-
PDF
- Split View
-
Views
-
Cite
Cite
Josef Parvizi, Antonio R. Damasio, Neuroanatomical correlates of brainstem coma, Brain, Volume 126, Issue 7, July 2003, Pages 1524–1536, https://doi.org/10.1093/brain/awg166
Close - Share Icon Share
Abstract
The brainstem tegmentum, including the reticular formation, contains distinct nuclei, each of which has a set of chemical, physiological and anatomical features. Damage to the brainstem tegmentum is known to cause coma, the most radical disturbance of consciousness. However, it has remained unclear which nuclei within the tegmentum are crucial for the maintenance of consciousness in humans. Accordingly, we initiated a retrospective study of MRIs obtained from 47 patients with brainstem stroke. The lesion boundaries were charted on patient MRIs and transferred onto a corresponding series of 4.7 T MRIs obtained from a control brainstem specimen that later was cut on a freezing microtome and analysed histologically. In addition, medical charts and available post‐mortem materials were used to obtain relevant clinical and anatomical data to verify the MRI readings in each case. We found that in the 38 patients who did not have coma, brainstem damage either was located outside the tegmentum (n = 29) or produced a very small and unilateral compromise of the tegmentum (n = 9). In contrast, in patients who had coma (n = 9), the lesions in the tegmentum were mostly bilateral (n = 7) and were located either in the pons alone (n = 4) or in the upper pons and the midbrain (n = 5). The maximum overlap territory of the lesions coincided with the location of the rostral raphe complex, locus coeruleus, laterodorsal tegmental nucleus, nucleus pontis oralis, parabrachial nucleus and the white matter in between these nuclei. We also found that four coma subjects developed hyperthermia and died in the absence of any infections. In these cases, the maximum lesion overlap was centred in the core of pontine tegmentum. Our findings suggest that lesions confined to the upper pons can cause coma in humans even in the absence of damage to the midbrain. The findings also point to the brainstem nuclei whose lesions are likely to be associated with loss of consciousness and fatal hyperthermia in humans.
Introduction
The foundation for the traditional account of the ascending reticular activating system (ARAS) and its role in the maintenance of consciousness can be traced back to two lines of experiments. In the beginning of the 20th century, Bremer (1935) showed that in lightly anaesthetized cats, the transection of the brainstem at the pontomesencephalic level caused coma, whereas a transection at the level of the spinomedullary junction did not. In another experiment, Moruzzi and Magoun (1949) showed that stimulation within the brainstem reticular formation of lightly anaesthetized cats resulted in high frequency/low amplitude (so‐called desynchronized) EEG, an electrophysiological correlate of the conscious state, whereas lesions of the same region of the reticular formation caused coma with low frequency/high amplitude (so‐called synchronized) EEG. These results led to the suggestion that the upper brainstem reticular formation is the origin of a system involved in activating the cerebral cortex, namely the ARAS, and that the process of cortical activation is indispensable for the maintenance of consciousness (Fig. 1A). Subsequent clinical observations showed that lesions in the upper brainstem reticular formation are a major cause of coma (Loeb, 1958; Loeb and Stirling Meyer, 1965; Chase et al., 1968). Eventually, Plum and Posner (1980) used a series of clinical and pathological observations to establish the now classical notion that coma in humans is caused by lesions occurring in the reticular formation territory extending from the upper third of the pons to the upper limits of the midbrain.
The classical notion of brainstem coma was coined at a time when the reticular formation was defined as including almost all the nuclei in the brainstem tegmentum (except for cranial nerve nuclei) and appeared functionally homogeneous. We now know that the reticular formation, rather than being the homogeneous mesh of neurons that its name implies, is an anatomical region which comprises different nuclei, each of which operates through distinct anatomical and physiological channels. Moreover, we know that the ARAS may operate from nuclei outside the conventional domain of the reticular formation (Fig. 1B). See Parvizi and Damasio (2001) for a review of anatomical details, Pace‐Schott and Hobson (2002) for a review of genetic and cellular details, and Caplan (1996) for clinical details. As shown in Fig. 1, several major groups of nuclei in the brainstem tegmentum have the means to modulate the electrophysiological activity of the cerebral cortex and thus contribute to the maintenance of consciousness. Their modulation of the electrophysiological activity of the rostral structures is orchestrated by other structures such as the thalamus and the basal forebrain. What remain to be determined are (i) which specific nuclei in the brainstem tegmentum are involved in the maintenance of consciousness in humans; and (ii) whether the nuclei presumed to be involved are contributing equally to the maintenance of consciousness in humans.
We explored these two questions in the current study by taking advantage of the available structural neuroimaging tools. Mindful of the limited spatial resolution of these techniques, we aimed at identifying the boundaries of brainstem lesions that cause loss of consciousness in humans, and then determining where these lesions overlapped. Following the mapping of the maximum overlap lesion area, an inference was made regarding the identity of nuclei and pathways that fell within the overlap territory in a normal brainstem specimen, thus producing an informed guess as to the identity of brainstem structures whose damage is most frequently correlated with coma.
We note that the aim of the study was not to attempt to identify brainstem nuclei with the current imaging techniques. Our goal was to identify the brainstem territory that is damaged in most patients with coma, and then make an inference about the identity of nuclei that fall within that lesion territory.
Material and methods
First, a normal brainstem specimen was selected randomly from a collection of 610 brains from the University of Iowa brain collections at Dr Gary Van Hoesen’s laboratory. The donor had died of stomach cancer without any neurological or psychiatric history. The brainstem specimen was dissected from the rest of the brain and sent to the National Laboratory of Nuclear Imaging at the University of Illinois at Urbana–Champaign. Here, a high resolution MRI was obtained in a 4.7 T scanner with a 33 cm bore magnet and a Varian/SISCO operating console. A multi‐slice spin‐echo Fourier transform sequence was obtained with a thickness of 0.75 mm MRIs and an in‐plane resolution of ∼0.1 mm. These high resolution images were all obtained in a plane perpendicular to the long axis of the stem. The major landmarks of the brainstem could easily be identified on the final images due to the near‐microscopic resolution of the scans.
After the MRIs had been obtained, frozen sections of the same brainstem were obtained in a plane identical to the MRI cuts, and 50 µm thick sections were collected at 0.5 mm intervals (Fig. 2). A series of sections were stained for Nissl bodies and with myelin stain, or immunostained with antibodies against calcium‐binding proteins for histological analysis. Selected sections were immunolabelled against tyrosine hydroxylase for identification of catecholaminergic cell groups. Paxinos and Huang (1995), and Olszewski and Baxter’s (1982) atlases were used for the identification of brainstem nuclei and pathways. Paxinos and Huang (1995) nomenclature is used here.
After staining, the histological sections were scanned into a computer, and the location of nuclei and pathways was mapped on each histological scan. The high resolution MRIs and the histological sections were aligned to allow the mapping of nuclei and pathways onto the high resolution MRIs. This template brainstem, formed of high resolution MRIs and histological slices, was used in the following steps of the study.
MRI mapping of cases with brainstem lesions
After obtaining permission from the University of Iowa Institutional Review Board, we identified 236 subjects who previously had been admitted with the diagnosis of stroke and had undergone MRI studies using the Stroke Registry of the Department of Neurology and the In‐Patient Registry of the University of Iowa Hospital and Clinics. All patients were either deceased or had been discharged. Exclusion criteria were: (i) absence of lesions in the brainstem; (ii) presence of extensive damage to other brain structures; (iii) unavailable MRIs; (iv) poor quality MRIs; or (v) presence of conflicting information between our MRI reading, the radiological report and the review of clinical information with respect to data pertaining to intact/compromised function of key anatomical structures such as cranial nerves and ascending/descending fibre tracts (see later). The medical records were also used to obtain data regarding the duration of coma, age and gender. The records did not include behavioural or cognitive measurements regarding the degree of impaired consciousness. The application of these criteria reduced the number of suitable subjects to 47 (19.9%).
The lesions were traced on each MRI slice in which they were apparent (Fig. 3). Although the MRIs did not have the same anatomical resolution of the template series, the lesion outlines could be identified and traced without problems. The patient MRIs were mostly T1 and/or T2 images, and in some cases FLAIR images were also available. Once the lesion boundaries were traced, the MRIs were aligned with the corresponding slices of the high resolution MRIs and the lesion boundary transferred onto the high resolution templates. Scout scans, saggital and coronal slices were helpful guides to perform a correct transfer; so were the characteristic landmarks of the brainstem such as the cranial nerves, the pyramids, the belly of the basis pontis, the cerebral peduncles, the superior and inferior colliculi, and the shape of the periaqueductal grey (PAG). In addition, the MRI readings could be confirmed according to the available clinical information in the following way. The clinical data were reviewed to assess the integrity or dysfunction of landmark brainstem structures such as cranial nerve nuclei or ascending sensory and descending motor tracts. These data were useful to judge the concordance between the extent of the lesions presumed from the MRIs and the actual neurological signs. For example, in MRI cases presumed to have lesions only in the upper pontine but not the midbrain tegmentum, the normal function of cranial nerves III and IV (which are located in the upper and lower midbrain, respectively) should be consistent with the MRI readings, which was indeed the case. Likewise, the status of cranial nerves V–XII was used to check on readings concerning the pontine and medullary sectors of the brainstem.
The transferred lesion images were studied with respect to the involvement of different nuclei in the brainstem. The identification of damaged nuclei was qualitative, i.e. finding whether a nucleus location coincided with the lesion territory. No quantification of the degree of nuclear damage was attempted because this is a task beyond the reach of current state of the art technology.
In two patients whose post‐mortem material was available, microscopical sections were studied under the microscope, and autopsy reports were reviewed. On sections stained with Nissl stain and a silver impregnation method, the boundaries of lesions could be easily detected based on the presence of signs suggesting haemorrhage or infarction (e.g. the presence of red blood cells, central neuronal loss, axonal degeneration, macrophages and tract degeneration).
We note that in our study, a distinction was made between coma and state of hypersomnolence. We defined coma as a state of deep sleep with and without stimulations, whereas hypersomnolence was defined as a tendency to sleep when unstimulated but arousable when stimulated. We did not have behavioural and cognitive measurements regarding the quality of consciousness in patients who had not become comatose or had gained consciousness after being in a coma. However, we do not think that knowing about the precise quality of consciousness in our patients was necessary to make an inference regarding the anatomical correlates of coma defined as a total loss of consciousness.
