-
PDF
- Split View
-
Views
-
Cite
Cite
Chantal François, David Grabli, Kevin McCairn, Caroline Jan, Carine Karachi, Etienne-C. Hirsch, Jean Féger, Léon Tremblay, Behavioural disorders induced by external globus pallidus dysfunction in primates II. Anatomical study, Brain, Volume 127, Issue 9, September 2004, Pages 2055–2070, https://doi.org/10.1093/brain/awh239
Close - Share Icon Share
Abstract
The anatomical organization of the basal ganglia supports their involvement in movement and behavioural disorders. Thus dyskinesia, attention deficit with or without hyperactivity, and stereotyped behaviour can be induced by microinjections of bicuculline, a GABAergic antagonist, into different parts of the external globus pallidus (GPe) in monkeys. The aim of the present study was to determine the anatomo-functional circuits inside the basal ganglia which are specifically related to each of these behavioural changes. For that, axonal tracers were injected in the same pallidal sites where abnormal behaviours have previously been obtained by bicuculline microinjections. The labelling was mapped in the different basal ganglia and matched with the topography of the cortico-striato-pallidal projections already reported in the literature and with the distribution of calbindin immunoreactivity. Our results first show that the pallidal sites related to dyskinesia, attention deficit with or without hyperactivity, and stereotyped behaviour, were respectively in motor, associative and limbic territories, defined as weak, moderate and intensive calbindin immunoreactivity. The same relationship was observed between the distribution of the labelling in the different basal ganglia after tracer injections performed in these different pallidal sites and the anatomo-functional territories. Thus regarding the origin of the circuits within the striatum, tracer injections performed in the dyskinesia site labelled neurons located in the posterior sensorimotor putamen, those performed in the hyperactivity and/or attention deficit labelled neurons in the laterodorsal putamen and caudate nucleus, regions corresponding to associative and anterior motor territories, while those performed in the stereotyped behaviour site labelled neurons in the ventral limbic striatum. Regarding the GPe output on the basal ganglia, the different circuits also appeared underlined by different anatomo-functional territories, even if a partial overlap exists. Each of these anatomical circuits systematically involves both the internal globus pallidus (GPi) and the substantia nigra pars reticulata (SNr) but, whereas movement circuit is mainly related to the GPi, stereotyped behaviour is mainly related to the SNr. Additionally, subregions of the subthalamic nucleus were also systematically involved, depending on the movement or behavioural disorder produced. These results demonstrate that distinct circuits involving different anatomo-functional territories of the basal ganglia, with partial overlap, participate in different behavioural disorders in monkeys. It seems likely that these neuronal circuits are involved in pathologies like Tourette's syndrome, attention deficit/hyperactivity disorders and obsessional compulsive troubles. This study provides the basis for further researches with a therapeutical viewpoint.
Introduction
It is well established that the origin and distribution of corticostriatal inputs allow us to subdivide the striatum into three anatomo-functional territories. They are referred to respectively as (i) sensorimotor territory processing sensorial and motor information; (ii) associative territory processing cognitive information; and (iii) limbic territory processing emotional and motivational information (Alexander et al., 1986; Parent and Hazrati, 1995b; Haber et al., 2000). These anatomo-functional territories occupy distinct regions without clear-cut boundaries between each of them (Parent and Hazrati, 1995a; Haber et al., 2000). This partition in three territories is maintained within the basal ganglia, especially in the external globus pallidus (GPe) regarding the distribution of the striatopallidal projections (Parent et al., 1984; Smith and Parent, 1986; Haber et al., 1990; Saint-Cyr et al., 1990; Hedreen and DeLong, 1991; François et al., 1994a). Such anatomical organization allows us to speculate that activities of some cortical area will affect the neuronal activity in the corresponding striatal and pallidal territories.
Functional imaging studies (PET or functional MRI) in humans suggest that abnormal activities in different parts of the cortex and basal ganglia are present in various behavioural disorders. It was reported that patients expressing attention deficit/hyperactivity disorder (ADHD) have a reduced metabolism in the prefrontal cortex and in the associative part of the striatum (Castellanos et al., 1996; Filipek et al., 1997; Rubia et al., 1999; Castellanos, 2001) and in the pallidum as a unit (Aylward et al., 1996; Castellanos et al., 1996). Tourette's syndrome which is characterized by motor (tics) and behavioural disorders such as obsessive-compulsive disorder and ADHD (for a review, see Jankovic, 2001) is marked by abnormalities in various parts of the cortex, striatum and thalamus (Scarone et al., 1992; Peterson et al., 1993; Singer et al., 1993; Hyde et al., 1995; Eidelberg et al., 1997; Moriarty et al., 1997; Peterson, 2001). Thus in these various behavioural disorders, the dysfunction seems limited to the cortex and striatum, whereas the involvement of the other components of the basal ganglia is not obviously mentioned. Only one clinical study reported cases of obsessive-compulsive disorder with an emphasis on the involvement of a subcortical structure other than the striatum, since these troubles are attributed to a bilateral lesion restricted to the pallidum (Laplane et al., 1984, 1989).
Generally, the functional imaging approach has been, until now, without sufficient resolution to distinguish small anatomical structures or subterritories. Moreover, this approach shows the structures where abnormal changes in activity occur, but it does not allow us to determine which of them is at the origin of such changes. In a complementary way, a pharmacological approach using microinjection of a suitable pharmacological agent in animals allows us to induce a local disturbance of the neuronal activity within a given structure. Then, it can be stated that a functional relationship of causality exists between this part of the structure where a dysfunction is induced and the expression of abnormal movements or behavioural disorders. Indeed, we previously observed in monkeys that microinjections of bicuculline, an antagonist of GABAergic receptor, provoked abnormal movement (dyskinesia) and behavioural disorders (hyperactivity, attention deficit or stereotypy) dependent on the injection site into the anatomo-functional part of the GPe (see Grabli et al., 2004).
The aim of the present study was to determine the anatomical relationships between the origin of the inputs and the distribution of the output pathways which are related to the neuronal population localized in the GPe where microinjections of bicuculline have been able to induce abnormal movements or behavioural disorders in monkeys. To circumvent this question, injections of axonal tracers were performed in different sites of the GPe where the most characteristic effects have been obtained. This approach allowed us to determine which anatomo-functional circuit is implicated in the different behavioural disorders. The axonal tracers used ensured retrograde tracing in the striatum, and thus opened the way to correlate our results with the numerous data in the literature concerning the corticostriatal projection and functional MRI results in human pathology. The anterograde transport of tracers allowed us to compare the detailed patterns of axonal ending distribution at both the output structures of the basal ganglia represented by the internal globus pallidus (GPi) and the pars reticulata of the substantia nigra (SNr). We also focused our attention on the subthalamic nucleus (STN), another output structure of the GPe. The delineation of anatomo-functional territories in the GPe, as well as in different basal ganglia structures, has been performed according to the distribution of calbindin (Cb) immunohistochemistry, which was shown to be correlated with the delineation of anatomo-functional territories in the striatopallidal complex (François et al., 1994b; Karachi et al., 2002). More generally, the delineation of anatomo-functional territories was compared with afferent territories already published in the literature using a tract-tracing method. These results have been previously reported in an abstract form (François et al., 2002) and are completed by their behavioural counterparts in Grabli et al. (2004).
Materials and methods
Subjects
Three adult male African green monkeys (Cercopithecus aethiops; H, A and B) were used in both behavioural and anatomical studies and have been supplemented with the anatomical observations performed on one macaque (Macaca mulatta MM33). They weighed between 5 and 7 kg and were aged between 5 and 10 years, as determined by their age at entry in captivity, dentition and hair appearance. All studies were carried out in accordance with European Communities Council Directive of 1986 (86/609/EEC). The animals were kept under standard conditions (12-h light cycles, 23°C and 50% humidity).
Microinjections of bicuculline and behavioural analysis
The behavioural study described in Grabli et al. (2004) was conducted to assess the effects of microinjection of bicuculline into the different functional territories of the GPe. Briefly, to perform these microinjections and finally the injections of tracers, a stainless steel chamber containing a perforated grid was first positioned above the animal's dura. For that, monkeys were first tranquillized with ketamine hydrochloride (10 mg/kg), then anaesthetized through intratracheal intubation (fluothane 1%, nitrogen protoxide 50% and oxygen 50%). The chamber was positioned with reference to the anterior and posterior commissures (AC and PC, respectively) visualized by ventriculography (Percheron et al., 1986). Since the chamber was fixed on the skull with reference to the ventricular system of coordinates, this device allowed us to perform all microinjections in reference to the stereotaxic coordinates of the various parts of the GPe (for more precise description of the experimental set-up device, see Grabli et al., 2004).
