-
PDF
- Split View
-
Views
-
Cite
Cite
A. Valentín, M. Anderson, G. Alarcón, J. J. García Seoane, R. Selway, C. D. Binnie, C. E. Polkey, Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo, Brain, Volume 125, Issue 8, August 2002, Pages 1709–1718, https://doi.org/10.1093/brain/awf187
Close - Share Icon Share
Abstract
The aim of the present study was to investigate in vivo cortical excitability in the human brain. We studied 45 consecutive patients with refractory epilepsy in whom subdural or intracerebral electrodes were implanted for assessment prior to epilepsy surgery. We compared cortical responses to single pulse stimulation (up to 8 mA, 1 ms duration) in areas where seizure onset occurred, with responses recorded elsewhere. Two main types of responses were seen: (i) ‘early responses’, spikes and/or slow waves starting within 100 ms after the stimulus which were observed in most regions in all patients; and (ii) ‘delayed responses’, spikes or sharp waves occurring between 100 ms and 1 s after stimulation which were seen in some regions in 27 patients. The distributions of early and delayed responses were compared with the topography of seizure onset. Whereas early responses were seen in most regions and seem to be a normal response of the cortex to single pulse stimulation, the distributions of delayed responses were significantly associated with the regions where seizure onset occurred. We conclude that the presence of delayed responses can identify regions of hyperexcitable cortex in the human brain. The study of delayed responses may improve our understanding of the physiology and dynamics of neuronal circuits in epileptic tissue and may have an immediate clinical application in assessment of candidates for surgical treatment of epilepsy.
Introduction
A cortical imbalance between excitatory and inhibitory mechanisms is thought to be the pathophysiological basis for human partial epilepsy. It is currently accepted that epileptic seizures are due to a sudden excessive synchronization of neuronal activity in areas of altered cortical excitability. Animal models of epilepsy have shown that an enhancement of excitatory mechanisms and/or a decrement in inhibition results in long lasting cellular electrical discharges which take place spontaneously or as a response to electrical cortical stimulation (Gardner et al., 1974; Jefferys, 1989; Empson et al., 1993; Delgado‐Escueta et al., 1999; Kohling et al., 2000). In patients with partial epilepsy, an imbalance between excitability and inhibition would be expected in cortical areas which possess the capacity to generate spontaneous epileptic seizures. Sub‐acute recordings with intracranial electrodes are sometimes performed in epileptic patients being assessed for surgery in order to identify the onset of spontaneous seizures and localise the cortical regions responsible for their generation. Such intracranial recordings provide a unique opportunity to study cortical excitability and its relation to seizure onset. We hypothesize that measurement of cortical excitability may help identify and establish the limits of epileptogenic cortex in the human brain.
Trains of electrical pulses lasting for several seconds have long been used in order to induce patients’ habitual attacks and/or to map the cortical threshold for after‐discharges (Bidwell and Sherrington, 1889; Parker and Gotch, 1893; Cushing, 1909; Horsley, 1909; Penfield and Jasper, 1954; Chauvel et al., 1993; Luciano et al., 1993; Schulz et al., 1997). However, habitual seizures can be induced by secondary activation of epileptogenic cortex remote from the area stimulated electrically and determination of the threshold required to evoke after‐discharges has yielded contradictory results (Ajmone‐Marsan, 1972; Cherlow et al., 1977; Lesser et al., 1984a, b, 1991; Luders et al., 1988). This is not surprising, as cortical stimulation with relatively long trains of current pulses (1–5 s) is likely to produce massive and widespread cortical activation. An alternative would be to study the balance between excitability and inhibition with brief single pulses, which activate only a limited and localized population of neurones. Cortical responses evoked by single or paired pulse electrical stimulation applied directly to the human cortex have been studied previously in patients with epilepsy to identify cortical connections of the human temporal lobe (Brazier, 1964; Rutecki et al., 1989; Wilson et al., 1990; Buser et al., 1992) and to look for differences between epileptogenic and non epileptogenic regions (Wilson and Engel, 1993; Wilson et al., 1998).
In the present work, we studied 45 patients assessed with chronic intracranial recordings for surgical treatment of epilepsy. The distribution of cortical responses to single pulse stimulation was compared with the distribution of seizure onset. We define epileptogenic cortex as cortex that has the capacity to originate spontaneous seizures, presumably because it is abnormally hyperexcitable. The concept of epileptogenic cortex overlaps with, but it is not necessarily the same as the ‘epileptogenic zone’ defined by Lüders and colleagues (Lüders et al., 1992), as we have not specifically studied surgical outcome. As a result from the present study, we have developed a method to identify interictally the topography and extent of hyperexcitable cortex which might be potentially epileptogenic.