Results
The results are presented in detail in Tables 1–3 and Fig. 4 and 5.
In summary, none of the 47 cases included in the study had lesions in the hypothalamus, basal forebrain or bilateral cerebral cortex. This was due to the fact that we had, in advance, excluded cases in which brainstem lesions were accompanied by lesions in neural structures whose dysfunction could also impair consciousness. In other words, we were confident that coma in our subjects was due exclusively to the compromise of the brainstem and not the other brain structures.
Among the cases included in the study, 38 had brainstem stroke without loss of consciousness. In these cases, lesions did not cluster at any particular rostrocaudal level of the brainstem. They were scattered in the medulla, pons or in the midbrain. However, regardless of their rostrocaudal level, lesions in the majority of these cases (n = 29) were clustered in the anterior portion of the brainstem, sparing the tegmentum but involving structures such as the cerebral peduncles, the pontine nuclei of the basis pontis or the pyramidal tracts. In only five cases, a small portion of the tegmentum was affected unilaterally, but the lesions were always in the most anterior portion of the tegmentum affecting structures such as the substantia nigra or the red nucleus.
In contrast, in cases with coma (n = 9), the brainstem lesions were clustered at a specific rostrocaudal level of the brainstem, namely the pontomesencephalic tegmentum. These lesions involved the posterior aspect of the tegmentum (Fig. 4). Lesions in coma cases affected either the midbrain and pontine tegmentum together (n = 5), or only the upper pontine tegmentum without involving the midbrain (n = 4). None of the cases with coma had lesions in the medulla, and no cases contained lesions confined to the midbrain only. On the other hand, the upper pons was always damaged in all cases with loss of consciousness.
In cases with coma, the maximum overlap of lesion territories was centred in the core of the upper pontine tegmentum involving the raphe complex (NR), locus coeruleus (LC), laterodorsal tegmental nucleus (LDT), pedunculopontine tegmental nucleus (PPTg), nucleus pontis oralis (PnO) and the parabrachial nucleus (PBN). The local connections between these nuclei and the ascending central tegmental tract 2 were also severed.
The duration of coma in our cases varied significantly: longer duration of coma was associated with bilateral lesions, and shorter duration was associated with unilateral lesions (Tables 1 and 3). Patients T.G. and I.P. were the only coma patients who had acquired unilateral lesions of the tegmentum, and both had the shortest duration of coma (Table 1). The longest duration of coma was seen in patient M.M. who was comatose for 7 days before he died without gaining consciousness. MRI readings showed a peculiar horseshoe‐shaped lesion affecting the upper pontine tegmentum posteriorly on both sides sparing the midline territories anteriorly (Fig. 4). Post‐mortem analysis confirmed the MRI readings and indicated a thrombotic basilar artery with an infarct area in the upper pons that affected the lateral tegmental regions including the corticospinal tracts bilaterally.
In addition to patient M.M., patients P.H., V.S. and V.R. also died without gaining consciousness (Tables 1 and 3). To our surprise, we found that all four cases died in a state of hyperthermia (temperature >40°C) in the absence of any explainable source of fever. In these four cases, brainstem lesions were either in the pons alone (n = 2) or in the pons and the midbrain (n = 2). As noted, patient M.M. died after 7 days of coma due to his brainstem lesion. Patients P.H., V.S. and V.R. had 2, 5 and 5 days of coma, respectively. Only patient P.H. had haemorrhagic stroke, whereas the other three had non‐haemorrhagic infarcts. Of note is that these patients developed Cheyne–Stoke respiration and one had pathological sneezing, all suggesting a pontine compromise (Miller et al., 1994). Additional data about the clinical status of the other coma patients are presented in Table 3.
Discussion
We believe that the current study adds to our knowledge of the neural correlates of brainstem coma in humans on several grounds. First, it suggests that structures in the upper pontine tegmentum, compared with the midbrain structures, may have a more crucial role in maintaining consciousness than previously thought. Secondly, it suggests the possible identity of nuclei that are damaged in patients with brainstem coma. Thirdly, our study suggests a neuroanatomical correlate for hyperthermia following brainstem stroke, and raises the possibility that some brainstem lesions are fatal perhaps due to the compromise of the components of the brainstem involved in temperature regulation.
The neuroanatomical correlates of brainstem coma
Our findings are compatible with the classical notion of Plum and Posner (1980) of brainstem coma, which identified a crucial territory of the brainstem extending from the upper third of the pons to the upper midbrain. However, our study complements that notion by finding that the maximum overlap of coma‐causing lesions is centred in the upper pons, and that lesions confined to the pons alone can cause coma even in the absence of midbrain damage. Similar to our findings, Chase et al. (1968) correlated the EEG changes and the state of consciousness with the post‐mortem findings and, without specifying in more anatomical details, concluded that all cases with coma had bilateral damage to the reticular formation of the pons.
The fact that midbrain lesions cause coma does not necessarily indicate that coma is due to midbrain dysfunction per se. It could be due to, for instance, the disruption of pathways passing through the midbrain such as those that originate in the upper pons and target the rostral structures, or those that descend from the rostral structures and target the pontine structures. In keeping with this, a recent study in cats showed that a cell‐specific lesion with ibotenic acid in the core of the midbrain reticular formation did not cause alterations in the EEG pattern beyond the first postoperative day (Denoyer et al., 1991). This study did not examine the effect of similar lesions to the upper pons.
In brief, our finding is in agreement with the classical notion of brainstem coma, but it narrows down the crucial brainstem territory and points to the possibility that, compared with the midbrain, the human upper pons may play a more crucial role in the maintenance of consciousness than the classical notion implies. As outlined ahead, there is a large body of anatomical and physiological evidence from animal studies supporting the role of upper pontine structures in modulating the global activity of the cerebral cortex.
In our study, the maximum lesion overlap area in patients with coma coincided with the location of the nuclei PnO, LC, NR, LDT and PBN. As shown in Fig. 1, studies in non‐human mammals have shown that these nuclei have the anatomical and physiological means to induce global changes in the electrophysiological activity of the brain.
The PnO sends presumably glutamatergic projections to the intralaminar nuclei of the thalamus, which in turn project to various cortical regions (Brodal, 1957; Steriade et al., 1988; Newman and Ginsberg, 1994). It is noteworthy that anatomical studies in the early 1950s showed that the PnO is also a major recipient of cortical projections to the ‘reticular formation’ (Brodal, 1957). More recent studies show that the PnO receives major descending inputs from several rostral structures (Robertson and Feiner, 1982; Shammah‐Lagnado et al., 1987; Royce et al., 1991).
The LC and the NR, respectively, are the source of direct noradrenergic and serotonergic projections to most of the cortical mantle (Moore and Bloom, 1979). They also project to the basal forebrain where widespread projections to the cerebral cortex originate (Smiley et al., 1999). In a recent study, it was found that the dopaminergic cells in the ventral PAG and NR are active during wakefulness, and their lesion causes 20% more sleep (Lu et al., 2002). Perhaps some of the evidence about the involvement of the NR in the maintenance of consciousness is due to the involvement of these dopaminergic cells that happen to be located close to the serotonergic cells of the NR.
The LDT contains cholinergic neurons (Mesulam et al., 1989), which project to several thalamic nuclei including the reticular nucleus of the thalamus (Steriade et al., 1988) and to basal forebrain regions such as the substantia innominata (Muller et al., 1993). The reticular nucleus of the thalamus, in turn, sends inhibitory projections to other thalamic nuclei (Scheibel et al., 1966; Steriade and Deschenes, 1984; Barth and MacDonald, 1996), thereby functioning as a pacemaker for the thalamic oscillations, which hallmark non‐rapid eye movement sleep. It is known that the activity of the brainstem cholinergic system blocks the generation of these spindles and thereby initiates a wakeful state (Steriade, 1993).
The PBN is known as an afferent integration site that is involved in many aspects of homeostasis, including thermal, fluid, electrolyte and energy balance (Caille et al., 1981; Dick et al., 1994; Saper and Breder, 1994; Menani et al., 1995; Mizusawa et al., 1995; Buritova et al., 1998). There is also evidence that the PBN projects to the intralaminar thalamic nuclei (Bester et al., 1999; Krout and Loewy, 2000a). Moreover, there are projections from the PBN to the basal forebrain and other brainstem nuclei such as the classical reticular nuclei involved in activating the cerebral cortex (Fulwiler and Saper, 1984; Alden et al., 1994). Thus, the PBN has the anatomical means to modulate the activity of the cerebral cortex via either the thalamus, the basal forebrain or brainstem nuclei. In a recent study by Munk et al. (1996), stimulation of the PBN in cats was found to induce maximal changes in the electrophysiological activity of cerebral cortex. Of note is the fact that the PBN is one of the major relay stations for the ascending pathways from the vagus complex in the medulla. There is evidence, from classical (Moruzzi, 1963) and recent experiments (e.g. Schachter and Saper, 1998), that the vagal complex can modulate the global activity of the cerebral cortex. It is likely that the vagal effect is mediated, at least partially, through the PBN.
In summary, it is conceivable that brainstem lesions compromise the function of the PnO, LC, NR, LDT and PBN and impair their influence on rostral structures, thus halting their orchestrated modulation of the global activity of the brain. This impairment could be due to a direct lesion centred in the upper pons from where these nuclei operate, or to a lesion in the midbrain through which the ascending pathways from these nuclei must travel. A third possibility is that a lesion in the midbrain disrupts the pathways that descend from the rostral structures and target the upper pons. Targeted studies are needed to address this issue. Other future studies are also needed to determine whether loss of consciousness caused by brainstem lesions is associated with the dysfunction of one or several neurotransmitter systems such as the noradrenergic, serotonergic, dopaminergic, cholinergic or glutamatergic systems. If so, one could imagine that patients with brainstem coma would benefit from neurochemical interventions aimed at these neuromodulators or neurotransmitters.
