-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Saleh, Michael Schroeter, Cornelia Jonkmanns, Hans‐Peter Hartung, Ulrich Mödder, Sebastian Jander, In vivo MRI of brain inflammation in human ischaemic stroke, Brain, Volume 127, Issue 7, July 2004, Pages 1670–1677, https://doi.org/10.1093/brain/awh191
Close - Share Icon Share
Abstract
Inflammation contributes to brain damage caused by ischaemic stroke. Macrophages, as the prevailing inflammatory cell population in stroke lesions, can be visualized using ultrasmall superparamagnetic iron oxide (USPIO) as a cell‐specific contrast agent for MRI. In this single‐centre open‐labelled clinical phase II study we tested the potential of USPIO‐enhanced MRI for macrophage imaging in human ischaemic stroke lesions. In a series of 10 consecutive patients, USPIO contrast agent was infused at the end of the first week after symptom onset. Two follow‐up MRI scans were performed 24–36 h and 48–72 h after infusion. Two distinct components of USPIO‐related signal changes were discernible, one associated with blood vessels and one representing parenchymal enhancement. Vessel‐associated changes appeared as signal loss on T2/T2*‐weighted images and decreased from the first to second scan after USPIO infusion, most likely reflecting a transient blood pool effect of the contrast agent. Conversely, parenchymal enhancement was mainly evident on T1‐weighted images, increased over time, and matched with the expected distribution of macrophages. Importantly, USPIO‐induced signal alterations throughout differed from signatures of conventional gadolinium‐enhanced MRI, thus being independent from breakdown of the blood–brain barrier. We suggest that increasing USPIO‐enhancement on T1‐weighted images indicates brain infiltration by USPIO‐laden macrophages. Thus, USPIO‐enhanced MRI may provide an in vivo surrogate marker of cellular inflammation in stroke and other CNS pathologies.
Introduction
Brain inflammation contributes to the pathogenesis of neurodegeneration in common neurological diseases such as stroke and Alzheimer’s disease (Allan and Rothwell, 2001). In ischaemic stroke lesions, the inflammatory response is dominated by macrophages derived from both resident microglia and circulating monocytic precursors (Stoll et al., 1998). Activated macrophages produce a wide spectrum of cytotoxic substances and may thereby exacerbate the ischaemic tissue damage (Banati et al., 1993). Therefore, brain inflammation is a potential therapeutic target in subacute ischaemic stroke for which there is currently no other treatment option available (Becker, 2001).
Non‐invasive monitoring of inflammatory lesion activity via gadolinium‐enhanced MRI has greatly contributed to the development of immunomodulatory therapy in the human autoimmune disease, multiple sclerosis (Barkhof et al., 2003). However, work in experimental stroke models showed that multimodal MRI comprising diffusion‐ and perfusion‐weighted, as well as gadolinium‐enhanced imaging, is not able to discriminate inflamed from non‐inflamed infarct subareas (Schroeter et al., 2001). Ultrasmall superparamagnetic iron oxide (USPIO) particles have been recently introduced as a cell‐specific MRI contrast agent taken up by macrophages (Weissleder et al., 1990; Wang et al., 2001). In experimental stroke lesions macrophages could be visualized using USPIO‐enhanced MRI (Rausch et al., 2001). Based on these findings, we designed an open‐labelled clinical phase II single centre study to test the potential of USPIO‐enhanced MRI for the noninvasive visualization of macrophage influx into human ischaemic stroke lesions. In our study, we performed USPIO‐enhanced MRI 6–8 days after stroke at the stage of most pronounced phagocyte activity identified in experimental stroke studies (Stoll et al., 1998).
Patients and methods
Patients
We enrolled 10 consecutive adult neurological inpatients (nine male, one female; mean age 56.3 years; range 49–71 years) presenting with typical clinical signs of stroke. From all patients oral and written informed consent was obtained prior to inclusion. Major exclusion criteria were cerebral haemorrhage evident on initial MRI (see below), ambiguous time of symptom onset, lesion size below 1 cm3 at diffusion‐weighted imaging on initial stroke imaging, enrolment into other clinical studies, having received gadolinium complexes within 24 h or iron particles within 6 months before, known allergy to dextran or drugs containing iron salts and contraindications to MR imaging. The study design was approved by the Ethikkommission an der Medizinishcen Fakultät der Heinrich‐Heine‐Univerßität, Düsseldorf.
MRI
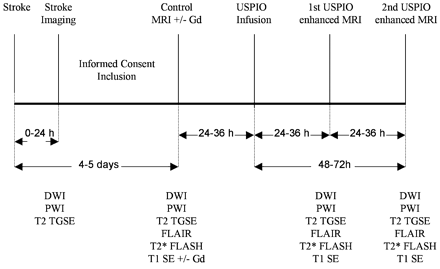
An outline of the trial schedule is provided in Fig. 1. To delineate the extent and location of ischaemic brain damage, all patients underwent multimodal stroke imaging (Neumann‐Haefelin et al., 1999) within 24 h of symptom onset. Stroke imaging comprised diffusion‐weighted imaging (DWI; see below for details), perfusion‐weighted imaging (PWI), and a T2‐weighted turbo spin‐echo gradient‐echo sequence (TGSE). To define the pattern of gadolinium‐based contrast medium enhancement, a first follow‐up MRI was performed 4–5 days after symptom onset before and after the administration of gadopentetate dimeglumine (Magnevist®, Schering, Berlin, Germany; 15 ml bolus at 5 ml/s, immediately followed by 15 ml 0.9% sodium chloride at the same rate). This was followed by a single USPIO infusion 24–36 h later. The USPIO contrast agent (ferumoxtran, AMI‐227, Sinerem®) was provided by Guerbet (Roissy, France). It was administered intravenously in a single dose (2.6 mg iron/kg body weight) by drip infusion through an infusion filter (0.22‐µm pore size) at a rate of 4 ml/min. Two additional follow‐up MRI scans were performed 24–36 and 48–72 h after USPIO infusion. These scans included conventional T1‐weighted spin‐echo and T2‐weighted TGSE sequences and T2*‐weighted gradient echo sequences. All MRI examinations were performed on a 1.5‐T magnet (Vision; Siemens, Erlangen, Germany) using a standard quadrature head coil operating in the receive mode.
DWI was performed with a single‐shot echo‐planar sequence with two diffusion‐weighted b values (of 0 and 1000 s/mm2) in three dimensions in space‐readout, phase‐encoding, and section‐selection directions, to result in four images per selection. The imaging parameters were an echo time (TE) of 100 ms, 20 transverse sections acquired, a 5‐mm section thickness, a 1.5‐mm intersection gap, a 96 × 128 matrix, a 240‐mm field of view (FOV), and imaging time of 26 s.
PWI was performed with serial T2*‐weighted single‐shot echo‐planar sequences, with imaging of 12 sections 40 times every 2 s. The parameters were a TE of 54 ms, 12 sections with 5‐mm thickness acquired, a 1.5‐mm intersection gap, a 128 × 128 matrix, and a 240‐mm FOV.
For T2‐weighted imaging, we used a TGSE with a repetition time (TR) of 7040 ms, TE of 115 ms, echo train length of 69, 20 transverse sections aquired parallel to the base of the skull, 5‐mm section thickness, 1.5‐mm intersection gap, 160° flip angle, 230‐mm FOV, 345 × 512 matrix, and imaging time of 1 min 17 s.
For T1‐weighted imaging, we used a spin‐echo sequence with a TR of 560 ms, TE of 17 ms, 20 transverse sections parallel to the base of the skull, 5‐mm section thickness, 1.5‐mm intersection gap, 70° flip angle, 230‐mm FOV, 192 × 256 matrix and imaging time of 3 min 38 s.
Fluid‐attenuated inversion recovery (FLAIR) imaging was performed with an immersion recovery–turbo spin‐echo (IR‐TSE) sequence with a TR of 9000 ms, a TE of 105 ms and an inversion time of 2200 ms, 20 transverse sections parallel to the base of the skull, 5‐mm section thickness, 1.5‐mm intersection gap, 180° flip angle, 230‐mm FOV, 173 × 256 matrix and imaging time of 3 min 9 s.
For susceptibility‐weighting we used a multi‐echo fast low‐angle shot (FLASH) sequence with a TR of 1000 ms, TE of 15, 35, 56 ms, 20 transverse sections parallel to the base of the skull, 5‐mm section thickness, 1.5‐mm intersection gap, 15° flip angle, 240‐mm FOV, 144 × 256 matrix and imaging time of 2 min 26 s.
Image analysis
In order to assess USPIO‐induced signal changes on T1‐ and T2*‐weighted images over time we performed region of interest (ROI) measurements of the infarcted brain parenchyma as defined by hyperintensity on the corresponding FLAIR images. Results of ROI measurements of the ischaemic lesion were normalized to the signal of the cerebrospinal fluid according the formula SInorm = (SIUSPIO–SICSF)/SICSF where SInorm is the normalized signal intensity, SIUSPIO the signal intensity of USPIO‐enhancement, and SICSF the signal intensity of cerebrospinal fluid. The results of ROI measurements on the first and second scan after USPIO administration were compared using a non‐parametric Wilcoxon signed rank test with the aid of SPSS statistical package version 11.0.1 (SPSS, Chicago, IL, USA).
Results
Baseline characteristics of study population
In line with the clinical presentation, initial stroke imaging obtained within 24 h after symptom onset revealed acute ischaemic lesions in the middle cerebral artery territory in eight patients, in the middle and posterior cerebral artery territory in one patient, and in the basilar artery territory in one patient. USPIO infusions were administered between days 5 and 6 after stroke and were well tolerated by all patients. For at least 48 h after the infusion, patients stayed hospitalized under neurological observation. No unexpected deterioration of their clinical status was observed.
Spatiotemporal pattern of USPIO‐related signal changes
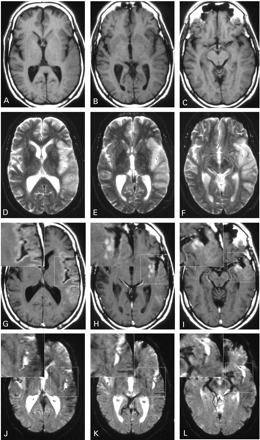
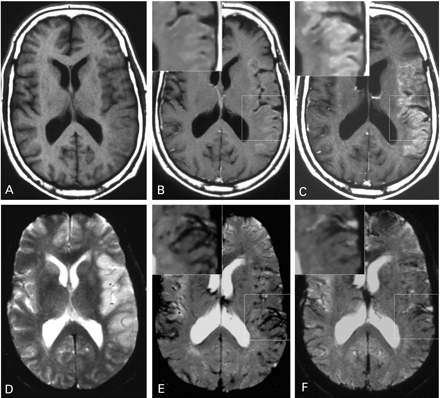
The follow‐up MRI scans showed secondary haemorrhagic transformation of the infarct in one patient who was therefore excluded from further analysis. In all remaining nine patients, consistent USPIO‐induced signal alterations in the ischaemic area were evident on both T1‐ and T2/T2*‐weighted images (Fig. 2). T1‐weighted images displayed USPIO enhancement mainly as hyperintense band‐like signals in the periphery of the infarcted parenchyma (Figs 2G and 4B and C). On several occasions, additional small foci of spot‐like enhancement were observed (Fig. 2H and I). On T2/T2*‐weighted images (Figs 2J–L and 4E and F), signal loss was evident due to the magnetic susceptibility effects of the USPIO contrast agent. Overall, the distribution of signal changes differed between T1‐ and T2*‐weighted sequences with more extensive alterations evident on T1‐weighted images (compare G–I with J–L in Fig. 2).
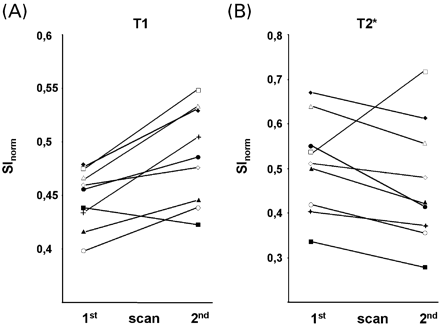
To study the temporal dynamics of USPIO enhancement, we performed two scans at 24–36 h and 48–72 h after USPIO administration, respectively (Fig. 1), and quantified the extent of signal changes via ROI measurements of the infarcted parenchyma (Fig. 3). T1 effects showed a statistically significant increase from the first to second scan after infusion (P = 0.011; Fig. 3A). Conversely, T2* effects tended to decrease, although the difference between the signal intensities of the lesions on the first and second scan did not reach statistical significance (P = 0.11; Fig. 3B). Morphologically, the increasing hyperintense signal on T1‐weighted images throughout appeared as parenchymal enhancement in the periphery of the infarct (Fig. 4A–C). This pattern was compatible with the distribution of macrophages demonstrated in experimental brain infarcts and autopsy material from human stroke cases (Stoll et al., 1998). In contrast, the T2* signal loss was mostly associated with blood vessels (Fig. 4D–F).
Relationship between USPIO and gadolinium enhancement
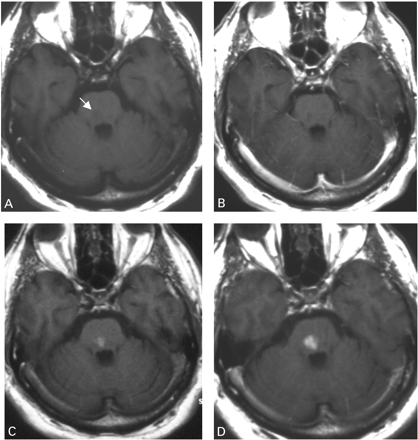
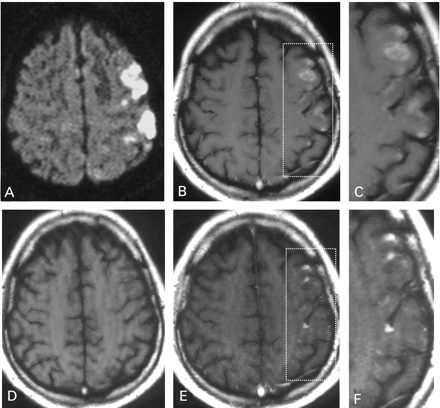
In contrast to the USPIO‐induced signal alterations found in all patients examined, gadolinium enhancement was present in only six patients and absent in three patients (gadolinium not administered in one patient). Differential USPIO and gadolinium enhancement was most conspicuous in a pontine infarct exhibiting complete lack of gadolinium enhancement but strong uptake of USPIO contrast agent (Fig. 5). In those infarcts showing gadolinium enhancement to at least some degree, its spatial distribution throughout differed from that of USPIO enhancement (Fig. 6).
Discussion
In our study, we have for the first time used USPIO‐enhanced MRI for the cellular imaging of human ischaemic stroke lesions. USPIO is a cell‐specific MRI contrast agent taken up by macrophages (Weissleder et al., 1990). USPIO‐induced signal alterations in the ischaemic area were evident on both T1‐ and T2/T2*‐weighted images, reflecting the T1‐ and T2‐relaxivity of the contrast agent (Wang et al., 2001). Interestingly, however, the time course and distribution of signal alterations differed between T1‐ and T2/T2*‐weighted sequences. Overall, the T2*‐signal void tended to decrease, whereas the T1‐hyperintensity increased from the first to second scan after USPIO infusion. In humans, the blood‐pool half‐life of USPIO has been calculated to be 24–36 h (McLachlan et al., 1994). The most likely explanation of our findings would therefore be that the T2* signal void is mainly due to a blood‐pool effect of USPIO and that its decrease indicates macrophage‐mediated clearance of the contrast agent from the circulation. In line with this assumption, signal loss on T2/T2*‐weighted images was predominantly associated with blood vessels. Conversely, the pattern of parenchymal T1 hyperintensity was consistent with the distribution of macrophages demonstrated in human and experimental stroke lesions (Clark et al., 1993; Jander et al., 1998; Stoll et al., 1998; Schroeter et al., 1999). Experimental ischaemia studies furthermore revealed close matching of USPIO‐induced signal alterations with the distribution of iron‐laden macrophages on histological brain sections (Rausch et al., 2001; Kleinschnitz et al., 2003; Schroeter et al., 2004). Thus, the increasing T1 enhancement observed from the first to second scan after USPIO infusion most likely indicates progressive invasion of the lesion parenchyma by USPIO‐laden macrophages.
The phagocytic response to focal brain ischaemia involves both activation of resident microglia and infiltration of blood‐derived macrophages (Stoll et al., 1998). Microglia activation occurs rapidly within 24 h after ischaemia, whereas the recruitment of blood‐borne macrophages is delayed by several days (Schroeter et al., 1997). In our present study, we administered the USPIO contrast agent at the end of the first week after stroke. At this time point, most of the phagocytes in the infarctions are derived from circulating monocytes/macrophages (Schroeter et al., 1997; Schilling et al., 2003; Tanaka et al., 2003). Interestingly, following experimental axotomy iron oxide particles did not accumulate in CNS fibre tracts undergoing secondary Wallerian degeneration, which is characterized by activation of resident microglia but a lack of haematogenous macrophage recruitment (Bendszus and Stoll, 2003). It therefore appears that the USPIO‐related T1‐enhancement found in our study is mainly caused by haematogenous macrophages which take up USPIO in the periphery and secondarily invade the ischaemic brain tissue. Thereby, USPIO‐enhanced MRI potentially differs from positron emission tomography with [11C]11195 which detects activated microglia in addition to haematogenous macrophages (Banati et al., 2000; Pappata et al., 2000). Of course, additional studies with USPIO administration at earlier stages after ischaemia will be necessary to clarify the relative contribution of resident microglia versus haematogenous macrophages to USPIO uptake into human ischaemic stroke lesions.
In our study, USPIO‐related signal alterations throughout differed from signatures of conventional gadolinium‐ enhanced MRI. Thus, USPIO‐enhancement does not simply reflect blood–brain barrier disruption as a non‐specific epiphenomenon of ischaemic tissue damage but provides new pathophysiological information which cannot be obtained using conventional MRI techniques. At least in experimental ischaemia, the USPIO contrast agent did not cause obvious adverse effects on clinical outcome and lesion size (Doerfler et al., 2000). Thus, USPIO‐enhanced MRI may find widespread application in the study of human CNS disease. Apart from their role in brain ischaemia, macrophages are key players in immune‐mediated tissue damage caused by multiple sclerosis (Huitinga et al., 1990) and are also essential for mounting a protective host response to infection (Polfliet et al., 2001). USPIO‐enhanced MRI may therefore contribute to the development of treatment strategies targeting macrophage recruitment and activation in a wide range of inflammatory CNS pathologies.
Acknowledgements
We wish to thank Dr Bruno Bonnemain (Laboratoire Guerbet) for kindly providing the USPIO contrast agent, Professor David Miller (London) for critical reading of the text and helpful suggestions, Professor Mario Siebler (Düsseldorf) for help with patient recruitment, and Philipp Zickler (Düsseldorf) for computing Fig. 3.
Fig. 1 Graphical outline of the trial schedule.
Fig. 2 Images of a 54‐year‐old man with infarction of the left middle cerebral artery territory, illustrating the different spatial distribution of the USPIO enhancement on T1‐weighted (A–C and G–I) versus T2*‐weighted images (D–F and J–L). Non‐enhanced images 5 days after stroke display slight swelling of the infarcted area with hypointense signal on T1‐weighted images (A–C) and hyperintense signal on T2*‐weighted images (D–F). At 48 h after USPIO infusion (G–L) T1‐weighted images show hyperintense parenchymal enhancement (G–I), whereas T2*‐weighted images display signal loss mainly associated with blood vessels (J–L). High magnification insets clearly demonstrate the differential distribution of the signal alterations on T1‐ (G–I) and T2*‐weighted images (J–L).
Fig. 3 ROI measurements of USPIO‐induced signal changes in infarcted parenchyma on T1‐ (A) and T2*‐weighted images (B) obtained 24–36 h (first scan) and 48–72 h (second scan) after USPIO infusion. SInorm represents the signal intensity of USPIO‐enhancement normalized to the signal intensity of cerebrospinal fluid. Solid lines connect values of one individual patient at the respective scans after infusion. T1‐enhancement significantly increased (P = 0.011), whereas T2* signal loss tended to decrease from the first to the second scan after infusion (P = 0.11).
Fig. 4 Same patient as in Fig. 2, showing the differential development of USPIO‐related signal changes over time. T1‐weighted images 24 (B) and 48 (C) h after USPIO infusion show increasing hyperintense signal enhancement in the periphery of the infarcted parenchyma. Non‐enhanced T2*‐weighted images 5 days after stroke display hyperintense demarcation of the infarcted territory (D). T2*‐weighted images 24 (E) and 48 (F) h after USPIO infusion show that the appearance of the lesion changed from hyperintense to hypointense due to USPIO perfusion. T2* signal changes are vessel‐associated and decrease over time (E and F). Note that in E vessel‐associated signal loss is also present outside the infarcted cortex (e.g. contralateral hemisphere).
Fig. 5 Non‐enhanced T1‐weighted image (A) of a 61‐year‐old patient displays a hypointense brainstem infarct (arrow) 5 days after stroke. A corresponding gadolinium‐enhanced T1‐weighted image (B) reveals no signs of blood–brain barrier disruption. Twenty‐four (C) and 48 (D) h after USPIO infusion T1‐weighted images show increasing hyperintense enhancement of the infarcted parenchyma.
Fig. 6 Images of a 52‐year‐old man with infarction within the left middle cerebral artery territory. A diffusion‐weighted image (A) displays the ischaemic area 2 h after symptom onset. The non‐enhanced T1‐weighted image obtained 5 days later (D) shows slight hypointensity in the corresponding infarction. The spatial distribution of hyperintensity on the USPIO‐enhanced images (B and C) is clearly different from the distribution on gadolinium‐enhancement (E and F). (C and F) Representations of high magnification images of left parietal cortical and subcortical regions as outlined in B and E.
References
Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity.
Barkhof F, Rocca M, Francis G, Van Waesberghe JH, Uitdehaag BM, Hommes OR, et al. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon beta1a.
Becker KJ. Targeting the central nervous system inflammatory response in ischemic stroke.
Bendszus M, Stoll G. Caught in the act: in vivo mapping of macrophage infiltration in nerve injury by magnetic resonance imaging.
Clark RK, Lee EV, Fish CJ, White RF, Price WJ, Jonak ZL, et al. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study.
Doerfler A, Engelhorn T, Heiland S, Knauth M, Wanke I, Forsting M. MR contrast agents in acute experimental cerebral ischemia: potential adverse impacts on neurologic outcome and infarction size.
Huitinga I, Van Rooijen N, De Groot CJA, Uitdehaag BMJ, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages.
Jander S, Schroeter M, D’Urso D, Gillen C, Witte OW, Stoll G. Focal ischaemia of the rat brain elicits an unusual inflammatory response: early appearance of CD8+ macrophages/microglia.
Kleinschnitz C, Bendszus M, Frank M, Solymosi L, Toyka KV, Stoll G. In vivo monitoring of macrophage infiltration in experimental ischemic brain lesions by magnetic resonance imaging.
McLachlan SJ, Morris MR, Lucas MA, Fisco RA, Eakins MN, Fowler DR, et al. Phase I clinical evaluation of a new iron oxide MR contrast agent.
Neumann‐Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Mödder U, et al. Diffusion‐ and perfusion‐weighted MRI. The DWI/PWI mismatch region in acute stroke.
Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, et al. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK11195.
Polfliet MM, Zwijnenburg PJ, van Furth AM, van der PT, Dopp EA, Renardel de Lavalette C, et al. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis.
Rausch M, Sauter A, Frohlich J, Neubacher U, Radu EW, Rudin M. Dynamic patterns of USPIO enhancement can be observed in macrophages after ischemic brain damage.
Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice.
Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of the rat cerebral cortex: the role of resident microglia.
Schroeter M, Jander S, Witte OW, Stoll G. Heterogeneity of the microglial response in photochemically induced focal ischemia of the rat cerebral cortex.
Schroeter M, Franke C, Stoll G, Hoehn M. Dynamic changes of magnetic resonance imaging abnormalities in relation to inflammation and glial responses after photothrombotic cerebral infarction in the rat brain.
Schroeter M, Saleh A, Hoehn M, Jander S. Histochemical detection of ultrasmall superparamagnetic iron oxide (USPIO) contrast medium uptake in experimental brain ischemia.
Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions.
Tanaka R, Komine‐Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, et al. Migration of enhanced green fluorescent protein expressing bone marrow‐derived microglia/macrophage into the mouse brain following permanent focal ischemia.
Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging.
- magnetic resonance imaging
- inflammatory cells
- contrast media
- inflammation
- ischemic stroke
- blood-brain barrier
- traumatic brain injuries
- phase 2 clinical trials
- macrophages
- brain
- diagnostic imaging
- surrogate markers
- brain mri
- ferumoxtran
- infusion procedures
- symptom onset
- transverse spin relaxation time