In a first step, abnormal movements and behavioural disorders were induced by microinjections of bicuculline in several parts of the GPe in the two hemispheres of each monkey. Then microinjection sites related to the most typical changes were selected for this anatomical study.
Injections of axonal tracers
Injections of axonal tracers were performed in each GPe of the three African green monkeys studied when all behavioural studies were achieved. They consisted of 0.1–0.2 µl of wheat germ agglutinin conjugated to horseradish peroxidase (WGA-HRP; Sigma, St Louis, MO, USA) 10% in 0.1 M phosphate buffered saline (PBS, 0.01 M, pH 7.4), or of 0.5–0.8 µl of biotin dextran amine (BDA) diluted (10%) in PBS (0.01 M, pH 7.4) injected using the same specific device used for bicuculline micro-injection. Moreover, in order to compensate the weakness of injection in one site (site no. 37 in monkey H), BDA was also injected in the GPe of the experimental case MM33 which was not involved in the behavioural studies. In that case, the injection site was exactly at the same coordinates as those of monkey H.
Three days (for HRP-WGA tracer) and 10 days (for BDA tracer) after injections, the animals were deeply anaesthetized and replaced in the stereotaxic frame as described above. Two vertical and two horizontal metal rods (one in each hemisphere) were introduced into the brain perpendicular and parallel, respectively, to the plane passing through the two ventricular landmarks. These landmarks allowed us to define two ventricular planes, transverse and horizontal, in order to analyse the brains post-mortem according to the stereotaxic landmarks. The monkeys were then perfused transcardially with 400 ml of saline (0.9% at 37°C) followed by 5 l of 4% paraformaldehyde (in 0.1 M PBS, pH 7.4 at 4°C) and 1 l of PBS with 5% sucrose. The brains were removed from the skull, rinsed in PBS complemented with 10% sucrose for 1 day and 20% sucrose for 1 day, then frozen and cut into 50-µm-thick sections transversely with reference to the ventricular anterior and posterior commissures on a freezing microtome.
Histological processing
On all monkeys, Cb was revealed on regularly interspaced (one in five) free-floating sections using a monoclonal antiserum raised against Cb (Sigma) diluted to 1 : 1.000 for 48 h at 4°C under gentle agitation. The primary antibodies were revealed using the ABC method (diluted 1 : 100; Vector Laboratories, Burlingame, CA, USA) using diaminobenzidine tetrahydrochloride (DAB; 0.05%) as peroxidase chromogen.
For the revelation of BDA, all sections were pretreated with 1% Triton X-100 in PBS, then incubated using avidin–biotin complex staining (ABC, Elite, Vector Laboratories) in PBS with 1% Triton for 48 h at 4°C. Sections were treated with nickel (0.2%)-DAB (0.05%) as peroxidase chromagen. Cell bodies could easily be visualized in our material as biocytin occurs in trace in eukaryotic organisms (Smith, 1992). WGA-HRP was revealed using the method previously described by Mesulam (1978). Briefly, sections were abundantly rinsed in 0.1 M PBS, then in acetate buffer. Preincubation was performed in 0.1% nitroferricyanure (Sigma) and 0.005% 3,3-5,5 tetramethyl-benzidine (Sigma) solution for 15 min, and hydrogen peroxide was added at a concentration of 0.02–0.04% for about 15 min. Sections were then counterstained with green methyl solution (0.25%).
Cartographic methods
Contours of cerebral structures were traced under the microscope with the aid of an XY plotter connected to the stage of the microscope. The anteroposterior position of each section was given by the anteriority of each section along the AC–PC axis, taking AC as the origin of the system of axes. The dorsoventral axis was given by the holes corresponding to the horizontal rods. The mediolateral axis was perpendicular to the dorsoventral axis. All sections were thus transformed into maps drawn in relation to the AC–PC system of coordinates, and the contours of structures mapped in different monkeys could be directly compared. The limits of anatomo-functional territories shown in published figures for the striatopallidal projection (Parent et al., 1984; Smith and Parent, 1986; Haber et al., 1990; Saint-Cyr et al., 1990; Hedreen and DeLong, 1991; Hazrati and Parent, 1992; Flaherty and Graybiel, 1994; François et al., 1994a) were transferred onto the corresponding cerebral maps of our series drawn from Cb material.
All cartographic data obtained from the left hemisphere of monkeys were transferred to the right hemisphere for an easier comparison. The illustrations were scanned into a computer from camera lucida drawings or charts and finished using Adobe Photoshop or Microsoft PowerPoint software.
Results
Overview of abnormal movement and behavioural disorders induced by bicuculline microinjections into the GPe
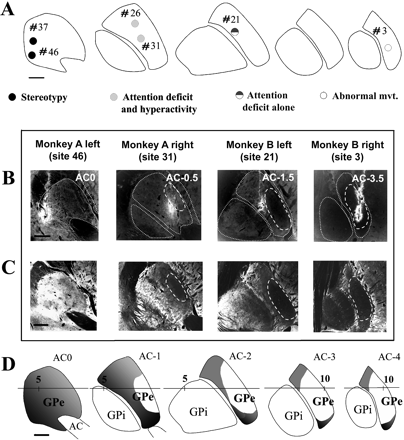
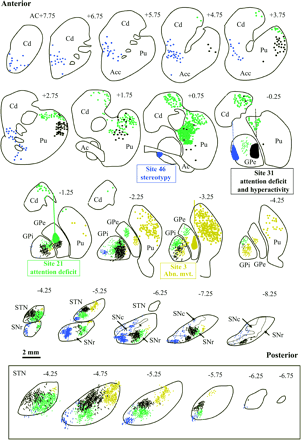
In the three monkeys studied, microinjections of bicuculline performed into various parts of the GPe induced three different types of behavioural disorders. Thus injections in the posterior and lateral part of the GPe induced abnormal movement referred to as dyskinesia, those performed in the antero-dorsal part of the GPe produced hyperactivity and/or attention deficit, while stereotyped behaviours where obtained after injections in the anterior and medioventral portion of the GPe. For the present study, different sites of bicuculline microinjections that produced the most characteristic behavioural changes were chosen to perform injections of axonal tracer. The localization of these microinjection sites is illustrated in Fig. 1A, and their behavioural effects induced in the different monkeys are summarized in Table 1.
Series of anteroposterior sequences of regularly interspaced transverse sections located with reference to the anterior commissural reference point (AC) and centred on the pallidum. (A) Localization of sites of bicuculline microinjections within the GPe that produced the most characteristic behavioural changes and that were chosen to perform injections of axonal tracer. These pallidal sites elicited either stereotyped behaviour, or hyperactivity with attention deficit, or attention deficit alone, or abnormal movements. (B) Dark-field photomicrographs of axonal tracer injections into four different cases (monkey A, left and right hemisphere, monkey B left and right hemisphere). (C) Dark-field photomicrographs of Cb-immunoreactive material. The distribution of immunoreactivity was directly compared with the delineation of the functional territories of the striatopallidal complex (D) obtained by superimposition of data from tracing studies in the literature (Parent et al., 1984; Smith and Parent, 1986; Haber et al., 1990; Saint-Cyr et al., 1990; Hedreen and DeLong, 1991; Hazrati and Parent, 1992; Flaherty and Graybiel, 1994; François et al., 1994a) onto the same anteroposterior sequence of transverse sections. The sensorimotor territory is represented in white, the associative territory in grey and the limbic territory in black. AC = anterior commissure; GPe = external globus pallidus; GPi = internal globus pallidus.