Duration of coma in patients with brainstem stroke
Our study included cases with various lengths of coma. This design made it possible for us to compare brainstem lesions that cause short lapses of consciousness with those that cause longer duration of coma. We found that the shortest duration of coma was associated with unilateral lesions, especially when the lesions were non‐haemorrhagic (patients T.G. and I.P. compared with patient H.R.). Comparing the unilateral lesions in non‐coma patients with the unilateral lesions in coma patients revealed the following: lesions in comatose patients with unilateral lesions were close to the midline and the posterior tegmentum, while the lesions in non‐comatose patients were centred in the lateral and anterior tegmentum. None of our cases had persistent vegetative state or loss of consciousness beyond 1 week. The maximum length of coma was 7 days in patient M.M. who died without recovering consciousness.
The fact that none of our cases had duration of coma longer than 7 days could be attributed to the design of our study. As noted, from a pool of 236 subjects, we included only 19.9% of the cases. Some of the excluded cases had, in addition to brainstem lesions, extensive cortical damage or lesions in structures known to be important for the maintenance of consciousness (i.e. hypothalamus, thalamus or basal forebrain). It is possible that the lack of cases with long duration of coma among our subjects had to do with the fact that our cases were somewhat ‘pure’ brainstem cases. Perhaps in patients with lesions confined to only brainstem, the plasticity of the nervous system allows the remaining rostral structures to maintain the level of vigilance needed to regain consciousness. In this respect, it is noteworthy that some of the classical experiments in non‐human mammals showed that even crude electrolytic lesions in the brainstem do not cause an irreversible loss of consciousness. In these experiments, cats, dogs and monkeys with brainstem lesions recovered consciousness after days or weeks of coma.
Another reason may have to do with the fact that in our study, cases with the most extensive brainstem lesions did not survive beyond 7 days. It is possible that they could have had longer duration of coma had they survived.
Brainstem stroke and fatal hyperthermia
In our study, we found that four cases had died without recovering consciousness and that they all had died in a hyperthermic state in the absence of any obvious cause for their fever. Although we cannot rule out the possibility that these patients had developed a diffuse inflammation of the CNS following their stroke, we believe a more plausible explanation for hyperthermia in these patients pertains to the location of their lesion. Our analysis showed that the lesions in these four subjects overlapped in the upper pontine tegmentum (Fig. 5). Accordingly, patients who died of hyperthermia had signs of pontine compromise such as pinpoint pupils and Cheyne–Stoke respiration. This finding is in accordance with the existing clinical data concerning post‐stroke hyperthermia. Although hyperthermia can be caused by hydrocephalus (Talman et al., 1988) or lesions anywhere between the upper pons and the hypothalamus (Simpson et al., 1993), there is evidence suggesting a strong correlation between fatal hyperthermia and pontine compromise (Wijdicks and St Louis, 1997). Experimental evidence also suggests a crucial role for the upper pons in temperature regulation (e.g. Shibata et al., 1987). It is noteworthy that, in the study of rodents performed by Shibata and colleagues, hyperthermia secondary to pontine damage could be treated effectively with the use of propranolol. Thus, it is a possibility that in patients with lesions to the lateral pontine tegmentum, prophylactic pharmacotherapy or pre‐emptive temperature adjustments might reduce their risk of mortality.
Acknowledgements
We wish to thank Doug Morris and the National Laboratory of Nuclear Imaging at the University of Illinois, Urbana‐Champaign for acquiring the 4.7 T MRIs, Drs Katharine Saunders Louis for help in acquiring the initial data, Harold Adams for help in finding data from patients with brainstem stroke, Gary Van Hoesen for making available the control brainstem, Hanna Damasio for help with processing MRI images, and Dr Louis Caplan for his thoughtful review of the manuscript and his important suggestions. This project was supported in part by a grant from the Mathers Foundation.
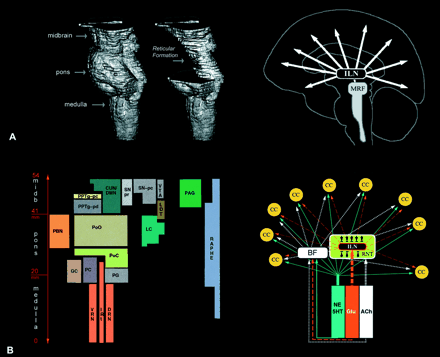
Fig. 1 The reticular formation and the ARAS. (A) The brainstem encompasses medulla oblongata, pons and midbrain. Earlier histological studies indicated that the central and dorsal part of the brainstem extending from the lower medulla to the level of the upper midbrain had the appearance of a ‘reticulum’. Therefore, this region was labelled as the reticular formation. The two images on the left are Brainvox three‐dimensional reconstructions of MRIs obtained from the specimen that was used as the template brainstem in our study (see Material and methods). For this reconstruction we used low resolution. The cartoon on the right reflects the classical view that the mesencephalic reticular formation (MRF) is the origin of ARAS that operates through the intralaminar nuclei of the thalamus (ILN) and activates widespread regions of the cerebral cortex. (B) An outline of the anatomical heterogeneity of the reticular formation and of the multiplicity of channels through which the ARAS operates. The brainstem reticular formation consists of many different nuclei. A nucleus is a three‐dimensional collection of neurons with idiosyncratic chemical and electrophysiological as well as anatomical features. All nuclei are aligned in parallel to the long axis of the brainstem. As this figure illustrates, each nucleus has its own location within the brainstem. The size and the shape of the columns, as shown here, reflect the relative area of the brainstem occupied by the nucleus. The cartoon on the right shows the neurotransmitters that may be involved in modulating the electrophysiological activity of the cerebral cortex. Based on the histochemical features, functional properties, and anatomical connections of these nuclei, we have grouped them into the following categories of nuclei. Classical reticular nuclei include the nucleus cuneiforme (CUN), the deep mesencephalic nucleus (DMN), the non‐cholinergic portion of the pedunculopontine tegmental nucleus (PPTg‐pd) and the PnO. These nuclei are located in the core of the brainstem in a relatively cell‐poor but interlaced region, which first suggested the term reticular formation (hence the term classical reticular). Monoaminergic nuclei encompass noradrenergic LC, serotonergic raphe, and dopaminergic substantia nigra (SN) and ventral tegmental area (VTA) (Moore, 1980). There are direct noradrenergic and serotonergic projections to most of the cortical mantle (Moore and Bloom, 1979). The dopaminergic projections from the SN and the VTA project extensively to the putamen, caudate nucleus, nucleus accumbens and the thalamus (van Domburg and ten Donkelaar, 1991). There are also direct dopaminergic projections from the brainstem to many cortical areas, with a predominance towards the prefrontal cortex, the cingulate cortex and the insular cortex (Porrino and Goldman‐Rakic, 1982). Moreover, there are projections from brainstem dopaminergic, noradrenergic and probably serotonergic nuclei to the basal forebrain where widespread cortical projections originate (Smiley et al., 1999). The physiological involvement of the serotonergic and noradrenergic systems in modulating the global activity of cortex, and in supporting increased attentiveness and behavioural response to environmental stimuli, is well documented (Clark et al., 1987; Jacobs et al., 1990; Aston‐Jones et al., 1991; Azmitia and Whitaker‐Azmitia, 1991; Berridge et al., 1993; Geyer, 1996; Bloom, 1997; Cahill and McGaugh, 1998; Rico and Cavada, 1998). Cholinergic nuclei include the LDT and the cholinergic portion of the PPTg (PPTg‐pc) (Mesulam et al., 1989). These cholinergic nuclei project to several thalamic nuclei including the reticular nucleus of the thalamus (Pare et al., 1988), and to basal forebrain regions such as the substantia innominata (Muller et al., 1993; Steriade, 1993). The reticular nucleus of the thalamus projects to other thalamic nuclei (Scheibel et al., 1966), and inhibits their activity (Steriade and Deschenes, 1984; Barth and MacDonald, 1996), thereby functioning as a pacemaker for the thalamic spindle oscillations which hallmark non‐REM sleep (Steriade and Deschenes; 1984; Steriade et al., 1986, 1993; Steriade, 1992, 1993). Activity of the brainstem cholinergic system blocks the generation of these spindles and thereby initiates the wakeful state (Steriade, 1993). The PBN and the PAG nuclei are known for their involvement in visceral functions, but there is evidence suggesting that they too might be capable of modulating the global activity of the cerebral cortex. For instance, both the PAG (Brodal, 1957; Jones and Yang, 1985; Krout and Loewy, 2000b) and the PBN (Bester et al., 1999; Krout and Loewy, 2000a) project to the intralaminar thalamic nuclei. Moreover, there are projections from the PBN (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Alden et al., 1994; Knyihar‐Csillik et al., 1999) and the PAG (Mantyh, 1983; Beitz, 1990) to the basal forebrain and other brainstem nuclei such as the classical reticular nuclei involved in activating the cerebral cortex. In a recent study by Munk et al. (1996), the stimulation of the PBN was found to induce maximal changes in the electrophysiological activity of cortex. It should be noted that all mentioned nuclei here are under strong influence from rostral structures in the brain (e.g. see Holstege et al., 1985 for anatomical and French et al., 1953 for physiological evidence).
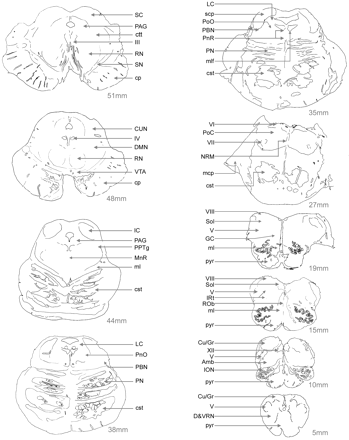
Fig. 2 The human brainstem in horizontal sections. The control brainstem specimen used in our study was cut on a freezing microtome into 50 µm thick sections. In this figure, only 10 of those sections were charted for the location of different nuclei and pathways. The first slide on the upper left is ∼51 mm rostral to the spinomedullary junction and ∼3 mm caudal to the most rostral section of the midbrain where the mesencephalon ends and the diencephalic territory begins. The last section on the lower right is from the level of pyramidal decussation, ∼5 mm rostral to the spinomedullary junction. Abbreviations: Amb = ambiguus nucleus; cp = cerebral peduncle; cst = corticospinal tract; CU/GR = cuneate and gracile nuclei; CUN = cuneiform nucleus; D&VRN = dorsal and ventral reticular nuclei of the medulla; DMN = deep mesencephalic nucleus; GC = gigantocellular nucleus; IC = inferior colliculus; III = cranial nerve nucleus III (oculomotor); ION = inferior olivary nucleus; IRt = intermediate reticular zone; IV = cranial nerve nucleus IV (trochlear); mcp = middle cerebellar peduncle; ml = medial lemniscus; mlf = medial longitudinal fasciculus; MnR = median raphe nucleus; NRM = raphe magnus nucleus; PN = pontine nuclei of the basis pontis; PnR = pontine raphe nucleus; PoC = pontis caudalis nucleus; PoO = pontis oralis nucleus; pyr = pyramids; RN = red nucleus; Rob = raphe obscurus nucleus; SC = superior colliculus; scp = superior cerebellar peduncle; SN = substantia negra; Sol = solitary nucleus; V = cranial nerve nucleus V (trigeminal); VI = cranial nerve nucleus VI (abducens); VII = cranial nerve nucleus VII (facial); VIII = cranial nerve nucleus VIII (vestibular); VTA = ventral tegmental area; XII = cranial nerve nucleus XII (hypoglossal).