Methods
Subjects
We studied 45 consecutive patients (17 males, 28 females, mean age 33.8 years, range 14–58 years) who were being evaluated as candidates for resective surgery for the treat ment of their epilepsy in the Department of Clinical Neurophysiology at King’s College Hospital, London. Patients suffered from medically refractory epilepsy and were admitted for video‐EEG monitoring with intracranial electrodes because reliable localization of the epileptogenic zone was not achieved by non‐invasive tests. All patients were fully informed of the nature of the research and gave informed consent, according to the Declaration of Helsinki. The experimental procedure was approved by the ethical committee of King’s College Hospital (reference number 99–017).
Electrode placement
Subdural or intracerebral (depth) electrodes were implanted in the 45 patients. The type, number and location of the electrodes were determined by the suspected location of the epileptogenic zone in each patient according to previous findings from clinical history, neuroimaging, neuropsychology and scalp EEG recordings. The selection criteria and implantation procedure were as described previously (Elwes, 2000).
Subdural electrodes
Electrode strips or mats (AdTech Medical Instruments Corp., WI, USA) were used in 30 patients. Each strip consisted of a single row of 4–8 platinum disk electrodes spaced at 10 mm between centres. The disks were embedded in a 0.7 mm thick polyurethane strip which overlapped the edges leaving a diameter of 2.3 mm exposed, recessed approximately 0.1 mm from the surface plane. Mats contained similar 8, 20 or 32 platinum electrode rectangular arrays with 10 mm centre‐to‐centre distances within rows.
Twenty‐four of the 30 patients had bilateral subdural strips slid under the inferior aspect of the temporal lobe so that the deepest electrodes in each strip laid in contact with medial temporal structures and the parahippocampal gyri. Fifteen of these 24 patients had additional subdural strips or mats located over the lateral temporal cortex, frontal or occipital lobes. Five patients had electrodes located over the lateral convexity of the temporal lobe and over the frontal or occipital lobes. One patient had electrodes restricted to the frontal lobes. Eight patients had electrodes restricted to one hemisphere.
Intracerebral (depth) electrodes
In 15 patients, multicontact flexible bundles of depth electrodes (AdTech Medical Instruments Corp., WI, USA) were implanted stereotactically under MRI guidance in both hemispheres. The electrodes consisted of 6–10 cylindrical 2.3 mm long platinum contacts separated by 5 mm between centres of adjacent electrodes of the same bundle. Nine of these 15 patients had electrodes located in frontal and mesial temporal structures, and four patients had electrodes restricted to mesial temporal structures. One patient had electrodes restricted to the frontal lobe and one had electrodes in occipital and mesial temporal structures.
EEG recording and determination of ictal onset
Recording of intracranial EEG started when the patient had recovered from electrode implantation, usually 24–48 h after surgery. Cable telemetry with up to 64 recording channels was used for data acquisition with simultaneous video monitoring. A Telefactor Beehive‐Beekeeper system (Astro‐Med, RI, USA) was used in 42 patients. Data were digitized at 200 Hz and band pass filtered (high pass cut‐off frequency at 0.3 Hz and low pass cut‐off frequency at 70 Hz). The system input range was 2 mV and data were digitized with a 12 bit analogue‐to‐digital converter (an amplitude resolution of 0.488 µV). A Medelec‐Profile system (Medelec, Oxford Instruments, Old Woking, UK) was used in the remaining three patients. Data were digitized at 256 Hz and band pass filtered (0.05–67 Hz). The input range was 10 mV and data were digitized with a 22 bit analogue‐to‐digital converter (an amplitude resolution of 0.153 µV). The sampling frequencies used allow a time resolution of 4–5 ms, which was adequate for the duration and latency of observed responses (in the order of tens or hundreds of milliseconds). Data were recorded as common reference to Pz or to an intracranial electrode and displayed in a variety of montages including common average reference. Channels showing large spikes, artefacts or responses were removed from the average when common average reference was used.
Between 1 and 20 seizures (median = 5) were recorded in each patient during the period of telemetry (3–22 days, median = 9 days). Ictal onset was identified independently by two accredited electroencephalographers.
The site of seizure onset was determined by the number and position of the electrodes which showed the first electroencephalographic ictal changes. Seizure onset was identified as a clear ictal EEG pattern consisting of regular spikes, rhythmic sharp waves, spike‐and‐slow wave complexes, sharp and slow wave complexes, regular delta or theta activities, sharpened delta or theta activities, or low‐amplitude activity in the beta range. Diffuse electrodecremental events were noted but not considered for analysis, as they do not seem to be of localising value (Alarcon et al., 1995). Individual seizures were classified in three types depending on the extension of electroencephalographic changes at seizure onset: (i) focal, if only one or two adjacent electrodes were involved; (ii) regional, if more than two electrodes were involved; and (iii) diffuse, if electroencephalographic changes at seizure onset were bilateral or involved all recording channels.
Patients were classified as: (i) focal, if only focal seizures were recorded and seizure onset showed similar topography in all seizures; (ii) regional, if all seizures had a regional onset; (iii) focal/regional, if some seizures were focal and others showed a regional onset; and (iv) diffuse, if each seizure showed a diffuse or bilateral onset. No patient showed other combinations of seizure onsets.
