-
PDF
- Split View
-
Views
-
Cite
Cite
Timm Rosburg, Peter Trautner, Thomas Dietl, Oleg A. Korzyukov, Nashaat N. Boutros, Carlo Schaller, Christian Erich Elger, Martin Kurthen, Subdural recordings of the mismatch negativity (MMN) in patients with focal epilepsy, Brain, Volume 128, Issue 4, April 2005, Pages 819–828, https://doi.org/10.1093/brain/awh442
Close - Share Icon Share
Abstract
Mismatch negativity (MMN) is elicited by discernible changes in an otherwise regular stream of auditory stimulation and reflects a pre-attentive detection mechanism. In the current study, auditory evoked potentials were recorded intracranially and electrode contacts sensitive for stimulus deviance were selected in order to further elucidate the contribution of different brain areas to MMN generation. Data were obtained from patients with frontal and temporal lobe epilepsy undergoing a presurgical evaluation by subdural and depth electrodes. In 13 of 29 patients under investigation an intracranial MMN could be observed, while in four other patients a response recovery of the N100 was revealed, mimicking an MMN. Most electrodes with an MMN signal were located in or close to the superior temporal lobe. In two patients an MMN was observed at electrode contacts over the lateral inferior frontal cortex and in one patient at a frontal interhemispheric electrode strip, giving evidence for a participation of the frontal gyrus in MMN generation. Current findings have, however, to be interpreted with caution owing to the placement and limited extension of the used electrode arrays.
Introduction
Mismatch negativity (MMN) is an event-related potential (ERP) component, elicited by discernible changes in a repetitive auditory stimulation. The MMN is regarded as the result of a pre-attentive comparison process, as it can be obtained also in absence of directed attention. Usually the MMN is calculated as the difference between the cortical response evoked by deviant and repeated (‘standard’) tones and becomes visible in EEG data as a fronto-central negativity in a latency range between 100 and 250 ms. Towards the mastoids an inversion of the polarity is observed that can be regarded as evidence for the generation of the MMN in the temporal lobe. This assumption was corroborated quite early by magnetoencephalography (MEG) studies showing that the cortical generator of the MMN probably originates from the primary auditory cortex or its vicinity (Hari et al., 1984; Sams et al., 1985).
Besides these bilateral temporal lobe generators additional sources of the MMN have been proposed (Giard et al., 1990; Kasai et al., 1999; Levänen et al., 1996; Rinne et al., 2000; Celsis et al., 1999; Liasis et al., 2001; Waberski et al., 2001; Jemel et al., 2002; Müller et al., 2002b; Opitz et al., 2002; Park et al., 2002; Sevostianov et al., 2002; Doeller et al., 2003; Liebenthal et al., 2003; Schall et al., 2003). Those sources were located nearly exclusively in the frontal and right parietal lobe, but, nevertheless, the findings were not equivocal. In a first report on frontal MMN activity Giard and co-workers (1990) analysed EEG data by means of a scalp current density mapping. They reported that the frontal generator had a right hemispheric dominance, independently of the side of stimulation.
Other evidence came from a lesion study. Alho et al. (1994) revealed a reduction of the MMN in patients with frontal lobe lesions which was most pronounced over the side of the lesion. EEG but not MEG seems to be capable to detect the frontal MMN activity. This was underlined by the study of Rinne et al. (2000) who recorded EEG and MEG simultaneously. The frontal MMN generator was observable only in the EEG data and peaked about 8 ms later than the temporal source. The authors concluded from the divergence between EEG and MEG data that the frontal activity is evoked by a radial source or by a source deep in the brain. For both kinds of sources MEG is clearly less sensitive than EEG. The exact localization of the frontal generator, however, diverges between EEG studies. Waberski et al. (2001) located the frontal MMN activity in the cingulum. Jemel et al. (2002) reconstructed the frontal activity by two sources, one located in the left cingulate and one in the right inferior frontal cortex.
Overall, the contribution of the frontal source to the MMN signal seems to be quite small. Frodl-Bauch et al. (1997) found the temporal sources sufficient to explain the MMN data, just keeping 3.2% of the data variance unexplained. In their PET study, Tervaniemi et al. (2000) revealed a statistically significant activation only in regions of the temporal lobe (but not in the frontal lobe) despite the large sample size of 30 subjects. Similarly, temporal but no frontal lobe activation was observed in a number of functional magnetic resonance imaging (fMRI) studies (Opitz et al., 1999; Wible et al., 2001; Mathiak et al., 2002; Liebenthal et al., 2003). Other fMRI studies gave evidence for a participation of the frontal cortex, but with some variation in localization. Celsis et al. (1999) reported an activation of the right inferior frontal cortex (Brodmann area BA 44) in one of three conditions. Opitz et al. (2002) obtained also an activation of the right inferior frontal gyrus. The latter finding was confirmed by a follow-up study, but was confined to frequency deviance (Doeller et al., 2003). A more extended activation, including the right inferior, middle and superior frontal gyrus, was observed by Schall et al. (2003). Contrasting to other studies, a left inferior frontal activation was obtained in the PET study of Müller et al. (2002b). No activation of the inferior frontal lobe, but of the dorsolateral prefrontal cortex and the anterior cingulum was reported by Sevostianov et al. (2002).
To sum the current findings up, the (right) inferior frontal gyrus and/or the cingulum seem to be the most likely candidates for the frontal generation of the MMN. The functional role of a frontal MMN generator is unresolved as yet. In their initial observation Giard et al. (1990) assumed that the frontal generator relates to an automatic attention switching, while the temporal generator reflects a sensory memory mechanism. Others hypothesized that the frontal generator might serve the function of contrast enhancement (Opitz et al., 2002), but empirical evidence for this hypothesis is lacking. Currently, there is no clear-cut reason for the assumption that a frontal contribution to the MMN can be observed only for certain kinds of deviants or for certain experimental protocols. With respect to fMRI results one has to be aware of the low temporal resolution of the method, leaving the possibility that some parts of the observed activation could refer to other stages of information processing rather than to the MMN.
