification would also help account for why patients with typical cerebellar signs (e.g. dysdiadochokinesia and dyscoordination) might not exhibit PLC.
Therapeutic effect of antidepressants in PLC
The condition of C.B. improved markedly after the administration of an SSRI, supporting the recent findings that PLC is alleviated by the administration of SSRIs (Andersen et al., 1993). There is also evidence that tricyclic antidepressants can help patients with PLC (Robinson et al., 1993). A parsimonious explanation for the beneficial effect of SSRIs and of other antidepressants on PLC would be that, by altering the operation of higher order cortical areas involved in cognitive processing, the drugs would alter the cognitive context enough to raise the low threshold at which stimuli engage the system in PLC, reducing, in short, emotional lability. The benefit of the drugs, however, might also be due to a direct action on the induction sites, or on the cerebellum itself. Serotonergic receptors are widespread in many brain regions (Feldman et al., 1997), especially in the paralimbic regions that we have designated as emotion–induction and emotion–effector sites (Fig. 4). There are also serotonergic projections to the cerebellum (Bishop and Ho, 1985; Kerr and Bishop, 1991; Trouillas, 1993).
Concluding remarks
In conclusion, we present an alternative view of PLC that takes into account the novel neuroanatomical findings in a typical PLC case, new knowledge regarding the neurobiology of emotion and feeling and recent evidence that the cerebellum is not a purely motor structure, but is in fact interposed in the broader normal organization of cognitive processing (Schmahmann, 1997b). Although our view is more consonant with currently available evidence, it raises questions that only further neuroanatomical, neurophysiological and neuroimaging studies can answer.
Neuropsychological findings
| Function . | Test . | Performance . | Interpretation . |
|---|---|---|---|
| Neuropsychological evaluation included the following standardized measures, with C.B.'s performance referenced to age- and education-matched normative data: WAIS-III = Wechsler Adult Intelligence Scale—Third Edition; Rey AVLT = Rey Auditory Verbal Learning Test; RMT = Warrington Recognition Memory Test; Benton VRT = Benton Visual Retention Test, Administration A; CFT = Rey-Osterrieth Complex Figure Test, Copy and Recall; WRAT-3 = Wide Range Achievement Test—Third Edition; MAE = Multilingual Aphasia Examination; subtests COWA = Controlled Oral Word Association; Visual Naming and Sentence Repetition; Facial Recognition Test; JOLO = Judgment of Line Orientation; Grooved Pegboard; Trail making Test; WCST = Wisconsin Card Sorting Test; and Tower of Hanoi. See the discussion in the text for our explanation for the impairment in the Tower of Hanoi task. | |||
| Verbal reasoning | WAIS-III | (Scaled scores) | |
| Vocabulary | 8 | Average | |
| Similarities | 8 | Average | |
| Arithmetic | 9 | Average | |
| Digit Span | 8 | Average | |
| Information | 8 | Average | |
| Nonverbal reasoning | WAIS-III | ||
| Picture Completion | 14 | High average | |
| Digit–Symbol | 7 | Low average | |
| Block Design | 9 | Average | |
| Matrix Reason | 9 | Average | |
| Verbal memory | Rey AVLT | Raw scores | |
| Trials 1–5 | 5–6–8–10–11 | Normal | |
| 30′ recall | 7 | Low normal | |
| RMT Words | 10% | Low normal | |
| Visual memory | Benton VRT | ||
| No. correct | 5 | Mildly impaired | |
| No. errors | 7 | Mildly impaired | |
| CFT 30′ Recall | 5% | Impaired | |
| RMT Faces | 15% | Low normal | |
| Academic achievement | WRAT-3 | ||
| Reading | 63% | Normal | |
| Spelling | 55% | Normal | |
| Arithmetic | 34% | Normal | |
| Word finding | MAE COWA | 43% | Normal |
| Visual naming | MAE | 75% | Normal |
| Sentence repetition | MAE | 43% | Normal |
| Visual discrimination | Facial Recognition | 71% | Normal |
| Spatial perception | JOLO | 45% | Normal |
| Visuoconstruction | CFT Copy | 45% | Normal |
| Visuomotor coordination | Grooved Pegboard | ||
| Right hand | 16 % | Low normal | |
| Left hand) | 16 % | Low normal | |