Bicuculline effects at tracer injection sites compared with the baseline behavioural profile for each monkey
| M . | Condition . | Ab Mvt . | Behaviours (mean duration: s/3 min) . | . | . | . | . | . | . | . | Task . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | . | |||||||
| B | Control | 0 | 168 ± 2 | 1 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 1 ± 0.4 | 0 ± 0.1 | 0 ± 0.0 | N | |||||||
| Ab Mvt (3) | Leg | 156 ± 4 | 1 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | 1 ± 1.0 | 0 ± 0.0 | 0 ± 0.0 | N | ||||||||
| AD (21) | 0 | 171 ± 1 | 0 ± 0.0 | 0.3 ± 0.2 | 1.5 ± 0.6 | 0.8 ± 0.5 | 1 ± 0.5 | 0 ± 0.0 | 0 ± 0.0 | SP | ||||||||
| A | Control | 0 | 94 ± 4 | 42 ± 2.3 | 6.0 ± 1.0 | 0.5 ± 0.1 | 0.9 ± 0.1 | 3 ± 0.8 | 9 ± 1.4 | 4 ± 0.7 | N | |||||||
| AD&Hyp (31) | 0 | 35 ± 9↓↓ | 55 ± 11.3↑ | 0.2 ± 0.2 | 7.0 ± 2.3↑↑↑ | 8.0 ± 6.5↑↑ | 5 ± 5.0 | 21 ± 11.3↑↑ | 11 ± 6.0 | SP | ||||||||
| Stereo (46) | 0 | 12 ± 11↓↓↓ | 8 ± 6.0↓↓ | 0.6 ± 0.6 | 2.0 ± 2.0 | 3.0 ± 3.0 | 152 ± 18.0↑↑↑ | 0 ± 0.0 | 0 ± 0.0 | N | ||||||||
| H | Control | 0 | 119 ± 8 | 4 ± 0.9 | 3.0 ± 1.0 | 5.0 ± 1.2 | 0.3 ± 0.1 | 35 ± 5.6 | 2 ± 1.0 | 5 ± 1.7 | N | |||||||
| AD&Hyp (26) | 0 | 110 ± 23 | 0 ± 0.0 | 0.0 ± 0.0 | 8.0 ± 2.4↑ | 0.0 ± 0.0 | 43 ± 15.6 | 0 ± 0.1 | 15 ± 8.0↑ | SP | ||||||||
| Stereo (37) | 0 | 32 ± 14↓ | 4 ± 2.0 | 0.0 ± 0.0 | 0.6 ± 0.4↓ | 0.0 ± 0.0 | 104 ± 19.5↑↑↑ | 0 ± 0.0 | 28 ± 10.8↑ | N | ||||||||
| M . | Condition . | Ab Mvt . | Behaviours (mean duration: s/3 min) . | . | . | . | . | . | . | . | Task . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | . | |||||||
| B | Control | 0 | 168 ± 2 | 1 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 1 ± 0.4 | 0 ± 0.1 | 0 ± 0.0 | N | |||||||
| Ab Mvt (3) | Leg | 156 ± 4 | 1 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | 1 ± 1.0 | 0 ± 0.0 | 0 ± 0.0 | N | ||||||||
| AD (21) | 0 | 171 ± 1 | 0 ± 0.0 | 0.3 ± 0.2 | 1.5 ± 0.6 | 0.8 ± 0.5 | 1 ± 0.5 | 0 ± 0.0 | 0 ± 0.0 | SP | ||||||||
| A | Control | 0 | 94 ± 4 | 42 ± 2.3 | 6.0 ± 1.0 | 0.5 ± 0.1 | 0.9 ± 0.1 | 3 ± 0.8 | 9 ± 1.4 | 4 ± 0.7 | N | |||||||
| AD&Hyp (31) | 0 | 35 ± 9↓↓ | 55 ± 11.3↑ | 0.2 ± 0.2 | 7.0 ± 2.3↑↑↑ | 8.0 ± 6.5↑↑ | 5 ± 5.0 | 21 ± 11.3↑↑ | 11 ± 6.0 | SP | ||||||||
| Stereo (46) | 0 | 12 ± 11↓↓↓ | 8 ± 6.0↓↓ | 0.6 ± 0.6 | 2.0 ± 2.0 | 3.0 ± 3.0 | 152 ± 18.0↑↑↑ | 0 ± 0.0 | 0 ± 0.0 | N | ||||||||
| H | Control | 0 | 119 ± 8 | 4 ± 0.9 | 3.0 ± 1.0 | 5.0 ± 1.2 | 0.3 ± 0.1 | 35 ± 5.6 | 2 ± 1.0 | 5 ± 1.7 | N | |||||||
| AD&Hyp (26) | 0 | 110 ± 23 | 0 ± 0.0 | 0.0 ± 0.0 | 8.0 ± 2.4↑ | 0.0 ± 0.0 | 43 ± 15.6 | 0 ± 0.1 | 15 ± 8.0↑ | SP | ||||||||
| Stereo (37) | 0 | 32 ± 14↓ | 4 ± 2.0 | 0.0 ± 0.0 | 0.6 ± 0.4↓ | 0.0 ± 0.0 | 104 ± 19.5↑↑↑ | 0 ± 0.0 | 28 ± 10.8↑ | N | ||||||||
The mean duration (± SEM) of each behaviour induced by bicuculline injection was obtained from five measurements of 3-min periods during the post-injection period. For control sessions, values were obtained during the corresponding post-injection period and pooled from several sessions (Monkey B: n = 14; monkey A: n = 19; monkey H: n = 5). Statistical analysis between control and injections was done using a two-way ANOVA followed by appropriate post hoc comparisons. Significant modifications for each injection effect are illustrated in bold (↓ ↑ SP Leg). (↑↑↑: P < 0.001; ↑↑: P < 0.01 and ↑: P < 0.05). M = monkey; Ab Mvt = abnormal movement; N = normal; SP = spatial perturbation. Behaviours: (1) resting; (2) hand examination; (3) grooming; (4) leg movements; (5) arm movements; (6) licking/biting fingers; (7) touching equipment; (8) tail examination.
Bicuculline effects at tracer injection sites compared with the baseline behavioural profile for each monkey
| M . | Condition . | Ab Mvt . | Behaviours (mean duration: s/3 min) . | . | . | . | . | . | . | . | Task . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | . | |||||||
| B | Control | 0 | 168 ± 2 | 1 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 1 ± 0.4 | 0 ± 0.1 | 0 ± 0.0 | N | |||||||
| Ab Mvt (3) | Leg | 156 ± 4 | 1 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | 1 ± 1.0 | 0 ± 0.0 | 0 ± 0.0 | N | ||||||||
| AD (21) | 0 | 171 ± 1 | 0 ± 0.0 | 0.3 ± 0.2 | 1.5 ± 0.6 | 0.8 ± 0.5 | 1 ± 0.5 | 0 ± 0.0 | 0 ± 0.0 | SP | ||||||||
| A | Control | 0 | 94 ± 4 | 42 ± 2.3 | 6.0 ± 1.0 | 0.5 ± 0.1 | 0.9 ± 0.1 | 3 ± 0.8 | 9 ± 1.4 | 4 ± 0.7 | N | |||||||
| AD&Hyp (31) | 0 | 35 ± 9↓↓ | 55 ± 11.3↑ | 0.2 ± 0.2 | 7.0 ± 2.3↑↑↑ | 8.0 ± 6.5↑↑ | 5 ± 5.0 | 21 ± 11.3↑↑ | 11 ± 6.0 | SP | ||||||||
| Stereo (46) | 0 | 12 ± 11↓↓↓ | 8 ± 6.0↓↓ | 0.6 ± 0.6 | 2.0 ± 2.0 | 3.0 ± 3.0 | 152 ± 18.0↑↑↑ | 0 ± 0.0 | 0 ± 0.0 | N | ||||||||
| H | Control | 0 | 119 ± 8 | 4 ± 0.9 | 3.0 ± 1.0 | 5.0 ± 1.2 | 0.3 ± 0.1 | 35 ± 5.6 | 2 ± 1.0 | 5 ± 1.7 | N | |||||||
| AD&Hyp (26) | 0 | 110 ± 23 | 0 ± 0.0 | 0.0 ± 0.0 | 8.0 ± 2.4↑ | 0.0 ± 0.0 | 43 ± 15.6 | 0 ± 0.1 | 15 ± 8.0↑ | SP | ||||||||
| Stereo (37) | 0 | 32 ± 14↓ | 4 ± 2.0 | 0.0 ± 0.0 | 0.6 ± 0.4↓ | 0.0 ± 0.0 | 104 ± 19.5↑↑↑ | 0 ± 0.0 | 28 ± 10.8↑ | N | ||||||||
| M . | Condition . | Ab Mvt . | Behaviours (mean duration: s/3 min) . | . | . | . | . | . | . | . | Task . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | . | |||||||
| B | Control | 0 | 168 ± 2 | 1 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 1 ± 0.4 | 0 ± 0.1 | 0 ± 0.0 | N | |||||||
| Ab Mvt (3) | Leg | 156 ± 4 | 1 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.3 | 1 ± 1.0 | 0 ± 0.0 | 0 ± 0.0 | N | ||||||||
| AD (21) | 0 | 171 ± 1 | 0 ± 0.0 | 0.3 ± 0.2 | 1.5 ± 0.6 | 0.8 ± 0.5 | 1 ± 0.5 | 0 ± 0.0 | 0 ± 0.0 | SP | ||||||||
| A | Control | 0 | 94 ± 4 | 42 ± 2.3 | 6.0 ± 1.0 | 0.5 ± 0.1 | 0.9 ± 0.1 | 3 ± 0.8 | 9 ± 1.4 | 4 ± 0.7 | N | |||||||
| AD&Hyp (31) | 0 | 35 ± 9↓↓ | 55 ± 11.3↑ | 0.2 ± 0.2 | 7.0 ± 2.3↑↑↑ | 8.0 ± 6.5↑↑ | 5 ± 5.0 | 21 ± 11.3↑↑ | 11 ± 6.0 | SP | ||||||||
| Stereo (46) | 0 | 12 ± 11↓↓↓ | 8 ± 6.0↓↓ | 0.6 ± 0.6 | 2.0 ± 2.0 | 3.0 ± 3.0 | 152 ± 18.0↑↑↑ | 0 ± 0.0 | 0 ± 0.0 | N | ||||||||
| H | Control | 0 | 119 ± 8 | 4 ± 0.9 | 3.0 ± 1.0 | 5.0 ± 1.2 | 0.3 ± 0.1 | 35 ± 5.6 | 2 ± 1.0 | 5 ± 1.7 | N | |||||||
| AD&Hyp (26) | 0 | 110 ± 23 | 0 ± 0.0 | 0.0 ± 0.0 | 8.0 ± 2.4↑ | 0.0 ± 0.0 | 43 ± 15.6 | 0 ± 0.1 | 15 ± 8.0↑ | SP | ||||||||
| Stereo (37) | 0 | 32 ± 14↓ | 4 ± 2.0 | 0.0 ± 0.0 | 0.6 ± 0.4↓ | 0.0 ± 0.0 | 104 ± 19.5↑↑↑ | 0 ± 0.0 | 28 ± 10.8↑ | N | ||||||||