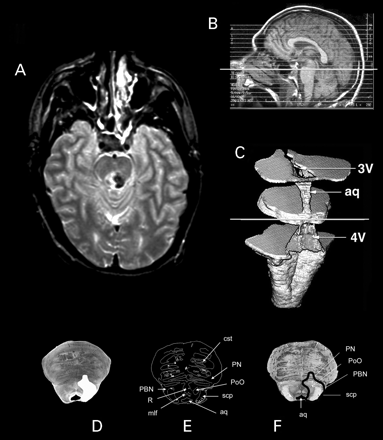
Fig. 3 Methods. (A) In our study, we first charted the lesion territory on patient MRIs. Using the scout scans (B), three‐dimensional reconstruction of the brainstem MRIs (C) and the characteristic shape of brainstem landmarks on each horizontal image, we found the corresponding 4.7 T template MRI (D) obtained from the control brainstem specimen. Once the boundaries of lesions were transferred onto the template MRIs (E), these were aligned in parallel to the histological sections (F) that were obtained from the template brainstem, and an inference was made about the identity of nuclei that fall within the lesion territory. Note the high resolution of the 4.7 T MRIs.
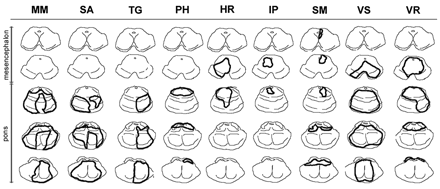
Fig. 4 Brainstem lesions in patients with coma. This figure shows the lesion boundaries on each patient with coma. Midbrain sections are in the first two rows, and pontine sections are in the remaining three rows. As seen in this figure, the brainstem damage in the first four patients spared the midbrain.
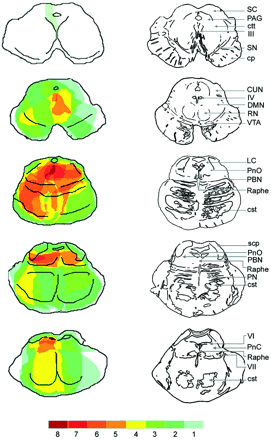
Fig. 5 The maximum area of lesion overlap in patients with coma and fatal hyperthermia. Once the MRI chartings were transferred to the template brainstem MRI atlas, the overlapping regions were coded with different colours. Dark red indicates the region where the lesion territories of eight patients overlapped. Note that the maximum overlap of lesion for patients with coma is in the core of the upper pontine tegmentum.
Coma subjects
| Number | Name | Gender | Age | Lesion location | Nuclei affected | Type of MRI | Other lesions | Type of lesion | Co‐existing medical condition | Duration of coma |
| 1 | M.M. | M | 75 | Pons | b‐LC, b‐NR, b‐LDT, b‐PBN | T2 | Right occipital lobe | Infarct | Hypertension and diabetes mellitus | 7 days coma, then fatal hyperthermia |
| 2 | S.A. | M | 57 | Pons | b‐PnO, b‐LC, b‐NR, b‐PBN | T1 and T2 | None | Infarct | COPD and hypertension | 3 days coma, later locked‐in‐syndrome |
| 3 | T.G. | F | 56 | Pons | r‐PnO, r‐LC, r‐NR, r‐PBN | T2 | White matter plaques | Infarct | 40 years history of multiple sclerosis | 2 h coma before recovery |
| 4 | P.H. | M | 61 | Pons | b‐PnO, b‐LC, b‐NR, b‐LDT, b‐PPTg, b‐PBN | T1 and T2 | Cerebellum | Haemorrhage | Diabetes mellitus | 2 days coma,and then fatal hyperthermia |
| 5 | H.R. | M | 31 | Pons and midbrain | l‐CUN/DMN, b‐PnO, l‐LC, b‐NR, l‐LDT, l‐PPTg, l‐PAG | T2 | Parietal lobe | Haemorrhage | None | Coma for 2 days and fluctuating hypersomnolence for 8 days before complete recovery |
| 6 | I.P. | F | 68 | Pons and midbrain | l‐CUN/DMN, l‐NR, l‐LDT, l‐PAG | T1 andT2 | Cerebellum | Infarct | Hypertensionand recurrent pulmonary embolism | 3 h coma before recovery |
| 7 | S.M. | F | 73 | Pons and midbrain | r‐CUN/DMN, b‐PnO, b‐LC, b‐NR, r‐LDT, b‐PBN | T1 and T2 | Left internal capsule, parietal cortex and cerebellum | Vasculitic infarct | Treated hyperthyroidism | 3 days coma before recovery |
| 8 | V.S. | M | 33 | Pons and midbrain | r‐CUN/DMN, b‐PnO, b‐LC, b‐NR, b‐LDT, r‐PPTg, b‐PBN, b‐PAG | T1 and FLAIR | Left cerebellum | Infarct | None | 5 days coma, then fatal hyperthermia |
| 9 | V.R. | M | 53 | Pons and midbrain | b‐CUN/DMN, b‐PnO, b‐LC, b‐NR, b‐LDT, b‐PPTg, b‐PBN, b‐PAG | T2 | Cerebellum | Infarct | Prostate cancer, bilateral hydronephrosis, and hypotension | 5 days coma, then fatal hyperthermia |
| Number | Name | Gender | Age | Lesion location | Nuclei affected | Type of MRI | Other lesions | Type of lesion | Co‐existing medical condition | Duration of coma |
| 1 | M.M. | M | 75 | Pons | b‐LC, b‐NR, b‐LDT, b‐PBN | T2 | Right occipital lobe | Infarct | Hypertension and diabetes mellitus | 7 days coma, then fatal hyperthermia |
| 2 | S.A. | M | 57 | Pons | b‐PnO, b‐LC, b‐NR, b‐PBN | T1 and T2 | None | Infarct | COPD and hypertension | 3 days coma, later locked‐in‐syndrome |
| 3 | T.G. | F | 56 | Pons | r‐PnO, r‐LC, r‐NR, r‐PBN | T2 | White matter plaques | Infarct | 40 years history of multiple sclerosis | 2 h coma before recovery |
| 4 | P.H. | M | 61 | Pons | b‐PnO, b‐LC, b‐NR, b‐LDT, b‐PPTg, b‐PBN | T1 and T2 | Cerebellum | Haemorrhage | Diabetes mellitus | 2 days coma,and then fatal hyperthermia |
| 5 | H.R. | M | 31 | Pons and midbrain | l‐CUN/DMN, b‐PnO, l‐LC, b‐NR, l‐LDT, l‐PPTg, l‐PAG | T2 | Parietal lobe | Haemorrhage | None | Coma for 2 days and fluctuating hypersomnolence for 8 days before complete recovery |
| 6 | I.P. | F | 68 | Pons and midbrain | l‐CUN/DMN, l‐NR, l‐LDT, l‐PAG | T1 andT2 | Cerebellum | Infarct | Hypertensionand recurrent pulmonary embolism | 3 h coma before recovery |
| 7 | S.M. | F | 73 | Pons and midbrain | r‐CUN/DMN, b‐PnO, b‐LC, b‐NR, r‐LDT, b‐PBN | T1 and T2 | Left internal capsule, parietal cortex and cerebellum | Vasculitic infarct | Treated hyperthyroidism | 3 days coma before recovery |
| 8 | V.S. | M | 33 | Pons and midbrain | r‐CUN/DMN, b‐PnO, b‐LC, b‐NR, b‐LDT, r‐PPTg, b‐PBN, b‐PAG | T1 and FLAIR | Left cerebellum | Infarct | None | 5 days coma, then fatal hyperthermia |
| 9 | V.R. | M | 53 | Pons and midbrain | b‐CUN/DMN, b‐PnO, b‐LC, b‐NR, b‐LDT, b‐PPTg, b‐PBN, b‐PAG | T2 | Cerebellum | Infarct | Prostate cancer, bilateral hydronephrosis, and hypotension | 5 days coma, then fatal hyperthermia |
b = bilaterally; l = left; r = right; F = female; M = male; COPD = chronic obstructive pulmonary disease; CUN/DMN = cuneiform/deep mesencephalic nucleus.