Whether the parietal lobe is involved in MMN generation remains open. Evidence for a participation of the right parietal lobe in MMN generation stems mainly from electrophysiological studies (EEG: Kasai et al., 1999; and MEG: Levänen et al., 1996), while only in one fMRI study such activation could be shown (Schall et al., 2003). A contribution of left parietal lobe to the MMN as proposed by the study of Park et al. (2002) was criticized because of methodological weaknesses (Rosburg, 2004). Besides the temporal, and possible frontal and parietal contribution to MMN generation, a single animal study also reported on a MMN like signal in the non-primary thalamus (Kraus et al., 1994). In humans, an investigation of the thalamic involvement in MMN generation is yet lacking.
Obtaining more information about brain areas involved in MMN generation has a significant impact on clinical ERP research. In schizophrenia but also in other neuropsychiatric diseases the MMN was found to be reduced (Rosburg et al., 2004b). The identification of MMN generators possibly helps to characterize the anatomical correlates of an MMN reduction. Intracranial recordings are among the most favourable tools to investigate the contribution of different brain areas to MMN generation, but the study of presurgical patients with intracranial electrodes represents a rare opportunity and only few studies exist. In the majority of these studies, an involvement of areas in the temporal lobe in MMN generation was shown (Halgren et al., 1995; Kropotov et al., 1995; Liasis et al., 2000), most presumably of BA 22 (Kropotov et al., 2000). The involvement of the temporal regions in MMN generation was also verified in invasive animal recordings (e.g. Csepe et al., 1987; Javitt et al., 1995). Similar to non-invasive methods, evidence for a frontal contribution in MMN generation by means of invasive recordings is much sparser and relies solely on a single case study of a 6-year-old child (Liasis et al., 2001). In that study the MMN was assumed to originate frontally from BA 45. The aim of the current study was to determine cortical areas, possibly involved in the generation of the MMN, by means of intracranial recordings in a larger sample of epilepsy patients.
Methods
Participants
The study was conducted in a group of 29 epilepsy patients, undergoing presurgical evaluation with subdural grid/strip and hippocampal depth electrodes. Patients were included only if no hearing deficits were apparent. The average age of the included patients (13 males) was 37.2 years (range 16–61). The group represented a subsample of a larger study on sensory gating still under conduction. Patients were thoroughly informed about the purposes of the study and gave written informed consent. The study was approved by the local ethics committee of the University of Bonn.
Stimulation
Subjects were stimulated by earphones with 100 trains of six clicks. The first five stimuli had a frequency of 1500 Hz and 6.6 ms duration (including 1.5 ms rising and falling time). The sixth stimulus was deviating in both frequency (2000 Hz) and duration (12.8 ms). The clicks were separated by 500 ms and the trains by 8000 ms. Subjects were instructed to sit relaxed on their chair, to avoid eye movements and to keep awake. The deviating click required no behavioural response.
Recording and data analysis
The number of intracranial electrodes and their positions varied between patients. The electrode placements of each patient are listed in Table 1 and exemplary electrode placements are displayed in Figs 1, 3, 4 and 5. The electrode placement was determined solely by the purpose of presurgical evaluation. Electrophysiological data were recorded at a sampling rate of 1000 Hz with the digital EPAS system (Schwarzer, Munich, Germany) and its implemented Harmonie EEG software (Stellate, Quebec, Canada) in a sound shielded room. The left and right mastoids served as references. Besides, in the majority of patients, surface EEG was recorded with six electrodes (Cz, C3, C4, T5, T6, Oz). Recordings were visually inspected for artefacts and down-sampled to 200 Hz. EEG segments of 700 ms duration with 200 ms prestimulus period as baseline were averaged for the first, for the second to fifth and for the sixth stimulus position in the train, resulting in three averaged ERPs: ERP1, ERP2…5 and ERP6. Averaged data were filtered from 1 to 20 Hz with a slope of 24 dB/oct each and baseline corrected. The MMN was calculated as difference potential between ERP6 and ERP2…5 (ERPMMN = ERP6 – ERP2…5), as the first click in a train evoked a much stronger N100 than the succeeding stimuli.