The mean duration (± SEM) of each behaviour induced by bicuculline injection was obtained from five measurements of 3-min periods during the post-injection period. For control sessions, values were obtained during the corresponding post-injection period and pooled from several sessions (Monkey B: n = 14; monkey A: n = 19; monkey H: n = 5). Statistical analysis between control and injections was done using a two-way ANOVA followed by appropriate post hoc comparisons. Significant modifications for each injection effect are illustrated in bold (↓ ↑ SP Leg). (↑↑↑: P < 0.001; ↑↑: P < 0.01 and ↑: P < 0.05). M = monkey; Ab Mvt = abnormal movement; N = normal; SP = spatial perturbation. Behaviours: (1) resting; (2) hand examination; (3) grooming; (4) leg movements; (5) arm movements; (6) licking/biting fingers; (7) touching equipment; (8) tail examination.
The effect obtained from site no. 3 in the right GPe of monkey B (Fig. 1A) was leg dyskinesia without any behavioural modification (Table 1). This dyskinesia did not modify the execution of the task. In the same monkey, site no. 21 in the left GPe (Fig. 1A) was retained as the injection of bicuculline induced only a change in the spatial strategy in the execution of the task, a trouble that we related to an attention deficit. In monkey A, we have retained site no. 31 in the right GPe, as it was associated with the production of a hyperactivity with spatial strategy modification in the task, and site no. 46 in the left GPe which produced stereotypy (Table 1). Finally, in the third monkey, H, we chose to reproduce the results obtained from monkey A, and thus selected site no. 26 right GPe which produced hyperactivity with spatial strategy modification and site no. 37 left GPe which produced stereotypy (Fig. 1A and Table 1). However, in the last case (site no. 37), the injection of tracer performed was very small and weak, and we have thus chosen to add another experimental case (MM33) in which the axonal tracer was injected at the same location in the GPe as in monkey H, but which was not involved in the behavioural studies.
Relationship between the localization of the injection sites and the pallidal territories
All injection sites of tracers performed in the GPe (Fig. 1B) were compared with the distribution of Cb immunoreactivity as seen on adjacent sections (Fig. 1C) and with the delineation of anatomo-functional territories already reported in the literature (Fig. 1D). The Cb immunoreactivity was absent in the posterior and lateral part of the GPe, and gradually increased in the more anterior and medial part to reach a maximal intensity in the most anterior and ventral parts (Fig. 1C).
The most posterior site of tracer injection where we produced abnormal movements (site no. 3) was laterally located (AC-3.5; Fig. 1B), in a region characterized by a very weak staining of Cb immunoreactivity (Fig. 1C), and corresponding to the sensorimotor territory (Fig. 1D). The site in relation with the expression of an attention deficit without hyperactivity (site no. 21) was more anteriorly located in the GPe than in the preceding case (AC-1.5; Fig. 1B). It lies in a region characterized by a moderate staining in Cb reactivity (Fig. 1C) and corresponding to the associative territory of the GPe at the limit with the sensorimotor territory (Fig. 1D). The sites in relation to the expression of an attention deficit with hyperactivity (sites nos 26 and 31) were localized more anteriorly in the GPe (AC-0.5; Fig. 1B) than the other site described previously. This GPe region was moderately to weakly stained in Cb reactivity (Fig. 1C) and corresponded to the associative territory for one site (no. 26) close to the most anterior part of the sensorimotor territory for the other site (no. 31), the transition between both being progressive (Fig. 1C and D). Finally, the sites chosen for tracer injection where microinjections elicited a stereotyped behaviour (stereotypy sites nos 37 and 46) were the most anteriorly and medially located in the GPe (AC 0; Fig. 1B), in a region densely stained in Cb reactivity (Fig. 1C) and which corresponded to the limbic territory (Fig. 1D).
General labelling features
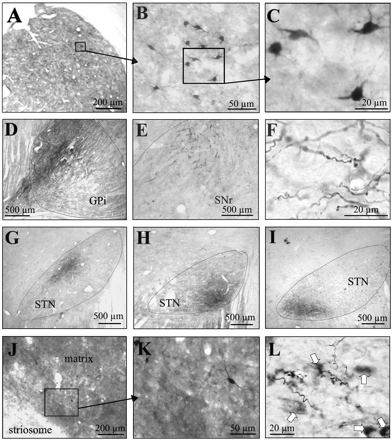
In all cases, retrogradely labelled cell bodies were found in the striatum (Fig. 2A) where they were labelled in a Golgi-like manner with filling of the whole dendritic trees (Fig. 2B and C). Anterogradely labelled terminal axons were distributed in the two output structures of the basal ganglia represented by the GPi (Fig. 2D) and SNr (Fig. 2E). These labelled terminal axons were characterized by numerous varicosities borne by very thin, branched terminations (Fig. 2F). Intensely labelled axons were also distributed within the STN (Fig. 2G, H and I). The retrograde labelling observed in the STN as well as the anterograde labelling in the striatum has not been considered in the present study.
Light photomicrographs showing examples of retrograde and anterograde labelling in different basal ganglia. (A, B, C) Retrogradely labelled neurons in the putamen at different magnifications. (D) Anterograde labelled axons in the internal globus pallidus and (E) in the substantia nigra pars reticulata. (F) High magnification of anterogradely labelled varicose fibres in the internal globus pallidus. (G, H, I) Anterograde labelling in different portions of the subthalamic nucleus depending on the localization of the tracer within the external globus pallidus. (G) represents the pattern of distribution following injection in hyperactivity with attention deficit site (no. 31), (H) from injection in attention deficit site alone (no. 21) and (I) from injection in stereotypy site (no. 46). (J, K) Example of retrogradely labelled neurons in the matrix of the striatum. (L) Anterogradely labelled varicose fibres in the pars compacta of the substantia nigra following tracer injection in the ventral part of the GPe where microinjections elicited stereotypy. Arrows represent nigral cell bodies. Cd = caudate nucleus; GPe = external globus pallidus; GPi = internal globus pallidus; SNr = substantia nigra pars reticulata; STN = subthalamic nucleus.
Retrograde and anterograde labelling relative to each site within the GPe
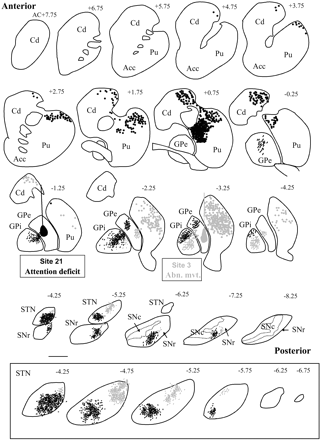
After injection of axonal tracer into site no. 3 of the GPe that produced movement disorder, numerous retrogradely labelled cells were distributed in the posterior and dorsal part of the putamen (Fig. 3, grey). Anterogradely labelled terminal axons were mainly distributed in posterior and central portions of the GPi, whereas in the SNr, the other output of the basal ganglia, the labelling was obviously less dense and was distributed in a restricted anterolateral portion anteriorly and in a central part more posteriorly. Anterogradely labelled terminals were also largely distributed in the dorsolateral part of the STN.
Distribution of labelling after injections of tracer into the sites of the external globus pallidus where microinjections elicited abnormal leg movements (Abn. mvt.) (no. 3, grey) and an attention deficit without hyperactivity (no. 21, black) in monkey B. Transverse sections are regularly interspaced from anterior to posterior and anteriorly located in reference to the anterior commissure (AC). Note that in two cases, labelled cell bodies (dots) were distributed in different parts of the putamen and of the caudate nucleus. Similarly anterogradely labelled axons (sinuous lines) were seen in different parts of the internal globus pallidus, substantia nigra and subthalamic nucleus. The insert shows a high-power view of labelled terminal axons in the subthalamic nucleus. AC = anterior commissure; Acc = nucleus accumbens; Cd = caudate nucleus; GPe = external globus pallidus; GPi = internal globus pallidus; Pu = putamen; SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata; STN = subthalamic nucleus.
After injection of axonal tracer in site no. 21 that produced attention deficit site without hyperactivity, numerous retrogradely labelled cells were distributed in the striatum (Fig. 3, black). They mainly occupied the dorsal portion of the putamen and of the caudate nucleus (Cd) from 4.75 anterior to AC and 1.25 posterior to the AC. Anterogradely labelled terminal axons were quite equally distributed in the two output structures from their anterior to their posterior extent. In the GPi, they were observable in the lateral part anteriorly (AC-1.25) and in its dorsal part posteriorly (AC-3.25), while in the SNr, they were present in a ventral portion. Within the STN, labelled terminals were mainly encountered in the ventral portion.
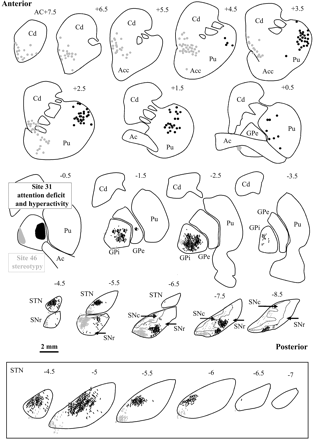
When injections of tracer were performed in site no. 26 or no. 31 that produced hyperactivity and an attention deficit, the distribution of retrograde and anterograde labelling was obviously similar. Thus only case no. 31 in monkey A was illustrated (Fig. 4, black). Retrogradely labelled cells were located in the striatum occupying roughly the same anteroposterior extent as in the preceding case (anterior AC+4.75 to posterior AC-0.25). However in that case, they were mainly found in the dorsolateral part of the anterior putamen. Anterogradely labelled terminal axons were distributed equally in the two output structures of the basal ganglia, occupying their central portion from anterior to posterior. Terminal axons also occupied the mediodorsal portion of the anterior STN.
Distribution of labelling after injections of axonal tracer into the sites of the external globus pallidus where microinjections elicited an hyperactivity associated to attention deficit (no. 31, black) and stereotypy (no. 46, grey) in monkey A. Transverse sections are regularly interspaced from anterior to posterior and anteriorly located in reference to the anterior commissure. Note that in two cases, labelled cell bodies (dots) were distributed in different parts of the putamen and of the caudate nucleus. Similarly anterogradely labelled axons (sinuous lines) were seen in different parts of the internal globus pallidus, substantia nigra and subthalamic nucleus. The insert shows a high-power view of labelled terminal axons in the subthalamic nucleus. Same abbreviations as in Fig. 3.
Finally injection of axonal tracers made in site no. 37 or no. 46 that produced stereotypy resulted in the same distribution of labelling and only case no. 46 in monkey A was illustrated (Fig. 4, grey). In these cases, retrogradely labelled cells were found in the anterior part of the striatum, and more particularly at the limit between the dorsal portion of the nucleus accumbens and the ventral part of the head of the caudate nucleus. As observed on striatal sections which were double labelled for tracer and Cb, the labelled cells were mainly located in the matrix, thus outside striosomes (Fig. 2J and K). Anterogradely labelled terminal axons were restricted to a small anterior and medial portion of the GPi. On the contrary, these terminals were more densely packed in the other output structure, the SNr, where they occupied a large part of the anteromedial portion. Labelled terminal axons were also observed in the STN, but they were restricted to the most anteromedial part. Contrary to the other cases, injections of tracer within a pallidal site which induced stereotypy also resulted in numerous labelled terminals in the whole extent of the SN pars compacta (Figs 2L and 4, grey).
Comparison of the different neuronal networks
In order to compare these different neuronal networks underlying the input and output connections of the subregions of the GPe described above, maps of the labelling distribution obtained in different cases were transferred onto one hemisphere of the monkey B (Fig. 5). Although the data were derived from different experimental cases, they were deemed to be comparable since all of the cerebral maps were related to the AC–PC stereotaxic coordinates system. From posterior to anterior portions of the basal ganglia, it appeared that the regions linked to abnormal movement circuit (yellow), were the most caudally and laterally located in all the basal ganglia. Moreover, the GPi appeared to be more linked to movement disorder than the SNr. The two circuits underlying hyperactivity and/or attention deficit were both more anteriorly located. The circuit linked to the production of hyperactivity with attention deficit (black) tended to be more ventrally located in the striatum and GPi than the circuit underlying attention deficit circuit alone (green) while it occupied more dorsal portions of the SNr and STN. However, in all structures considered, the hyperactivity with attention deficit circuit was partly superimposed on the attention deficit circuit alone, as would be expected, but also on the movement disorder circuit, especially in the GPi (AC-2.25; Fig. 5) and in the SNr (CA-6.25; Fig. 5). Finally, the regions linked to stereotypy (blue) were the most anteromedially located in all basal ganglia with no superimposition with another circuit in any part of the basal ganglia. Moreover, the SN as a whole (pars reticulata and compacta) appeared to be more linked to stereotypy than the GPi.
Comparison between the neuronal circuits which produced either abnormal movements (yellow), attention deficit alone (green), hyperactivity and attention deficit (grey) and stereotypy (blue), after tracer injections into different portions of the external globus pallidus. These circuits involve different portions of the caudate nucleus and putamen, of the internal globus pallidus, subthalamic nucleus and substantia nigra. Note a partial overlap between the different neuronal networks, especially in the internal globus pallidus and substantia nigra. Same abbreviations as in Fig. 3.
Discussion
By using axonal tracer injected in restricted parts of the GPe, where transitory behavioural disorders have previously been produced by microinjection of bicuculline, we describe the regions of the basal ganglia underlying these different behavioural disorders. Basic principles that emerge are that (i) the different behavioural disorders produced in monkeys are underlined by different anatomical circuits within the basal ganglia; (ii) these circuits involve different subregions of the basal ganglia and appeared largely separated even if a partial overlap exists within the output structures represented by the GPi and SNr; (iii) each of these anatomical circuits systematically involves both the GPi and the SNr but not equally; (iv) different subregions of the STN were also involved depending on the movement or behavioural disorder produced.
Different structures of the basal ganglia related to the GPe
The present tract-tracing study first confirms previous data in monkeys concerning the retrograde labelling of cells in the striatum and anterograde labelling of terminals within the GPi, SNr and STN after tracer injection into the GPe (Nauta and Mehler, 1966; Carpenter et al., 1968, 1981; Hazrati et al., 1990; Shink et al., 1996). Indeed it had already been reported that the pallidal terminals display large varicosities that were often closely apposed to the cell bodies and proximal dendrites of STN, GPi and SNr (Sato et al., 2000). The existence of preferential synaptic contacts with cell bodies and proximal dendrites of SNr and entopeduncular neurons in rats (the equivalent of the GPi of primates) has been demonstrated using electron microscopy (Smith and Bolam, 1991; Bolam and Smith, 1992). Altogether these data suggest that all parts of the GPe project to the GPi and SNr as well as to the STN. The present study confirms such an organization, but also indicates that different subregions of the GPe project to different anatomo-functional territories of the basal ganglia. In such an organization, the most anteromedial portion of the GPe appeared particular as it was the only one subregion of the GPe which projects not only to the SNr but also to the whole extent of the pars compacta. As previously shown in monkeys, this projection appeared not topographically organized (Haber et al., 1993).
The GPe was reported to also project to structures such as the thalamic reticular nucleus and the pedunculopontine nucleus, which are located outside of the basal ganglia. However, such projections are very sparse and even questioned (Sato et al., 2000). This suggests that the different anatomo-functional territories of the basal ganglia that were labelled in our study are the main cerebral structures involved in the behavioural effect produced. Our data are based on the delineation of distinct anatomo-functional territories in the basal ganglia as already done in the literature on the basis of tract-tracing data and using the distribution of Cb immunoreactivity. Thus in the striatum and in the two pallidal segments, but also in the SNr, the limbic territories were delineated as regions of intense immunoreactivity, the associative territories as those of moderate Cb immunoreactivity, and the sensorimotor territories as those of very weak immunoreactivity, confirming previous data from our laboratory (François et al., 1994b; Karachi et al., 2002). All these different anatomo-functional territories of the pallidum and SNr appeared implicated in circuits related to the expression of abnormal movements and behavioural disorders. This is also the case for the STN which thus appeared subdivided into different functional subregions. This result may explain the role played by the STN not only in motor control but also in cognitive and motivational processes as reported in clinical and experimental studies (Baunez and Robbins, 1997, 1999; Alegret et al., 2001; Mallet et al., 2002; Funkiewiez et al., 2003).
In our study, it is not possible to determine if the extent of the bicuculline injection site, and thus its physiological action, can be superimposed on that of the axonal tracer injection sites. However, injection of tracers performed within two different sites of a given anatomo-functional territory of the GPe where microinjections of bicuculline produced a given behavioural effect, resulted in similar distribution of labelling within the subregions of the basal ganglia. This thus reinforces the result that every behavioural effect produced is underlined by a given anatomo-functional subregion within the basal ganglia.
Anatomical circuits underlying the movement and behavioural disorders
Regions related to movement disorder
The present data first confirm that after injection of axonal tracer into the posterior and central portion of the GPe where microinjections produced leg dyskinesia, retrogradely labelled cells were distributed in the posterior part of the putamen. This striatal region is known to receive inputs mainly from the motor, premotor and somatosensory cortex (Künzle, 1975; Flaherty and Graybiel, 1991; Inase et al., 1996; Takada et al., 1998; Tokuno et al., 1999). These two pallidal and striatal regions contain very weak Cb immunoreactivity, which is a characteristic feature of sensorimotor territories (François et al., 1994b; Karachi et al., 2002). Moreover, we observed that the sensorimotor region of the GPe projects to posterolateral parts of the SNr, but above all to posterior portions of the GPi, all regions which are known to receive inputs from the striatal sensorimotor territories (Parent et al., 1984; Smith and Parent, 1986; Flaherty and Graybiel, 1991, 1994; Hedreen and DeLong, 1991; Hazrati and Parent, 1992; François et al., 1994a; Kaneda et al., 2002). Electrophysiological evidence in support of the main sensorimotor role of the GPi was provided by recording cells after stimulation of motor cortical areas (Yoshida et al., 1993) and by retrograde transynaptic labelling of herpes virus from motor cortical areas (Hoover and Strick, 1999). The dorsolateral portion of the STN where we found labelled GPe terminations is also known to receive motor cortical inputs (Nambu et al., 1996). It can thus be concluded that movement disorder that can be induced by microinjection of bicuculline in the GPe involves motor territories of all the basal ganglia.
Regions related to hyperactivity and/or attention deficit
The striatal region occupied by neurons which project to the GPe region where microinjections elicited spatial perturbation in the task, suggesting attention deficit, without hyperactivity (see Grabli et al., 2004) were located anteriorly in dorsal portions of the putamen and of the caudate nucleus. These striatal regions are reported to receive cortical inputs from different areas: the associative parietal and dorsolateral cortex (Yeterian and Pandya, 1991, 1993), the anterior part of the motor and premotor cortex (Inase et al., 1996, 1999; Takada et al., 1998), but above all the supplementary and pre-supplementary motor areas (Inase et al., 1999; Kaneda et al., 2002), the supplementary eye field (Shook et al., 1991; Parthasarathy et al., 1992, Cui et al., 2003) and the motor cingulate cortex (Takada et al., 2001). All these cortical areas anterior to the primary motor cortex participate in cognitive processes in addition to their well-known role in movement planning and execution. In particular, the anterior part of the premotor cortex, the dorsolateral prefrontal cortex and the supplementary eye field which mainly project to the caudate nucleus were reported to be specialized, among others, in spatial attention (Corbetta et al., 1991; Corbetta and Shulman, 1998; Boussaoud, 2001).
Within the GPi, axonal fibres were located in the lateral part anteriorly, and in its dorsal part posteriorly, while in the SNr they were present in a ventral portion. These regions, which appeared moderately stained on Cb immunoreactive sections, were reported to receive striatal inputs from the most posterior part of the associative territories, and from the most anterior part of the sensorimotor territories (Parent et al., 1984; Smith and Parent, 1986; Hedreen and DeLong, 1991; Hazrati and Parent, 1992; Flaherty and Graybiel, 1994; François et al., 1994a), and more specifically from striatal regions linked to the supplementary motor area (Kaneda et al., 2002). These regions contain neurons which are known to project via the thalamus back to the associative prefrontal cortex as well as to the supplementary and pre-supplementary motor cortex (Middleton and Strick, 2000, 2002). Within the STN, axonal fibres were confined to the anterior and ventral portion, in a region known to receive cortical inputs mainly from the supplementary and pre-supplementary motor areas (Takada et al., 2001) but also, partly, from pre-supplementary and supplementary eye field (Shook et al., 1991). Thus our results certainly indicate that the attention deficit circuit alone is underlined by subregions of the basal ganglia linked to the cortical areas which play a major role in both motor planning and attention processes.
Comparatively, hyperactivity with attention deficit circuit involves roughly the same portions of the striatum, but the labelling was slightly more ventrally located in the putamen and did not reach the caudate nucleus. This striatal zone not only receives cortical projection from the most anterior part of the secondary motor areas such as the supplementary motor cortex but also from the rostral cingulate motor cortex as recently reported (Takada et al., 2001). Within the GPi, GPe labelled axons occupied a more ventral portion, while in the SNr they were more dorsally located than in the attention deficit circuit alone. As for the striatum, these regions, and particularly that located in the GPi, tended to involve the anterior part of the sensorimotor territories. The region implicated in the STN was also more dorsally located than in the preceding case and thus implicated a region which was reported to receive inputs from the rostral cingulate motor cortex (Takada et al., 2001). It may thus be concluded from our results that the attention deficit with hyperactivity circuit is underlined by subregions of the basal ganglia linked not only to the supplementary and pre-supplementary motor areas but also to the rostral cingulate motor cortex. These secondary motor cortical areas are implicated not only in the execution of the movement but also in higher-order cognitive aspects of the movement such as motor selection or error detection (Akkal et al., 2002; Isomura et al., 2003).
Even if it is difficult to compare attention deficit with hyperactivity observed in monkeys with ADHD disorder described in humans, it is interesting to note that functional abnormalities in humans were visualized not only in prefrontal cortical areas (Amen et al., 1993; Amen and Carmichael, 1997; Spalletta et al., 2001) and more specifically in the anterior cingulate cortex (Bush et al., 1999), but also in the caudate nucleus (Castellanos et al., 1996; Filipek et al., 1997; Rubia et al., 1999; Castellanos, 2001), in both caudate nucleus and putamen (Vaidya et al., 1998; Teicher et al., 2000) and in the pallidum as a unit (Aylward et al., 1996; Castellanos et al., 1996). Our findings and these observations in humans strongly suggest that ADHD as a whole represents a deficiency in parts of the basal ganglia linked to associative prefrontal cortex and to secondary motor cortical areas involved in attention processes and motor planning.
Regions related to stereotyped behaviour
The striatal region labelled after injection of axonal tracer in the region of the GPe where microinjections elicited a persistent repetition of a single behaviour was ventromedial in the striatum, in a region located at the limit between the dorsal portion of the nucleus accumbens and the ventral part of the head of the caudate nucleus. Even if neither cytoarchitectonic nor immunohistochemical methods allows us to isolate the nucleus accumbens from the other part of the striatum, this striatal region is known to be the major structure that receives limbic inputs from the orbitofrontal, anterior cingulate and insular cortices (Kunishio and Haber, 1994; Haber et al., 1995; Chikama et al., 1997; Ferry et al., 2000), amygdala and hippocampus (Russchen et al., 1985; Friedman et al., 2002; Fudge and Haber, 2002), all regions which are known to process emotional and motivational information. The labelled GPe fibres originating in the site related to stereotypy occupied anterior and ventromedial located regions of the GPi but above all of the SNr, regions which correspond to limbic territories as seen on Cb-immunoreactive sections and after comparison with a tracing study (Haber et al., 1990). However it is clear from our results that the SNr is more linked to limbic structures than the GPi. These regions of the output structures of the basal ganglia are known to project to the orbital frontal cortex via the medial part of the thalamus (Ilinsky et al., 1985; Haber et al., 1993). In the STN, which is free of Cb, the labelling region was also restricted to the most rostromedial part of the nucleus, which confirms the existence of a limbic pallidosubthalamic territory, as recently described in our laboratory (C. Karachi, in press). Our present anatomical evidence thus supports the early suggestion that the pallidum could be involved in obsessive-compulsive disorder as it was reported that bilateral lesions in this structure can produce striking obsessive-compulsive disorder-like behaviour (Laplane et al., 1984, 1989).
Our data also confirm that one particular output target of the ventral limbic GPe is represented by the dopaminergic neurons of the SN pars compacta (Gerfen, 1992; Haber et al., 1993; Lynd-Balta and Haber, 1994). These dopaminergic neurons are considered to be the key for focusing attention on significant and rewarding stimuli (Schultz, 1997). This suggests an influence of the limbic GPe on the whole extent of the striatum via the dopaminergic nigro-striatal projection (Haber et al., 1993). Moreover, it was recently demonstrated in the rat that striosomes, one of the two compartments of the striatum which is thought to also project to the SNc (Graybiel, 1990; Gerfen, 1992), appear active when the animal performs repetitive, stereotyped behaviours in response to dopamine receptor agonists (Canales and Graybiel, 2000). The fact that, in our study, neurons, labelled after injection in the site where stereotypy was induced, were mainly found in the matrix of the ventral striatum not in striosomes, suggests that the output pathway of the matrix is also involved in the production of stereotyped behaviour.
This stereotyped behaviour that was produced in our monkeys resembles tics and compulsive disorders described in patients with Tourette's syndrome or with obsessive-compulsive disorder. Imaging studies from patients with obsessive-compulsive disorder and with Tourette's syndrome reported abnormalities of volumes in the caudate nucleus (Scarone et al., 1992; Peterson et al., 1993; Singer et al., 1993; Hyde et al., 1995; Moriarty et al., 1997; Peterson, 2001). Abnormal neuronal activity has also been reported at rest in the caudate nucleus but also in the ventral globus pallidus, orbito-frontal and cingulate cortex (Eidelberg et al., 1997; Rauch and Baxter, 1998; Saxena et al., 1998; Peterson, 2001; Rauch et al., 2001). Interestingly it was reported that infusion of sera from patients with Tourette's syndrome into the ventral striatum of rats increased the rate of oral stereotypies (Taylor et al., 2002). Together, these data and our present results provide strong evidence for an implication of the limbic parts of all the basal ganglia in producing stereotyped behaviour.
Partial superimposition of the different neuronal networks
There was no evidence of superimposition of the different neuronal networks that produced abnormal behaviours and movements within the striatum and the STN, except in limited zones between the hyperactivity associated to attention deficit circuit and the attention deficit circuit alone. On the other hand, within the output structures of the basal ganglia, regions which appeared linked to attention deficit alone overlapped with some portions of the hyperactivity and attention deficit circuits, as can be expected, but also with the movement disorder circuit. This highly suggests the existence of a gradient between movement, hyperactivity and attention deficit circuits in the GPi and in the SNr. The notion that such a gradient exists is supported by the existence of very long dendrites especially in these two structures (Yelnik et al., 1984, 1987), which allows convergence of information coming from different functional striatal territories on a single neuron. This was observed for pallidal neurons located in an intermediate zone between the associative and the sensorimotor territories and which respond to the stimulation of both the caudate nucleus and the putamen (Tremblay and Filion, 1989), and this may also be the case at the interface between the limbic and associative territories. Such a pattern gives the possibility of integration of functionally diverse information, at least within the output structures of the basal ganglia.
Conclusion
Our approach allowed us to demonstrate that the different behavioural disorders produced in monkeys by microinjections of bicuculline in different portions of the GPe are underlined by different anatomical circuits within the basal ganglia. This provides strong evidence for the involvement of different subregions of the basal ganglia (the two output structures: GPi and SNr, and the STN) in producing abnormal movement and behaviours. It can thus be hypothesized that different portions of the basal ganglia organized in anatomo-functional circuits play a role in the pathophysiology of syndromes such as obsessive-compulsive disorder, Tourette's syndrome or ADHD. In such a hypothesis, these different subregions of the basal ganglia could be considered as therapeutic targets. Thus, high frequency stimulation, which is well known to improve motor disorders of Parkinson's disease when it is centred in the sensorimotor territory of the STN (Yelnik et al., 2003) could also improve behavioural disorders when other anatomo-functional subregions are targeted.
We wish to thank Dominique Tandé for expert participation in surgical operations and immunohistochemical procedures, and Nicholas Barton for checking the English. This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM, France) and a grant from The Tourette Syndrome Association (USA).
References
Akkal D, Bioulac B, Audin J, Burbaud P. Comparison of neuronal activity in the rostral supplementary and cingulate motor areas during a task with cognitive and motor demands.
Alegret M, Junque C, Valldeoriola F, Vendrell P, Pilleri M, Rumia J, et al. Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex.
Amen DG, Carmichael BD. High-resolution brain SPECT imaging in ADHD.
Amen DG, Paldi F, Thisted RA. Brain SPECT imaging.
Aylward EH, Reiss AL, Reader MJ, Singer HS, Brown JE, Denckla MB. Basal ganglia volumes in children with attention-deficit hyperactivity disorder.
Baunez C, Robbins TW. Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats.
Baunez C, Robbins TW. Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time task in rats.
Bolam JP, Smith Y. The striatum and the globus pallidus send convergent synaptic inputs onto single cells in the entopeduncular nucleus of the rat: a double anterograde labelling study combined with postembedding immunocytochemistry for GABA.
Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop.
Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy.
Carpenter MB, Fraser RAR, Shriver JE. The organization of pallidosubthalamic fibers in the monkey.
Carpenter MB, Batton RR 3rd, Carleton SC, Keller JT. Interconnections and organization of pallidal and subthalamic nucleus neurons in the monkey.
Castellanos FX. Neural substrates of attention-deficit hyperactivity disorder.
Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder.
Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate.
Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search.
Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography.
Cui DM, Yan YJ, Lynch JC. Pursuit subregion of the frontal eye field projects to the caudate nucleus in monkeys.
Eidelberg D, Moeller JR, Antonini A, Kazumata K, Dhawan V, Budman C, et al. The metabolic anatomy of Tourette's syndrome.
Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks.
Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls.
Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations.
Flaherty AW, Graybiel AM. Input-output organization of the sensorimotor striatum in the squirrel monkey.
François C, Yelnik J, Percheron G, Fénelon G. Topographic distribution of the axonal endings from the sensorimotor and associative striatum in the macaque pallidum and substantia nigra.
François C, Yelnik J, Percheron G, Tandé D. Calbindin D-28k as a marker for the associative cortical territory of the striatum in macaque.
François C, Jan C, McCairn K, Grabli D, Hirsch EC, Féger J, et al. A primate model of Tourette's syndrome: anatomical analysis of the basal ganglia territories involved in hyperactivity disorder with attention deficit (HD/AD) and stereotypy [abstract]. Soc Neurosci Abstr
Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain.
Fudge JL, Haber SN. Defining the caudal ventral striatum in primates: cellular and histochemical features.
Funkiewiez A, Ardouin C, Krack P, Fraix V, Van Blercom N, Xie J, et al. Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson's disease.
Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization.
Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia.
Grabli D, McCarin K, Hirsch EC, Agid Y, Féger J, François C, et al. Behavioural disorders induced by external globus pallidus dysfunction in primates: I Behavioural study.
Haber SN, Lynd E, Klein C, Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study.
Haber SN, Lynd-Balta E, Mitchell SJ. The organization of the descending ventral pallidal projections in the monkey.
Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia.
Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum.
Hazrati LN, Parent A. The striatopallidal projection displays a high degree of anatomical specificity in the primate.
Hazrati LN, Parent A, Mitchell S, Haber SN. Evidence for interconnections between the two segments of the globus pallidus in primates: a PHA-L anterograde tracing study.
Hedreen JC, DeLong MR. Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque.
Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1.
Hyde TM, Stacey ME, Coppola R, Handel SF, Rickler KC, Weinberger DR. Cerebral morphometric abnormalities in Tourette's syndrome: a quantitative MRI study of monozygotic twins.
Ilinsky IA, Jouandet ML, Goldman-Rakic PS. Organization of the nigrothalamocortical system in the rhesus monkey.
Inase M, Sakai ST, Tanji J. Overlapping corticostriatal projections from the supplementary motor area and the primary motor cortex in the macaque monkey: an anterograde double labeling study.
Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area.
Isomura Y, Ito Y, Akazawa T, Nambu A, Takada M. Neural coding of ‘attention for action’ and ‘response selection’ in primate anterior cingulate cortex.
Kaneda K, Nambu A, Tokuno H, Takada M. Differential processing patterns of motor information via striatopallidal and striatonigral projections.
Karachi C, François C, Parain K, Bardinet E, Tandé D, Hirsch EC, et al. Three-dimensional cartography of functional territories in the human striatopallidal complex using calbindin immunoreactivity.
Karachi C, Yelnik J, Tandé D, Tremblay L, Hirsch EC, François C. An anatomical substrate for non motor functions of the subthalamic nucleus in primates. Mov Disord, in press.
Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input.
Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. an autoradiographic study in Macaca fascicularis.
Laplane D, Baulac M, Widlocher D, Dubois B. Pure psychic akinesia with bilateral lesions of basal ganglia.
Laplane D, Levasseur M, Pillon B, Dubois B, Baulac M, Mazoyer B, et al. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study.
Lynd-Balta E, Haber SN. Primate striatonigral projections: a comparison of the sensorimotor-related striatum and the ventral striatum.
Mallet L, Mesnage V, Houeto JL, Pelissolo A, Yelnik J, Behar C, et al. Compulsions, Parkinson's disease, and stimulation.
Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinognic blue reaction product with superior sensitivity for visualizing neural afferents and efferents.
Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits.
Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate.
Moriarty J, Varma AR, Stevens J, Fish M, Trimble MR, Robertson MM. A volumetric MRI study of Gilles de la Tourette's syndrome.
Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: Evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area.
Parent A, Hazrati LN. Functional anatomy of the basal ganglia. 1. The cortico-basal ganglia-thalamo-cortical loop.
Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry.
Parent A, Bouchard C, Smith Y. The striatopallidal and striatonigral projections: two distinct fiber systems in primate.
Parthasarathy HB, Schall JD, Graybiel AM. Distributed but convergent ordering of corticostriatal projections: analysis of the frontal eye field and the supplementary eye field in the macaque monkey.
Percheron G, François C, Yelnik J. Instruments and techniques for the stereotactic surgery based on the CA-CP ventricular system of coordinates in monkeys.
Peterson BS. Neuroimaging studies of Tourette syndrome: a decade of progress.
Peterson B, Riddle MA, Cohen DJ, Katz LD, Smith JC, Hardin MT, et al. Reduced basal ganglia volumes in Tourette's syndrome using three-dimensional reconstruction techniques from magnetic resonance images.
Rauch SL, Baxter LR. Neuroimaging of OCD and related disorders. In: Jenike MA, Baer L, Minichiello WE, editors. Obsessive-compulsive disorders: practical management. 3rd ed. St Louis: Mosby;
Rauch SL, Whalen PJ, Curran T, Shin LM, Coffey BJ, Savage CR, et al. Probing striato-thalamic function in obsessive-compulsive disorder and Tourette syndrome using neuroimaging methods.
Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study.
Saint-Cyr JA, Ungerleider LG, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey.
Sato F, Lavallee P, Levesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate.
Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder.
Scarone S, Colombo C, Livian S, Abbruzzese M, Ronchi P, Locatelli M, et al. Increased right caudate nucleus size in obsessive-compulsive disorder: detection with magnetic resonance imaging.
Schultz W. Dopamine neurons and their role in reward mechanisms.
Shink E, Bevan MD, Bolam JP, Smith Y. The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey.
Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field. II. Comparative aspects of connections with the thalamus, corpus striatum, and related forebrain nuclei.
Singer HS, Reiss AL, Brown JE, Aylward EH, Shih B, Chee E, et al. Volumetric MRI changes in basal ganglia of children with Tourette's syndrome.
Smith Y. Anterograde tracing with PHA-L and biocytin at the electron microscopic level. In: Bolam JP, Rickwood D, Hames BD, editors. Experimental neuroanatomy. A practical approach. New York: Plenum Press;
Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study.
Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saïmiri sciureus).
Spalletta G, Pasini A, Pau F, Guido G, Menghini L, Caltagirone C. Prefrontal blood flow dysregulation in drug naive ADHD children without structural abnormalities.
Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal input zones from the supplementary motor area overlap those from the contra-rather than ipsilateral primary motor cortex.
Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, et al. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey.
Taylor JR, Morshed SA, Parveen S, Mercadante MT, Scahill L, Peterson BS, et al. An animal model of Tourette's syndrome.
Teicher MH, Anderson CM, Polcari A, Glod CA, Maas LC, Renshaw PF. Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry.
Tokuno H, Inase M, Nambu A, Akazawa T, Miyachi S, Takada M. Corticostriatal projections from distal and proximal forelimb representations of the monkey primary motor cortex.
Tremblay L, Filion M. Responses of pallidal neurons to striatal stimulation in intact waking monkeys.
Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study.
Yelnik J, Percheron G, François C. A Golgi analysis of the primate globus pallidus. II. Quantitative morphology and spatial orientation of dendritic arborizations.
Yelnik J, François C, Percheron G, Heyner S. Golgi study of the primate substantia nigra. I. Quantitative morphology and typology of nigral neurons.
Yelnik J, Damier P, Demeret S, Gervais D, Bardinet E, Bejjani BP, et al. Localization of stimulating electrodes in patients with Parkinson disease by using a three-dimensional atlas-magnetic resonance imaging coregistration method.
Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys.
Yeterian EH, Pandya DN. Striatal connections of the parietal association cortices in rhesus monkeys.
- signal-to-noise ratio
- basal ganglia
- dyskinesia
- bicuculline
- caudate nucleus
- cercopithecidae
- corpus striatum
- globus pallidus
- glucose-6-phosphate isomerase
- glycosylphosphatidylinositols
- microinjections
- neurons
- primates
- putamen
- stereotyped behavior
- substantia nigra
- subthalamic nucleus
- tourette syndrome
- behavioral change
- behavior disorders
- pars reticulata