Coma subjects
| Number | Name | Gender | Age | Lesion location | Nuclei affected | Type of MRI | Other lesions | Type of lesion | Co‐existing medical condition | Duration of coma |
| 1 | M.M. | M | 75 | Pons | b‐LC, b‐NR, b‐LDT, b‐PBN | T2 | Right occipital lobe | Infarct | Hypertension and diabetes mellitus | 7 days coma, then fatal hyperthermia |
| 2 | S.A. | M | 57 | Pons | b‐PnO, b‐LC, b‐NR, b‐PBN | T1 and T2 | None | Infarct | COPD and hypertension | 3 days coma, later locked‐in‐syndrome |
| 3 | T.G. | F | 56 | Pons | r‐PnO, r‐LC, r‐NR, r‐PBN | T2 | White matter plaques | Infarct | 40 years history of multiple sclerosis | 2 h coma before recovery |
| 4 | P.H. | M | 61 | Pons | b‐PnO, b‐LC, b‐NR, b‐LDT, b‐PPTg, b‐PBN | T1 and T2 | Cerebellum | Haemorrhage | Diabetes mellitus | 2 days coma,and then fatal hyperthermia |
| 5 | H.R. | M | 31 | Pons and midbrain | l‐CUN/DMN, b‐PnO, l‐LC, b‐NR, l‐LDT, l‐PPTg, l‐PAG | T2 | Parietal lobe | Haemorrhage | None | Coma for 2 days and fluctuating hypersomnolence for 8 days before complete recovery |
| 6 | I.P. | F | 68 | Pons and midbrain | l‐CUN/DMN, l‐NR, l‐LDT, l‐PAG | T1 andT2 | Cerebellum | Infarct | Hypertensionand recurrent pulmonary embolism | 3 h coma before recovery |
| 7 | S.M. | F | 73 | Pons and midbrain | r‐CUN/DMN, b‐PnO, b‐LC, b‐NR, r‐LDT, b‐PBN | T1 and T2 | Left internal capsule, parietal cortex and cerebellum | Vasculitic infarct | Treated hyperthyroidism | 3 days coma before recovery |
| 8 | V.S. | M | 33 | Pons and midbrain | r‐CUN/DMN, b‐PnO, b‐LC, b‐NR, b‐LDT, r‐PPTg, b‐PBN, b‐PAG | T1 and FLAIR | Left cerebellum | Infarct | None | 5 days coma, then fatal hyperthermia |
| 9 | V.R. | M | 53 | Pons and midbrain | b‐CUN/DMN, b‐PnO, b‐LC, b‐NR, b‐LDT, b‐PPTg, b‐PBN, b‐PAG | T2 | Cerebellum | Infarct | Prostate cancer, bilateral hydronephrosis, and hypotension | 5 days coma, then fatal hyperthermia |
| Number | Name | Gender | Age | Lesion location | Nuclei affected | Type of MRI | Other lesions | Type of lesion | Co‐existing medical condition | Duration of coma |
| 1 | M.M. | M | 75 | Pons | b‐LC, b‐NR, b‐LDT, b‐PBN | T2 | Right occipital lobe | Infarct | Hypertension and diabetes mellitus | 7 days coma, then fatal hyperthermia |
| 2 | S.A. | M | 57 | Pons | b‐PnO, b‐LC, b‐NR, b‐PBN | T1 and T2 | None | Infarct | COPD and hypertension | 3 days coma, later locked‐in‐syndrome |
| 3 | T.G. | F | 56 | Pons | r‐PnO, r‐LC, r‐NR, r‐PBN | T2 | White matter plaques | Infarct | 40 years history of multiple sclerosis | 2 h coma before recovery |
| 4 | P.H. | M | 61 | Pons | b‐PnO, b‐LC, b‐NR, b‐LDT, b‐PPTg, b‐PBN | T1 and T2 | Cerebellum | Haemorrhage | Diabetes mellitus | 2 days coma,and then fatal hyperthermia |
| 5 | H.R. | M | 31 | Pons and midbrain | l‐CUN/DMN, b‐PnO, l‐LC, b‐NR, l‐LDT, l‐PPTg, l‐PAG | T2 | Parietal lobe | Haemorrhage | None | Coma for 2 days and fluctuating hypersomnolence for 8 days before complete recovery |
| 6 | I.P. | F | 68 | Pons and midbrain | l‐CUN/DMN, l‐NR, l‐LDT, l‐PAG | T1 andT2 | Cerebellum | Infarct | Hypertensionand recurrent pulmonary embolism | 3 h coma before recovery |
| 7 | S.M. | F | 73 | Pons and midbrain | r‐CUN/DMN, b‐PnO, b‐LC, b‐NR, r‐LDT, b‐PBN | T1 and T2 | Left internal capsule, parietal cortex and cerebellum | Vasculitic infarct | Treated hyperthyroidism | 3 days coma before recovery |
| 8 | V.S. | M | 33 | Pons and midbrain | r‐CUN/DMN, b‐PnO, b‐LC, b‐NR, b‐LDT, r‐PPTg, b‐PBN, b‐PAG | T1 and FLAIR | Left cerebellum | Infarct | None | 5 days coma, then fatal hyperthermia |
| 9 | V.R. | M | 53 | Pons and midbrain | b‐CUN/DMN, b‐PnO, b‐LC, b‐NR, b‐LDT, b‐PPTg, b‐PBN, b‐PAG | T2 | Cerebellum | Infarct | Prostate cancer, bilateral hydronephrosis, and hypotension | 5 days coma, then fatal hyperthermia |
b = bilaterally; l = left; r = right; F = female; M = male; COPD = chronic obstructive pulmonary disease; CUN/DMN = cuneiform/deep mesencephalic nucleus.
Compromised systems in patients with brainstem coma
| Systems | Nuclei | Left only | Right only | Bilateral |
| Classical reticular nuclei | CUN/DMN | 2 | 1 | 1 |
| PPTg pars dissipatus | 2 | 1 | 4 | |
| PnO | 1 | 1 | 6 | |
| Noradrenergic | LC | 0 | 1 | 6 |
| Serotonergic | Raphe (NR)* | 1 | 1 | 7 |
| Medullary only | 0 | 0 | 0 | |
| Pontine only | 0 | 1 | 3 | |
| Pontine and mesencephalic | 1 | 0 | 4 | |
| Mesencephalic only | 0 | 0 | 0 | |
| Dopaminergic | VTA | 1 | 0 | 2 |
| Cholinergic | LDT | 1 | 1 | 5 |
| PPTg pars compacta | 2 | 1 | 4 | |
| PBN and PAG | PBN | 1 | 1 | 6 |
| PAG | 1 | 1 | 2 |
| Systems | Nuclei | Left only | Right only | Bilateral |
| Classical reticular nuclei | CUN/DMN | 2 | 1 | 1 |
| PPTg pars dissipatus | 2 | 1 | 4 | |
| PnO | 1 | 1 | 6 | |
| Noradrenergic | LC | 0 | 1 | 6 |
| Serotonergic | Raphe (NR)* | 1 | 1 | 7 |
| Medullary only | 0 | 0 | 0 | |
| Pontine only | 0 | 1 | 3 | |
| Pontine and mesencephalic | 1 | 0 | 4 | |
| Mesencephalic only | 0 | 0 | 0 | |
| Dopaminergic | VTA | 1 | 0 | 2 |
| Cholinergic | LDT | 1 | 1 | 5 |
| PPTg pars compacta | 2 | 1 | 4 | |
| PBN and PAG | PBN | 1 | 1 | 6 |
| PAG | 1 | 1 | 2 |
*The raphe complex comprises several nuclei along the caudorostral length of the brainstem starting from the lower medulla and continuing to the level of the upper midbrain (see Fig. 1). The other nuclei are located either in the pons or in the midbrain only, and therefore not specified further by their caudorostral level. Abbreviations as in Fig. 1.
Compromised systems in patients with brainstem coma
| Systems | Nuclei | Left only | Right only | Bilateral |
| Classical reticular nuclei | CUN/DMN | 2 | 1 | 1 |
| PPTg pars dissipatus | 2 | 1 | 4 | |
| PnO | 1 | 1 | 6 | |
| Noradrenergic | LC | 0 | 1 | 6 |
| Serotonergic | Raphe (NR)* | 1 | 1 | 7 |
| Medullary only | 0 | 0 | 0 | |
| Pontine only | 0 | 1 | 3 | |
| Pontine and mesencephalic | 1 | 0 | 4 | |
| Mesencephalic only | 0 | 0 | 0 | |
| Dopaminergic | VTA | 1 | 0 | 2 |
| Cholinergic | LDT | 1 | 1 | 5 |
| PPTg pars compacta | 2 | 1 | 4 | |
| PBN and PAG | PBN | 1 | 1 | 6 |
| PAG | 1 | 1 | 2 |
| Systems | Nuclei | Left only | Right only | Bilateral |
| Classical reticular nuclei | CUN/DMN | 2 | 1 | 1 |
| PPTg pars dissipatus | 2 | 1 | 4 | |
| PnO | 1 | 1 | 6 | |
| Noradrenergic | LC | 0 | 1 | 6 |
| Serotonergic | Raphe (NR)* | 1 | 1 | 7 |
| Medullary only | 0 | 0 | 0 | |
| Pontine only | 0 | 1 | 3 | |
| Pontine and mesencephalic | 1 | 0 | 4 | |
| Mesencephalic only | 0 | 0 | 0 | |
| Dopaminergic | VTA | 1 | 0 | 2 |
| Cholinergic | LDT | 1 | 1 | 5 |
| PPTg pars compacta | 2 | 1 | 4 | |
| PBN and PAG | PBN | 1 | 1 | 6 |
| PAG | 1 | 1 | 2 |
*The raphe complex comprises several nuclei along the caudorostral length of the brainstem starting from the lower medulla and continuing to the level of the upper midbrain (see Fig. 1). The other nuclei are located either in the pons or in the midbrain only, and therefore not specified further by their caudorostral level. Abbreviations as in Fig. 1.