Experimental protocol
Single pulse electrical stimulation was performed between adjacent electrodes using a constant‐current neurostimulator approved for use in human subjects (Medelec ST10 Sensor, Oxford Instruments, Old Woking, UK). Electrical stimulation was carried out with single pulses of 0.3 or 1 ms duration and a current intensity of 1–8 mA (4 mA being the intensity most often used). Monophasic pulses were chosen in order to increase the localising accuracy of electrical stimulation. According to standard practice in clinical neurophysiology, it was assumed that neuronal stimulation occurred mainly at the cathode. A single pulse was delivered every 10 s and EEG responses to each pulse were recorded by the electrodes not used for stimulation.
To simplify the wording in this paper, the term ‘stimulus’ or ‘stimulation’ will be used to designate each single pulse and the term ‘trial’ will be used to designate a batch of several identical single pulses applied to the same pair of electrodes, every 10 s, with the same polarity. Trials used in this study usually comprised 10 stimuli. For each pair of adjacent electrodes, stimulation trials of opposite polarity were carried out. In subdural recordings, pairs of contiguous electrodes were used successively so that every electrode was the negative pole (i.e. the stimulating electrode) in at least one trial. During each trial, recording from the electrodes used for stimulation was suspended. When the presence of delayed responses (see below) was suspected, stimulation was repeated (up to 40 stimuli) in order to confirm (or reject) the presence of delayed response on a larger number of stimuli. Unless side effects were present (see below), the stimulation intensity was the same for all the trials carried out in each patient. In each patient, all available electrodes were used to stimulate in at least one trial, except in the case of intracerebral recordings where only pairs of electrodes located in grey matter (according to MRI studies obtained with the electrodes implanted) were used to stimulate.
Each pulse produced charge density at the electrodes (Jayakar, 1993) ranging between 8–193 µC/cm2/pulse (most commonly 96.5 µC/cm2/pulse). Stimulation parameters were chosen to avoid tissue damage as similar charge densities produce no detectable histopathological abnormalities in humans (Rutecki et al., 1989) and no kindling appears to occur in animals at stimulation rates of 0.1 Hz, even after weeks of daily stimulation (Goddard et al., 1969). As the period between consecutive pulses was 10 s, the period free of current was at least 10 000 times longer than the period when current was applied. This allowed ample time for electrodes to depolarize after each pulse and for the brain to recover its baseline state. Therefore, monophasic pulses were considered safe. As we used single pulse stimulation, the total charge delivery per second was ∼100 times smaller than that used routinely during cortical functional mapping in humans for presurgical assessment (Ojemann, 1979; Black and Ronner, 1987; Alarcon and Binnie, 1995). Thirty patients showed no behavioural, sensory or motor response to stimulation with the described protocol. Thirteen patients showed a unilateral contraction of ipsilateral orbicularis oculi and/or tingling of the ipsilateral cheek during stimulation of the deepest contacts of subtemporal strips at intensities above 3 mA, probably due to electrical stimulation of the trigeminal or facial nerves. Two patients had a tingling sensation in the contralateral limbs when medial frontal cortex was stimulated. If any of these effects occurred, stimulus intensity was decreased or stimulation at the corresponding site was abandoned.
Statistical analysis
As delayed responses often resembled spontaneous interictal discharges and were not seen after every stimulus, the association between stimulation and delayed responses was established by comparing the occurrence of spikes during one second before and one second after each stimulation. It was assumed that spikes were related to stimulation if the number of stimuli showing spikes during the following second was greater than the number of stimuli showing spikes during the previous second with P < 0.05 (one‐tailed sign test).
Results
Types of responses to single pulse stimulation
Two types of responses were evoked by the stimuli:
(i) Early responses. These responses consisted of a sharp deflection immediately following the stimulus artefact or occasionally merging with it. This initial deflection was followed by one or two slow waves of alternating polarity (Fig. 1A and B). Early responses were seen in all patients when stimulating at most locations and were therefore considered to be a normal response of the cortex to stimulation. The amplitude of early responses depended on stimulation intensity and tended to show maximal amplitude at electrodes closest to the stimulus. However, early responses could sometimes be observed at electrodes located several cm away from stimulating electrodes (Fig. 1A and B). Eight patients showed bilateral early responses of large amplitude and long duration when medial frontal structures were stimulated. The polarity, morphology and latencies of early responses were variable among patients and stimulating regions. A more detailed study of early responses will be the subject of a further report.
(ii) Delayed responses. These were seen in 27 out of 45 patients. They consisted of spikes and sharp waves, which resembled epileptiform discharges, and were seen with a latency >100 ms and <1 s after the stimulus artefact (Figs 1B, 2A and B, 3B and 4B). Delayed responses were not always seen after every identical stimulus. Occurrence rates varied between 10–90%, depending on the patient and stimulation site. A one‐tailed sign test was used to establish the association between stimulation and delayed responses. When the stimulus intensity was increased, the morphology of delayed responses was not clearly modified, but a higher probability for their occurrence was observed and, in some patients, bursts of spikes could be evoked with a single stimulus (Figs 2A and B, 4B).