| Executive functions | Trail making B | 66 s | Normal |
| WCST # Categories | 6 | Normal | |
| WCST # Persev. Error | 13 | Normal | |
| Tower of Hanoi Trial 1 | 66 | Normal | |
| Tower of Hanoi Trial 2 | 65 | Normal | |
| Tower of Hanoi Trial 3 | 120 | Impaired | |
| Tower of Hanoi Trial 4 | 120 | Impaired | |
| Function . | Test . | Performance . | Interpretation . |
|---|---|---|---|
| Neuropsychological evaluation included the following standardized measures, with C.B.'s performance referenced to age- and education-matched normative data: WAIS-III = Wechsler Adult Intelligence Scale—Third Edition; Rey AVLT = Rey Auditory Verbal Learning Test; RMT = Warrington Recognition Memory Test; Benton VRT = Benton Visual Retention Test, Administration A; CFT = Rey-Osterrieth Complex Figure Test, Copy and Recall; WRAT-3 = Wide Range Achievement Test—Third Edition; MAE = Multilingual Aphasia Examination; subtests COWA = Controlled Oral Word Association; Visual Naming and Sentence Repetition; Facial Recognition Test; JOLO = Judgment of Line Orientation; Grooved Pegboard; Trail making Test; WCST = Wisconsin Card Sorting Test; and Tower of Hanoi. See the discussion in the text for our explanation for the impairment in the Tower of Hanoi task. | |||
| Verbal reasoning | WAIS-III | (Scaled scores) | |
| Vocabulary | 8 | Average | |
| Similarities | 8 | Average | |
| Arithmetic | 9 | Average | |
| Digit Span | 8 | Average | |
| Information | 8 | Average | |
| Nonverbal reasoning | WAIS-III | ||
| Picture Completion | 14 | High average | |
| Digit–Symbol | 7 | Low average | |
| Block Design | 9 | Average | |
| Matrix Reason | 9 | Average | |
| Verbal memory | Rey AVLT | Raw scores | |
| Trials 1–5 | 5–6–8–10–11 | Normal | |
| 30′ recall | 7 | Low normal | |
| RMT Words | 10% | Low normal | |
| Visual memory | Benton VRT | ||
| No. correct | 5 | Mildly impaired | |
| No. errors | 7 | Mildly impaired | |
| CFT 30′ Recall | 5% | Impaired | |
| RMT Faces | 15% | Low normal | |
| Academic achievement | WRAT-3 | ||
| Reading | 63% | Normal | |
| Spelling | 55% | Normal | |
| Arithmetic | 34% | Normal | |
| Word finding | MAE COWA | 43% | Normal |
| Visual naming | MAE | 75% | Normal |
| Sentence repetition | MAE | 43% | Normal |
| Visual discrimination | Facial Recognition | 71% | Normal |
| Spatial perception | JOLO | 45% | Normal |
| Visuoconstruction | CFT Copy | 45% | Normal |
| Visuomotor coordination | Grooved Pegboard | ||
| Right hand | 16 % | Low normal | |
| Left hand) | 16 % | Low normal | |
| Executive functions | Trail making B | 66 s | Normal |
| WCST # Categories | 6 | Normal | |
| WCST # Persev. Error | 13 | Normal | |
| Tower of Hanoi Trial 1 | 66 | Normal | |
| Tower of Hanoi Trial 2 | 65 | Normal | |
| Tower of Hanoi Trial 3 | 120 | Impaired | |
| Tower of Hanoi Trial 4 | 120 | Impaired | |
Neuropsychological findings
| Function . | Test . | Performance . | Interpretation . |
|---|---|---|---|
| Neuropsychological evaluation included the following standardized measures, with C.B.'s performance referenced to age- and education-matched normative data: WAIS-III = Wechsler Adult Intelligence Scale—Third Edition; Rey AVLT = Rey Auditory Verbal Learning Test; RMT = Warrington Recognition Memory Test; Benton VRT = Benton Visual Retention Test, Administration A; CFT = Rey-Osterrieth Complex Figure Test, Copy and Recall; WRAT-3 = Wide Range Achievement Test—Third Edition; MAE = Multilingual Aphasia Examination; subtests COWA = Controlled Oral Word Association; Visual Naming and Sentence Repetition; Facial Recognition Test; JOLO = Judgment of Line Orientation; Grooved Pegboard; Trail making Test; WCST = Wisconsin Card Sorting Test; and Tower of Hanoi. See the discussion in the text for our explanation for the impairment in the Tower of Hanoi task. | |||
| Verbal reasoning | WAIS-III | (Scaled scores) | |
| Vocabulary | 8 | Average | |
| Similarities | 8 | Average | |
| Arithmetic | 9 | Average | |
| Digit Span | 8 | Average | |
| Information | 8 | Average | |
| Nonverbal reasoning | WAIS-III | ||
| Picture Completion | 14 | High average | |
| Digit–Symbol | 7 | Low average | |
| Block Design | 9 | Average | |
| Matrix Reason | 9 | Average | |
| Verbal memory | Rey AVLT | Raw scores | |
| Trials 1–5 | 5–6–8–10–11 | Normal | |
| 30′ recall | 7 | Low normal | |
| RMT Words | 10% | Low normal | |
| Visual memory | Benton VRT | ||
| No. correct | 5 | Mildly impaired | |
| No. errors | 7 | Mildly impaired | |
| CFT 30′ Recall | 5% | Impaired | |
| RMT Faces | 15% | Low normal | |
| Academic achievement | WRAT-3 | ||
| Reading | 63% | Normal | |
| Spelling | 55% | Normal | |
| Arithmetic | 34% | Normal | |
| Word finding | MAE COWA | 43% | Normal |
| Visual naming | MAE | 75% | Normal |
| Sentence repetition | MAE | 43% | Normal |
| Visual discrimination | Facial Recognition | 71% | Normal |
| Spatial perception | JOLO | 45% | Normal |
| Visuoconstruction | CFT Copy | 45% | Normal |
| Visuomotor coordination | Grooved Pegboard | ||
| Right hand | 16 % | Low normal | |
| Left hand) | 16 % | Low normal | |
| Executive functions | Trail making B | 66 s | Normal |
| WCST # Categories | 6 | Normal | |
| WCST # Persev. Error | 13 | Normal | |
| Tower of Hanoi Trial 1 | 66 | Normal | |
| Tower of Hanoi Trial 2 | 65 | Normal | |
| Tower of Hanoi Trial 3 | 120 | Impaired | |
| Tower of Hanoi Trial 4 | 120 | Impaired | |
| Function . | Test . | Performance . | Interpretation . |
|---|---|---|---|
| Neuropsychological evaluation included the following standardized measures, with C.B.'s performance referenced to age- and education-matched normative data: WAIS-III = Wechsler Adult Intelligence Scale—Third Edition; Rey AVLT = Rey Auditory Verbal Learning Test; RMT = Warrington Recognition Memory Test; Benton VRT = Benton Visual Retention Test, Administration A; CFT = Rey-Osterrieth Complex Figure Test, Copy and Recall; WRAT-3 = Wide Range Achievement Test—Third Edition; MAE = Multilingual Aphasia Examination; subtests COWA = Controlled Oral Word Association; Visual Naming and Sentence Repetition; Facial Recognition Test; JOLO = Judgment of Line Orientation; Grooved Pegboard; Trail making Test; WCST = Wisconsin Card Sorting Test; and Tower of Hanoi. See the discussion in the text for our explanation for the impairment in the Tower of Hanoi task. | |||
| Verbal reasoning | WAIS-III | (Scaled scores) | |
| Vocabulary | 8 | Average | |
| Similarities | 8 | Average | |
| Arithmetic | 9 | Average | |
| Digit Span | 8 | Average | |
| Information | 8 | Average | |
| Nonverbal reasoning | WAIS-III | ||
| Picture Completion | 14 | High average | |
| Digit–Symbol | 7 | Low average | |
| Block Design | 9 | Average | |
| Matrix Reason | 9 | Average | |
| Verbal memory | Rey AVLT | Raw scores | |
| Trials 1–5 | 5–6–8–10–11 | Normal | |
| 30′ recall | 7 | Low normal | |
| RMT Words | 10% | Low normal | |
| Visual memory | Benton VRT | ||
| No. correct | 5 | Mildly impaired | |
| No. errors | 7 | Mildly impaired | |
| CFT 30′ Recall | 5% | Impaired | |
| RMT Faces | 15% | Low normal | |
| Academic achievement | WRAT-3 | ||
| Reading | 63% | Normal | |
| Spelling | 55% | Normal | |
| Arithmetic | 34% | Normal | |
| Word finding | MAE COWA | 43% | Normal |
| Visual naming | MAE | 75% | Normal |
| Sentence repetition | MAE | 43% | Normal |
| Visual discrimination | Facial Recognition | 71% | Normal |
| Spatial perception | JOLO | 45% | Normal |
| Visuoconstruction | CFT Copy | 45% | Normal |
| Visuomotor coordination | Grooved Pegboard | ||
| Right hand | 16 % | Low normal | |
| Left hand) | 16 % | Low normal | |
| Executive functions | Trail making B | 66 s | Normal |
| WCST # Categories | 6 | Normal | |
| WCST # Persev. Error | 13 | Normal | |
| Tower of Hanoi Trial 1 | 66 | Normal | |
| Tower of Hanoi Trial 2 | 65 | Normal | |
| Tower of Hanoi Trial 3 | 120 | Impaired | |
| Tower of Hanoi Trial 4 | 120 | Impaired | |
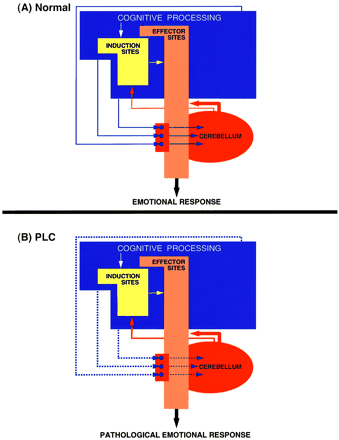
The traditional view. According to the traditional view, laughter and crying would be triggered normally via two separate neuroanatomical pathways to the brainstem laughter and crying centre (LCC), and pathological laughter and crying is caused by lesions of the voluntary paths to the LCC. We believe this view is problematic.
Lesions in patient C.B. The MRIs show the neuroanatomical correlates of the lesions of Patient C.B. in the axial (first column), coronal (second column) and parasagittal planes (third column). In total, five small lesions were detected: first lesion (1) was 2 × 1 × 1 mm in size at the pontomesencephalic junction, affecting the left cerebral peduncle in its lateral segment where descending tracts from the occipital, parietal and temporal cortices travel (Martin, 1996; Afifi and Bergman, 1998) (see Fig. 3). The second lesion (2) was 4 × 3 × 3 mm in size in the midline basis pontis and at the level of mid-to-upper pons. This region contains pontine nuclei of the left and the crossing fibres from the pontine nuclei of the right basis pontis. This region of the basis pontis receives input principally from the prefrontal cortex (Schmahmann and Pandya, 1997a) and the anterior cingulate gyrus (Vilensky and Van Hoesen, 1981). The third and fourth lesions (3 and 4) are almost continuous (i.e. 3–4 mm apart), both affecting the middle cerebellar peduncles from its brainstem root until the white matter below the superior semilunar lobule. These two lesions were together 12 mm in axial plane and varied from 2 to 5 mm in coronal and parasagittal planes. Finally, the fifth lesion (5) was 6 × 2 × 2 mm in size affecting, also on the right cerebellum, the white matter of the inferior semilunar lobule. In summary, all five lesions disrupt the topographically organized input from the higher association areas to the cerebellum. MRIs were taken 14 months and 4 days after C.B.'s stroke.
The cerebellum is interconnected with telencephalic structures. The descending pathways to the cerebellum (blue lines) originate in many telencephalic structures including the motor and limbic cortices as well as cortical association areas (AA). These pathways descend in topographically organized manner in the internal capsule (ic) and continue in the cerebral peduncle (cp) toward the basis pontis (bp) where they synapse topographically with the nuclei of the basis pontis. Neurones in the nuclei of basis pontis give rise to decussating pontocerebellar pathways that project within the middle cerebellar peduncle (mcp) toward the cerebellar targets. The ascending branch (red lines) consists of fibres from the deep cerebellar nuclei to the contralateral thalamus (T) and also to the red nucleus (r) from which many descending (small arrows) projections to the brainstem nuclei arise. The cerebellar projections to the thalamus are relayed to motor areas of the cerebral cortex. However, there are relatively minor projections from the cerebellum to other thalamic nuclei, which in turn project to the limbic and prefrontal cortices. The transversal sections of the middle panel show that the corticopontine pathways originated in the prefrontal cortex (PFC) and the temporal, parietal and occipital association areas (other) travel in the most anterior and posterior aspects of the internal capsule and in the most medial and lateral portions of the cerebral peduncles, respectively. The middle sector of the internal capsule contains fibres from the primary motor cortices directed, via the corticospinal tract (CST), mainly toward the brainstem and spinal motor structures (Afifi and Bergman, 1998). Three of C.B.'s five lesions are shown with dark patches on the right panel. The lesions of patient C.B. are located in the left cp (lesion 1), midline bp (lesion 2) and right mcp (lesion number 3). Lesions number 4 and 5 are, as shown in Fig. 2, located in the white matter of the inferior and superior semilunar lobules.