Short summary of clinical data regarding the patients with brainstem coma
| Case | Clinical data |
| M.M. | Was brought to the emergency department because of sudden onset of dizziness. Upon admission, he developed left hand and facial numbness and later facial contortion. These symptoms lasted 5 min, and the patient became totally unresponsive and remained that way until he died 7 days later. He had bilateral Babinski sign, decorticate posturing without withdrawal, occasional sneezing and irregular breathing. He had intact corneal reflexes and reactive and equal pupils. However, he did not have oculocephalic or cold caloric response bilaterally, gag response or any spontaneous movements. Patient M.M. died on day 7 of unexplained hyperthermia. MRI readings showed a peculiar horseshoe‐shaped lesion affecting the upper pontine tegmentum posteriorly on both sides, sparing the midline territories anteriorly (Fig. 4). The damage in the lower pons affected the entire right tegmentum, the basis pontis, and also extended across the midline and involved the left tegmentum partially. Post‐mortem analysis confirmed the MRI readings and indicated a thrombotic basilar artery with an infarct area affecting the corticospinal tracts bilaterally at the level of the upper pons. |
| S.A. | Upon admission to the hospital, he had loss of response to commands or painful stimuli, symmetrical and equally reactive pupils, absent eye movements, facial symmetry, intact gag reflex and complete paralysis of all extremities with decreased tone. He remained in this state until day 4 when he opened his eyes and could blink to yes/no questions. His condition improved and he gained consciousness responding to all questions by moving his eyes vertically. He remained in persistent locked‐in syndrome. MRI readings suggested the involvement of the pons. |
| T.G. | Was brought to the emergency department in her home town because of sudden onset of left arm and facial weakness with difficulty swallowing. Upon admission, her condition worsened and she became totally unresponsive. CT of the head excluded haemorrhage, and she was transferred to the UIHC. During the transfer, she gradually became alert but could not speak. By then, her total loss of consciousness had lasted approximately a couple of hours. By the time of arrival at the UIHC, she could open her eyes to command and had positive corneal reflexes. Her lateral gaze was impaired on the right. She had bilateral facial weakness but could grimace when tickled bilaterally. Her tongue movement was slow, and the rest of the motor exam showed spasticity in the right lower extremity and left upper extremity. On sensory exam, she could wince to deep painful stimuli applied to all extremities. MRI examination on day 1 of admission showed an infarct in the right pons. |
| P.H. | Admitted to a local hospital in a somnolent state. However, he could be aroused to follow commands. He could grip with his left, but not right, hand. He had anarthria, irregular pupils, decreased response to threat on the left, absent left lateral gaze and decreased sensation on the right. Moreover, he could wrinkle his forehead bilaterally, respond to voice and move his tongue randomly. Later during the first day of admission, he became increasingly unconscious (Glasgow Coma Scale = 3) and remained in that state. He was then transferred to the UIHC and remained there until day 3 when he developed fever and died in a hyperthermic state. EEG showed continuous δ slow waves with interspersed or superimposed β and α activities. No reactive EEG changes were noted. MRI on day 2 of admission showed haemorrhagic stroke in the upper pons. Gross brain examination at autopsy showed that the haemorrhagic stroke had isolated the brainstem from supratentorial structures at the pontomesencephalic level. Examination of the serial microscopical sections revealed areas of haemorrhage dissecting into the pontine tegmentum and through the cerebellum. |
| H.R. | Was found unresponsive on the ground outside his girlfriend’s house. He had arrived around midnight and knocked on the door of his girlfriend who had not opened the door since she had thought him to be intoxicated. At the local emergency department, the patient scored 7 on the Glascow Coma Scale. He withdrew to painful stimuli on the left side but not on the right. He had right hemiparesis, right facial asymmetry, decreased right corneal reflex, intact gag reflex and intact Doll’s eyes to both vertical and horizontal testing. He remained unresponsive until day 3 of admission when he occasionally opened his eyes and moved his limbs spontaneously. However, he did not respond to any commands yet. On day 4, he responded intermittently to commands and answered only the question of what his name was. His level of consciousness fluctuated for 9 days before he became gradually and increasingly alert and verbally responsive. The brainstem MRI on day 2 of admission showed haemorrhagic stroke in the upper pons and lower midbrain. |
| I.P. | Had a sudden loss of consciousness 30 min prior to her transfer to the emergency room. She remained totally unresponsive for another 2.5 h before she gradually became alert and responsive although she had developed diplopia and difficulties opening the left eyelid. Later, her level of consciousness was assessed as unimpaired, but her gaze defect persisted. The MRIs showed a lesion centred in the midline region of the mesencephalic tegmentum extending to the upper pons. |
| S.M. | Was admitted to hospital because of decreased alertness. On physical examinations, she had deviation of eyes and head to left, flaccid on the left and rhythmic movements of the left side of mouth, a slight increase in the tone on the right, hyperreflexia, positive Babinski and rigidity on the right. Her consciousness was deteriorated and she went into coma, being barely responsive to painful stimuli. She gradually opened her eyes on day 4, but was unresponsive to commands. On day 5, she was alert and responded to questions with mumbling; she was able to open her eyes, and the pupils were equal and reactive; she could not stick out her tongue. On day 7, she became responsive, and later recovered cognitively with persistent motor defects. MRIs showed a pontomesencephalic lesion as well as some lacunar infarcts in the left internal capsule and parietal cortex and the cerebellum secondary to vasculitis. |
| V.S. | Admitted to the hospital because of acute headache, dizziness, nausea and ataxia, and numbness of the left lip. At admission, he made odd muttering sounds. He had disconjugate eye movements, decerebrate reflex pattern and bilateral Babinski sign. His corneal reflex was absent bilaterally. His muscle tone was also flaccid. MRI showed an infarct affecting the pons and the midbrain as well as the left anterior medial hemisphere of the cerebellum. The diagnoses of basilar artery thrombosis and dissection of left vertebral artery was suggested, and the patient died on day 5 of admission of unexplained fever. |
| VR | Admitted in the early morning for bacterial endocarditis. Later that day, he had sudden onset of neurological signs. The patient was alert and awake but had slurred speech, left facial droop, left sided weakness, left Babinski sign, no medial or lateral movements on the left eye and ptosis and impaired lateral movements on the right eye, intact facial sensation; the tongue deviated to the left. The patient’s condition worsened gradually overnight, and on day 2 of admission, he was unresponsive to all stimuli with no oculocephalic or cold caloric eye movements. His corneal and gag reflexes were absent, and the pupils were in mid position and fixed. MRIs obtained that day showed bilateral lesions in the upper pons extending to the right basis pontis and into the lower midbrain tegmentum. He remained in coma for 5 days until he died in fever of unknown cause. |
| Case | Clinical data |
| M.M. | Was brought to the emergency department because of sudden onset of dizziness. Upon admission, he developed left hand and facial numbness and later facial contortion. These symptoms lasted 5 min, and the patient became totally unresponsive and remained that way until he died 7 days later. He had bilateral Babinski sign, decorticate posturing without withdrawal, occasional sneezing and irregular breathing. He had intact corneal reflexes and reactive and equal pupils. However, he did not have oculocephalic or cold caloric response bilaterally, gag response or any spontaneous movements. Patient M.M. died on day 7 of unexplained hyperthermia. MRI readings showed a peculiar horseshoe‐shaped lesion affecting the upper pontine tegmentum posteriorly on both sides, sparing the midline territories anteriorly (Fig. 4). The damage in the lower pons affected the entire right tegmentum, the basis pontis, and also extended across the midline and involved the left tegmentum partially. Post‐mortem analysis confirmed the MRI readings and indicated a thrombotic basilar artery with an infarct area affecting the corticospinal tracts bilaterally at the level of the upper pons. |
| S.A. | Upon admission to the hospital, he had loss of response to commands or painful stimuli, symmetrical and equally reactive pupils, absent eye movements, facial symmetry, intact gag reflex and complete paralysis of all extremities with decreased tone. He remained in this state until day 4 when he opened his eyes and could blink to yes/no questions. His condition improved and he gained consciousness responding to all questions by moving his eyes vertically. He remained in persistent locked‐in syndrome. MRI readings suggested the involvement of the pons. |
| T.G. | Was brought to the emergency department in her home town because of sudden onset of left arm and facial weakness with difficulty swallowing. Upon admission, her condition worsened and she became totally unresponsive. CT of the head excluded haemorrhage, and she was transferred to the UIHC. During the transfer, she gradually became alert but could not speak. By then, her total loss of consciousness had lasted approximately a couple of hours. By the time of arrival at the UIHC, she could open her eyes to command and had positive corneal reflexes. Her lateral gaze was impaired on the right. She had bilateral facial weakness but could grimace when tickled bilaterally. Her tongue movement was slow, and the rest of the motor exam showed spasticity in the right lower extremity and left upper extremity. On sensory exam, she could wince to deep painful stimuli applied to all extremities. MRI examination on day 1 of admission showed an infarct in the right pons. |
| P.H. | Admitted to a local hospital in a somnolent state. However, he could be aroused to follow commands. He could grip with his left, but not right, hand. He had anarthria, irregular pupils, decreased response to threat on the left, absent left lateral gaze and decreased sensation on the right. Moreover, he could wrinkle his forehead bilaterally, respond to voice and move his tongue randomly. Later during the first day of admission, he became increasingly unconscious (Glasgow Coma Scale = 3) and remained in that state. He was then transferred to the UIHC and remained there until day 3 when he developed fever and died in a hyperthermic state. EEG showed continuous δ slow waves with interspersed or superimposed β and α activities. No reactive EEG changes were noted. MRI on day 2 of admission showed haemorrhagic stroke in the upper pons. Gross brain examination at autopsy showed that the haemorrhagic stroke had isolated the brainstem from supratentorial structures at the pontomesencephalic level. Examination of the serial microscopical sections revealed areas of haemorrhage dissecting into the pontine tegmentum and through the cerebellum. |
| H.R. | Was found unresponsive on the ground outside his girlfriend’s house. He had arrived around midnight and knocked on the door of his girlfriend who had not opened the door since she had thought him to be intoxicated. At the local emergency department, the patient scored 7 on the Glascow Coma Scale. He withdrew to painful stimuli on the left side but not on the right. He had right hemiparesis, right facial asymmetry, decreased right corneal reflex, intact gag reflex and intact Doll’s eyes to both vertical and horizontal testing. He remained unresponsive until day 3 of admission when he occasionally opened his eyes and moved his limbs spontaneously. However, he did not respond to any commands yet. On day 4, he responded intermittently to commands and answered only the question of what his name was. His level of consciousness fluctuated for 9 days before he became gradually and increasingly alert and verbally responsive. The brainstem MRI on day 2 of admission showed haemorrhagic stroke in the upper pons and lower midbrain. |
| I.P. | Had a sudden loss of consciousness 30 min prior to her transfer to the emergency room. She remained totally unresponsive for another 2.5 h before she gradually became alert and responsive although she had developed diplopia and difficulties opening the left eyelid. Later, her level of consciousness was assessed as unimpaired, but her gaze defect persisted. The MRIs showed a lesion centred in the midline region of the mesencephalic tegmentum extending to the upper pons. |
| S.M. | Was admitted to hospital because of decreased alertness. On physical examinations, she had deviation of eyes and head to left, flaccid on the left and rhythmic movements of the left side of mouth, a slight increase in the tone on the right, hyperreflexia, positive Babinski and rigidity on the right. Her consciousness was deteriorated and she went into coma, being barely responsive to painful stimuli. She gradually opened her eyes on day 4, but was unresponsive to commands. On day 5, she was alert and responded to questions with mumbling; she was able to open her eyes, and the pupils were equal and reactive; she could not stick out her tongue. On day 7, she became responsive, and later recovered cognitively with persistent motor defects. MRIs showed a pontomesencephalic lesion as well as some lacunar infarcts in the left internal capsule and parietal cortex and the cerebellum secondary to vasculitis. |
| V.S. | Admitted to the hospital because of acute headache, dizziness, nausea and ataxia, and numbness of the left lip. At admission, he made odd muttering sounds. He had disconjugate eye movements, decerebrate reflex pattern and bilateral Babinski sign. His corneal reflex was absent bilaterally. His muscle tone was also flaccid. MRI showed an infarct affecting the pons and the midbrain as well as the left anterior medial hemisphere of the cerebellum. The diagnoses of basilar artery thrombosis and dissection of left vertebral artery was suggested, and the patient died on day 5 of admission of unexplained fever. |
| VR | Admitted in the early morning for bacterial endocarditis. Later that day, he had sudden onset of neurological signs. The patient was alert and awake but had slurred speech, left facial droop, left sided weakness, left Babinski sign, no medial or lateral movements on the left eye and ptosis and impaired lateral movements on the right eye, intact facial sensation; the tongue deviated to the left. The patient’s condition worsened gradually overnight, and on day 2 of admission, he was unresponsive to all stimuli with no oculocephalic or cold caloric eye movements. His corneal and gag reflexes were absent, and the pupils were in mid position and fixed. MRIs obtained that day showed bilateral lesions in the upper pons extending to the right basis pontis and into the lower midbrain tegmentum. He remained in coma for 5 days until he died in fever of unknown cause. |
UIHC = University of Iowa Hospital and Clinics.