The electrode placements of each patient participating in the study
| Patient . | Left . | . | . | . | . | Right . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | f . | t . | p . | tm . | tb . | f . | t . | p . | tm . | tb . | ih . | ||||||||
| 105 | 3 × 8 | 10 | 3 × 4 | ||||||||||||||||
| 106 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 1 × 4 | |||||||||||||
| 107 | 8 × 8, 1 × 8 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 108 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 109 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 110* | 1 × 4 | 2 × 4 | 8 × 8, 2 × 8 | 3 × 4 | |||||||||||||||
| 111 | 4 × 8 | 10 | 2 × 4 | ||||||||||||||||
| 114 | 1 × 8 | 10 | 2 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||||
| 116 | 4 × 8 | ||||||||||||||||||
| 117* | 8 ×8 | 2 × 4 | |||||||||||||||||
| 118* | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 119 | 1 × 4 | 2 × 8 | 10 | 2 × 4 | 1 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||
| 120* | 3 × 8, 8 × 8, 1 × 8 | 10 | 3 × 4 | 1 × 4 | |||||||||||||||
| 123* | 8 × 8 | ||||||||||||||||||
| 125 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 126* | 8 × 8 | ||||||||||||||||||
| 128* | 8 × 8 | 2 × 4 | |||||||||||||||||
| 129 | 8 × 8 | 10 | |||||||||||||||||
| 130* | 8 × 8 | ||||||||||||||||||
| 503 | 2 × 9 | 2 × 4 | 4 × 5 | 2 × 4 | |||||||||||||||
| 504 | 2 × 9 | 10 | 2 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||||
| 508 | 4 × 8 | 3 × 4 | |||||||||||||||||
| 509 | 2 × 8, 4 × 8 | 2 × 4, 1 × 6 | |||||||||||||||||
| (104) | 1 × 8 | 1 × 4 | 10 | 2 × 4 | 1 × 8 | 10 | |||||||||||||
| (112) | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (121) | 4 × 8 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (122) | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (127) | 2 × 9 | 10 | |||||||||||||||||
| (501) | 10 | 10 | |||||||||||||||||
| Patient . | Left . | . | . | . | . | Right . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | f . | t . | p . | tm . | tb . | f . | t . | p . | tm . | tb . | ih . | ||||||||
| 105 | 3 × 8 | 10 | 3 × 4 | ||||||||||||||||
| 106 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 1 × 4 | |||||||||||||
| 107 | 8 × 8, 1 × 8 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 108 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 109 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 110* | 1 × 4 | 2 × 4 | 8 × 8, 2 × 8 | 3 × 4 | |||||||||||||||
| 111 | 4 × 8 | 10 | 2 × 4 | ||||||||||||||||
| 114 | 1 × 8 | 10 | 2 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||||
| 116 | 4 × 8 | ||||||||||||||||||
| 117* | 8 ×8 | 2 × 4 | |||||||||||||||||
| 118* | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 119 | 1 × 4 | 2 × 8 | 10 | 2 × 4 | 1 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||
| 120* | 3 × 8, 8 × 8, 1 × 8 | 10 | 3 × 4 | 1 × 4 | |||||||||||||||
| 123* | 8 × 8 | ||||||||||||||||||
| 125 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 126* | 8 × 8 | ||||||||||||||||||
| 128* | 8 × 8 | 2 × 4 | |||||||||||||||||
| 129 | 8 × 8 | 10 | |||||||||||||||||
| 130* | 8 × 8 | ||||||||||||||||||
| 503 | 2 × 9 | 2 × 4 | 4 × 5 | 2 × 4 | |||||||||||||||
| 504 | 2 × 9 | 10 | 2 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||||
| 508 | 4 × 8 | 3 × 4 | |||||||||||||||||
| 509 | 2 × 8, 4 × 8 | 2 × 4, 1 × 6 | |||||||||||||||||
| (104) | 1 × 8 | 1 × 4 | 10 | 2 × 4 | 1 × 8 | 10 | |||||||||||||
| (112) | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (121) | 4 × 8 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (122) | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (127) | 2 × 9 | 10 | |||||||||||||||||
| (501) | 10 | 10 | |||||||||||||||||
The number in the table refers to the number of electrodes at frontal (f), temporal (t), parietal (p), temporomesial (tm), temporobasal (tb) or interhemispheric (ih) contacts; numbers between two columns indicate that frontal and temporal or temporal and parietal areas were covered by electrodes; a patient code in brackets indicates that no N100/MMN signal was observable in this patient; codes of patients whose data are depicted in Figure 1 are marked with an asterisk.
The electrode placements of each patient participating in the study
| Patient . | Left . | . | . | . | . | Right . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | f . | t . | p . | tm . | tb . | f . | t . | p . | tm . | tb . | ih . | ||||||||
| 105 | 3 × 8 | 10 | 3 × 4 | ||||||||||||||||
| 106 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 1 × 4 | |||||||||||||
| 107 | 8 × 8, 1 × 8 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 108 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 109 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 110* | 1 × 4 | 2 × 4 | 8 × 8, 2 × 8 | 3 × 4 | |||||||||||||||
| 111 | 4 × 8 | 10 | 2 × 4 | ||||||||||||||||
| 114 | 1 × 8 | 10 | 2 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||||
| 116 | 4 × 8 | ||||||||||||||||||
| 117* | 8 ×8 | 2 × 4 | |||||||||||||||||
| 118* | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 119 | 1 × 4 | 2 × 8 | 10 | 2 × 4 | 1 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||
| 120* | 3 × 8, 8 × 8, 1 × 8 | 10 | 3 × 4 | 1 × 4 | |||||||||||||||
| 123* | 8 × 8 | ||||||||||||||||||
| 125 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 126* | 8 × 8 | ||||||||||||||||||
| 128* | 8 × 8 | 2 × 4 | |||||||||||||||||
| 129 | 8 × 8 | 10 | |||||||||||||||||
| 130* | 8 × 8 | ||||||||||||||||||
| 503 | 2 × 9 | 2 × 4 | 4 × 5 | 2 × 4 | |||||||||||||||
| 504 | 2 × 9 | 10 | 2 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||||
| 508 | 4 × 8 | 3 × 4 | |||||||||||||||||
| 509 | 2 × 8, 4 × 8 | 2 × 4, 1 × 6 | |||||||||||||||||
| (104) | 1 × 8 | 1 × 4 | 10 | 2 × 4 | 1 × 8 | 10 | |||||||||||||
| (112) | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (121) | 4 × 8 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (122) | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (127) | 2 × 9 | 10 | |||||||||||||||||