Delayed responses were seen only within 3 cm from the stimulating electrodes (Fig. 3A) in 15 patients and were recorded with >3 cm from the stimulating electrodes within the same hemisphere in 11 patients (Figs 1B, 2A and 3B). In addition, one patient showed delayed responses in medial temporal structures when the stimulus was applied to the contralateral medial temporal structures.
Delayed responses were classified as focal if only one or two electrodes were involved, and regional if seen in more than two electrodes within the same hemisphere. When delayed responses were seen independently in both hemispheres, they were classified as bilateral. No diffuse delayed responses were seen.
No differences were seen in either response type depending on the electrode modality (subdural or depth) used.
Topography of seizure onset
Seizure onset was located within the temporal lobe in 11 out of 12 patients who showed exclusively focal seizures. Among the 22 patients who showed exclusively regional seizure onset, this was confined to the temporal lobe in 16 patients. Seizure onset was temporal in three of the four patients who showed both focal and regional seizure onsets. Six patients showed diffuse seizure onset. Only nine patients showed focal or regional seizure onset in structures outside the temporal lobe.
Relationship between early responses and seizure onset
No obvious relationship was found between the distribution of early responses and the topography of seizure onset. A more detailed study regarding the relations between the morphology of early responses and the topography of seizure onset will be the subject of a further report.
Relation between delayed responses and patient type
A total of 255 seizures were recorded from the 45 patients. The relationship between delayed responses and patient type is shown in Table 1. Fifteen of the 27 patients with delayed responses showed exclusively focal delayed responses, two showed focal and regional delayed responses and 10 showed only regional delayed responses. Thus, 17 patients showed focal delayed responses. Three patients showed bilateral independent delayed responses, and two of them had only seizures with onsets restricted to one side. All 16 patients who showed no delayed responses had regional or diffuse seizure onsets.
Interestingly, among the 23 regional patients, 10 showed focal or regional delayed responses at the sites of seizure onset, only one showed regional delayed responses outside the area of seizure onset, and 12 had no delayed responses.
Relation between delayed responses and seizure onset
For those sites that showed delayed responses, the relation between the distribution and topography of delayed responses and of seizure onset is shown in Table 2 for the 27 patients who showed delayed responses. As seven patients showed delayed responses at more than one location, the total number of occasions in which delayed responses were seen was 34. Delayed responses were seen within the temporal lobe on 28 occasions and outside the temporal lobe on only six occasions. Diffuse delayed responses were not seen. Interestingly, on all 17 occasions when focal temporal delayed responses were seen, they occurred in regions that were involved in seizure onset (Fig. 3). In addition, among the 11 occasions when regional temporal delayed responses were seen, in nine they occurred at electrodes involved in seizure onset (Fig. 4). Out of the 28 occasions where delayed responses were seen in the temporal lobe, delayed responses occurred outside the region where seizure onset was seen in two patients only. In these two patients, seizure onset was restricted to one temporal lobe whereas delayed responses were seen independently in both temporal lobes. Of the 28 occasions when delayed responses were seen in the temporal lobe, 15 were restricted to mesial temporal structures. Apart from topography, no clear differences were seen between delayed responses seen over the temporal neocortex or at mesial temporal structures.
Among the six occasions in which delayed responses were seen outside the temporal lobe, seizure onset occurred in regions showing delayed responses in four. In the remaining two cases, delayed responses were seen at electrodes located in the same lobe as those electrodes involved at seizure onset.
No relation was found between the topography of seizure onset and the location of the stimulating electrodes when delayed responses were seen.
In summary, there was a significant association between the location of focal delayed responses and the topography of seizure onset in temporal lobe epilepsy. Too few patients showed extratemporal delayed responses for statistical comparison.
Surgery and pathology
In the last 2 years, 23 out of the total of 45 patients have had resective surgery for the treatment of their epilepsy. Four patients had extratemporal resections and 19 had en bloc temporal lobectomies. Among the four patients with extratemporal resections, three had focal cortical dysplasia (FCD) and one had a low‐grade glioma. Of the 19 patients who had temporal lobectomies, eight had mesial temporal sclerosis (MTS), three had FCD, two had dysembryoplastic neuroepithelial tumours (DNET), two had low‐grade gliomas, one had MTS and DNET, one had MTS and FCD, one had a periventricular grey matter heterotopia, one had an old infarct and four showed non‐specific changes.
Discussion
We have observed two different types of cortical responses to single pulse stimulation. Early responses could be recorded when stimulating at most sites, and their amplitude was related to stimulation intensity. They seem to be normal responses of the human cortex to stimulation. Similar results have been reported in human medial temporal structures by other authors (Brazier, 1964; Rutecki et al., 1989; Wilson et al., 1990; Buser et al., 1992). However, as far as we know, delayed responses to single pulse stimulation have not been described previously in humans.