Short summary of clinical data regarding the patients with brainstem coma
| Case | Clinical data |
| M.M. | Was brought to the emergency department because of sudden onset of dizziness. Upon admission, he developed left hand and facial numbness and later facial contortion. These symptoms lasted 5 min, and the patient became totally unresponsive and remained that way until he died 7 days later. He had bilateral Babinski sign, decorticate posturing without withdrawal, occasional sneezing and irregular breathing. He had intact corneal reflexes and reactive and equal pupils. However, he did not have oculocephalic or cold caloric response bilaterally, gag response or any spontaneous movements. Patient M.M. died on day 7 of unexplained hyperthermia. MRI readings showed a peculiar horseshoe‐shaped lesion affecting the upper pontine tegmentum posteriorly on both sides, sparing the midline territories anteriorly (Fig. 4). The damage in the lower pons affected the entire right tegmentum, the basis pontis, and also extended across the midline and involved the left tegmentum partially. Post‐mortem analysis confirmed the MRI readings and indicated a thrombotic basilar artery with an infarct area affecting the corticospinal tracts bilaterally at the level of the upper pons. |
| S.A. | Upon admission to the hospital, he had loss of response to commands or painful stimuli, symmetrical and equally reactive pupils, absent eye movements, facial symmetry, intact gag reflex and complete paralysis of all extremities with decreased tone. He remained in this state until day 4 when he opened his eyes and could blink to yes/no questions. His condition improved and he gained consciousness responding to all questions by moving his eyes vertically. He remained in persistent locked‐in syndrome. MRI readings suggested the involvement of the pons. |
| T.G. | Was brought to the emergency department in her home town because of sudden onset of left arm and facial weakness with difficulty swallowing. Upon admission, her condition worsened and she became totally unresponsive. CT of the head excluded haemorrhage, and she was transferred to the UIHC. During the transfer, she gradually became alert but could not speak. By then, her total loss of consciousness had lasted approximately a couple of hours. By the time of arrival at the UIHC, she could open her eyes to command and had positive corneal reflexes. Her lateral gaze was impaired on the right. She had bilateral facial weakness but could grimace when tickled bilaterally. Her tongue movement was slow, and the rest of the motor exam showed spasticity in the right lower extremity and left upper extremity. On sensory exam, she could wince to deep painful stimuli applied to all extremities. MRI examination on day 1 of admission showed an infarct in the right pons. |
| P.H. | Admitted to a local hospital in a somnolent state. However, he could be aroused to follow commands. He could grip with his left, but not right, hand. He had anarthria, irregular pupils, decreased response to threat on the left, absent left lateral gaze and decreased sensation on the right. Moreover, he could wrinkle his forehead bilaterally, respond to voice and move his tongue randomly. Later during the first day of admission, he became increasingly unconscious (Glasgow Coma Scale = 3) and remained in that state. He was then transferred to the UIHC and remained there until day 3 when he developed fever and died in a hyperthermic state. EEG showed continuous δ slow waves with interspersed or superimposed β and α activities. No reactive EEG changes were noted. MRI on day 2 of admission showed haemorrhagic stroke in the upper pons. Gross brain examination at autopsy showed that the haemorrhagic stroke had isolated the brainstem from supratentorial structures at the pontomesencephalic level. Examination of the serial microscopical sections revealed areas of haemorrhage dissecting into the pontine tegmentum and through the cerebellum. |
| H.R. | Was found unresponsive on the ground outside his girlfriend’s house. He had arrived around midnight and knocked on the door of his girlfriend who had not opened the door since she had thought him to be intoxicated. At the local emergency department, the patient scored 7 on the Glascow Coma Scale. He withdrew to painful stimuli on the left side but not on the right. He had right hemiparesis, right facial asymmetry, decreased right corneal reflex, intact gag reflex and intact Doll’s eyes to both vertical and horizontal testing. He remained unresponsive until day 3 of admission when he occasionally opened his eyes and moved his limbs spontaneously. However, he did not respond to any commands yet. On day 4, he responded intermittently to commands and answered only the question of what his name was. His level of consciousness fluctuated for 9 days before he became gradually and increasingly alert and verbally responsive. The brainstem MRI on day 2 of admission showed haemorrhagic stroke in the upper pons and lower midbrain. |
| I.P. | Had a sudden loss of consciousness 30 min prior to her transfer to the emergency room. She remained totally unresponsive for another 2.5 h before she gradually became alert and responsive although she had developed diplopia and difficulties opening the left eyelid. Later, her level of consciousness was assessed as unimpaired, but her gaze defect persisted. The MRIs showed a lesion centred in the midline region of the mesencephalic tegmentum extending to the upper pons. |
| S.M. | Was admitted to hospital because of decreased alertness. On physical examinations, she had deviation of eyes and head to left, flaccid on the left and rhythmic movements of the left side of mouth, a slight increase in the tone on the right, hyperreflexia, positive Babinski and rigidity on the right. Her consciousness was deteriorated and she went into coma, being barely responsive to painful stimuli. She gradually opened her eyes on day 4, but was unresponsive to commands. On day 5, she was alert and responded to questions with mumbling; she was able to open her eyes, and the pupils were equal and reactive; she could not stick out her tongue. On day 7, she became responsive, and later recovered cognitively with persistent motor defects. MRIs showed a pontomesencephalic lesion as well as some lacunar infarcts in the left internal capsule and parietal cortex and the cerebellum secondary to vasculitis. |
| V.S. | Admitted to the hospital because of acute headache, dizziness, nausea and ataxia, and numbness of the left lip. At admission, he made odd muttering sounds. He had disconjugate eye movements, decerebrate reflex pattern and bilateral Babinski sign. His corneal reflex was absent bilaterally. His muscle tone was also flaccid. MRI showed an infarct affecting the pons and the midbrain as well as the left anterior medial hemisphere of the cerebellum. The diagnoses of basilar artery thrombosis and dissection of left vertebral artery was suggested, and the patient died on day 5 of admission of unexplained fever. |
| VR | Admitted in the early morning for bacterial endocarditis. Later that day, he had sudden onset of neurological signs. The patient was alert and awake but had slurred speech, left facial droop, left sided weakness, left Babinski sign, no medial or lateral movements on the left eye and ptosis and impaired lateral movements on the right eye, intact facial sensation; the tongue deviated to the left. The patient’s condition worsened gradually overnight, and on day 2 of admission, he was unresponsive to all stimuli with no oculocephalic or cold caloric eye movements. His corneal and gag reflexes were absent, and the pupils were in mid position and fixed. MRIs obtained that day showed bilateral lesions in the upper pons extending to the right basis pontis and into the lower midbrain tegmentum. He remained in coma for 5 days until he died in fever of unknown cause. |
| Case | Clinical data |
| M.M. | Was brought to the emergency department because of sudden onset of dizziness. Upon admission, he developed left hand and facial numbness and later facial contortion. These symptoms lasted 5 min, and the patient became totally unresponsive and remained that way until he died 7 days later. He had bilateral Babinski sign, decorticate posturing without withdrawal, occasional sneezing and irregular breathing. He had intact corneal reflexes and reactive and equal pupils. However, he did not have oculocephalic or cold caloric response bilaterally, gag response or any spontaneous movements. Patient M.M. died on day 7 of unexplained hyperthermia. MRI readings showed a peculiar horseshoe‐shaped lesion affecting the upper pontine tegmentum posteriorly on both sides, sparing the midline territories anteriorly (Fig. 4). The damage in the lower pons affected the entire right tegmentum, the basis pontis, and also extended across the midline and involved the left tegmentum partially. Post‐mortem analysis confirmed the MRI readings and indicated a thrombotic basilar artery with an infarct area affecting the corticospinal tracts bilaterally at the level of the upper pons. |
| S.A. | Upon admission to the hospital, he had loss of response to commands or painful stimuli, symmetrical and equally reactive pupils, absent eye movements, facial symmetry, intact gag reflex and complete paralysis of all extremities with decreased tone. He remained in this state until day 4 when he opened his eyes and could blink to yes/no questions. His condition improved and he gained consciousness responding to all questions by moving his eyes vertically. He remained in persistent locked‐in syndrome. MRI readings suggested the involvement of the pons. |
| T.G. | Was brought to the emergency department in her home town because of sudden onset of left arm and facial weakness with difficulty swallowing. Upon admission, her condition worsened and she became totally unresponsive. CT of the head excluded haemorrhage, and she was transferred to the UIHC. During the transfer, she gradually became alert but could not speak. By then, her total loss of consciousness had lasted approximately a couple of hours. By the time of arrival at the UIHC, she could open her eyes to command and had positive corneal reflexes. Her lateral gaze was impaired on the right. She had bilateral facial weakness but could grimace when tickled bilaterally. Her tongue movement was slow, and the rest of the motor exam showed spasticity in the right lower extremity and left upper extremity. On sensory exam, she could wince to deep painful stimuli applied to all extremities. MRI examination on day 1 of admission showed an infarct in the right pons. |