| (501) | 10 | 10 | |||||||||||||||||
| Patient . | Left . | . | . | . | . | Right . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | f . | t . | p . | tm . | tb . | f . | t . | p . | tm . | tb . | ih . | ||||||||
| 105 | 3 × 8 | 10 | 3 × 4 | ||||||||||||||||
| 106 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 1 × 4 | |||||||||||||
| 107 | 8 × 8, 1 × 8 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 108 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 109 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 110* | 1 × 4 | 2 × 4 | 8 × 8, 2 × 8 | 3 × 4 | |||||||||||||||
| 111 | 4 × 8 | 10 | 2 × 4 | ||||||||||||||||
| 114 | 1 × 8 | 10 | 2 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||||
| 116 | 4 × 8 | ||||||||||||||||||
| 117* | 8 ×8 | 2 × 4 | |||||||||||||||||
| 118* | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 119 | 1 × 4 | 2 × 8 | 10 | 2 × 4 | 1 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||
| 120* | 3 × 8, 8 × 8, 1 × 8 | 10 | 3 × 4 | 1 × 4 | |||||||||||||||
| 123* | 8 × 8 | ||||||||||||||||||
| 125 | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| 126* | 8 × 8 | ||||||||||||||||||
| 128* | 8 × 8 | 2 × 4 | |||||||||||||||||
| 129 | 8 × 8 | 10 | |||||||||||||||||
| 130* | 8 × 8 | ||||||||||||||||||
| 503 | 2 × 9 | 2 × 4 | 4 × 5 | 2 × 4 | |||||||||||||||
| 504 | 2 × 9 | 10 | 2 × 4 | 2 × 8 | 10 | 2 × 4 | |||||||||||||
| 508 | 4 × 8 | 3 × 4 | |||||||||||||||||
| 509 | 2 × 8, 4 × 8 | 2 × 4, 1 × 6 | |||||||||||||||||
| (104) | 1 × 8 | 1 × 4 | 10 | 2 × 4 | 1 × 8 | 10 | |||||||||||||
| (112) | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (121) | 4 × 8 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (122) | 1 × 4 | 10 | 2 × 4 | 1 × 4 | 10 | 2 × 4 | |||||||||||||
| (127) | 2 × 9 | 10 | |||||||||||||||||
| (501) | 10 | 10 | |||||||||||||||||
The number in the table refers to the number of electrodes at frontal (f), temporal (t), parietal (p), temporomesial (tm), temporobasal (tb) or interhemispheric (ih) contacts; numbers between two columns indicate that frontal and temporal or temporal and parietal areas were covered by electrodes; a patient code in brackets indicates that no N100/MMN signal was observable in this patient; codes of patients whose data are depicted in Figure 1 are marked with an asterisk.
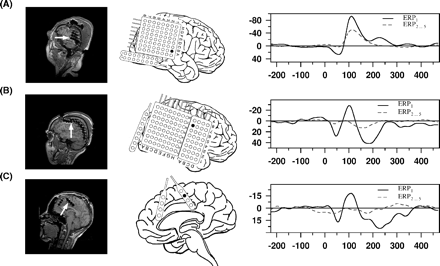
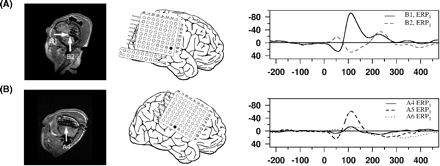
Individual intracranial data: in the MRIs, implanted electrodes become observable as artefacts (left column), and the electrode(s) selected for analysis are marked by white arrow(s). In the middle column, the schematic electrode placement of the individual is presented and selected electrodes are indicated by a filled circle. In the right column, the ERPs at the specified position(s) are depicted; for clarity only two ERPs are shown per individual; each row represents a data set of one individual: (A) temporal N100 of Patient 110, (B) fronto-central N100 of Patient 120, and (C) frontal interhemispheric N100 of Patient 128 (ERP1 versus ERP2…5).
The electrode positions exhibiting the two ERP components of interest, namely the N100 and MMN, were determined by visual inspection of ERP1 and ERPMMN, respectively. The N100 was expected to peak in a latency window from 80 to 140 ms. This late offset of the time window was chosen because most patients were on anti-convulsive medication at the time of measurement and this medication could possibly slow down long latency ERP components. Also the time course of the ERP with polarity inversion before and after the N100 and the ERP at the Cz electrode were taken into account for the definition of the N100 peak. The MMN was expected to peak later than the N100 up to a latency of 250 ms. Peaks in both latency ranges were regarded only as evident if they were discernible from earlier or later brain activity and their amplitude clearly surmounted baseline activity. If two or more neighbouring electrodes exhibited a peak at a similar latency, only the electrode with the largest amplitude was chosen for the analysis since in that case the observed peak was assumed to originate from a common source, best represented by the electrode with the highest amplitude. The selected electrodes will be referred to as N100 and MMN electrodes.
To validate the selected electrodes and waveforms, grand averages were calculated for these electrodes in or close to the temporal lobe. Peak amplitudes and latencies were measured for the N100 at the individual peaks of the ERPs at N100 electrodes and at the peak latency of ERP6 or, if not possible at the peak latency of the ERPMMN, at the MMN electrodes. ERP1 and ERP2…5 mostly had no discernible peak in this latency range. The amplitudes and latencies were compared between ERPs by means of a repeated measurement analysis of variance (ANOVA) and post hoc paired t tests. The location of the electrodes was verified from axial and coronal, 2 mm sliced T2-weighted and 3 mm sliced FLAIR magnetic resonance images routinely acquired after electrode implantation.
Results
An N100 component could be detected in 18 patients. It peaked in the surface recordings approximately at 100 ms (Cz) and intracranially at 105 ms. Intracranial electrodes exhibiting an N100 were located either in the region of the temporal lobe (n = 18), fronto-central region (n = 2) or in the frontal interhemispheric fissure (n = 2) (for exemplary data see Fig. 1). If an N100 was observable in ERP1 at a frontal contact, it was always strongly suppressed in ERP2…5 and merely distinguishable from the noise level (Fig. 1b and c). In two patients with, and four patients without, an intracranial N100, a positive deflection was observed in the latency range (‘P100’). The locations of electrodes exhibiting a P100 were considerably spread: in the anterior and posterior superior temporal gyrus, in the posterior mesial temporal gyrus and in the temporobasal region.