The presence of delayed responses in areas that originate focal seizures is of interest with regard to the pathophysiological basis of human partial epilepsy. As delayed responses are evoked with a latency of between 100 ms and 1 s, a likely pathophysiological explanation is the presence of a cortical loop lasting for some hundreds of milliseconds. A long‐lasting activation of a subset of neurones in the epileptogenic regions may therefore be responsible for delayed responses. Indeed, in vitro experiments using disinhibited neocortical slices treated with low doses (0.8–0.9 µM) of the gamma aminobuytric acid (GABA) receptor blocker, bicuculline, have shown field potentials with latencies >150 ms as a response to single pulse stimulation (Chagnac‐Amitai and Connors, 1989). Similar results have been observed when hippocampal slices were exposed to Mg2+ free medium (Kohling et al., 2000) and after local injection of tetanus toxin (Jefferys, 1989; Empson et al., 1993). If activation of delayed responses is strong enough to become a stimulus in its own right, then delayed loops could re‐activate repeatedly giving rise to a run of spikes and, ultimately, a seizure. Thus, our findings suggest that temporal lobe epilepsy may not be due to a global, non‐specific increased excitability of all cortical neurones, but to an increased excitability of cells responsible for triggering delayed responses. Identification of such cellular mechanisms appears necessary and may aid in the distinction between different types of epilepsies according to their pathophysiology. Furthermore, the study of the mechanisms underlying the occurrence of delayed responses could help in understanding the physiology and dynamics of neuronal circuits in epileptic tissue.
The presence of delayed responses in regions located several centimetres away from the stimulating electrodes was interpreted as evidence of physiological connections between the cortical areas underlying the stimulating electrodes and those electrodes where delayed responses were seen. This suggests that delayed responses are a response to afferents from the stimulated area. Unfortunately, it was not possible to record through the electrodes used for stimulation and the responses of the cortex immediately underlying the stimulating electrodes remain unknown.
A number of important practical implications derive from our results. Delayed responses can predict interictally the topography of seizure onset with sufficient specificity to be of potential use in clinical practice. Indeed, in temporal lobe epilepsy, delayed responses were virtually as reliable in the identification of epileptogenic cortex as the study of seizure onset. Additional clinical information could be obtained by comparing the topography of delayed responses and seizure onset. An important question often arises when interpreting intracranial recordings in those patients where seizure onset is detected simultaneously by many electrodes (regional or diffuse onset, in our terminology). A widespread seizure onset may be due to the existence of a large epileptogenic zone or to rapid propagation of ictal activity from a more discrete seizure onset located distant from any recording electrode. In such cases, the occurrence of delayed responses at the region that showed ictal onset would suggest that the electrodes are located close to functionally abnormal cortex, whereas the absence of delayed responses would suggest a seizure onset arising either at a focus elsewhere or diffusely. In addition, delayed responses to single pulse stimulation may be a method to identify epileptogenic cortex in those patients who do not have enough seizures during the period of video monitoring, or in any case, can support ictal data. This technique could also be useful to guide implantation of intracranial electrodes in order to maximize the likelihood of identifying a focal seizure onset at a later stage during video‐telemetry. Furthermore, single pulse stimulation can be performed during acute electrocorticography in patients who have not undergone invasive recordings to confirm localization and to decide to proceed with resection or, in the case that no delayed responses were seen, to implant electrodes and carry out telemetry.
Acknowledgements
We wish to thank Dr Robert C. D. Elwes for his contribution during presurgical assessment of the patients studied. This project has been funded by The Fund for Epilepsy.
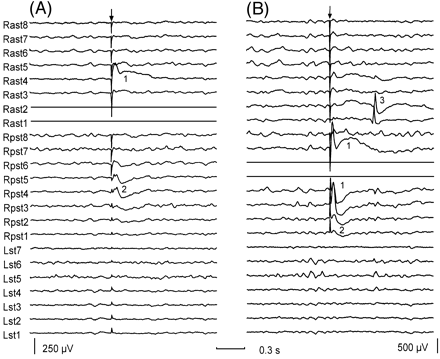
Fig. 1 Early and delayed responses evoked by single pulse electrical stimulation. The patient showed focal seizures starting at electrode 1 of the right anterior subtemporal strip (Rast1). (A) Early responses seen when stimulating the deepest electrodes of the right anterior subtemporal region (Rast1, Rast2, shown as flat traces). (B) Early and delayed responses during stimulation through electrodes located at the right posterior subtemporal region (Rpst4, Rpst5). Numbers inserted in the figure indicate different response types: (1) early responses seen at electrodes located close to the stimulating electrodes; (2) early responses seen at electrodes located >3 cm away from the stimulating electrodes; and (3) delayed responses seen with a latency of >100 ms. The arrows indicate the stimulation artefact. Both recordings have similar time calibration but different gain. For each subdural strip, electrode 1 was the most distal electrode to the insertion burr hole. Abbreviations: Lst = left subtemporal strip; Rast = right anterior subtemporal strip; Rpst = right posterior subtemporal strip.
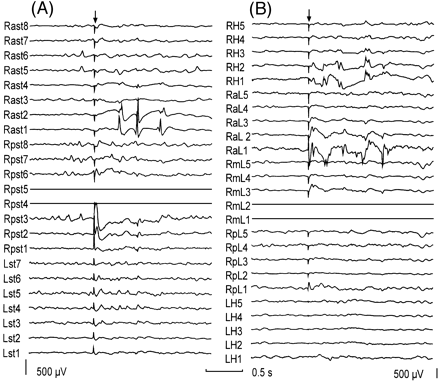
Fig. 2 Bursts of delayed responses evoked by a single stimulus. (A) Burst of delayed responses seen at Rast1 and Rast2 during stimulation through Rpst4 and Rpst5 in the same patient as in Fig. 1. (B) Bursts of delayed responses recorded by depth electrodes located in the hippocampus (RaL1, 2 and RH1, 2) in a different patient with a lesion located at the lateral convexity of the temporal lobe. The arrow indicates the stimulation artefact. Both recordings have the same time calibration but different gain. For each subdural strip or depth electrode bundle, the most distal electrode to the insertion burr hole was electrode 1. In B, contacts 1 and 2 of each depth electrode bundle are located at mesial temporal structures. Electrodes used for stimulation are shown as flat traces. Abbreviations: LH = left hippocampus electrode; Lst = left subtemporal strip; RaL = right electrode anterior to the lesion; Rast = right anterior subtemporal; RH = right hippocampus electrode; RmL = right electrode medial to the lesion; RpL = right electrode posterior to the lesion; Rpst = right posterior subtemporal strip.
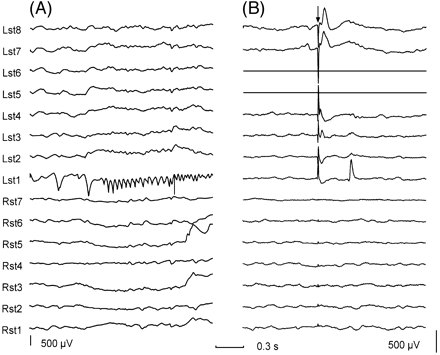
Fig. 3 Relation between focal seizure onset and focal delayed responses. (A) A subdural recording of a focal seizure onset. Note the fast activity building up in the electrode 1 of the left subtemporal region (Lst1). (B) Delayed response seen at Lst1 during stimulation through electrodes 6 and 5 of the left subtemporal strip (Lst5, Lst6). There are also sharp early responses observed at Lst8, Lst7, Lst4, Lst3, with maximal amplitude at Lst8. Note that seizure onset occurs at the site where delayed responses were seen rather than at the site of stimulation which, in this case, is located 4 cm away. The arrow indicates the stimulation artefact. Both recordings have the same time calibration but different gain. For each subdural strip, electrode 1 was the most distal electrode to the insertion burr hole. Electrodes used for stimulation are shown as flat traces. Abbreviations: Lst = left subtemporal strip; Rst = right subtemporal strip.
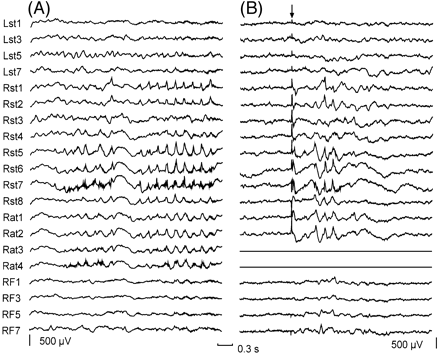
Fig. 4 Relation between regional seizure onset and regional delayed responses. (A) A subdural recording of a regional seizure onset. Note that most electrodes of the right subtemporal region and all electrodes of the right anterior temporal strip are involved. (B) Delayed responses seen at the same location during stimulation through electrodes 3 and 4 of the right anterior temporal strip (Rat3, Rat4). The arrow indicates the stimulation artefact. Both recordings have the same time and amplitude calibrations. For each subdural strip, electrode 1 was the most distal electrode to the insertion burr hole. Electrodes used for stimulation are shown as flat traces. Abbreviations: Lst = left subtemporal strip; Rat = right anterior temporal strip; Rst = right subtemporal strip.
Cross tabulation between delayed responses and patient type
| Delayed responses | Patient type | ||||
| Focal | Focal/regional | Regional | Diffuse | Total patients | |
| Focal DR | 12 | 1 | 2 | 0 | 15 |
| Focal + regional DR | 0 | 1 | 1 | 0 | 2 |
| Regional DR | 0 | 1 | 6 | 0 | 7 |
| Bilateral DR | 0 | 1 | 2 | 0 | 3 |
| None | 0 | 0 | 12 | 6 | 18 |
| Total patients | 12 | 4 | 23 | 6 | 45 |
| Delayed responses | Patient type | ||||
| Focal | Focal/regional | Regional | Diffuse | Total patients | |
| Focal DR | 12 | 1 | 2 | 0 | 15 |
| Focal + regional DR | 0 | 1 | 1 | 0 | 2 |
| Regional DR | 0 | 1 | 6 | 0 | 7 |
| Bilateral DR | 0 | 1 | 2 | 0 | 3 |
| None | 0 | 0 | 12 | 6 | 18 |
| Total patients | 12 | 4 | 23 | 6 | 45 |
DR = delayed responses; bilateral DR = independent DR seen on both hemispheres; Focal + regional DR = patients showing focal and regional DR when stimulating at different sites; Focal/regional = patients with seizures showing focal and regional onsets.
Cross tabulation between delayed responses and patient type
| Delayed responses | Patient type | ||||
| Focal | Focal/regional | Regional | Diffuse | Total patients | |
| Focal DR | 12 | 1 | 2 | 0 | 15 |
| Focal + regional DR | 0 | 1 | 1 | 0 | 2 |
| Regional DR | 0 | 1 | 6 | 0 | 7 |
| Bilateral DR | 0 | 1 | 2 | 0 | 3 |
| None | 0 | 0 | 12 | 6 | 18 |
| Total patients | 12 | 4 | 23 | 6 | 45 |
| Delayed responses | Patient type | ||||
| Focal | Focal/regional | Regional | Diffuse | Total patients | |
| Focal DR | 12 | 1 | 2 | 0 | 15 |
| Focal + regional DR | 0 | 1 | 1 | 0 | 2 |
| Regional DR | 0 | 1 | 6 | 0 | 7 |
| Bilateral DR | 0 | 1 | 2 | 0 | 3 |
| None | 0 | 0 | 12 | 6 | 18 |
| Total patients | 12 | 4 | 23 | 6 | 45 |
DR = delayed responses; bilateral DR = independent DR seen on both hemispheres; Focal + regional DR = patients showing focal and regional DR when stimulating at different sites; Focal/regional = patients with seizures showing focal and regional onsets.
Relation between delayed responses and types of seizure onset in the 27 patients who showed delayed responses*
| Delayed response type and location | Type of seizure onset and relation with topography of delayed responses | |||||
| Focal onset | Regional onset | Diffuse | Total | |||
| At site where DR seen | Elsewhere | Involving site where DR seen | Elsewhere | |||
| Focal temporal | 14 | 0 | 3 | 0 | 0 | 17 |
| Regional temporal | 0 | 0 | 9 | 2 | 0 | 11 |
| Focal extratemporal | 0 | 1 | 1 | 0 | 0 | 2 |
| Regional extratemporal | 1 | 0 | 2 | 1 | 0 | 4 |
| Total | 15 | 1 | 15 | 3 | 0 | 34 |
| Delayed response type and location | Type of seizure onset and relation with topography of delayed responses | |||||
| Focal onset | Regional onset | Diffuse | Total | |||
| At site where DR seen | Elsewhere | Involving site where DR seen | Elsewhere | |||
| Focal temporal | 14 | 0 | 3 | 0 | 0 | 17 |
| Regional temporal | 0 | 0 | 9 | 2 | 0 | 11 |
| Focal extratemporal | 0 | 1 | 1 | 0 | 0 | 2 |
| Regional extratemporal | 1 | 0 | 2 | 1 | 0 | 4 |
| Total | 15 | 1 | 15 | 3 | 0 | 34 |
*Five patients showed more than one type of delayed responses: two had regional delayed responses at two sites and three had focal and regional delayed responses at two sites. In addition, two patients showed focal or regional delayed responses in sites involved in focal and regional seizure onsets. DR = delayed responses.
Relation between delayed responses and types of seizure onset in the 27 patients who showed delayed responses*
| Delayed response type and location | Type of seizure onset and relation with topography of delayed responses | |||||
| Focal onset | Regional onset | Diffuse | Total | |||
| At site where DR seen | Elsewhere | Involving site where DR seen | Elsewhere | |||
| Focal temporal | 14 | 0 | 3 | 0 | 0 | 17 |
| Regional temporal | 0 | 0 | 9 | 2 | 0 | 11 |
| Focal extratemporal | 0 | 1 | 1 | 0 | 0 | 2 |
| Regional extratemporal | 1 | 0 | 2 | 1 | 0 | 4 |
| Total | 15 | 1 | 15 | 3 | 0 | 34 |
| Delayed response type and location | Type of seizure onset and relation with topography of delayed responses | |||||
| Focal onset | Regional onset | Diffuse | Total | |||
| At site where DR seen | Elsewhere | Involving site where DR seen | Elsewhere | |||
| Focal temporal | 14 | 0 | 3 | 0 | 0 | 17 |
| Regional temporal | 0 | 0 | 9 | 2 | 0 | 11 |
| Focal extratemporal | 0 | 1 | 1 | 0 | 0 | 2 |
| Regional extratemporal | 1 | 0 | 2 | 1 | 0 | 4 |
| Total | 15 | 1 | 15 | 3 | 0 | 34 |
*Five patients showed more than one type of delayed responses: two had regional delayed responses at two sites and three had focal and regional delayed responses at two sites. In addition, two patients showed focal or regional delayed responses in sites involved in focal and regional seizure onsets. DR = delayed responses.
References
Ajmone‐Marsan C. Focal electrical stimulation. In: Purpura DP, Penry JK, Tower DB, Woodbury DM, Walter RD, editors. Experimental models of epilepsy. New York: Raven Press;
Alarcon G and Binnie CD. Functional presurgical assessment in partial epilepsy: stimulation procedures and sodium amytal test [abstract].
Alarcon G, Binnie CD, Elwes RD, Polkey CE. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy.
Bidwell LA, Sherrington CS. Focal epilepsy: trephining and removal of small haemorrhagic focus: no improvement; removal of part of leg centre after electrical stimulation: improvement.
Black PM, Ronner SF. Cortical mapping for defining the limits of tumor resection.
Brazier M. Evoked responses recorded from the depths of the human brain.
Buser P, Bancaud J, Chauvel P. Callosal transfer between mesial frontal areas in frontal lobe epilepsies.
Chagnac‐Amitai Y, Connors BW. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex.
Chauvel P, Landre E, Trottier S, Vignel JP, Biraben A, Devaux B, et al. Electrical stimulation with intracerebral electrodes to evoke seizures.
Cherlow DG, Dymond AM, Crandall PH, Walter RD, Serafetinides EA. Evoked response and after‐discharge thresholds to electrical stimulation in temporal lobe epileptics.
Cushing H. A note upon the faradic stimulation of the postcentral gyrus in conscious patients.
Delgado‐Escueta AV, Wilson WA, Olsen RW, Porter RJ, Pietsch SG. Jasper’s mechanisms of the epilepsies. Advances in neurology, Vol. 79. Philadelphia: Lippincott Williams & Wilkins;
Elwes RDC. Invasive neurophysiological evaluation. In: Oxbury JM, Polkey CE, Duchowny M, editors. Intractable focal epilepsy. London: WB Saunders;
Empson RM, Amitai Y, Jefferys JG, Gutnick MJ. Injection of tetanus toxin into the neocortex elicits persistent epileptiform activity but only transient impairment of GABA release.
Gardner CR, Gartside IB, Webster RA. Action of antiepileptic drugs on cortical induced epileptogenic activity. In: Harris P, Mawdsley C, editors. Epilepsy. Proceedings of the Hans Berger Centenary Symposium. Edinburgh: Churchill Livingstone;
Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation.
Jayakar P. Physiological principles of electrical stimulation. (Review).
Jefferys JG. Chronic epileptic foci in vitro in hippocampal slices from rats with the tetanus toxin epileptic syndrome.
Kohling R, Vreugdenhil M, Bracci E, Jefferys JG. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor‐mediated oscillations.
Lesser RP, Lüders H, Klem G, Dinner DS, Morris HH. A comparison of the variability of afterdischarge and functional alteration thresholds in man: results of extraoperative testing [abstract].
Lesser RP, Lüders H, Klem G, Dinner DS, Morris HH, Hahn J. Cortical afterdischarge and functional response thresholds: results of extraoperative testing.
Lesser RP, Gordon B, Fisher R, Hart J, Uematsu S. Subdural grid electrodes in surgery of epilepsy. In: Luders H, editor. Epilepsy surgery. New York: Raven Press;
Luciano D, Devinsky O, Pannizzo F. Electrocorticography during cortical stimulation. (Review).
Lüders H, Awad I, Burgess R, Wyllie E, Van Ness P. Subdural electrodes in the presurgical evaluation for surgery of epilepsy. (Review).
Lüders H, Lesser RP, Dinner DS, MOrris HH, Wyllie E, Godoy J. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy.
Ojemann GA. Individual variability in cortical localization of language.
Parker R, Gotch F. A case of focal epilepsy: trephining: electrical stimulation and excision of focus: primary healing: improvement.
Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Boston: Little, Brown;
Rutecki PA, Grossman RG, Armstrong D, Irish‐Loewen S. Electrophysiological connections between the hippocampus and entorhinal cortex in patients with complex partial seizures.
Schulz R, Luders HO, Tuxhorn I, Ebner A, Holthausen H, Hoppe M, et al. Localization of epileptic auras induced on stimulation by subdural electrodes.
Wilson CL, Engel J. Electrical stimulation of the human epileptic limbic cortex. (Review).
Wilson CL, Isokawa M, Babb TL, Crandall PH. Functional connections in the human temporal lobe. I. Analysis of limbic system pathways using neuronal responses evoked by electrical stimulation.
Author notes
1Division of Neuroscience, Guy’s, King’s and St. Thomas’ School of Medicine, King’s College Hospital, London, UK and 2Facultad de Medicina, Universidad Complutense, Madrid, Spain