The alternative view. We suggest that PLC is caused by a dysfunction in circuits that involve the cerebellum. In normal circumstances (A) the profile, intensity and duration of an emotional response are modulated according to the cognitive/social context in which the triggering stimulus appears. For this to occur, telencephalic structures in which emotionally-competent stimuli as well as the relevant cognitive/social context are processed (blue box) signal their computation to the cerebellum where, according to the context, a certain profile and level of emotional response is computed. In turn, the cerebellum will signal its computation to the emotion-effector (and perhaps to the emotion-induction) sites (red lines). Examples of effector sites include the motor cortices, the hypothalamus, the periaqueductal grey matter and the cranial nerve nuclei, whereas the induction sites include the ventromedial prefrontal cortex, the anterior cingulate cortex, the extended amygdala and the ventral striatum. According to this view, PLC (B) is caused by a disruption (marked by dotted lines) of topographically organized pathways from higher association areas to the cerebellum that are involved in the adjustment of laughter and crying responses according to the specific contexts in which a triggering stimulus appears.
References
Albrecht U, Sutcliffe JS, Cattanach BM, Beechey CV, Armstrong D, Eichele G, et al. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons.
Andersen G, Vestergaard K, Riis JO. Citalopram for post-stroke pathological crying.
Arroyo S, Lesser RP, Gordon B, Uematsu S, Hart J, Schwerdt P, et al. Mirth, laughter and gelastic seizures.
Bassetti C, Bogousslavsky J, Barth A, Regli F. Isolated infarcts of the pons.
Bhatjiwale MG, Nadkarni TD, Desai KI, Goel A. Pathological laughter as a presenting symptom of massive trigeminal neuromas: report of four cases.
Bishop GA, Ho RH. The distribution and origin of serotonin immunoreactivity in the rat cerebellum.
Brodal P, Bjaalie JG. Salient anatomic features of the cortico-ponto-cerebellar pathway. [Review].
Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, et al. Distinctive patterns of subcortical and cortical brain activity during the feeling of self-generated emotions.
Damasio H, Frank R. Three-dimensional in vivo mapping of brain lesions in humans.
Dietrichs E. Cerebellar autonomic function: direct hypothalamocerebellar pathway.
Feinstein A, Feinstein K, Gray T, O'Connor P. Prevalence and neurobehavioral correlates of pathological laughing and crying in multiple sclerosis.
Feinstein A, O'Connor P, Gray T, Feinstein K. Pathological laughing and crying in multiple sclerosis: a preliminary report suggesting a role for the prefrontal cortex.
Feldman RS, Meyer JS, Quenzer LF. Principles of neuropsychopharmacology. Sunderland MA: Sinauer Associates; 1997.
Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging.
Gavrilescu T, Kase CS. Clinical stroke syndromes: clinical-anatomical correlations. [Review].
Goel V, Grafman J. Are the frontal lobes implicated in `planning' functions? Interpreting data from the Tower of Hanoi.
Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, et al. Parallel neural networks for learning sequential procedures. [Review].
Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging.
Kerr CW, Bishop GA. Topographical organization in the origin of serotoninergic projections to different regions of the cat cerebellar cortex.
Kim JS, Choi-Kwon S. Poststroke depression and emotional incontinence: correlation with lesion location.
Lal AP, Chandy MJ. Pathological laughter and brain stem glioma [letter].
Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population.
MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention [Review].
Matsuoka S, Yokota A, Yasukouchi H, Harada A, Kadoya C, Wada S, et al. Clival chordoma associated with pathological laughter. Case report.
Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate.
Poeck K. Pathophysiology of emotional disorders associated with brain damage. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology, Vol. 3. Amsterdam: Elsevier; 1985: 343–67.
Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. [Review].
Provine RR. Contagious yawning and laughter: significance for sensory feature detection, motor pattern generation, imitation, and the evolution of social behavior. In: Heyes CM, Galef B, editors. Social learning in animals: the roots of culture. New York: Academic Press; 1996. p. 179.
Robinson RG, Parikh RM, Lipsey JR, Starkstein SE, Price TR. Pathological laughing and crying following stroke: validation of a measurement scale and a double-blind treatment study.
Schmahmann JD. The cerebellum and cognition (Int Rev Neurobiol, Vol. 41). San Diego: Academic Press, 1997b.
Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. [Review].
Schmahmann JD, Pandya DN. The cerebrocerebellar system. [Review].
Shafqat S, Elkind MS, Chiocca EA, Takeoka M, Koroshetz WJ. Petroclival meningioma presenting with pathological laughter.
Thach WT. Combination, complementarity and automatic control: a role for the cerebellum in learning movement coordination. [Review].
Trouillas P. The cerebellar serotoninergic system and its possible involvement in cerebellar ataxia. [Review].
Vilensky JA, van Hoesen GW. Corticopontine projections from the cingulate cortex in the rhesus monkey.
Wali GM. `Fou rire prodromique' heralding a brainstem stroke.