At temporal N100 electrodes, the N100 peak amplitudes were significantly higher in ERP1 as compared to ERP2…5 (Fig. 2A, F2,34 = 21.570, P < 0.001; t17 = 6.366, P < 0.001), while the difference between the N100 of ERP1 and ERP6 marginally failed to reach significance (t17 = 2.038, not significant). The N100 amplitudes of ERP6 significantly surmounted the N100 amplitudes of ERP2…5 (t17 = 4.156, P < 0.001), indicating a response recovery of the N100 by the sound deviation. N100 latencies did not differ between the three averages (F2,34 = 2.108, not significant).
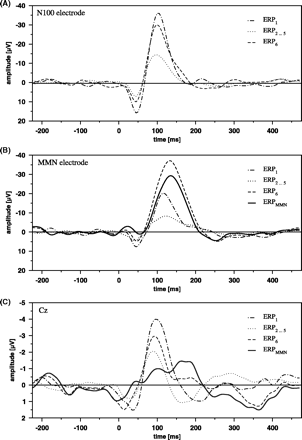
The ERPs, averaged for the first stimulus (ERP1), the second to fifth stimulus (ERP2…5) and sixth (deviating) stimulus (ERP6), at the temporal N100 electrode (A), temporal MMN electrode (B) and Cz (C). For Cz and MMN electrode, the MMN is also depicted as difference potential between ERP6 and ERP2…5. The averages at the MMN electrode are based on 12 subjects, at the N100 electrode on 18 subjects and at Cz on 22 subjects.
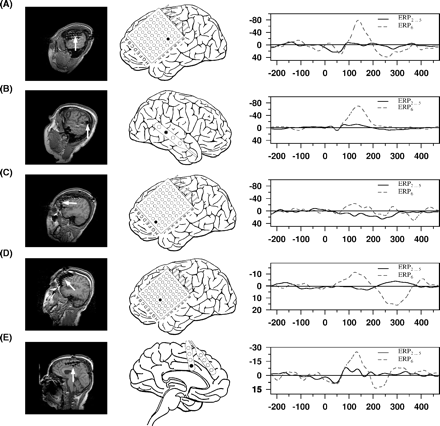
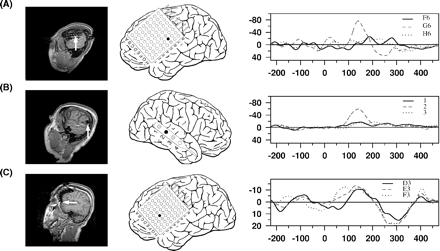
ERPMMN at Cz was characterized by a bimodal peak, one representing a modulation of the N100 at ∼100 ms and one the genuine MMN at ∼165 ms. Its amplitude was relatively small at both time points (Fig. 2C, 0.997 μV and 1.454 μV, based on 22 subjects exhibiting an intracranial N100 or MMN). In intracranial recordings an MMN as difference potential between ERP6 and ERP2…5 was detectable in 17 patients. Similar to the N100 electrodes most electrodes with an MMN were located in the region of the temporal lobe (exemplary data see Fig. 3A and B). The time course of the intracranial MMN was somewhat different as compared with extracranial data. In the grand average, the intracranial MMN had only a single peak, reaching its maximum at ∼140 ms, i.e. before the MMN peak was observed at Cz. An MMN like deflection was found in two patients on the left lateral inferior frontal cortex (Fig. 3C and D) and in another patient in the frontal interhemispheric fissure (Fig. 3E).
Individual data on the effects of tone deviation, as compared to ERP of the repeated stimulus (ERP6versus ERP2…5); please note that in this figure the MMN is not illustrated as difference potential; the structure of the figure is the same as in Fig. 1; (A) temporal MMN of Patient 126, (B) temporal MMN of Patient 118, (C) frontal MMN of Patient 126, (D) frontal MMN of Patient 123, (E) frontal interhemispheric MMN of Patient 117.
At temporal MMN electrodes, the mean amplitude of ERP6 at its maximum was significantly higher than those of ERP1 and ERP2…5 (Fig. 2B, F2,30 = 26.449, P < 0.001; t15 = 4.157, P < 0.001 and t15 = 7.389, P < 0.001, respectively). Also the mean amplitude of ERP1 surmounted the ERP2…5 at these electrode positions significantly (t15 = 2.677, P < 0.05). In some patients (n = 4) the ERP6 resembled ERP1 and in those cases the generated difference potentials (ERPMMN) could possibly contain activity from non-refractory neurons rather than a genuine mismatch response. After exclusion of these subjects the difference between ERP1 and ERP2…5 decreased and was no longer significant (t11 = 1.276, not significant), while the differences between ERP6 and ERP1, as well as between ERP6 and ERP2…5 remained significant (F2,22 = 28.445, P < 0.001; t11 = 5.493, P < 0.001 and t11 = 6.025, P < 0.001).
Discussion
An intracranially recorded MMN could be observed in 13 of 29 patients. The portion of subjects with an MMN was comparable to the portion of subjects exhibiting an N100. The observation of both auditory evoked potentials depends on the exact placement of the electrodes and this placement was determined by clinical considerations. One major limitation of the current study is the experimental design. The experiment was not designed with the primary intention to elicit an MMN, but to investigate the habituation of the P50 (Rosburg et al., 2004d; for the experimental rationale see Boutros et al., 1997, 1999). Stimuli with a very short duration are not very favourable for the elicitation of both the N100 and MMN (Paavilainen et al., 1993; Aleksandrov et al., 2003). It might be on the first glance surprising that an MMN was elicited by the short acoustic stimuli applied in our study, as a minimum duration of 10 ms for intensity deviants and of 30 ms for frequency deviants was found to be as necessary for the elicitation of an MMN (Paavilainen et al., 1993; Aleksandrov et al., 2003). The here applied deviant can be regarded as a triple deviant: first, it was of longer duration and, secondly, as a side-effect more intense than the standard. Thirdly and most importantly, the longer duration of the deviant permitted the perception of its pitch, while the standard was usually perceived as click (with no apparent frequency information). The large difference between the standard and deviant stimulus has, however, the unwanted effect that part of the difference potential between ERP6 and ERP2…5 does not represents purely a genuine MMN, but stems from non-refractory neurons, generating the N100. Prior research has shown that part of the activity elicited by deviant stimuli might be referred to refractoriness, especially in the ascending slope of the MMN (pitch deviants: Jacobsen and Schröger, 2001; duration deviants: Jacobsen and Schröger, 2003; intensity deviants: Jacobsen et al., 2003). In lack of a proper control condition, applying standards as deviants and vice versa, data have to be interpreted with some precautions.
In four subjects, ERP6 resembled ERP1 in latency and shape at the MMN electrode. In those cases, the difference potential between ERP6 and ERP2…5 was not assumed to reflect a MMN, but activity of non-refractory neurons. After exclusion of these subjects, the difference between ERP1 and ERP2…5 at the MMN electrode did not reach statistical significance anymore, but ERP1 still exhibited a substantial signal. This might indicate that the difference potential between ERP6 and ERP2…5 is still contaminated by activity of non-refractory neurons. However, while the N100 amplitudes at the N100 electrode (and Cz) have the order ERP1 > ERP6 > ERP2…5, the order of the peak amplitudes at the MMN electrode is different (ERP6 > ERP1 > ERP2…5) (Fig. 2) and gives evidence that the difference potential ERPMMN reflects to a larger amount a genuine MMN. The ERPMMN crossed on average the zero line at 200 ms, comparable to previous studies (Tiitinen et al., 1994). There is no clear latency correspondence between ERPMMN as recorded at Cz and at the MMN electrode. ERPMMN peaks at the intracranial electrode right between the two peaks, which became visible in the surface recordings at Cz. On a tentative basis, one might refer the difference in timing to a contamination of the surface ERP by N200b, as it is followed by a positive deflection, maybe reflecting a P300b. Neither the possible impact of an N200b nor of a P300b can be verified for the current data set, as both would require a topographical analysis. Another possible explanation could be that ERPMMN, as measured at the MMN electrode, reflects only a partial process of the MMN, but again this remains speculative on basis of the current data.
The application of a fixed order in the train (sixth tone always deviating) was not assumed to have a major impact on the observation of an MMN, as expectancy was reported to have no effect on the elicitation of an MMN (Ritter et al., 1999). The MMN can be elicited in the absence of attention and in the majority of studies attention did not modulate the MMN amplitude significantly (e.g. Alho et al., 1989; Kaukoranta et al., 1989), but a modulation of the MMN amplitude by attention factors cannot completely be ruled out (Alain and Woods, 1997; Woldorff et al., 1998) and might be observed especially when deviants and standards are difficult to discriminate (Müller et al., 2002a). In the current study, also the anticonvulsant medication of the patients could possibly have influenced the amplitude and latency of the MMN. Different classes of substances are prescribed as anticonvulsants and effects of anticonvulsants such as carbamazepine, phenytoin or valproic acid on MMN have not been investigated as yet. Benzodiazepines which are also used as antiepileptic medication were shown to reduce the MMN amplitude (Rosburg et al., 2004c) and one might also expect a general dampening of ERP components by anticonvulsants. However, neither drugs nor attention effects were ever reported to alter the topography of the MMN systematically, but N200b and P300b, i.e. additional activity after 200 ms, might be observed if subjects pay attention to stimulus material.
Previous studies by means of non-invasive electrophysiological recordings have already given good evidence for a bilateral temporal generator, but a confirmation by invasive recordings rested on few studies (Halgren et al., 1995; Kropotov et al., 1995, 2000; Liasis et al., 2000). In line with these previous findings, most MMN deflections were currently observed over the temporal lobe. Evidence for temporal (and frontal) generators participating in MMN generation also stems from fMRI studies. In general, fMRI is well suited for the detection of brain areas involved in the process of deviance detection due to its spatial resolution, but one has to be aware that its low temporal resolution does not allow a clear differentiation between processes associated with a response recovery of the N100, genuine MMN and later components which are associated with more conscious discrimination processes (especially P300a). The response recovery of the N100 (and other components) has to be regarded as a fundamental problem of MMN studies by fMRI, as most of them apply deviants with a large difference to the standards to overcome the problem of a noisy environment in the scanner and these deviants result very likely in a response recovery.
The exact determination of the brain regions involved in the generation of the N100 and MMN is, however, also handicapped in the current study. Depth electrodes in the auditory cortex allow a much more precise localization of the temporal MMN generator, as indicated by the studies of Godey et al. (2001), but these electrode placements are not implemented in our clinical diagnosis procedure. Furthermore, owing to the insufficient coverage of the auditory cortex with electrodes in the current study, a source reconstruction algorithm was not regarded as a very useful way of data analysis. A negative potential with relatively steep gradient to neighbouring electrodes could be observed for the N100 and MMN in the majority of recordings (exemplary data in Figs 4B, 5A and B). A negative potential with no neighbouring positive potential indicates most likely a source with respect to the brain surface radial orientation. The closer a radial source to the brain surface, the steeper gradients can be expected. A tangential source would result in a bipolar potential structure, and simultaneous positive and negative potentials in close vicinity to each other can be regarded as an indicator for a tangential source close to the brain surface. Overall, tangential and radial sources can hardly be differentiated in the current recordings. In two cases a bipolar potential over the temporal lobe was detected, once for the N100 and once for the MMN. In one patient a neighbouring electrode to the electrode with the maximal N100 response exhibited a simultaneous positive potential (Fig. 4A). In that case we would expect the generator between both electrodes slightly under the brain surface. In another patient the superior and middle temporal gyrus was covered with electrodes and a bipolar structure was detected for the MMN, but no potential at all for the N100. Positive deflections could simply not have been observed because they might occur at the middle and inferior temporal gyri which were nearly never covered by arrays. As the observation of N100 outnumbered the observations of P100 and the negative potentials were often stronger than their positive counterparts, one may conclude that the observed negative potentials at intracranial electrodes are more likely to originate from primarily radial sources. For the N100 both a radial and tangential component have been described previously (Näätänen and Picton, 1987). However, even for the tangential source in the temporal lobe, the model of a single and/or stationary source might be insufficient for the description of the N100 (and MMN) (Lütkenhöner, 2003; Rosburg et al., 2002, 2004a). Therefore, current findings have to be interpreted with caution not only owing to the placement and extension of the used electrode arrays.
Individual data of an N100 (A) with and (B) without a phase reversal on the neighbouring electrodes (Patients 110 and 130, respectively); a phase reversal at neighbouring electrodes was, however, an exceptional observation.
Individual data for the MMN as difference potential (ERP6 – ERP2…5) at the MMN electrode and two neighboring leads: (A) at temporal electrodes in Patient 126, (B) at temporal electrodes in Patient 118, and (C) at frontal electrodes in Patient 123. The neighbouring leads are only indicated in the schematic electrode view by grey filled circles.
These limitations are also true for the frontal MMN generator. In some of the patients, the frontal brain was covered by a subdural grid and in two a frontal MMN-like deflection was observed on the surface of the left lateral inferior frontal cortex (Fig. 3C and D). This activation was spatially separated from the temporal MMN. The involvement of left frontal structures is somewhat surprising with respect to earlier studies, predominantly reporting a right frontal activation (e.g. Giard et al., 1990; Opitz et al., 2002), but also in other studies an involvement of left frontal structures was shown (Rinne et al., 2000; Müller et al., 2002b). The current observation of a negative intracranial MMN potential with no simultaneous positive potential at neighbouring electrodes possibly indicates a radial source. A radial orientation would be in line with the insensitivity of MEG to detect activity generated by this source (Rinne et al., 2000). In one patient (126) the negative deflection was found at the edge of the electrode array (Fig. 3C). In the second patient (123), the frontal MMN exhibited a spatially broad negative deflection, making it likely that the potential is generated in deeper structures of the frontal cortex (Figs 3D and 5C). For reasons outlined above, the current study is not capable to clarify the exact configuration and location of the frontal MMN source.
In one patient an MMN like response was detected at an interhemispheric electrode position in close vicinity to the cingulum (Fig. 3E), giving possible evidence for a participation of the cingulum in MMN generation. However, for this subject it is difficult to rule out that a response recovery of the N100 has contributed to the observed signal. An involvement of the cingulum has been suggested for both MMN and N100 generation (Mulert et al., 2001; Waberski et al., 2001). Unfortunately, the current sample included only three patients with interhemispheric electrodes at frontal sites, not allowing any systematic investigation.
No evidence could be found for a participation of the parietal lobe in MMN generation. Up to now, such a participation was reported in three studies (Levänen et al., 1996; Kasai et al., 1999; Schall et al., 2003). Our negative finding might be referred to the low number of patients with electrodes placed over this area (Table 1). Other possible reasons might be that the contribution of parietal lobe to MMN is rather small and/or depends on the experimental conditions. In recent studies the parietal MMN source was activated clearly after the temporal and frontal sources (Kasai et al., 1999; Levänen et al., 1996), leaving the possibility that the parietal lobe source is associated to other processes than pre-attentive mismatch detection.
Taken together, the current findings confirmed the generation of the MMN in the temporal lobe by means of subdural recordings and gave some additional evidence for a frontal MMN component, while a contribution of the parietal lobe was not detectable in the current data. However, further series with intracranial recordings are necessary to clarify the frontal contribution to MMN generation, as a frontal MMN was observed in 2 of 9 patients with a frontal electrode grid and 1 of 3 patients with interhemispheric electrode strips only. Future studies in our group will include a larger sample of patients with interhemispheric electrodes and apply a modified experimental design, reducing a possible impact of N100 response recovery and attention on results that cannot be ruled out explicitly in the current study.
Supported by the NIH under grant number R01 MH063476.
References
Alain C, Woods DL. Attention modulates auditory pattern memory as indexed by event-related brain potentials.
Aleksandrov AA, Babanin ME, Stankevich LN. Mechanisms of generation of mismatch negativity and their role in the recognition of brief auditory stimuli.
Alho K, Sams M, Paavilainen P, Reinikainen K, Näätänen R. Event-related brain potentials reflecting processing of relevant and irrelevant stimuli during selective listening.
Alho K, Woods DL, Algazi A, Knight RT, Näätänen R. Lesions of frontal cortex diminish the auditory mismatch negativity.
Boutros NN, Bonnet KA, Millana R, Liu J. A parametric study of the N40 auditory evoked response in rats.
Boutros NN, Belger A, Campbell D, D'Souza C, Krystal J. Comparison of four components of sensory gating in schizophrenia and normal subjects: a preliminary report.
Celsis P, Boulanouar K, Doyon B, Ranjeva JP, Berry I, Nespoulous JL, et al. Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones.
Csepe V, Karmos G, Molnar M. Evoked potential correlates of stimulus deviance during wakefulness and sleep in cat–animal model of mismatch negativity.
Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schröger E. Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence.
Frodl-Bauch T, Kathmann N, Möller HJ, Hegerl U. Dipole localization and test-retest reliability of frequency and duration mismatch negativity generator processes.
Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study.
Godey B, Schwartz D, de Graaf JB, Chauvel P, Liegeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients.
Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, et al. Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe.
Hari R, Hämäläinen M, Ilmoniemi R, Kaukoranta E, Reinikainen K, Salminen J, et al. Responses of the primary auditory cortex to pitch changes in a sequence of tone pips: neuromagnetic recordings in man.
Jacobsen T, Horenkamp T, Schröger E. Preattentive memory-based comparison of sound intensity.
Jacobsen T, Schröger E. Is there pre-attentive memory-based comparison of pitch?
Jacobsen T, Schröger E. Measuring duration mismatch negativity.
Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation.
Jemel B, Achenbach C, Müller BW, Ropcke B, Oades RD. Mismatch negativity results from bilateral asymmetric dipole sources in the frontal and temporal lobes.
Kasai K, Nakagome K, Itoh K, Koshida I, Hata A, Iwanami A, et al. Multiple generators in the auditory automatic discrimination process in humans.
Kaukoranta E, Sams M, Hari R, Hamalainen M, Näätänen R. Reactions of human auditory cortex to a change in tone duration.
Kraus N, McGee T, Littman T, Nicol T, King C. Nonprimary auditory thalamic representation of acoustic change.
Kropotov JD, Alho K, Näätänen R, Ponomarev VA, Kropotova OV, Anichkov AD, et al. Human auditory-cortex mechanisms of preattentive sound discrimination.
Kropotov JD, Näätänen R, Sevostianov AV, Alho K, Reinikainen K, Kropotova OV. Mismatch negativity to auditory stimulus change recorded directly from the human temporal cortex.
Levänen S, Ahonen A, Hari R, McEvoy L, Sams M. Deviant auditory stimuli activate human left and right auditory cortex differently.
Liasis A, Towell A, Boyd S. Intracranial evidence for differential encoding of frequency and duration discrimination responses.
Liasis A, Towell A, Alho K, Boyd S. Intracranial identification of an electric frontal-cortex response to auditory stimulus change: a case study.
Liebenthal E, Ellingson ML, Spanaki MV, Prieto TE, Ropella KM, Binder JR. Simultaneous ERP and fMRI of the auditory cortex in a passive oddball paradigm.
Lütkenhöner B. Single-dipole analyses of the N100m are not suitable for characterizing the cortical representation of pitch.
Mathiak K, Rapp A, Kircher TTJ, Grodd W, Hertrich I, Weiskopf N, et al. Mismatch responses to randomized gradient switching noise as reflected by fMRI and whole-head magnetoencephalography.
Mulert C, Gallinat J, Pascual-Marqui R, Dorn H, Frick K, Schlattmann P, et al. Reduced event-related current density in the anterior cingulate cortex in schizophrenia.
Müller BW, Achenbach C, Oades RD, Bender S, Schall U. Modulation of mismatch negativity by stimulus deviance and modality of attention.
Müller BW, Juptner M, Jentzen W, Müller SP. Cortical activation to auditory mismatch elicited by frequency deviant and complex novel sounds: a PET study.
Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure.
Opitz B, Mecklinger A, Von-Cramon DY, Kruggel F. Combining electrophysiological and hemodynamic measures of the auditory oddball.
Opitz B, Rinne T, Mecklinger A, von-Cramon DY, Schröger E. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results.
Paavilainen P, Jiang D, Lavikainen J, Näätänen R. Stimulus duration and the sensory memory trace: an event-related potential study.
Park HJ, Kwon JS, Youn T, Pae JS, Kim JJ, Kim MS, et al. Statistical parametric mapping of LORETA using high density EEG and individual MRI: application to mismatch negativities in schizophrenia.
Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Näätänen R. Separate time behaviors of the temporal and frontal mismatch negativity sources.
Ritter W, Sussman E, Deacon D, Cowan N, Vaughan HG Jr. Two cognitive systems simultaneously prepared for opposite events.
Rosburg T, Haueisen J, Sauer H. Stimulus duration influences the dipole location shift within the auditory evoked field component N100m.
Rosburg T, Haueisen J. Kreitschmann-Andermahr I. The dipole location shift within the auditory evoked neuromagnetic field components N100m and mismatch negativity (MMNm).
Rosburg T, Kreitschmann-Andermahr I, Sauer H. [Mismatch negativity in schizophrenia research. An indicator of early processing disorders of acoustic information.]
Rosburg T, Marinou V, Haueisen J, Smesny S, Sauer H. Effects of lorazepam on the neuromagnetic mismatch negativity (MMNm) and auditory evoked field component N100m.
Rosburg T, Trautner P, Korzyukov OA, Boutros NN, Schaller C, Elger CE, Kurthen M. Short term habituation of the intracranially recorded auditory evoked potentials P50 and N100.
Sams M, Hämäläinen M, Antervo A, Kaukoranta E, Reinikainen K, Hari R. Cerebral neuromagnetic responses evoked by short auditory stimuli.
Schall U, Johnston P, Todd J, Ward PB, Michie PT. Functional neuroanatomy of auditory mismatch processing: an event-related fMRI study of duration-deviant oddballs.
Sevostianov A, Fromm S, Nechaev V, Horwitz B, Braun A. Effect of attention on central auditory processing: an fMRI study.
Tervaniemi M, Medvedev SV, Alho K, Pakhomov SV, Roudas MS, Van-Zuijen TL, et al. Lateralized automatic auditory processing of phonetic versus musical information: a PET study.
Waberski TD, Kreitschmann-Andermahr I, Kawohl W, Darvas F, Ryang Y, Gobbele R, et al. Spatio-temporal source imaging reveals subcomponents of the human auditory mismatch negativity in the cingulum and right inferior temporal gyrus.
Wible CG, Kubicki M, Yoo SS, Kacher DF, Salisbury DF, Anderson MC, et al. A functional magnetic resonance imaging study of auditory mismatch in schizophrenia.
Author notes
1Department of Epileptology and 2Department of Neurosurgery, University of Bonn, Germany and 3Yale University, School of Medicine, Department of Psychiatry, West Haven, USA