-
PDF
- Split View
-
Views
-
Cite
Cite
David Grabli, Kevin McCairn, Etienne C. Hirsch, Yves Agid, Jean Féger, Chantal François, Léon Tremblay, Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study, Brain, Volume 127, Issue 9, September 2004, Pages 2039–2054, https://doi.org/10.1093/brain/awh220
Close - Share Icon Share
Abstract
The current model of basal ganglia organization postulates the existence of a functional partitioning into sensorimotor, associative and limbic territories, implicated in motor, cognitive and emotional aspects of behaviour, respectively. This organization was proposed initially on the basis of the cortico-striatal projections and was extended to the various structures of the basal ganglia. While there is a considerable body of experimental evidence in support of an involvement of the basal ganglia sensorimotor territory in basic control of movements, evidence for the functional relevance of the non-motor territories has had to be based on a growing number of clinical observations due to the paucity of relevant animal studies. Previous studies in monkeys have, however, shown that a reversible and focal dysfunction induced by microinjections of bicuculline in the sensorimotor territory of the external globus pallidus (GPe) can generate abnormal movements. We therefore hypothesized that the same approach applied to the associative and limbic territories of the GPe would induce behavioural disorders rather than abnormal movements. To address this hypothesis, we performed microinjections of bicuculline, using the same concentration in each of the sensorimotor, associative and limbic territories of the GPe, as defined by striato-pallidal projections. Spontaneous behaviour and performance of a simple food-retrieving task during the effects of these microinjections were compared with data obtained in control conditions in the same monkeys. We found that bicuculline microinjections induced stereotypy when performed in the limbic part of the GPe, and attention deficit and/or hyperactivity when performed in the associative part. No movement disorders were observed during these behavioural disturbances. As previously described, abnormal movements were observed when bicuculline was injected into the sensorimotor territory of the GPe. The relationship between the localization of microinjection sites and the type of behavioural effect was similar for the three monkeys. Control microinjections of bicuculline into surrounding structures (striatum and internal globus pallidus) and saline injections into the GPe failed to induce any observable effect. These results support the hypotheses of functional diversity and territorial specificity in the GPe, in agreement with the parallel circuits organizational model of the basal ganglia. Furthermore, the behavioural effects shared similar features with symptoms observed in Tourette's syndrome, attention deficit/hyperactivity and compulsive disorders. Thus, our study provides experimental evidence for the involvement of the associative and limbic parts of the basal ganglia in these pathologies. These results may provide the basis for a primate model of these disorders.
Introduction
The involvement of the basal ganglia in movement control was clearly suggested by clinical observations of patients with movement disorders associated with lesions in these structures (Bhatia and Marsden, 1994; Lang and Lozano, 1998). This involvement was also highlighted by a number of primate studies (for a review, see Mink, 1996). Briefly, it was proposed that the function of the basal ganglia was not related to the generation of movement per se. These structures were considered more likely to be involved in the selection of context-dependent movements through a balance between the facilitation of desired movements and the inhibition of undesired competing ones (Mink, 1996).
The organization of cortico-striatal projections and its maintenance throughout the basal ganglia circuitry provides anatomical support in favour of a functional subdivision of the basal ganglia into sensorimotor, associative and limbic circuits (Alexander et al., 1986; Parent and Hazrati, 1995) devoted to transmitting sensorimotor, cognitive and motivational information, respectively. This enlargement of the function of the basal ganglia is supported by the identification, within these structures, of neuronal activities correlated to various cognitive or motivational aspects of conditioned tasks (Schultz et al., 1992; Hikosaka et al., 2002). In addition, several clinical studies have reported various cognitive deficits after lesions of the basal ganglia (Mendez et al., 1989; Dubois and Pillon, 1997). Consequently, the variety of the information processed by the basal ganglia suggests that they may be involved in the control of the motivational, attentional and memory processes required for goal-directed behaviours. A dysfunction within one of these systems may result in the appearance of abnormal behaviours. This hypothesis is supported by recent clinical studies focusing on the behavioural consequences of basal ganglia lesions (Laplane et al., 1981, 1982, 1984; Habib and Poncet, 1988; Mendez et al., 1989; Laplane, 1990; Dubois and Pillon, 1997). However, such clinical observations can only be partially taken into account with regard to the functional architecture of the basal ganglia since the precise localization and delineation of brain lesions are poorly reproducible. Among these observations, some of them describe symptoms that are similar to tics or obsessive–compulsive disorders (Laplane et al., 1989; Demirkol et al., 1999; Kwak and Jankovic, 2002). Similarly, functional imaging studies have suggested that the basal ganglia may be involved in purely behavioural disorders with no known anatomical lesions, such as Gilles de la Tourette's syndrome (TS) (Peterson, 2001), attention deficit/hyperactivity disorder (ADHD) (Castellanos, 2001) and OCD (Stein, 2002). However, compared with the body of evidence linking the basal ganglia with motor disturbances, few experimental studies in non-human primates have implicated the basal ganglia in the genesis of behavioural disorders.
We chose to address the involvement of the external globus pallidus (GPe) in the various functions of the basal ganglia, taking into account three elements. First, the topographical arrangement of the striatal afferent projections to the GPe, allowing the definition of three pallidal territories as motor, associative and limbic. Secondly, the GPe has been explored using various methodological approaches including single-unit recording (DeLong, 1990; Hamada et al., 1990; Anderson and Turner, 1991; Brotchie et al., 1991a, b; Wichmann et al., 1994; Mink, 1996), small lesions (Mink and Thach, 1991) and microinjections of pharmacological agents (Crossman et al., 1984, Crossman 1988; Matsumura et al., 1995; Inase et al., 1996; Wenger et al., 1999). However, all these investigations were concerned only with the motor aspect of this structure. Thirdly, previous experiments have shown that motor disturbances could be readily induced using microinjections of bicuculline into the sensorimotor part of the GPe (Crossman et al., 1984, 1988; Matsumura et al., 1995; Inase et al., 1996; Wenger et al., 1999).
Our working hypothesis can be summarized as follows: as dyskinesia can be induced by microinjections of bicuculline into the sensorimotor part of the GPe in the monkey (Crossman et al., 1988; Matsumura et al., 1995), we hypothesized that an identical neuronal dysfunction induced by similar microinjections into the associative or limbic parts of the GPe would result in behavioural disorders. Thus, this study was designed to evaluate, in monkeys, the effect of bicuculline microinjections performed in each of these three functional territories of the GPe. In order to characterize accurately the abnormal movements and behavioural disturbances induced by the microinjections, the effects were defined (i) according to the observation of the animal's spontaneous behaviour in a primate chair and in the homecage; and (ii) on the basis of changes in performance during a simple food retrieval task. This task allowed us to check whether the abnormal movements or behavioural disturbances prevented the monkey from performing the task or induced a change in the way it was executed.
These results have been reported previously in abstract form (Tremblay et al., 2002) and are complemented by their anatomical counterparts in the accompanying paper (François et al., 2004).
Material and methods
Subjects
Experiments were conducted on three male African green monkeys (Cercopithecus aethiops sabaeus) weighing 4–6 kg. Care and treatment of these monkeys, H, A and B, were in strict accordance with NIH guidelines (1996) and the recommendations of the EEC (86/609) and the French National Committee (87/848).
Surgical procedure
The surgical procedure was performed under gaseous anaesthesia provided through intratracheal intubation (Fluothane 1%, nitrogen monoxide, 50% and oxygen 50%) after induction with ketamine (10 mg/kg, i.m.) and atropine (0.1 mg/kg, i.m.). A rectangular stainless steel chamber (25 mm × 33 mm, Unimécanique, France) was implanted stereotactically on the interhemispheric line, over both the right and left hemispheres under aseptic conditions. The skull under the chamber was removed, but the animal's dura was left intact. The chamber was positioned with respect to the anterior (AC) and posterior commissure (PC) coordinates obtained by ventriculography.
For each experimental session, a perforated grid was inserted in the chamber to position the microinjection cannula. Spacing between the holes was 1 mm in each direction. This enabled us to determine the anteroposterior and lateral coordinates of each microinjection site according to a GPe stereotaxic representation obtained from the anatomical data of four other African green monkeys previously studied in our laboratory. The microinjection cannula (30 gauge) was mounted on a mechanical microdrive and connected to a 10 µl microsyringe (Hamilton) filled with the substance to be injected. In order to determine the depth of the GPe, a tungsten Teflon and Epoxylite-insulated microelectrode (stem with an outer diameter of 76 µm tapered down over 0.5 mm to exposed tips of 1.5–5 µm in diameter and 5–25 µm in length, impedance 1.5–6 MΩ) was inserted in the cannula. This microelectrode allowed us to perform extracellular single-unit recordings while the cannula was moved down. The signals were amplified (Amplifier DAMS, WPI, Sarasota, FL, USA) and displayed on an oscilloscope. The GPe was identified by its specific neuronal activity. The tip of the cannula was positioned at the chosen site before the beginning of the behavioural observation and was left in place during the entire experiment (∼90–120 min). When the microinjections were performed before the monkeys were returned to their homecage, the cannula was left at the chosen site for 10 min. The substance was delivered by pressure microinjection at a rate of 1 µl/min in steps of 0.5 µl. We typically used 1–1.7 µl of sterile bicuculline methiodide (Sigma) at a concentration of 15 µg/µl (29.5 mmol/l).
Most microinjections were made within the GPe at five antero-posterior levels from AC 0 to AC −4 mm. Some specific control microinjections were also made into the striatum and the internal globus pallidus (GPi). At some selected sites, we performed control microinjections of saline.
General experimental design
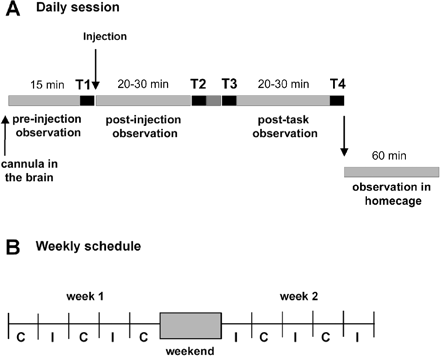
During the experimental sessions, the animals were seated in a primate chair with the head restrained. The schedule of daily experiments involved the following sequences (Fig. 1A). First, spontaneous behaviour and execution of task T1 were evaluated (control period, 15 min). After the microinjection of bicuculline, there was a first observation period of 20–30 min, two post-injection tasks (T2 and T3), the last observation period in the chair and the last task (T4). The monkeys were then returned to their homecage. Depending on the duration of the effect, the last task sessions were performed either during the end of the effect or during the recovery time. Control sessions were performed with microinjections of saline. Additional control sessions were also performed in which all the manipulations relating to the preparation of the microinjection were done but without the cannula in the brain of the monkey. These latter control sessions were to detect any possible contextual induction of an abnormal behaviour. They were divided into control, post-injection and post-task periods similarly to injection sessions. Some microinjections were performed just before the animals were returned to their homecage. In this condition, the monkeys could move freely.
(A) Daily schedules of experiments. Each session includes four task blocks (T1–T4, black line) and three observation periods of spontaneous behaviour. The first observation period and task block (T1) are performed after the cannula has been positioned at the injection site and before the microinjection of bicuculline. The period beginning after the microinjection is divided into two subperiods (post-injection and post-task) by the T2 and T3 task blocks. Behaviour is also recorded in the homecage for 60 min. Control sessions (without microinjection) are organized similarly. (B) Example of the experimental schedule over a 2-week period, showing the alternation between microinjection days (I) and control days (C). A maximum of two or three microinjections are performed during each week.
The weekly schedule (Fig. 1B) included no more than three microinjection days and at least two control days. The monkeys were accustomed to the daily procedure and learned the task before the surgical operation.
Analysis of behaviours in the primate chair
Spontaneous behaviours and task execution were observed and recorded during a 2 h experimental session using a quadravision video system. This system was designed to visualize behavioural sequences from four different directions: lateral, dorsal and two frontal (one general and the other focused on the monkey's face to record eye and mouth movements). The frequency and duration of each behaviour or abnormal movement were quantified after observation on videotapes. Nine kinds of behaviour and two types of abnormal movements were evaluated in 3 min segments over a 15 min control period before microinjection and during two periods of 20–30 min starting immediately after the microinjection. Behaviours were defined as follows: (i) resting (awake without limb movements); (ii) hand examination; (iii) grooming (cleaning its fur with fingers); (iv) leg or (v) arm movements (normal movements without a goal); (vi) licking and/or biting fingers; (vii) touching equipment; (viii) tail examination; (ix) others (rest with closed eyes or other behaviours), abnormal movement.
Spontaneous behaviours in the homecage
For monkeys H and A, microinjections were performed as described above and the cannula was left in place for at least 10 min. The cannula was then removed and the animals were returned to their homecage. For monkey B, cage observations were mainly made during the continuation of effects induced in the primate chair. For all three animals, control data in the homecage were obtained after control sessions in the chair. The observation period began when the animals were returned to their homecage and lasted 1 h (Fig. 1A). In this condition, the behaviours were observed through a video system providing a front view of the cage. These 1 h sessions were also videotaped for quantitative analysis.
Analysis of the activity in the homecage was performed using LabWatcher Software (View Point, Lyon, France). Ten kinds of behaviours were considered: (i) resting (awake without movements or sleeping); (ii) hand examination; (iii) grooming; (iv) exploration (moving in the cage except for taking food or circling); (v) circling to the left or (vi) right (unidirectional rotation behaviour without a goal); (vii) licking the bars of the cage; (viii) licking or biting fingers; (ix) searching for or eating food; and (x) visual tracking (head orientation movements). The duration of each behaviour was quantified by 12 sequences of 5 min over the 60 min period.
Simple food retrieval task
The monkeys were conditioned to perform a simple choice task in which they had to grasp and retrieve food from an 18-well board placed in front of them (Fig. 6A). The left and right sides of the board were clearly separated by a central plaque. During the training period, the monkeys learned to pick up rewards from the left and right part of the board with their left and right hand, respectively. We were therefore able to study the spatial strategy of reaching in the different experimental conditions. For statistical validity, 4–6 task sessions were conducted during each microinjection effect. We also identified different errors in the task: (i) returns to empty wells; (ii) cross-over hand errors; and (iii) non-taken rewards. We pooled data according to the type of effect and the monkey: control periods (34 sessions for monkey H and 24 sessions for monkey A), stereotypies (18 sessions from five microinjections for monkey H and 20 sessions from five microinjections for monkey A) and hyperactivity (20 sessions from five microinjections for monkey H and 28 sessions from seven microinjections for monkey A). Statistical analysis was then performed.
Statistical analysis
For the analysis of spontaneous behaviours in the primate chair, post-injection measurements were compared with pre-injection measurements from the same day and with post-injection measurements obtained during the preceding and following control days. For spontaneous behaviour in the homecage, post-injection measurements were compared with a group of control observations (monkey H, n = 4; monkey A, n = 5; monkey B, n = 8). Behavioural data were analysed by two-way ANOVA (analysis of variance) followed by appropriate post hoc comparisons. Changes in spatial strategy in the simple food retrieval task were analysed by two-way ANOVA. Mean numbers of errors in the task (returns to empty wells, cross-over hand errors and non-taken rewards) during the microinjection effect were compared with those of the control session using a Mann–Whitney test. Results with P < 0.05 were considered significant for all the analyses.
Histological procedures
At the end of the experiments, the monkeys were killed with an overdose of anaesthetic and were perfused transcardially with 1 l of saline (0.9% NaCl) followed by 5 l of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4, 4°C), then 1 l of PBS with 5% sucrose. The brains were removed from the skulls, immersed in PBS complemented with 10% sucrose for 1 day and with 20% sucrose for another day, then frozen and cut into 50 µm thick sections on a freezing microtome. Frontal sections were cut perpendicularly to the intercommissural plane according to a procedure described in the accompanying paper (François et al., 2004). The delineation of the sensorimotor, associative and limbic territories of the GPe were obtained from previous tracing studies (Haber et al., 1993; François et al., 1994) and transferred to a cartography of the GPe, showing five antero-posterior planes from the AC plane to 4 mm behind the AC (Fig. 2A). Trajectories and microinjection sites were reconstructed from cresyl violet-stained and calbindin-immunoreactive sections on the basis of their microdrive coordinates and gliosis associated with cannula tracks. Sites were then transferred to the cartography of the GPe and compared with the borders of the functional territories of the GPe.
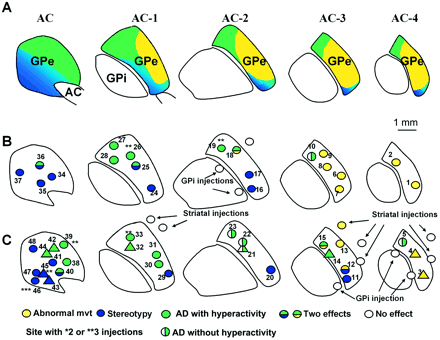
Functional territories and localization of microinjection sites in the GPe. (A) Representation of the associative (green), limbic (blue) and sensorimotor (yellow) territories within the GPe, as previously defined (Haber et al., 1993; François et al., 1994). An overview of the localization of microinjection sites and the nature of the responses obtained in monkey H (B), and in monkey A (circle) and monkey B (triangle) (C). AC = anterior commissure; AD = attention deficit; GPe = external globus pallidus; GPi = internal globus pallidus.
Results
Microinjections of bicuculline were performed in a total of 48 sites, listed in Table 1, at various levels throughout the GPe (20, 19 and nine sites in monkeys H, A and B, respectively). Thirteen of these sites, listed in Table 2, also received bicuculline microinjection before the monkeys were returned to the homecage in order to verify the effect on free-moving animals in this context. Some microinjections (n = 6) were repeated once or twice in order to check the reproducibility of the behavioural effects observed the first time. Figure 2 compares the effect of the microinjections in specific territories (Fig. 2B and C) and provides a schematic representation of the three anatomo-functional territories of the GPe (Fig. 2A).
Summary of injections performed in three monkeys in the primate chair
| Injection . | Side (vol µl) . | Abnormal movement . | Intensity . | Topography . | Abnormal behaviours . | Task . | Latency(s) . | Duration(s) . |
|---|---|---|---|---|---|---|---|---|
| 1 | L (1) | NA | ++ | Trunk | – | N | 3 | 72 |
| 2 | R (1.5) | Type 1/type 2 | +++a/++b | UL/UL | – | Motor pert | 5 | 92 |
| 3 | R (2) | Type 1 | LL | – | NA | 10 | 50 | |
| 4 | R (2) | Type 1 | LL | – | NA | 20 | 60 | |
| 5 | R (1.5) | – | – | – | – | Spat pert | NA | NA |
| 6 | L (1.5) | Type 1/type 2 | +a/++b | LL/UL | – | N | 3a40b | 33a40b |
| 7 | L (2) | Type 1 | ++++a | UL | – | Motor pert | 3 | 106 |
| 8 | L (1) | Type 1 | + | LL | – | N | 7 | 66 |
| 9 | L (1.5) | Type 1/type 1 | +++a/+++b | UL/LL | – | Motor pert | 6a8b | 88ab |
| 10 | L (1) | – | – | – | – | Spat pert | NA | NA |
| 11 | L (1.5) | – | – | – | Stereotypies | N | 14 | 60 |
| 12 | R (1.5) | Type 1 | ++a | LL | Stereotypiesb | N | 6a15b | 64ab |
| 13 | R (1.5) | Type 2 | ++++ | UL | – | N | 5 | 50 |
| 14 | R (1.7) | – | – | – | Hyperactivity | Spat pert | 15 | 85 |
| 15 | R (1.5) | Type 1 | ++a | LL | Hyperactivityb | Spat pert | 3a12b | 62ab |
| 16 | R (1) | – | – | – | Stereotypies | Spat pert | 4 | 125 |
| 17 | R (1) | – | – | – | Stereotypies | Not perf | 2 | 54 |
| 18 | R (1) | Type 2/type 2 | +++b/+++a | UL/LL | Hyperactivity | Spat pert | 70a88b | 71a40b |
| 19 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 72 |
| 20 | R (1.5) | – | – | – | Stereotypies | N | 3 | 70 |
| 21 | L (1.7) | – | – | – | – | Spat pert | NA | NA |
| 22 | R (2) | – | – | – | – | Spat pert | NA | NA |
| 23 | L (1.5) | – | – | – | – | Spat pert | NA | NA |
| 24 | L (1.7) | – | – | – | Stereotypies | N | 3 | 65 |
| 25 | L (1.5) | – | – | – | Hyperactivity/stereotypies | Spat pert | 4 | 66 |
| 26 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 4 | 67 |
| 27 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 60 |
| 28 | L (1.7) | – | – | – | Hyperactivity | Spat pert | 4 | 120 |
| 29 | L (1.5) | – | – | – | Stereotypies | N | 6 | 41 |
| 30 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 12 | 70 |
| 31 | R (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 48 |
| 32 | L (1.7) | – | – | – | Hyperactivity | Spat pert | 3 | 120 |
| 33 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 70 |
| 34 | L (1) | – | – | – | Stereotypies | N | 3 | 69 |
| 35 | R (1.5) | – | – | – | Stereotypies | N | 3 | 122 |
| 36 | R (2) | – | – | – | Hyperactivity/stereotypies | N | 2 | NA |
| 37 | R (1) | – | – | – | Stereotypies | N | 2 | NA |
| 38 | L (1.5) | – | – | – | Hyperactivity | NA | 2 | 15 |
| 39 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 40 |
| 40 | R (1.5) | – | – | – | Hyperactivity/stereotypies | N | 3 | 60 |
| 41 | L (1.5) | – | – | – | Stereotypies | N | 14 | 60 |
| 42 | L (1.7) | – | – | – | Hyperactivity | NA | 2 | 90 |
| 43 | L (1.7) | – | – | – | Stereotypies | NA | 2 | 120 |
| 44 | R (1.7) | – | – | – | Stereotypies | NA | 5 | 60 |
| 45 | R (1.7) | – | – | – | Stereotypies | NA | 10 | 120 |
| 46 | L (2) | – | – | – | Stereotypies | N | 1 | 90 |
| 47 | L (1.5) | – | – | – | Stereotypies | N | 3 | 90 |
| 48 | L (1.5) | – | – | – | Stereotypies | N | 3 | 90 |
| Injection . | Side (vol µl) . | Abnormal movement . | Intensity . | Topography . | Abnormal behaviours . | Task . | Latency(s) . | Duration(s) . |
|---|---|---|---|---|---|---|---|---|
| 1 | L (1) | NA | ++ | Trunk | – | N | 3 | 72 |
| 2 | R (1.5) | Type 1/type 2 | +++a/++b | UL/UL | – | Motor pert | 5 | 92 |
| 3 | R (2) | Type 1 | LL | – | NA | 10 | 50 | |
| 4 | R (2) | Type 1 | LL | – | NA | 20 | 60 | |
| 5 | R (1.5) | – | – | – | – | Spat pert | NA | NA |
| 6 | L (1.5) | Type 1/type 2 | +a/++b | LL/UL | – | N | 3a40b | 33a40b |
| 7 | L (2) | Type 1 | ++++a | UL | – | Motor pert | 3 | 106 |
| 8 | L (1) | Type 1 | + | LL | – | N | 7 | 66 |
| 9 | L (1.5) | Type 1/type 1 | +++a/+++b | UL/LL | – | Motor pert | 6a8b | 88ab |
| 10 | L (1) | – | – | – | – | Spat pert | NA | NA |
| 11 | L (1.5) | – | – | – | Stereotypies | N | 14 | 60 |
| 12 | R (1.5) | Type 1 | ++a | LL | Stereotypiesb | N | 6a15b | 64ab |
| 13 | R (1.5) | Type 2 | ++++ | UL | – | N | 5 | 50 |
| 14 | R (1.7) | – | – | – | Hyperactivity | Spat pert | 15 | 85 |
| 15 | R (1.5) | Type 1 | ++a | LL | Hyperactivityb | Spat pert | 3a12b | 62ab |
| 16 | R (1) | – | – | – | Stereotypies | Spat pert | 4 | 125 |
| 17 | R (1) | – | – | – | Stereotypies | Not perf | 2 | 54 |
| 18 | R (1) | Type 2/type 2 | +++b/+++a | UL/LL | Hyperactivity | Spat pert | 70a88b | 71a40b |
| 19 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 72 |
| 20 | R (1.5) | – | – | – | Stereotypies | N | 3 | 70 |
| 21 | L (1.7) | – | – | – | – | Spat pert | NA | NA |
| 22 | R (2) | – | – | – | – | Spat pert | NA | NA |
| 23 | L (1.5) | – | – | – | – | Spat pert | NA | NA |
| 24 | L (1.7) | – | – | – | Stereotypies | N | 3 | 65 |
| 25 | L (1.5) | – | – | – | Hyperactivity/stereotypies | Spat pert | 4 | 66 |
| 26 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 4 | 67 |
| 27 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 60 |
| 28 | L (1.7) | – | – | – | Hyperactivity | Spat pert | 4 | 120 |
| 29 | L (1.5) | – | – | – | Stereotypies | N | 6 | 41 |
| 30 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 12 | 70 |
| 31 | R (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 48 |
| 32 | L (1.7) | – | – | – | Hyperactivity | Spat pert | 3 | 120 |
| 33 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 70 |
| 34 | L (1) | – | – | – | Stereotypies | N | 3 | 69 |
| 35 | R (1.5) | – | – | – | Stereotypies | N | 3 | 122 |
| 36 | R (2) | – | – | – | Hyperactivity/stereotypies | N | 2 | NA |
| 37 | R (1) | – | – | – | Stereotypies | N | 2 | NA |
| 38 | L (1.5) | – | – | – | Hyperactivity | NA | 2 | 15 |
| 39 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 40 |
| 40 | R (1.5) | – | – | – | Hyperactivity/stereotypies | N | 3 | 60 |
| 41 | L (1.5) | – | – | – | Stereotypies | N | 14 | 60 |
| 42 | L (1.7) | – | – | – | Hyperactivity | NA | 2 | 90 |
| 43 | L (1.7) | – | – | – | Stereotypies | NA | 2 | 120 |
| 44 | R (1.7) | – | – | – | Stereotypies | NA | 5 | 60 |
| 45 | R (1.7) | – | – | – | Stereotypies | NA | 10 | 120 |
| 46 | L (2) | – | – | – | Stereotypies | N | 1 | 90 |
| 47 | L (1.5) | – | – | – | Stereotypies | N | 3 | 90 |
| 48 | L (1.5) | – | – | – | Stereotypies | N | 3 | 90 |
All injection sites are given with their corresponding effects, including abnormal movements (type, intensity and topography), abnormal behaviours and task execution. Latency and duration of the effects are provided where available and are referred to by a letter (a, b) when two effects co-existed. They could not be assessed (NA) when the effect was only a spatial perturbation during the task. Duration was not assessed for two injections that produced a strong effect, as the experiments were stopped before the end of the observation period. Type 1 and 2 abnormal movements were specified in the Results. L = left; R = right; LL = lower limb; UL = upper limb; N = normal; NA = not assessed; motor pert = motor perturbation; not perf = not performed; spat pert = spatial perturbation.
Summary of injections performed in three monkeys in the primate chair
| Injection . | Side (vol µl) . | Abnormal movement . | Intensity . | Topography . | Abnormal behaviours . | Task . | Latency(s) . | Duration(s) . |
|---|---|---|---|---|---|---|---|---|
| 1 | L (1) | NA | ++ | Trunk | – | N | 3 | 72 |
| 2 | R (1.5) | Type 1/type 2 | +++a/++b | UL/UL | – | Motor pert | 5 | 92 |
| 3 | R (2) | Type 1 | LL | – | NA | 10 | 50 | |
| 4 | R (2) | Type 1 | LL | – | NA | 20 | 60 | |
| 5 | R (1.5) | – | – | – | – | Spat pert | NA | NA |
| 6 | L (1.5) | Type 1/type 2 | +a/++b | LL/UL | – | N | 3a40b | 33a40b |
| 7 | L (2) | Type 1 | ++++a | UL | – | Motor pert | 3 | 106 |
| 8 | L (1) | Type 1 | + | LL | – | N | 7 | 66 |
| 9 | L (1.5) | Type 1/type 1 | +++a/+++b | UL/LL | – | Motor pert | 6a8b | 88ab |
| 10 | L (1) | – | – | – | – | Spat pert | NA | NA |
| 11 | L (1.5) | – | – | – | Stereotypies | N | 14 | 60 |
| 12 | R (1.5) | Type 1 | ++a | LL | Stereotypiesb | N | 6a15b | 64ab |
| 13 | R (1.5) | Type 2 | ++++ | UL | – | N | 5 | 50 |
| 14 | R (1.7) | – | – | – | Hyperactivity | Spat pert | 15 | 85 |
| 15 | R (1.5) | Type 1 | ++a | LL | Hyperactivityb | Spat pert | 3a12b | 62ab |
| 16 | R (1) | – | – | – | Stereotypies | Spat pert | 4 | 125 |
| 17 | R (1) | – | – | – | Stereotypies | Not perf | 2 | 54 |
| 18 | R (1) | Type 2/type 2 | +++b/+++a | UL/LL | Hyperactivity | Spat pert | 70a88b | 71a40b |
| 19 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 72 |
| 20 | R (1.5) | – | – | – | Stereotypies | N | 3 | 70 |
| 21 | L (1.7) | – | – | – | – | Spat pert | NA | NA |
| 22 | R (2) | – | – | – | – | Spat pert | NA | NA |
| 23 | L (1.5) | – | – | – | – | Spat pert | NA | NA |
| 24 | L (1.7) | – | – | – | Stereotypies | N | 3 | 65 |
| 25 | L (1.5) | – | – | – | Hyperactivity/stereotypies | Spat pert | 4 | 66 |
| 26 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 4 | 67 |
| 27 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 60 |
| 28 | L (1.7) | – | – | – | Hyperactivity | Spat pert | 4 | 120 |
| 29 | L (1.5) | – | – | – | Stereotypies | N | 6 | 41 |
| 30 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 12 | 70 |
| 31 | R (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 48 |
| 32 | L (1.7) | – | – | – | Hyperactivity | Spat pert | 3 | 120 |
| 33 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 70 |
| 34 | L (1) | – | – | – | Stereotypies | N | 3 | 69 |
| 35 | R (1.5) | – | – | – | Stereotypies | N | 3 | 122 |
| 36 | R (2) | – | – | – | Hyperactivity/stereotypies | N | 2 | NA |
| 37 | R (1) | – | – | – | Stereotypies | N | 2 | NA |
| 38 | L (1.5) | – | – | – | Hyperactivity | NA | 2 | 15 |
| 39 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 40 |
| 40 | R (1.5) | – | – | – | Hyperactivity/stereotypies | N | 3 | 60 |
| 41 | L (1.5) | – | – | – | Stereotypies | N | 14 | 60 |
| 42 | L (1.7) | – | – | – | Hyperactivity | NA | 2 | 90 |
| 43 | L (1.7) | – | – | – | Stereotypies | NA | 2 | 120 |
| 44 | R (1.7) | – | – | – | Stereotypies | NA | 5 | 60 |
| 45 | R (1.7) | – | – | – | Stereotypies | NA | 10 | 120 |
| 46 | L (2) | – | – | – | Stereotypies | N | 1 | 90 |
| 47 | L (1.5) | – | – | – | Stereotypies | N | 3 | 90 |
| 48 | L (1.5) | – | – | – | Stereotypies | N | 3 | 90 |
| Injection . | Side (vol µl) . | Abnormal movement . | Intensity . | Topography . | Abnormal behaviours . | Task . | Latency(s) . | Duration(s) . |
|---|---|---|---|---|---|---|---|---|
| 1 | L (1) | NA | ++ | Trunk | – | N | 3 | 72 |
| 2 | R (1.5) | Type 1/type 2 | +++a/++b | UL/UL | – | Motor pert | 5 | 92 |
| 3 | R (2) | Type 1 | LL | – | NA | 10 | 50 | |
| 4 | R (2) | Type 1 | LL | – | NA | 20 | 60 | |
| 5 | R (1.5) | – | – | – | – | Spat pert | NA | NA |
| 6 | L (1.5) | Type 1/type 2 | +a/++b | LL/UL | – | N | 3a40b | 33a40b |
| 7 | L (2) | Type 1 | ++++a | UL | – | Motor pert | 3 | 106 |
| 8 | L (1) | Type 1 | + | LL | – | N | 7 | 66 |
| 9 | L (1.5) | Type 1/type 1 | +++a/+++b | UL/LL | – | Motor pert | 6a8b | 88ab |
| 10 | L (1) | – | – | – | – | Spat pert | NA | NA |
| 11 | L (1.5) | – | – | – | Stereotypies | N | 14 | 60 |
| 12 | R (1.5) | Type 1 | ++a | LL | Stereotypiesb | N | 6a15b | 64ab |
| 13 | R (1.5) | Type 2 | ++++ | UL | – | N | 5 | 50 |
| 14 | R (1.7) | – | – | – | Hyperactivity | Spat pert | 15 | 85 |
| 15 | R (1.5) | Type 1 | ++a | LL | Hyperactivityb | Spat pert | 3a12b | 62ab |
| 16 | R (1) | – | – | – | Stereotypies | Spat pert | 4 | 125 |
| 17 | R (1) | – | – | – | Stereotypies | Not perf | 2 | 54 |
| 18 | R (1) | Type 2/type 2 | +++b/+++a | UL/LL | Hyperactivity | Spat pert | 70a88b | 71a40b |
| 19 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 72 |
| 20 | R (1.5) | – | – | – | Stereotypies | N | 3 | 70 |
| 21 | L (1.7) | – | – | – | – | Spat pert | NA | NA |
| 22 | R (2) | – | – | – | – | Spat pert | NA | NA |
| 23 | L (1.5) | – | – | – | – | Spat pert | NA | NA |
| 24 | L (1.7) | – | – | – | Stereotypies | N | 3 | 65 |
| 25 | L (1.5) | – | – | – | Hyperactivity/stereotypies | Spat pert | 4 | 66 |
| 26 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 4 | 67 |
| 27 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 60 |
| 28 | L (1.7) | – | – | – | Hyperactivity | Spat pert | 4 | 120 |
| 29 | L (1.5) | – | – | – | Stereotypies | N | 6 | 41 |
| 30 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 12 | 70 |
| 31 | R (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 48 |
| 32 | L (1.7) | – | – | – | Hyperactivity | Spat pert | 3 | 120 |
| 33 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 70 |
| 34 | L (1) | – | – | – | Stereotypies | N | 3 | 69 |
| 35 | R (1.5) | – | – | – | Stereotypies | N | 3 | 122 |
| 36 | R (2) | – | – | – | Hyperactivity/stereotypies | N | 2 | NA |
| 37 | R (1) | – | – | – | Stereotypies | N | 2 | NA |
| 38 | L (1.5) | – | – | – | Hyperactivity | NA | 2 | 15 |
| 39 | L (1.5) | – | – | – | Hyperactivity | Spat pert | 3 | 40 |
| 40 | R (1.5) | – | – | – | Hyperactivity/stereotypies | N | 3 | 60 |
| 41 | L (1.5) | – | – | – | Stereotypies | N | 14 | 60 |
| 42 | L (1.7) | – | – | – | Hyperactivity | NA | 2 | 90 |
| 43 | L (1.7) | – | – | – | Stereotypies | NA | 2 | 120 |
| 44 | R (1.7) | – | – | – | Stereotypies | NA | 5 | 60 |
| 45 | R (1.7) | – | – | – | Stereotypies | NA | 10 | 120 |
| 46 | L (2) | – | – | – | Stereotypies | N | 1 | 90 |
| 47 | L (1.5) | – | – | – | Stereotypies | N | 3 | 90 |
| 48 | L (1.5) | – | – | – | Stereotypies | N | 3 | 90 |
All injection sites are given with their corresponding effects, including abnormal movements (type, intensity and topography), abnormal behaviours and task execution. Latency and duration of the effects are provided where available and are referred to by a letter (a, b) when two effects co-existed. They could not be assessed (NA) when the effect was only a spatial perturbation during the task. Duration was not assessed for two injections that produced a strong effect, as the experiments were stopped before the end of the observation period. Type 1 and 2 abnormal movements were specified in the Results. L = left; R = right; LL = lower limb; UL = upper limb; N = normal; NA = not assessed; motor pert = motor perturbation; not perf = not performed; spat pert = spatial perturbation.
Summary of observations made in the homecage
| Category of effect in chair . | Site . | Monkey . | Side (vol µl) . | Main behaviours in homecage . |
|---|---|---|---|---|
| Stereotypy | 29 | A | L (1.5) | Licking/biting fingers, searching for food, tail exam. |
| Stereotypy | 46 | A | L (1.5) | Licking/biting fingers, licking bars, searching for food |
| Stereotypy | 43 | B | L (1.7) | Licking bars, licking/biting fingers, searching for food |
| Stereotypy | 17 | H | R (1) | Licking bars, tail exam., searching for food |
| Stereotypy | 34 | H | L (1.5) | Licking/biting fingers, searching for food |
| Stereotypy and hyperactivity | 36 | H | R (1) | Exploration, licking/biting fingers, circling contra. |
| Hyperactivity and dyskinesia | 15 | A | R (1.5) | Exploration, dyskinesia, circling contra. |
| Hyperactivity | 31 | A | R (2.3) | Exploration, licking bars, circling contra. |
| Hyperactivity | 14 | B | R (1.7) | Exploration, circling contra. |
| Hyperactivity | 21 | B | L (1.7) | Exploration, circling contra. |
| Hyperactivity | 32 | B | L (1.7) | Exploration, searching for food, circling contra. |
| Hyperactivity | 19 | H | L (1.5) | Exploration, circling contra. |
| Hyperactivity | 27 | H | L (1.5) | Exploration, circling contra. |
| Category of effect in chair . | Site . | Monkey . | Side (vol µl) . | Main behaviours in homecage . |
|---|---|---|---|---|
| Stereotypy | 29 | A | L (1.5) | Licking/biting fingers, searching for food, tail exam. |
| Stereotypy | 46 | A | L (1.5) | Licking/biting fingers, licking bars, searching for food |
| Stereotypy | 43 | B | L (1.7) | Licking bars, licking/biting fingers, searching for food |
| Stereotypy | 17 | H | R (1) | Licking bars, tail exam., searching for food |
| Stereotypy | 34 | H | L (1.5) | Licking/biting fingers, searching for food |
| Stereotypy and hyperactivity | 36 | H | R (1) | Exploration, licking/biting fingers, circling contra. |
| Hyperactivity and dyskinesia | 15 | A | R (1.5) | Exploration, dyskinesia, circling contra. |
| Hyperactivity | 31 | A | R (2.3) | Exploration, licking bars, circling contra. |
| Hyperactivity | 14 | B | R (1.7) | Exploration, circling contra. |
| Hyperactivity | 21 | B | L (1.7) | Exploration, circling contra. |
| Hyperactivity | 32 | B | L (1.7) | Exploration, searching for food, circling contra. |
| Hyperactivity | 19 | H | L (1.5) | Exploration, circling contra. |
| Hyperactivity | 27 | H | L (1.5) | Exploration, circling contra. |
Survey of behavioural effects observed in the homecage at selected site according to the type of effect produced in the primate chair. The main categories of behaviours observed are given for each site. The different behaviours are listed in order of importance. Circling contra. = circling towards the side contralateral to the injection site; tail exam. = tail examination; L = left; R = right.
Summary of observations made in the homecage
| Category of effect in chair . | Site . | Monkey . | Side (vol µl) . | Main behaviours in homecage . |
|---|---|---|---|---|
| Stereotypy | 29 | A | L (1.5) | Licking/biting fingers, searching for food, tail exam. |
| Stereotypy | 46 | A | L (1.5) | Licking/biting fingers, licking bars, searching for food |
| Stereotypy | 43 | B | L (1.7) | Licking bars, licking/biting fingers, searching for food |
| Stereotypy | 17 | H | R (1) | Licking bars, tail exam., searching for food |
| Stereotypy | 34 | H | L (1.5) | Licking/biting fingers, searching for food |
| Stereotypy and hyperactivity | 36 | H | R (1) | Exploration, licking/biting fingers, circling contra. |
| Hyperactivity and dyskinesia | 15 | A | R (1.5) | Exploration, dyskinesia, circling contra. |
| Hyperactivity | 31 | A | R (2.3) | Exploration, licking bars, circling contra. |
| Hyperactivity | 14 | B | R (1.7) | Exploration, circling contra. |
| Hyperactivity | 21 | B | L (1.7) | Exploration, circling contra. |
| Hyperactivity | 32 | B | L (1.7) | Exploration, searching for food, circling contra. |
| Hyperactivity | 19 | H | L (1.5) | Exploration, circling contra. |
| Hyperactivity | 27 | H | L (1.5) | Exploration, circling contra. |
| Category of effect in chair . | Site . | Monkey . | Side (vol µl) . | Main behaviours in homecage . |
|---|---|---|---|---|
| Stereotypy | 29 | A | L (1.5) | Licking/biting fingers, searching for food, tail exam. |
| Stereotypy | 46 | A | L (1.5) | Licking/biting fingers, licking bars, searching for food |
| Stereotypy | 43 | B | L (1.7) | Licking bars, licking/biting fingers, searching for food |
| Stereotypy | 17 | H | R (1) | Licking bars, tail exam., searching for food |
| Stereotypy | 34 | H | L (1.5) | Licking/biting fingers, searching for food |
| Stereotypy and hyperactivity | 36 | H | R (1) | Exploration, licking/biting fingers, circling contra. |
| Hyperactivity and dyskinesia | 15 | A | R (1.5) | Exploration, dyskinesia, circling contra. |
| Hyperactivity | 31 | A | R (2.3) | Exploration, licking bars, circling contra. |
| Hyperactivity | 14 | B | R (1.7) | Exploration, circling contra. |
| Hyperactivity | 21 | B | L (1.7) | Exploration, circling contra. |
| Hyperactivity | 32 | B | L (1.7) | Exploration, searching for food, circling contra. |
| Hyperactivity | 19 | H | L (1.5) | Exploration, circling contra. |
| Hyperactivity | 27 | H | L (1.5) | Exploration, circling contra. |
Survey of behavioural effects observed in the homecage at selected site according to the type of effect produced in the primate chair. The main categories of behaviours observed are given for each site. The different behaviours are listed in order of importance. Circling contra. = circling towards the side contralateral to the injection site; tail exam. = tail examination; L = left; R = right.
Effects of bicuculline microinjections
Spontaneous behaviours in the primate chair
The same three main categories of effects were observed in each of the three monkeys studied, as a function of the GPe territories into which the microinjections were made. The first type of effect, obtained for 12 microinjections (Fig. 2B and C: yellow circles; Table 1), was the production of movements that did not belong to the usual repertoire of these animals. Basically, two types of abnormal movements were observed. The most frequent (nine out of 12 injections) consisted of irregular and sustained flexion movements involving either one joint or two contiguous joints (type 1). Less frequently (four injections out of 12), the movements were more irregular and complex (type 2). They were characterized by internal and external rotation or flexion and extension movements involving an entire limb (three joints). The mean delay before onset of abnormal movements was 4.5 min (range 3–7) and the mean duration was 71 min (range 66–106). Movements occurred on the side contralateral to the microinjection site and involved the lower limb, the upper limb or both, without any clear somatotopy. The effective microinjection sites for the induction of abnormal movements (yellow circles, Fig. 2B and C) were restricted to the lateral and posterior part of the GPe.
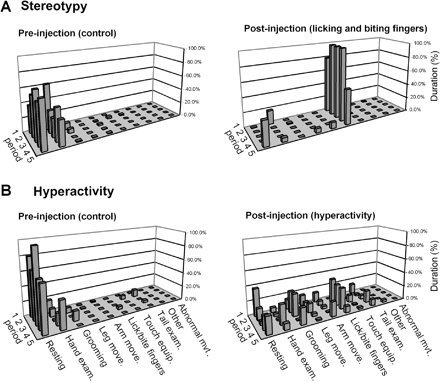
Two types of abnormal behaviours were obtained by microinjection in 37 other sites, mainly localized in the anterior parts of the GPe (Fig. 2 and Table 1). They were characterized by global perturbations of the natural behavioural organization of the animals in the absence of dyskinesia or by alteration of basic movement execution. One of these effects was the intense expression and persistent repetition of a particular behaviour belonging to the animal's usual repertoire, and which we referred to as stereotypy. An example of such a behavioural change is illustrated in Fig. 3A. Stereotypies mostly consisted of manipulating or licking a part of the body (often the tail or fingers). At the peak of symptomatic effect, the continuity and duration of these behaviours became clearly abnormal. All the monkeys could at such times produce behaviours that were never observed normally, such as biting their nails or tail. The same kinds of stereotypies were observed with all three monkeys when the microinjections were performed in comparable localizations within the ventro-medial part of the GPe (blue circles in Fig. 2B and C).
Examples of stereotypy (A) and behavioural hyperactivity (B) produced by bicuculline microinjections into the GPe in monkey A. Left panel: control period preceding the microinjection (five periods of 3 min) with normal behaviour, consisting of resting and examination of the hands. After a microinjection of bicuculline (site 46) into the limbic territory of the GPe (A, right panel), the monkey's behaviour was confined to two dominant behaviours: licking and biting the fingers (stereotypy). Following a bicuculline microinjection (site 33) into the associative territory of the GPe (B, right panel), behaviours were more variable, with rapid changes between the different types of behaviour (hyperactivity). Each line represents the different behaviours during an observation period of 3 min. Each behaviour is expressed as a percentage of this 3 min period devoted to this particular activity. Following these microinjections, the effect continued during the post-task period.
The other behavioural effect was a hyperactive state in which the animals expressed several behaviours from their normal repertoire, but with frequent changes, as illustrated in Fig. 3B. This abnormal behaviour involved arm as well as leg movements. The animals' activity was also directed at the closest parts of the experimental set-up, which they touched with their hands. Such abnormal behaviour was observed after microinjections in 17 sites localized in the dorsolateral part of the GPe (green circles in Fig. 2B and C).
Spontaneous behaviours in the homecage
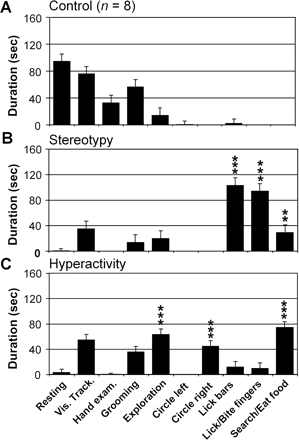
When effects were assessed in the homecage at sites that induced stereotypies, the expression of behaviours included abnormal repetition of licking or biting of the tail or fingers. An abnormal behaviour involving licking the cage bars, which was never observed outside the effect of the bicuculline microinjections, was also noted. In addition, compared with the control condition, the time spent searching for and eating food was increased (Fig. 4A and Table 2).
Examples of abnormal behaviours assessed in the homecage after bicuculline microinjections in monkey B. (A) Behavioural pattern in control conditions (n = 8). The monkey mainly displayed resting, visual tracking and grooming behaviours. (B) During expression of stereotypy after the microinjection into site 43, observation of the monkey in the homecage showed an increased expression of searching for or eating food behaviour and licking behaviour directed either at the environment (homecage bars) or at the monkey's own body (fingers). (C) During the expression of hyperactivity after the microinjection into site 32, exploration, contralateral circling and searching for and eating food were the main behaviours expressed. Each column of the histograms represents the duration of one behaviour over 12 consecutive periods of 5 min following a single microinjection. Control values obtained from eight control days were pooled (**P < 0.01; ***P < 0.001).
At sites that induced hyperactivity in the primate chair (Fig. 4B), the main behavioural change observed in the homecage consisted of an increase in exploratory activity, corresponding to the expression of hyperactivity involving the lower limb. The monkeys performed many vertical displacements between the floor and a platform in the upper half of the cage. In addition to these exploratory activities, the monkeys frequently touched the bars of the cage with their hands. Moreover, unidirectional rotation behaviour (circling) to the side contralateral to the microinjection could be observed during this period (Table 2).
In contrast, spontaneous behaviour in the homecage in control conditions for all the monkeys mostly consisted of visual tracking or resting on the platform and grooming for a short period (see example in Fig. 4A).
Perturbation of the simple food-retrieving task
This task was designed in order to study changes in the strategy regularly adopted by each monkey to pick up the food reward placed in the evenly spaced wells of the board. The animals were trained to use only the right hand to explore the right-hand side of the board, and vice versa. In the control condition, the animals always began by emptying the wells in the medial column on the preferred side (right in all three monkeys). The score for this task enabled us to objectivize changes in the spatial strategy and errors, such as returning to a previously emptied well.
Abnormal movements induced by microinjection of bicuculline did not disappear when this task had to be performed. The spatial organization of reward retrieval, and the low frequency of returns to empty wells and cross-over hand errors, were similar to the control condition when the lower limb was involved. Dyskinesia in the upper limb typically prevented task execution with the disabled arm, whereas the task could be performed with the opposite arm.
Stereotypies produced by microinjections disappeared when the monkeys had to perform the conditioned task, except for one microinjection (site 17) in monkey H, which induced stereotypies that were too severe for the task to be executed. For all the other microinjections, the task was carried out normally during the effect of bicuculline: the order of reward retrieval was not disrupted and the frequency of errors was at control levels (Fig. 5A and C).
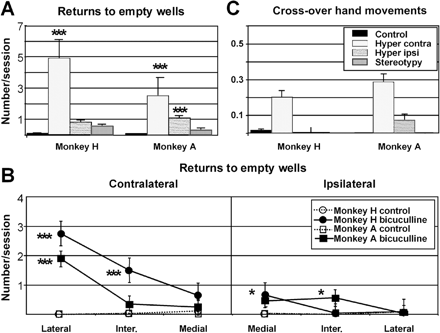
Errors on a simple choice task associated with hyperactivity. (A and B) Returns to empty wells; (C) cross-over hand movements in control conditions and after microinjections that induced hyperactivity. In A and C, the mean number of errors per session for the half-boards contralateral and ipsilateral to the microinjection site during hyperactivity are compared with the values obtained in control and stereotypy conditions. In B, the spatial distribution of returns to empty wells during hyperactivity is compared with the values obtained in control conditions (monkeys H and A are superimposed). There were significantly more returns to both contralateral (in the most lateral column) and ipsilateral (in the most medial column) empty wells during the hyperactivity effect. This effect was, however, greater on the contralateral side (A and B). In contrast, no effect was observed during microinjections inducing stereotypies. For both monkeys, the increase in cross-over hand movements was confined to the contralateral half-board. As there was no difference in the frequency of errors between the right and left sides for the control and stereotypy values, data from both sides were pooled. Results for monkey B, which had fewer microinjections than monkeys H and A, are not represented (*P < 0.05; **P < 0.01; ***P < 0.001).
In sharp contrast, for the microinjection sites where hyperactivity was produced, this hyperactivity was associated with perturbations in task execution, objectivized by a disorganization of the spatial strategy used to retrieve the rewards. After the microinjection, the wells located on the side contralateral to the microinjection were emptied from the lateral to the medial column (Fig. 6C and D). Then, the ipsilateral half-board was emptied from the medial to the lateral column. In some cases, during the peak effect, the monkeys completely ignored the pieces of food in the ipsilateral half-board (four microinjections out of five for monkey H, one out of seven for monkey A). Furthermore, the monkeys returned with abnormal frequency to empty wells, mainly in the lateral column of the half-board contralateral to the microinjection (Fig. 5A and B). In addition, hand movements were performed wrongly due to crossing of the medial part of the board in order to pick up the reward (Fig. 5C).
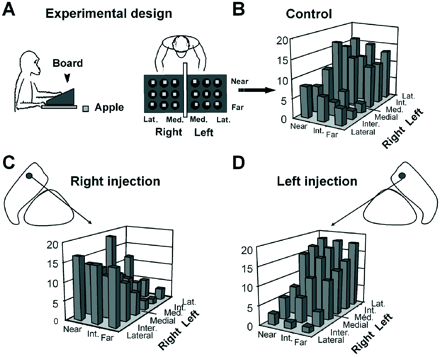
Effect of bicuculline on a simple food reward task. (A) Schematic representation of the behavioural task set-up showing the board with two separate groups of nine wells. (B) A normal spatial sequence of reward retrieval in monkey H with rewards being picked up from the medial to the lateral column, beginning on the right side and finishing on the left side. The level of each bar (low to high) indicates the chronological order in which the rewards were taken from the corresponding wells (mean of 30 control sessions). (C) Microinjection of bicuculline (site 18) into the right GPe in this monkey caused a shift in spatial strategy, the monkey starting at the far left side of the board, contralateral to the microinjection side. (D) Microinjections at symmetrical sites (site 19) into the left GPe induced opposite effects.
In each monkey, we also observed that some microinjection sites (n = 5) could perturb task execution in the absence of behavioural hyperactivity (Fig. 2).
Localization of microinjection sites
The localization of each microinjection site within the GPe was compared with the anatomical subdivision of the nucleus in the three functional territories (sensorimotor, associative or limbic) with reference to striatal inputs (Haber et al., 1993; François et al., 1994). The effective microinjection sites for the induction of dyskinesia (Fig. 1B and C: yellow circles) were located in the most posterior part of the GPe, i.e. in the posterior sensorimotor territory of the nucleus (Fig. 2A). Most of the sites that induced stereotypies were in the most anterior level of the GPe (plane AC in Fig. 2A), whereas the remainder were in the ventral part. The localization of microinjection sites was similar in the three monkeys (Fig. 2B and C). The spatial distribution of the microinjection sites eliciting stereotypies matched the limbic territory of the GPe as defined by its striatal afferent projections (Haber et al., 1993). The 17 microinjection sites inducing hyperactivity and spatial disorganization in the task were localized mostly in the dorsal part of the GPe and were within the associative territory (François et al., 1994) (Fig. 2). The five sites that induced perturbation in the task without behavioural hyperactivity were also located in the dorsal part of the GPe, but only in the posterior levels of the structure (Fig. 2).
Six microinjections induced the association of two kinds of effects: three microinjection sites, localized on the border between the sensorimotor and the associative or limbic territories, induced dyskinesia associated with behavioural disturbances; three other microinjection sites, localized in the anterior zone of the GPe on the border between the associative and limbic territories, produced an association of stereotypy and behavioural hyperactivity. For all these microinjections, however, the two different effects appeared with different post-injection latencies.
Microinjections outside the GPe and control microinjections
Saline microinjections into the GPe (n = 18) did not induce any discernible effects in any of the monkeys. The same volume of bicuculline as that used for the GPe was also injected into the striatum (n = 8) and into the GPi (n = 5), close to the GPe (Fig. 2B and C). No effects were observed with 12 control microinjections out of 13 (Fig. 2C). The only exception was in the striatum (yellow circle in Fig. 2C). This microinjection induced an abnormal movement different from that induced by microinjection into the nearest GPe site.
At selected sites (n = 6), more than one microinjection of bicuculline was performed (sites with two or three asterisks in Fig. 2B and C). Four of these sites, in which microinjections were performed twice in each of the three monkeys, reproducibly induced hyperactivity and attention deficit (monkey H, n = 2, sites 19 and 26; monkey A, n = 1, site 39; monkey B, n = 1, site 32). For the remaining two sites, the observed effects were stereotypies. They were expressed with the same amplitude as after the first microinjection (sites 45 and 46).
Evolution of spontaneous behaviour during control sessions
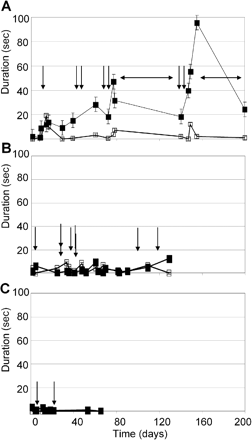
The behavioural pattern during control sessions mostly consisted of resting (monkeys H, A and B) and hand examination (monkey A). Throughout the experimental period, the frequency of behaviours involving stereotypies (including licking or biting fingers and tail examination) remained stable for two out of the three monkeys (monkeys A and B, Fig. 7B). For the third one (monkey H, Fig. 7C), the duration of the licking/biting fingers behaviour increased during control sessions performed close to the microinjection days that induced stereotypies. When experiments were interrupted, the level of this behaviour decreased. The levels observed in control sessions nevertheless remained below the levels obtained after microinjections. The level of tail examination, a behaviour that was also induced with stereotypies, was unaffected during the entire experimental period.
Evolution of spontaneous behaviour during control sessions over the entire experimental period. A, B and C show the time course of two kinds of behaviours observed during stereotypy effect, for monkeys H, A and B, respectively: licking/biting fingers (filled symbols) and tail examination (open symbols). Each value represents the mean duration of one behaviour over 6–12 three min periods during the post-injection and post-task periods. Abscissas show the time elapsed (in days) since the beginning of experimentation. Vertical arrows represent the moment of microinjections that induced stereotypies. (A) For monkey H, horizontal arrows show the two periods when experiments were interrupted. The level of expression of licking/biting fingers behaviour increased during control days close to microinjection days that induced stereotypy (filled symbols). The level of the other behaviour involved in the stereotypy effect (tail examination) remained stable throughout the entire period (open symbols). (B and C) For the other two monkeys (monkeys A and B), the expression of these behaviours was unchanged during the whole period.
Discussion
These results obtained in non-human primates show that, depending on the site of microinjection in the GPe, bicuculline can produce movement disorders or behavioural disorders, although in both cases the same alteration of neuronal activity was produced by the microinjection of this GABA antagonist. A reproducible relationship was found between the behaviours and the subdivisions of the GPe determined by the origin of their striatal inputs. Abnormal movements were observed exclusively after microinjections located in the posterior sensorimotor territory, whereas behavioural changes were induced by microinjections into the anterior part of the GPe. Stereotyped behaviours followed microinjections into the ventral part, corresponding to the limbic territory of the GPe; spatial perturbation in the task and hyperactivity were caused by microinjections into its dorsal part, corresponding to the associative territory.
Behavioural effects were induced by bicuculline in the GPe
Within the GPe, a small number of microinjections (n = 7) induced two distinct and successive effects among the three main categories described above. The first effect appeared after a short latency of <5 min, whereas the other occurred after a latency of ∼40 min. This temporal pattern suggests that bicuculline may diffuse within the GPe and act on functionally different neuronal populations. Consequently, the experimental design included several control microinjections into different structures close to the GPe: the GPi (n = 5) and the striatum (n = 8). An effect was observed with only one striatal site control microinjection and it differed from that induced by the nearest GPe site. It may thus be concluded that the three kinds of bicuculline effects observed in this study were related to a local action of the bicuculline in the GPe and not to diffusion to the GPi or striatum.
The mechanical effect of the cannula and/or the injected volume in the behavioural disturbances observed may have constituted another source of bias affecting our results. However, this possibility may be excluded due the absence of any behavioural effect after control procedures (with the cannula in situ but no fluid microinjection) and saline microinjections (same volume as that used for bicuculline microinjections). In addition, the presence of lesions induced by the repeated microinjections performed in each monkey was ruled out by the post-mortem histological analysis. Overall, the fact that the behavioural effects could be elicited reproducibly by two or three microinjections in the same site, together with the similarity of the effects observed in each of the three monkeys, provides strong evidence for the consistency of our behavioural results.
From movement to behavioural disorders
As previously described (Crossman et al., 1988; Matsumura et al., 1995), abnormal movements can be induced by bicuculline microinjections into the GPe and always occur on the side contralateral to the microinjection. Various kinds of abnormal movements have also been described in the monkey after GABAergic dysfunction of the sensorimotor circuit of the basal ganglia and thalamus: hemiballism in the subthalamic nucleus (Crossman et al., 1984), myoclonus in the putamen (Crossman et al., 1984, 1988) and dystonia in the GPi (Burbaud et al., 1998) or the ventral anterior part of the thalamus (Guehl et al., 2000; Macia et al., 2002). Overall, the final effectors of the abnormal movements produced by subcortical structures are mainly the motor and premotor cortices. GABAergic dysfunction at any level of this circuit including the cortex (Matsumura et al., 1991; Oishi et al., 1995) will result in a disruption of cortical activity, the type of effect depending on the GABAergic agent used (i.e. bicuculline or muscimol) and the structure primarily affected. These two main parameters (structure and pharmacological agent) may thus determine the various aspects of abnormal movements. Interestingly, the reproduction of normal premotor and motor cortical activity through prolonged direct electrical stimulation induces almost normal limb movements (Graziano et al., 2002).
The most striking result of our study was the induction, after bicuculline microinjections into the GPe, of a global disturbance of the monkey's behavioural organization without any alteration of basic control of movement. The pharmacological agent and the microinjection conditions were strictly identical to those that induced abnormal movements; only the localization of the microinjections sites differed.
Complex tics or compulsions
The first behavioural effect, defined as the intense and persistent repetition of a single behaviour, was referred to as stereotypy. This term was chosen with reference to the experimental literature relating to rats (Creese and Iversen, 1973; Joyce et al., 1983; Joyce and Iversen, 1984; Canales and Graybiel, 2000) and monkeys (Bryant et al., 1988) because it seemed appropriate to underline the repetitive character of this effect without making interpretative comparisons with human pathological behaviours.
The stereotypies that we observed clearly differed from the abnormal movements described above. The behavioural sequences were far more complex than those of dyskinesia and were in some respects similar to normal action observed in the animal's usual repertoire. After the microinjections, however, these sequences were clearly abnormal due to their repetition, duration and intensity, suggesting that they were not related to their usual context. Furthermore, in contrast to dyskinesia, stereotyped behaviours could be overcome, thereby allowing voluntary actions to be performed during the food retrieval task, which was carried out normally. Some of the microinjections performed in the anterior part of the GPe in the study by Matsumura et al. (1995) failed to induce dyskinesia but could also elicit abnormal behaviours, a finding that the authors did not include in their paper (Tremblay, personal communication).
In our study, stereotypies mainly appeared as highly repetitive licking or biting of fingers, behaviours that had never been observed before the first microinjection that induced them. There are at least two possible explanations for the expression of this abnormal behaviour. The bicuculline-induced dysfunction in these sites, localized in the limbic territory of the GPe, could induce the expression of stereotypies directly or indirectly through the induction of an anxious state. Two lines of evidence support the latter hypothesis. First, the most frequently produced stereotyped behaviour, namely biting of the fingers, frequently is related to an anxiogenic state in monkeys as well as in humans. Secondly, previous studies in monkeys have reported that anxiogenic environmental conditions (confinement and emotional deprivation) induced stereotypies (Bryant et al., 1988; Novak et al., 1998) associated with changes in basal ganglia architecture (Martin et al., 1991). When a monkey has been primed by the first microinjection effect inducing stereotypies, the experimental context may trigger an anxious state. At present, we have insufficient evidence to determine if stereotypies were directly or indirectly induced by the microinjection or related to the induction of an anxiogenic state, since our experimental design did not include anxiety markers. An important consideration in this respect is the risk of inducing a permanent state of anxiety in the monkey, an effect that would be contrary to the ethics of animal experimentation. On the other hand, these experiments are of importance since they could provide, through studies designed to characterize an anxiogenic state, a convincing argument for the implication of the basal ganglia in anxiety disorders, such as OCD.
An important aspect of our study is that stereotypies were induced in non-human primates and may thus be compared with repetitive pathological behaviours observed in human patients, including complex motor tics, compulsions and stereotypies. Tics are defined as sudden, repetitive, stereotyped movements that are misplaced in context. Complex tics, such as compulsions, are longer, more involved motor sequences that can easily be confused with goal-directed actions and sometimes include behaviours such as self-biting or touching (Leckman, 2002; Stein, 2002). Both of them tend to be more easily controlled when patients are involved in an attention-demanding task, as was the case with the stereotypies observed in our study. Compulsions are so close to complex motor tics that it may be difficult to distinguish between them reliably solely on the basis of their motor phenomenology (Leckman et al., 2001). They do, however, differ in terms of their respective psychic dimension: compulsions occur in response to an obsession and provide a release from the anxiety associated with this obsession (see Diagnostic and Statistical Manual of Mental Disorders, DSM IV). Tics are prompted by premonitory urges and are not associated with anxiety (Leckman et al., 1993). Human stereotypies are classically described as bizarre repetitive complex behavioural sequences observed mainly during schizophrenia or in children with mental retardation. As the psychic content underlying stereotyped behaviours remains hidden in animal studies, further attempts to classify the effects observed in our study with reference to human pathology would be speculative in the absence of any clear markers of anxiety. Our study may, however, provide important clues to the understanding of the repetitive aspect shared by all these abnormal behaviours.
In our study, stereotypies were correlated with a neuronal dysfunction restricted to the most anterior and ventral part of the GPe, which receives inputs mainly from the ventral limbic striatum and may thus be considered as the limbic territory of the GPe (Haber et al., 1993). Functional imaging studies in humans have suggested that the dysfunction of a neuronal network including the limbic part of the basal ganglia could explain these repetitive behaviours. The anatomic study (see accompanying paper by François et al., 2004) shows that the afferent and efferent connections of the microinjection sites that induced stereotypies match the neuronal network associated with OCD and TS (Castellanos, 2001; Peterson, 2001; Rauch et al., 2001). Our results, obtained in non-human primates, taken in conjunction with previous convergent experiments in rats (Canales and Graybiel, 2000; Taylor et al., 2002), provide experimental evidence to support the hypothesis of the implication of the limbic territories of the basal ganglia in human pathological repetitive behaviours (Graybiel and Rauch, 2000; Leckman and Riddle, 2000; Mink, 2001).
Attention disorder and/or hyperactivity
In the other abnormal behaviour, namely attention disorder and/or hyperactivity, the hallmark was the occurrence of a hyperactive state associated with an increased frequency of shifts from one behaviour to another. The multiple behaviours expressed in both contexts (chair and cage) involved the upper limbs (touching equipment or grooming) and the lower limbs (leg movements and exploration) and were always similar to those observed in the animal's normal repertoire. The fact that such hyperactivity was not observed during stereotypies or abnormal movements virtually rules out the possibility that it was due to a non-specific effect of the microinjections.
Interestingly, hyperactivity was in most cases associated with a set of specific effects on task performance that may reflect a spatial attention disturbance. First, we observed a deviation from the control spatial organization of reward retrieval towards a different pattern, which was identical for the three monkeys whatever their initial strategy. These perturbations seem to reflect a hyperattraction due to a positive attentional bias towards the contralateral space relative to the microinjection side, with a decreasing gradient from the most lateral part of the half-space contralateral to the microinjection side to the most lateral part of the ipsilateral half-space. These changes in task execution may be viewed as the exact opposite of the hemineglect observed in humans after hemispheric lesions, where the half-space contralateral to the lesion is neglected and there is a relative hyperattraction towards the ipsilateral side (Mesulam, 1999). As the effect induced by bicuculline microinjections was contralateral (Crossman et al., 1984, 1988; Matsumura et al., 1995), the alternative hypothesis of a primary neglect of the ipsilateral side seems less likely. Another aspect of task execution was the increased number of returns to empty wells. In line with the modification of spatial strategy, this error may also be considered as a clue to a hyperattraction towards behaviourally significant stimuli located in the half-board contralateral to the microinjection side. Alternatively, the perturbations during the task may have resulted from the motor hyperactivity of the hemibody contralateral to the microinjection side. However, the fact that cross-over hand movements were made more frequently with the ipsilateral arm and directed towards the half-board contralateral to the microinjection side, together with the observation that the monkeys specifically touched empty wells and no other part of the board, does not support this hypothesis. The possibility, for some microinjections, of inducing a perturbation of the task without inducing hyperactivity is further evidence for the independence of these behavioural effects.
Taken together, our results suggest that microinjections of bicuculline into the GPe could disrupt attentional processes, and underline the role of the GPe in attentional function. This interpretation is supported by the localization of the microinjection sites in the dorsal part of the GPe, which receives projections mainly from the associative striatal territories, which are specifically involved in attentional processes (Corbetta et al., 1991; Kermadi and Joseph, 1995; Miyashita et al., 1995; see also the accompanying paper by François et al., 2004).
The question of hyperactivity has been addressed in a few animal studies. As in the case of stereotypies, dopaminergic agents can induce locomotor hyperactivity in rodents (Davids et al., 2003). Some rodent models also display the association of an attention disorder and hyperactivity, with impaired sustained attention in specific tasks, impulsiveness and increased behavioural variability, sharing behavioural similarities with human ADHD (Sagvolden, 2000). However, the relationship between these symptoms and the neuronal correlates was not addressed in these models. Despite the absence of a specific paradigm to assess impulsivity, the hyperactivity observed in our study was similar to descriptions of spontaneously hypertensive rats and human ADHD. Moreover, our results emphasize the role of the associative and anterior sensorimotor circuits of the basal ganglia (see the accompanying paper by François et al., 2004) in the expression of this behavioural disorder, as previously suggested by functional MRI in humans (Vaidya et al., 1998; Bush et al., 1999; Rubia et al., 1999; Rubia, 2002).
Clues to the physiopathology of TS, ADHD and OCD
Interestingly, ADHD and OCD may exist as co-morbid conditions in TS. They share a common spectrum of symptoms (Robertson, 2000; Jankovic, 2001) and a similar neural basis, with strong evidence for a basal ganglia network dysfunction. Both animal studies and functional imaging results in humans have underlined the involvement of dopaminergic systems in repetitive behaviours (Iversen, 1973; Sahakian et al., 1975; Singer et al., 1991; Malison et al., 1995; Creese and Ernst et al., 1999a; Canales and Graybiel, 2000; Muller-Vahl et al., 2000; Peterson, 2001; Albin et al., 2003) and hyperactivity (Ernst et al., 1999b; Papa et al., 2000, 2002; Solanto, 2002). Our study has established a relationship between two dimensions of the TS symptomatic spectrum and a specific functional circuit of the basal ganglia: the expression of stereotyped behaviours involves the limbic network, whereas ADHD is related to an associative network. The fact that these two dimensions of abnormal behaviours are observed after dopaminergic (as previously described) and non-dopaminergic dysfunction (in our study) is not surprising: the dopaminergic system may be a regulatory system that modulates the expression of functional basal ganglia networks, which could be the effector systems for the production of hyperactive and compulsive behaviours. The involvement of both effector and modulatory systems in these structures was suggested previously by the observation that microinjections of bicuculline into the GPe (Matsumura et al., 1995) and increasing striatal dopamine (Filion et al., 1991) both induced dyskinesia in monkeys. It is generally accepted that modifications of dopaminergic activity in sensorimotor territories affect the expression of dyskinesia (DeLong, 1990). Modifications of dopaminergic activity in associative or limbic territories could affect the expression of stereotyped behaviours (compulsions) or attention deficit and hyperactivity, respectively.
Another important outcome of our study is to emphasize the role of the GPe in the production of involuntary or abnormal behaviours. This result is in line with current concepts about the function of this structure in motor control: as already suggested (Mink, 1996), the sensorimotor GPe acts as a selector to suppress unwanted movements during a voluntary action. On this basis, it has been suggested that a disruption of the neuronal activity in the associative and limbic GPe promotes the occurrence of unwanted behaviours in TS (Mink, 2001). Our results, obtained in the primate, provide the first experimental support for this hypothesis. However, further investigations will be necessary to elucidate the neuronal dysfunction and the cognitive mechanisms underlying these abnormal behaviours.
Conclusion
The similarity between the behaviours observed in our study and the spectrum of symptoms seen in human patients (Leckman, 2002) provides further experimental evidence for the implication of the basal ganglia networks in the pathophysiology of TS, ADHD and OCD, and suggests that the GPe may play a key role. In addition, our study suggests that the motor, cognitive and motivational/emotional disturbances observed in these pathologies could be produced by a single neuronal mechanism affecting specific functional networks within the basal ganglia. These results open the way to study the physiopathology of these behavioural disorders in a new primate model and to evaluate new treatments using pharmacological or neurosurgical (lesion or deep-brain stimulation) approaches.
We wish to thank Jérôme Yelnik and Dominique Tandé for expert participation in surgical operations and immunohistochemical procedures, Marie Vidailhet for her valuable advice, Nicholas Barton for checking the English, and Nicolas Wattiez for help with the behavioural analysis. This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM, France), Assistance Publique-Hôpitaux de Paris (AP-HP, France) and a grant from The Tourette Syndrome Association (USA).
References
Albin RL, Koeppe RA, Bohnen NI, Nichols TE, Meyer P, Wernette K, et al. Increased ventral striatal monoaminergic innervation in Tourette syndrome.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex.
Anderson ME, Turner RS. A quantitative analysis of pallidal discharge during targeted reaching movement in the monkey.
Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man.
Brotchie P, Iansek R, Horne MK. Motor function of the monkey globus pallidus. 1. Neuronal discharge and parameters of movement.
Brotchie P, Iansek R, Horne MK. Motor function of the monkey globus pallidus. 2. Cognitive aspects of movement and phasic neuronal activity.
Bryant CE, Rupniak NM, Iversen SD. Effects of different environmental enrichment devices on cage stereotypies and autoaggression in captive cynomolgus monkeys.
Burbaud P, Bonnet B, Guehl D, Lagueny A, Bioulac B. Movement disorders induced by gamma-aminobutyric agonist and antagonist injections into the internal globus pallidus and substantia nigra pars reticulata of the monkey.
Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop.
Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy.
Castellanos FX. Neural substrates of attention-deficit hyperactivity disorder.
Corbetta A, Jankovic M, Conter V, Fuga T, Crivellaro M, Masera G. Parenchymal brain leukemia. Case report: problems related to the diagnosis and treatment.
Creese I, Iversen SD. Blockage of amphetamine induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine.
Crossman AR, Sambrook MA, Jackson A. Experimental hemichorea/hemiballismus in the monkey. Studies on the intracerebral site of action in a drug-induced dyskinesia.
Crossman AR, Mitchell IJ, Sambrook MA, Jackson A. Chorea and myoclonus in the monkey induced by gamma-aminobutyric acid antagonism in the lentiform complex. The site of drug action and a hypothesis for the neural mechanisms of chorea.
Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder.
DeLong MR. Primate models of movement disorders of basal ganglia origin.
Demirkol A, Erdem H, Inan L, Yigit A, Guney M. Bilateral globus pallidus lesions in a patient with Tourette syndrome and related disorders.
Ernst M, Zametkin AJ, Jons PH, Matochik JA, Pascualvaca D, Cohen RM. High presynaptic dopaminergic activity in children with Tourette's disorder.
Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder.
Filion M, Tremblay L, Bedard PJ. Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism.
François C, Grabli D, McCairn K, Jan C, Karachi C, Hirsch EC, Féger J, Trembley L. Behavioural disorders induced by external globus pallidus dysfunction in primates II. Anatomical study.
François C, Yelnik J, Percheron G, Fenelon G. Topographic distribution of the axonal endings from the sensorimotor and associative striatum in the macaque pallidum and substantia nigra.
Graybiel AM, Rauch SL. Toward a neurobiology of obsessive–compulsive disorder.
Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex.
Guehl D, Burbaud P, Boraud T, Bioulac B. Bicuculline injections into the rostral and caudal motor thalamus of the monkey induce different types of dystonia.
Haber SN, Lynd-Balta E, Mitchell SJ. The organization of the descending ventral pallidal projections in the monkey.
Habib M, Poncet M. [Loss of vitality, of interest and of the affect (athymhormia syndrome) in lacunar lesions of the corpus striatum]. [French].
Hamada I, DeLong MR, Mano N. Activity of identified wrist-related pallidal neurons during step and ramp wrist movements in the monkey.
Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning.
Inase M, Buford JA, Anderson ME. Changes in the control of arm position, movement, and thalamic discharge during local inactivation in the globus pallidus of the monkey.
Joyce EM, Iversen SD. Dissociable effects of 6-OHDA-induced lesions of neostriatum on anorexia, locomotor activity and stereotypy: the role of behavioural competition.
Joyce EM, Stinus L, Iversen SD. Effect of injections of 6-OHDA into either nucleus accumbens septi or frontal cortex on spontaneous and drug-induced activity.
Kermadi I, Joseph JP. Activity in the caudate nucleus of monkey during spatial sequencing.
Laplane D, Widlocher D, Pillon B, Baulac M, Binoux F. [Obsessional-type compulsive behavior caused by bilateral circumscribed pallidostriatal necrosis. Encephalopathy caused by a wasp sting]. [French].
Laplane D, Baulac M, Pillon B, Panayotopoulou-Achimastos I. [Loss of psychic self-activation. Compulsive activity of obsessional type. Bilateral lenticular lesion (author's translation)]. [French].
Laplane D, Baulac M, Widlocher D, Dubois B. Pure psychic akinesia with bilateral lesions of basal ganglia.
Laplane D, Levasseur M, Pillon B, Dubois B, Baulac M, Mazoyer B, et al. Obsessive–compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study.
Leckman JF, Peterson BS, King RA, Scahill L, Cohen DJ. Phenomenology of tics and natural history of tic disorders.
Leckman JF, Riddle MA. Tourette's syndrome: when habit-forming systems form habits of their own?
Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome.
Macia F, Escola L, Guehl D, Michelet T, Bioulac B, Burbaud P. Neuronal activity in the monkey motor thalamus during bicuculline-induced dystonia.
Malison RT, McDougle CJ, van Dyck CH, Scahill L, Baldwin RM, Seibyl JP, et al. [123I]beta-CIT SPECT imaging of striatal dopamine transporter binding in Tourette's disorder.
Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions.
Matsumura M, Sawaguchi T, Oishi T, Ueki K, Kubota K. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex.
Matsumura M, Tremblay L, Richard H, Filion M. Activity of pallidal neurons in the monkey during dyskinesia induced by injection of bicuculline in the external pallidum.
Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions.
Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events.
Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs.
Mink JW. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis.
Mink JW, Thach WT. Basal ganglia motor control. III. Pallidal ablation: normal reaction time, muscle cocontraction, and slow movement.
Miyashita N, Hikosaka O, Kato M. Visual hemineglect induced by unilateral striatal dopamine deficiency in monkeys.
Muller-Vahl KR, Berding G, Brucke T, Kolbe H, Meyer GJ, Hundeshagen H, et al. Dopamine transporter binding in Gilles de la Tourette syndrome.
Novak MA, Kinsey JH, Jorgensen MJ, Hazen TJ. Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys.
Oishi T, Mikami A, Kubota K. Local injection of bicuculline into area 8 and area 6 of the rhesus monkey induces deficits in performance of a visual discrimination GO/NO–GO task.
Papa M, Sellitti S, Sadile AG. Remodeling of neural networks in the anterior forebrain of an animal model of hyperactivity and attention deficits as monitored by molecular imaging probes.
Papa M, Diewald L, Carey MP, Esposito FJ, Gironi Carnevale UA, Sadile AG. A rostro-caudal dissociation in the dorsal and ventral striatum of the juvenile SHR suggests an anterior hypo- and a posterior hyperfunctioning mesocorticolimbic system.
Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia–thalamo-cortical loop.
Peterson BS. Neuroimaging studies of Tourette syndrome: a decade of progress.
Rauch SL, Whalen PJ, Curran T, Shin LM, Coffey BJ, Savage CR, et al. Probing striato-thalamic function in obsessive–compulsive disorder and Tourette syndrome using neuroimaging methods.
Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment.
Rubia K. The dynamic approach to neurodevelopmental psychiatric disorders: use of fMRI combined with neuropsychology to elucidate the dynamics of psychiatric disorders, exemplified in ADHD and schizophrenia.
Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI.
Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD).
Sahakian BJ, Robbins TW, Morgan MJ, Iversen SD. The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats.
Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward.
Singer HS, Hahn IH, Moran TH. Abnormal dopamine uptake sites in postmortem striatum from patients with Tourette's syndrome.
Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research.
Taylor JR, Morshed SA, Parveen S, Mercadante MT, Scahill L, Peterson BS, et al. An animal model of Tourette's syndrome.
Tremblay L, Grabli D, McCairn K, Jan C, Hirsch E, Féger J, et al. A monkey model of Tourette's syndrome: induction of hyperactivity disorder with attention deficit (HD/AD) and stereotypy by microinjections of bicuculline in the external segment of globus pallidus. Soc Neurosci Abstr
Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study.
Wenger KK, Musch KL, Mink JW. Impaired reaching and grasping after focal inactivation of globus pallidus pars interna in the monkey.