| P.H. | Admitted to a local hospital in a somnolent state. However, he could be aroused to follow commands. He could grip with his left, but not right, hand. He had anarthria, irregular pupils, decreased response to threat on the left, absent left lateral gaze and decreased sensation on the right. Moreover, he could wrinkle his forehead bilaterally, respond to voice and move his tongue randomly. Later during the first day of admission, he became increasingly unconscious (Glasgow Coma Scale = 3) and remained in that state. He was then transferred to the UIHC and remained there until day 3 when he developed fever and died in a hyperthermic state. EEG showed continuous δ slow waves with interspersed or superimposed β and α activities. No reactive EEG changes were noted. MRI on day 2 of admission showed haemorrhagic stroke in the upper pons. Gross brain examination at autopsy showed that the haemorrhagic stroke had isolated the brainstem from supratentorial structures at the pontomesencephalic level. Examination of the serial microscopical sections revealed areas of haemorrhage dissecting into the pontine tegmentum and through the cerebellum. |
| H.R. | Was found unresponsive on the ground outside his girlfriend’s house. He had arrived around midnight and knocked on the door of his girlfriend who had not opened the door since she had thought him to be intoxicated. At the local emergency department, the patient scored 7 on the Glascow Coma Scale. He withdrew to painful stimuli on the left side but not on the right. He had right hemiparesis, right facial asymmetry, decreased right corneal reflex, intact gag reflex and intact Doll’s eyes to both vertical and horizontal testing. He remained unresponsive until day 3 of admission when he occasionally opened his eyes and moved his limbs spontaneously. However, he did not respond to any commands yet. On day 4, he responded intermittently to commands and answered only the question of what his name was. His level of consciousness fluctuated for 9 days before he became gradually and increasingly alert and verbally responsive. The brainstem MRI on day 2 of admission showed haemorrhagic stroke in the upper pons and lower midbrain. |
| I.P. | Had a sudden loss of consciousness 30 min prior to her transfer to the emergency room. She remained totally unresponsive for another 2.5 h before she gradually became alert and responsive although she had developed diplopia and difficulties opening the left eyelid. Later, her level of consciousness was assessed as unimpaired, but her gaze defect persisted. The MRIs showed a lesion centred in the midline region of the mesencephalic tegmentum extending to the upper pons. |
| S.M. | Was admitted to hospital because of decreased alertness. On physical examinations, she had deviation of eyes and head to left, flaccid on the left and rhythmic movements of the left side of mouth, a slight increase in the tone on the right, hyperreflexia, positive Babinski and rigidity on the right. Her consciousness was deteriorated and she went into coma, being barely responsive to painful stimuli. She gradually opened her eyes on day 4, but was unresponsive to commands. On day 5, she was alert and responded to questions with mumbling; she was able to open her eyes, and the pupils were equal and reactive; she could not stick out her tongue. On day 7, she became responsive, and later recovered cognitively with persistent motor defects. MRIs showed a pontomesencephalic lesion as well as some lacunar infarcts in the left internal capsule and parietal cortex and the cerebellum secondary to vasculitis. |
| V.S. | Admitted to the hospital because of acute headache, dizziness, nausea and ataxia, and numbness of the left lip. At admission, he made odd muttering sounds. He had disconjugate eye movements, decerebrate reflex pattern and bilateral Babinski sign. His corneal reflex was absent bilaterally. His muscle tone was also flaccid. MRI showed an infarct affecting the pons and the midbrain as well as the left anterior medial hemisphere of the cerebellum. The diagnoses of basilar artery thrombosis and dissection of left vertebral artery was suggested, and the patient died on day 5 of admission of unexplained fever. |
| VR | Admitted in the early morning for bacterial endocarditis. Later that day, he had sudden onset of neurological signs. The patient was alert and awake but had slurred speech, left facial droop, left sided weakness, left Babinski sign, no medial or lateral movements on the left eye and ptosis and impaired lateral movements on the right eye, intact facial sensation; the tongue deviated to the left. The patient’s condition worsened gradually overnight, and on day 2 of admission, he was unresponsive to all stimuli with no oculocephalic or cold caloric eye movements. His corneal and gag reflexes were absent, and the pupils were in mid position and fixed. MRIs obtained that day showed bilateral lesions in the upper pons extending to the right basis pontis and into the lower midbrain tegmentum. He remained in coma for 5 days until he died in fever of unknown cause. |
UIHC = University of Iowa Hospital and Clinics.
References
Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA‐L study in the rat.
Aston‐Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance.
Azmitia EC, Whitaker‐Azmitia PM. Awakening the sleeping giant: anatomy and plasticity of the brain serotonergic system.
Barth DS, MacDonald KD. Thalamic modulation of high‐frequency oscillating potentials in auditory cortex.
Beitz AJ. Central gray. In: Paxinos G, editor. The human nervous system. San Diego: Academic Press;
Berridge CW, Arnsten AF, Foote SL. Noradrenergic modulation of cognitive function: clinical implications of anatomical, electrophysiological and behavioural studies in animal models.
Bester H, Bourgeais L, Villanueva L, Besson JM, Bernard JF. Differential projections to the intralaminar and gustatory thalamus from the parabrachial area: a PHA‐L study in the rat.
Bloom FE. What is the role of general activating systems in cortical function? In: Rakic P, Singer W, editors. Neurobiology of neocortex. Chichester (UK): John Wiley;
Brodal A. The reticular formation of the brain stem: anatomical aspect and functional correlation. Edinburgh: Oliver & Boyd;
Buritova J, Besson JM, Bernard JF. Involvement of the spinoparabrachial pathway in inflammatory nociceptive processes: a c‐Fos protein study in the awake rat.
Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory.
Caille D, Vibert JF, Hugelin A. Apneusis and apnea after parabrachial or Kolliker–Fuse N. lesion; influence of wakefulness.
Caplan LR. Posterior circulation disease, clinical findings, diagnosis, and management. Cambridge (MA): Blackwell Science;
Chase TN, Moretti L, Prensky AL. Clinical and electroencephalographic manifestations of vascular lesions of the pons.
Clark CR, Geffen GM, Geffen LB. Catecholamines and attention. I: animal and clinical studies.
Denoyer M, Sallanon M, Buda C, Kitahama K, Jouvet M. Neurotoxic lesion of the mesencephalic reticular formation and/or the posterior hypothalamus does not alter waking in the cat.
Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats.
French JD, Verzeano M, Magoun HW. A neural basis of the anesthetic state.
Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat.
Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat.
Jacobs BL, Wilkinson LO, Fornal CA. The role of brain serotonin. A neurophysiologic perspective.
Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat.
Knyihar‐Csillik E, Boncz I, Sary G, Nemcsok J, Csillik B. Parabrachial origin of calcitonin gene‐related peptide‐immunoreactive axons innervating Meynert’s basal nucleus.
Krout KE, Loewy AD. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat.
Krout KE, Loewy AD. Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat.
Loeb C. Electroencephalographic changes during the state of coma.
Loeb C, Stirling Meyer J. Strokes due to vertebro‐basilar disease: infarction, vascular insufficiency, and hemorrhage of the brain stem and cerebellum. Springfield (IL): Charles C. Thomas;
Lu J, Chou TC, Saper CB. Identification of wake‐active dopaminergic neurons in the ventral periaqueductal gray [abstract].
Mantyh PW. Connections of midbrain periaqueductal gray in the monkey. I. Ascending efferent projections.
Menani JV, Beltz TG, Johnson AK. Effects of lidocaine injections into the lateral parabrachial nucleus on dipsogenic and pressor responses to central angiotensin II in rats.
Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons.
Miller AD, Nonaka S, Jakus J. Brain areas essential or non‐essential for emesis.
Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K. Role of the parabrachial nucleus in ventilatory responses of awake rats.
Moore RY. The reticular formation: monoamine neuron systems. In: Hobson JA, Brazier MAB, editors. The reticular formation revisisted. New York: Raven Press;
Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems.
Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG.
Muller CM, Lewandowski MH, Singer W. Structures mediating cholinergic reticular facilitation of cortical responses in the cat: effects of lesions in immunocytochemically characterized projections.
Munk MHJ, Roelfsema PR, König P, Engel A, Singer W. Role of reticular activation in the modulation of intracortical synchronization.
Newman DB, Ginsberg CY. Brainstem reticular nuclei that project to the thalamus in rats: a retrograde tracer study.
Pare D, Smith Y, Parent A, Steriade M. Projections of brainstem core cholinergic and non cholinergic neurons of cat to intralaminar and reticular thalamic nuclei.
Porrino LJ, Goldman‐Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP.
Rico B, Cavada C. Adrenergic innervation of the monkey thalamus: an immunohistochemical study.
Robertson RT, Feiner AR. Diencephalic projections from the pontine reticular formation: autoradiographic studies in the cat.
Royce GJ, Bromley S, Gracco C. Subcortical projections to the centromedian and parafascicular thalamic nuclei in the cat.
Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat.
Shammah‐Lagnado SJ, Negrao N, Silva BA, Ricardo JA. Afferent connections of the nuclei reticularis pontis oralis and caudalis: a horseradish peroxidase study in the rat.
Scheibel ME, Scheibel AB. The organization of the nucleus reticularis thalami: a Golgi study.
Shibata M, Benzi RH, Seydoux J, Girardier L. Hyperthermia induced by pre‐pontine knife‐cut: evidence for a tonic inhibition of non‐shivering thermogenesis in anaesthetized rat.
Simpson CW, Ruwe WD, Myers RD. Anatomical distribution of brainstem sites where PGE1 induces hyperthermia in macaque species.
Smiley JF, Subramanian M, Mesulam AM. Monoaminergic–cholinergic interactions in the primate basal forebrain.
Steriade M. Central core modulation of spontaneous oscillations and sensory transmission in thalamocortical systems.
Steriade M, Domich L, Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance.
Steriade M, Pare D, Parent A, Smith Y. Projections of cholinergic and non‐cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey.
Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain.
Talman WT, Florek G, Bullard DE. A hyperthermic syndrome in two subjects with acute hydrocephalus.
van Domburg PH, ten Donkelaar HJ. The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases.