-
PDF
- Split View
-
Views
-
Cite
Cite
Rodger J. Elble, Seth L. Pullman, Joseph Y. Matsumoto, Jan Raethjen, Günther Deuschl, Ron Tintner, Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales, Brain, Volume 129, Issue 10, October 2006, Pages 2660–2666, https://doi.org/10.1093/brain/awl190
Close - Share Icon Share
Abstract
Tremor rating scales (TRSs) are used commonly in the clinical assessment of tremor, but the relationship of a TRS to actual tremor amplitude has never been quantified. Consequently, the resolution of these scales is unknown, and the clinical significance of a 1-point change in TRS is uncertain. We therefore sought to determine the change in tremor amplitude that corresponds to a 1-point change in a typical 5-point TRS. Data from five laboratories were analysed, and 928 patients with various types of hand tremor were studied. Hand tremor was quantified with a graphics tablet in three different labs, an accelerometer in three labs and a mechanical-linkage device in one lab. Tremor in writing, drawing, horizontal posture, rest and finger–nose testing was graded using a variety of TRSs. The relationship between TRS scores and tremor amplitude was computed for each task and laboratory. A logarithmic relationship between a 5-point (0–4) TRS and tremor amplitude (T, measured in centimetres) was found in all five labs, despite widely varying rating scales and transducer methodology. Thus, T2/T1 = 10α(TRS2−TRS1). The value of α ranged from 0.414 to 0.441 for writing, 0.355–0.574 for spiral drawing, 0.441 to 0.488 for rest tremor, 0.266–0.577 for postural tremor and 0.306 for finger–nose testing. For α = 0.3, 0.4, 0.5, 0.6 and 0.7, the ratios T2/T1 for a 1-point decrease in TRS are 0.501, 0.398, 0.316, 0.251 and 0.200. Therefore, a 1-point change in TRS represents a substantial change in tremor amplitude. Knowledge of the relationship between TRS and precise measures of tremor is useful in interpreting the clinical significance of changes in TRS produced by disease or therapy.
Abbreviations
Introduction
Tremor rating scales (TRSs) such as the scale of Fahn et al. (1993) are used commonly in the clinical assessment of tremor, but the relationship of a TRS to actual tremor amplitude has never been quantified. Consequently, the resolution of the TRS and similar scales is unknown, and more importantly, it is virtually impossible to compare the results of clinical studies using a TRS with those using motion transducers such as accelerometers and digitizing tablets.
Material and methods
Four hundred and thirty patients met the consensus criteria for classic (definite) essential tremor (ET), developed by the Movement Disorder Society's Ad Hoc Committee on Tremor (Deuschl et al., 1998). These patients were studied in one of four laboratories run by the authors (Columbia University in New York, Mayo Clinic in Rochester Minnesota, Southern Illinois University School of Medicine (SIU) and Christian Albrechts University in Kiel Germany) and at Emory University in Atlanta, Georgia, where three patients were examined by 10 members of the Tremor Research Group. In addition, 498 patients with other tremor disorders were studied at Kiel and were diagnosed using the guidelines of the Movement Disorder Society's Ad Hoc Committee on Tremor (Deuschl et al., 1998).
Columbia
Tremor was assessed using the Fahn–Tolosa–Marín scale (Fahn et al., 1993) the same day as the testing in more than half the patients and within 4 weeks for the others. The Fahn–Tolosa–Marín rating scale uses the following criteria:
Grade 0 = No tremor.
Grade 1 = Slight, <0.5 cm; may be intermittent.
Grade 2 = Moderate, 0.5–1 cm; may be intermittent.
Grade 3 = Marked, 1–2 cm.
Grade 4 = Severe, >2 cm.
Accelerometry was used to quantify kinetic tremors in 49 adult (26 males) patients (ages: 63.2 ± 14.6, range: 27–86 years), as described previously (Louis and Pullman, 2001). The accelerometer was attached to the dorsum of the hand at the distal end of the middle carpus bone. All patients moved finger to nose in a horizontal plane with the arms abducted horizontally, elbows directed laterally and the wrists and index fingers straight.
Handwritten spirals were recorded from 52 adult (26 males) patients (ages: 64.6 ± 14.2, range: 25—85years) on a data acquisition graphics tablet (Wacom Technology, Vancouver, WA, USA), as described previously (Pullman, 1998). The tablet had an accuracy of ±0.25 mm, an output rate of 200 points/s. Patients were seated comfortably and instructed to start in the centre of a 10 × 10 cm square on white paper and freely draw spirals within the square. An electronic pen with ballpoint ink cartridge was held in a normal fashion without constraints to allow for full visual feedback of the spirals as they were drawn. Ten spirals were collected from each hand. For each patient, the spirals with greatest and least tremor were discarded, and tremor amplitudes from the remaining eight were averaged.
Mayo
Postural tremor was recorded from 22 adult patients (11 males; mean age: 70, range: 47–83) using a three-dimensional mechanical-linkage device that was attached to the tip of the index finger (Matsumoto et al., 1999). Three-dimensional displacement of the finger was recorded with three precision potentiometers. Patients were seated in a chair while their hand and forearm were held horizontally, with the elbow flexed 90° and the shoulder abducted 70–90°. The entire limb was free to move at all joints while the patients pointed their index finger at a 4 mm target, several centimetres in front of the finger.
Tremor was also assessed using a modified Fahn–Tolosa–Marín rating scale in which postural tremor was graded on an integer scale of 0 to 4 using the following criteria:
Grade 0 = No tremor.
Grade 1 = Slight, barely perceivable; may be intermittent.
Grade 2 = Moderate; amplitude < 2 cm; may be intermittent.
Grade 3 = Marked; amplitude 2–4 cm.
Grade 4 = Severe; amplitude > 4 cm.
The clinical rating scale was done first on each patient, and the instrumental measure was performed immediately thereafter.
Southern Illinois University School of Medicine (SIU)
Postural tremor was recorded for 60 s from the horizontally extended hand (palm down) of 137 adult patients (74 males), with an accelerometer (Elble et al., 1994). The patients' ages ranged from 18 to 102 years (mean ± SD = 60 ± 17). The accelerometer was mounted on a plastic splint that was strapped to the dorsum of the hand, with fingers extended. The forearm was pronated and supported so as to restrict motion to the wrist. Postural tremor was rated with the Fahn–Tolosa–Marín rating scale within 4 weeks of the accelerometric recordings.
Tremor in writing (series of cursive e and l) and drawing (Archimedes spiral) was recorded with a digitizing tablet from 30 adult patients (17 males), aged 20–84 years (mean ± SD = 60 ± 17), as described previously (Elble et al., 1996). Writing and drawing tremor were rated with the Fahn–Tolosa–Marín rating scale within 4 weeks of the tablet studies.
Kiel
Seven hundred and twenty-one patients (381 males) with tremor were studied. Two hundred and twenty-three (109 males; mean age: 57; range: 11–89) had ET, and 498 patients (272 males; mean age: 56; range: 10–89) had other conditions (parkinsonism: 49.4%, physiological tremor: 1.8%, enhanced physiological tremor: 11.2%, cerebellar tremor: 5.8%, dystonic tremor: 6.2%, Holmes tremor: 2.0%, myoclonus: 2.2%, neuropathic tremor: 1.8%, psychogenic tremor: 4.0%, orthostatic tremor: 1.2%, Wilson disease: 0.6% and uncertain diagnosis: 13.7%).
The methods of accelerometric measurement are described elsewhere (Timmer et al., 1996). Postural tremor was recorded for 30 s from the horizontally extended hand (palm down), with an accelerometer mounted on the dorsum of the hand, 9 cm distal to the processus styloideus ulnae. The horizontal forearm was supported, so as to restrict motion to the wrist. Rest tremor was recorded in the same manner, but the patients were instructed to relax and allow the hand to dangle over the edge of the forearm support. The accelerometric measurements were performed within five minutes before or after the rating scale assessments.
Rest tremor was rated by disability (social handicap): 0 = no social handicap 1 = occasional embarrassment in social settings; 2 = avoids certain social situations; 3 = avoids all social contacts. Postural tremor was rated by judging the amount of water spilled while pouring from a full test tube into another (0 = no tremor; 1 = <30% of the water is spilled; 2 = >30% of the water is spilled; 3 = pouring is not possible because of tremor). This rating scale has been used to assess ET patients previously and is described in detail elsewhere (Deuschl et al., 2000).
Twenty-nine patients with ET (18 males; mean age: 62; range: 30–80) were also assessed with the original Fahn–Tolosa–Marín rating scale.
Tremor Research Group
Three adult patients with mild, moderate and severe ET wrote a sentence with the dominant hand, drew a spiral with each hand (forearm unsupported) and held a pen vertically 1–3 mm over a dot on a piece of paper with each hand (forearm unsupported), while 10 members of the Tremor Research Group simultaneously graded the tremor in each of the five tasks. These 10 movement disorder specialists performed the ratings while another member of the group (Ron Tintner) conducted the examinations. The patients performed all tasks on paper that was mounted on a Wacom Intuos 2 digitizing tablet (Wacom Technology), which digitized the writing and drawings at 100 X–Y coordinates per second, with an accuracy of ±0.25 mm. The electronic ballpoint pen was wireless and comparable with a standard pen. The mean of the 10 ratings for each task was taken as the true rating for each patient. The following modification of the Fahn–Tolosa–Marín rating scale was used to rate the tremor in each task:
Archimedes spiral (6.5 cm outer diameter).
Grade 0 = No tremor.
Grade 1 = Very slight intermittent tremor—barely visible.
Grade 2 = Nearly continuous, mild tremor < 1 cm.
Grade 3 = Moderate tremor > 1 cm. Accomplishes the task with great difficulty. Largely illegible.
Grade 4 = Unable to complete drawing. Figure not recognizable.
Handwriting (‘Today is a nice day’).
Grade 0 = No tremor.
Grade 1 = Mildly abnormal. Slightly untidy, tremulous.
Grade 2 = Moderately abnormal. Legible, but with considerable tremor.
Grade 3 = Markedly abnormal. Illegible.
Grade 4 = Severely abnormal. Unable to keep pencil or pen on paper.
Hold pen vertically for 10 s as close as possible (ideally 1–2 mm) to a point on a sheet of paper, without touching the paper.
Grade 0 = No tremor.
Grade 1 = Tremor is barely visible.
Grade 1.5 = Tremor is visible, but <1 cm.
Grade 2 = Tremor is 1–3 cm amplitude.
Grade 2.5 = Tremor is 3–5 cm amplitude.
Grade 3 = Tremor is 5–10 cm amplitude.
Grade 3.5 = Tremor is 10–20 cm amplitude.
Grade 4 = Tremor is >20 cm amplitude.
The mean TRS scores and the tablet data for the five tasks were pooled from the three patients, and the relationship between TRS scores and tremor amplitudes was computed (n = 3 × 5 = 15 data points).
Data analysis
In all experiments, the digitized data from the motion transducers were acquired with a personal computer and custom software. At Columbia, hand tremor amplitudes were derived off-line by double integration of accelerometric data, after filtering low-frequency drift (<2 Hz) and averaging (Louis and Pullman, 2001). Tremor frequencies were calculated using a fast Fourier transform algorithm. At the other labs, the root-mean-square amplitude and frequency of tremor were computed from the digitized data using a fast Fourier transform (Elble et al., 1994; Elble et al., 1996; Timmer et al., 1996;,Matsumoto et al., 1999).
Curve fitting and statistical analyses were performed with Slide Write Plus version 6.1 (Advanced Graphics Software, Encinitas, CA, USA) and Systat 11 (Systat Software, Inc., Richmond, CA, USA) software. Patients with missing data were excluded. Earlier observations by two of us (Elble et al., 1996; Matsumoto et al., 1999) indicated a logarithmic relationship between TRS and tremor amplitude, consistent with Weber–Fechner relationship (Equation 2). We performed statistical curve fitting of our data to Equation 2 to compute the values of α and β. We also performed curve fitting to the Stevens power function (Equation 4) in case this provided a better representation of our data.
Results
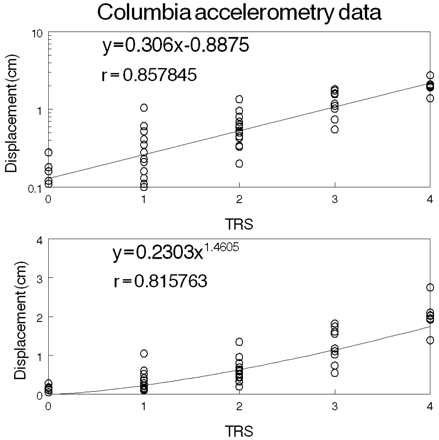
Scatter plots revealed a non-linear relationship between tremor amplitude and TRS data for all tremor measurements at the four laboratories and Tremor Research Group (Fig. 1). Equations 2 and 4 provided virtually equivalent approximations for our data. The correlation coefficients (r-values, Table 1) were nearly identical for the two equations.
The Columbia accelerometry data are plotted versus TRS and fitted to the Weber–Fechner equation (upper graph) and the Stevens equation (lower graph).
Summary of results
| Laboratory . | Method . | . | No. of patients . | log T = α · TRS + β . | T = a · TRSb . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | ||||
| . | . | . | . | α . | β . | r* . | a . | b . | r* . |
| Columbia | Accelerometry | Kinetic hand tremor | 49 | 0.306 | −0.888 | 0.858 | 0.230 | 1.46 | 0.816 |
| Columbia | Digitizing tablet Spiral | Hand tremor | 52 | 0.355 | −1.19 | 0.839 | 0.160 | 1.44 | 0.827 |
| Mayo | Linkage device | Postural finger tremor | 22 | 0.577 | −2.24 | 0.944 | 0.0123 | 3.14 | 0.950 |
| SIU | Digitizing tablet Cursive e | Hand tremor | 29 | 0.414 | −2.25 | 0.815 | 0.0144 | 1.67 | 0.797 |
| SIU | Digitizing tablet Cursive l | Hand tremor | 30 | 0.441 | −2.25 | 0.804 | 0.015 | 1.79 | 0.794 |
| SIU | Digitizing tablet Spiral | Hand tremor | 29 | 0.574 | −2.50 | 0.867 | 0.0114 | 2.23 | 0.842 |
| SIU | Accelerometry | Postural hand tremor | 137 | 0.545 | −2.05 | 0.612 | 0.0262 | 2.33 | 0.567 |
| Kiel | Accelerometry | Rest tremor (ET)** | 217 | 0.588 | −3.02 | 0.579 | 0.0024 | 3.43 | 0.594 |
| Kiel | Accelerometry | Postural tremor (ET)** | 221 | 0.355 | −2.64 | 0.407 | 0.0039 | 1.83 | 0.461 |
| Kiel | Accelerometry | Rest tremor (non-ET)** | 485 | 0.651 | −3.00 | 0.601 | 0.0035 | 2.94 | 0.499 |
| Kiel | Accelerometry | Postural tremor (non-ET)** | 493 | 0.419 | −2.70 | 0.437 | 0.0046 | 1.85 | 0.409 |
| Kiel | Accelerometry | Postural hand tremor (ET)*** | 29 | 0.417 | −1.192 | 0.544 | 0.326 | 0.916 | 0.318 |
| TRG | Digitizing tablet (multiple tasks) | Hand tremor | 3 | 0.502 | −1.49 | 0.883 | 0.0611 | 2.61 | 0.845 |
| Laboratory . | Method . | . | No. of patients . | log T = α · TRS + β . | T = a · TRSb . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | ||||
| . | . | . | . | α . | β . | r* . | a . | b . | r* . |
| Columbia | Accelerometry | Kinetic hand tremor | 49 | 0.306 | −0.888 | 0.858 | 0.230 | 1.46 | 0.816 |
| Columbia | Digitizing tablet Spiral | Hand tremor | 52 | 0.355 | −1.19 | 0.839 | 0.160 | 1.44 | 0.827 |
| Mayo | Linkage device | Postural finger tremor | 22 | 0.577 | −2.24 | 0.944 | 0.0123 | 3.14 | 0.950 |
| SIU | Digitizing tablet Cursive e | Hand tremor | 29 | 0.414 | −2.25 | 0.815 | 0.0144 | 1.67 | 0.797 |
| SIU | Digitizing tablet Cursive l | Hand tremor | 30 | 0.441 | −2.25 | 0.804 | 0.015 | 1.79 | 0.794 |
| SIU | Digitizing tablet Spiral | Hand tremor | 29 | 0.574 | −2.50 | 0.867 | 0.0114 | 2.23 | 0.842 |
| SIU | Accelerometry | Postural hand tremor | 137 | 0.545 | −2.05 | 0.612 | 0.0262 | 2.33 | 0.567 |
| Kiel | Accelerometry | Rest tremor (ET)** | 217 | 0.588 | −3.02 | 0.579 | 0.0024 | 3.43 | 0.594 |
| Kiel | Accelerometry | Postural tremor (ET)** | 221 | 0.355 | −2.64 | 0.407 | 0.0039 | 1.83 | 0.461 |
| Kiel | Accelerometry | Rest tremor (non-ET)** | 485 | 0.651 | −3.00 | 0.601 | 0.0035 | 2.94 | 0.499 |
| Kiel | Accelerometry | Postural tremor (non-ET)** | 493 | 0.419 | −2.70 | 0.437 | 0.0046 | 1.85 | 0.409 |
| Kiel | Accelerometry | Postural hand tremor (ET)*** | 29 | 0.417 | −1.192 | 0.544 | 0.326 | 0.916 | 0.318 |
| TRG | Digitizing tablet (multiple tasks) | Hand tremor | 3 | 0.502 | −1.49 | 0.883 | 0.0611 | 2.61 | 0.845 |
*P < 0.001 for all regression analyses; **4-point rating scale (ET = essential tremor); ***5-point Fahn–Tolosa–Marín rating scale.
Summary of results
| Laboratory . | Method . | . | No. of patients . | log T = α · TRS + β . | T = a · TRSb . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | ||||
| . | . | . | . | α . | β . | r* . | a . | b . | r* . |
| Columbia | Accelerometry | Kinetic hand tremor | 49 | 0.306 | −0.888 | 0.858 | 0.230 | 1.46 | 0.816 |
| Columbia | Digitizing tablet Spiral | Hand tremor | 52 | 0.355 | −1.19 | 0.839 | 0.160 | 1.44 | 0.827 |
| Mayo | Linkage device | Postural finger tremor | 22 | 0.577 | −2.24 | 0.944 | 0.0123 | 3.14 | 0.950 |
| SIU | Digitizing tablet Cursive e | Hand tremor | 29 | 0.414 | −2.25 | 0.815 | 0.0144 | 1.67 | 0.797 |
| SIU | Digitizing tablet Cursive l | Hand tremor | 30 | 0.441 | −2.25 | 0.804 | 0.015 | 1.79 | 0.794 |
| SIU | Digitizing tablet Spiral | Hand tremor | 29 | 0.574 | −2.50 | 0.867 | 0.0114 | 2.23 | 0.842 |
| SIU | Accelerometry | Postural hand tremor | 137 | 0.545 | −2.05 | 0.612 | 0.0262 | 2.33 | 0.567 |
| Kiel | Accelerometry | Rest tremor (ET)** | 217 | 0.588 | −3.02 | 0.579 | 0.0024 | 3.43 | 0.594 |
| Kiel | Accelerometry | Postural tremor (ET)** | 221 | 0.355 | −2.64 | 0.407 | 0.0039 | 1.83 | 0.461 |
| Kiel | Accelerometry | Rest tremor (non-ET)** | 485 | 0.651 | −3.00 | 0.601 | 0.0035 | 2.94 | 0.499 |
| Kiel | Accelerometry | Postural tremor (non-ET)** | 493 | 0.419 | −2.70 | 0.437 | 0.0046 | 1.85 | 0.409 |
| Kiel | Accelerometry | Postural hand tremor (ET)*** | 29 | 0.417 | −1.192 | 0.544 | 0.326 | 0.916 | 0.318 |
| TRG | Digitizing tablet (multiple tasks) | Hand tremor | 3 | 0.502 | −1.49 | 0.883 | 0.0611 | 2.61 | 0.845 |
| Laboratory . | Method . | . | No. of patients . | log T = α · TRS + β . | T = a · TRSb . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | ||||
| . | . | . | . | α . | β . | r* . | a . | b . | r* . |
| Columbia | Accelerometry | Kinetic hand tremor | 49 | 0.306 | −0.888 | 0.858 | 0.230 | 1.46 | 0.816 |
| Columbia | Digitizing tablet Spiral | Hand tremor | 52 | 0.355 | −1.19 | 0.839 | 0.160 | 1.44 | 0.827 |
| Mayo | Linkage device | Postural finger tremor | 22 | 0.577 | −2.24 | 0.944 | 0.0123 | 3.14 | 0.950 |
| SIU | Digitizing tablet Cursive e | Hand tremor | 29 | 0.414 | −2.25 | 0.815 | 0.0144 | 1.67 | 0.797 |
| SIU | Digitizing tablet Cursive l | Hand tremor | 30 | 0.441 | −2.25 | 0.804 | 0.015 | 1.79 | 0.794 |
| SIU | Digitizing tablet Spiral | Hand tremor | 29 | 0.574 | −2.50 | 0.867 | 0.0114 | 2.23 | 0.842 |
| SIU | Accelerometry | Postural hand tremor | 137 | 0.545 | −2.05 | 0.612 | 0.0262 | 2.33 | 0.567 |
| Kiel | Accelerometry | Rest tremor (ET)** | 217 | 0.588 | −3.02 | 0.579 | 0.0024 | 3.43 | 0.594 |
| Kiel | Accelerometry | Postural tremor (ET)** | 221 | 0.355 | −2.64 | 0.407 | 0.0039 | 1.83 | 0.461 |
| Kiel | Accelerometry | Rest tremor (non-ET)** | 485 | 0.651 | −3.00 | 0.601 | 0.0035 | 2.94 | 0.499 |
| Kiel | Accelerometry | Postural tremor (non-ET)** | 493 | 0.419 | −2.70 | 0.437 | 0.0046 | 1.85 | 0.409 |
| Kiel | Accelerometry | Postural hand tremor (ET)*** | 29 | 0.417 | −1.192 | 0.544 | 0.326 | 0.916 | 0.318 |
| TRG | Digitizing tablet (multiple tasks) | Hand tremor | 3 | 0.502 | −1.49 | 0.883 | 0.0611 | 2.61 | 0.845 |
*P < 0.001 for all regression analyses; **4-point rating scale (ET = essential tremor); ***5-point Fahn–Tolosa–Marín rating scale.
Table 2 contains the standard error for each estimate of α in Equation 2. Four of the Kiel values of α were computed using 4-point rating scales. The α for a 4-point TRS is 4/3 the α of a 5-point TRS. Therefore, the four values of α for the 4-point Kiel rating scales (0.588, 0.355, 0.651, 0.419 in Tables 1 and 2) correspond to values of 0.441, 0.266, 0.488, 0.314 for a 5-point scale.
Standard error for the estimates of α
| Laboratory . | Method . | . | log T = α · TRS + β . | |||
|---|---|---|---|---|---|---|
| . | . | . | . | |||
| . | . | . | α . | Standard error of α* . | Ratio T2 : T1 . | |
| . | . | . | . | . | . | |
| . | . | . | . | . | ΔTRS = 1 . | ΔTRS = −1 . |
| Columbia | Accelerometry | Kinetic hand tremor | 0.306 | 0.027 | 2.023 | 0.494 |
| Columbia | Digitizing tablet Spiral | Hand tremor | 0.355 | 0.033 | 2.265 | 0.442 |
| Mayo | Linkage device | Postural finger tremor | 0.577 | 0.045 | 3.776 | 0.265 |
| SIU | Digitizing tablet Cursive e | Hand tremor | 0.414 | 0.057 | 2.594 | 0.385 |
| SIU | Digitizing tablet Cursive l | Hand tremor | 0.425 | 0.060 | 2.661 | 0.376 |
| SIU | Digitizing tablet Spiral | Hand tremor | 0.574 | 0.063 | 3.750 | 0.267 |
| SIU | Accelerometry | Postural hand tremor | 0.545 | 0.061 | 3.508 | 0.285 |
| Kiel | Accelerometry | Rest tremor (ET)** | 0.588 | 0.056 | 3.873 | 0.258 |
| Kiel | Accelerometry | Postural tremor (ET)** | 0.355 | 0.054 | 2.265 | 0.442 |
| Kiel | Accelerometry | Rest tremor (non-ET)** | 0.651 | 0.039 | 4.477 | 0.223 |
| Kiel | Accelerometry | Postural tremor (non-ET)** | 0.419 | 0.039 | 2.624 | 0.381 |
| Kiel | Accelerometry | Postural hand tremor (ET)*** | 0.417 | 0.124 | 2.612 | 0.383 |
| TRG | Digitizing tablet multiple tasks) | Hand tremor | 0.502 | 0.074 | 3.177 | 0.315 |
| Laboratory . | Method . | . | log T = α · TRS + β . | |||
|---|---|---|---|---|---|---|
| . | . | . | . | |||
| . | . | . | α . | Standard error of α* . | Ratio T2 : T1 . | |
| . | . | . | . | . | . | |
| . | . | . | . | . | ΔTRS = 1 . | ΔTRS = −1 . |
| Columbia | Accelerometry | Kinetic hand tremor | 0.306 | 0.027 | 2.023 | 0.494 |
| Columbia | Digitizing tablet Spiral | Hand tremor | 0.355 | 0.033 | 2.265 | 0.442 |
| Mayo | Linkage device | Postural finger tremor | 0.577 | 0.045 | 3.776 | 0.265 |
| SIU | Digitizing tablet Cursive e | Hand tremor | 0.414 | 0.057 | 2.594 | 0.385 |
| SIU | Digitizing tablet Cursive l | Hand tremor | 0.425 | 0.060 | 2.661 | 0.376 |
| SIU | Digitizing tablet Spiral | Hand tremor | 0.574 | 0.063 | 3.750 | 0.267 |
| SIU | Accelerometry | Postural hand tremor | 0.545 | 0.061 | 3.508 | 0.285 |
| Kiel | Accelerometry | Rest tremor (ET)** | 0.588 | 0.056 | 3.873 | 0.258 |
| Kiel | Accelerometry | Postural tremor (ET)** | 0.355 | 0.054 | 2.265 | 0.442 |
| Kiel | Accelerometry | Rest tremor (non-ET)** | 0.651 | 0.039 | 4.477 | 0.223 |
| Kiel | Accelerometry | Postural tremor (non-ET)** | 0.419 | 0.039 | 2.624 | 0.381 |
| Kiel | Accelerometry | Postural hand tremor (ET)*** | 0.417 | 0.124 | 2.612 | 0.383 |
| TRG | Digitizing tablet multiple tasks) | Hand tremor | 0.502 | 0.074 | 3.177 | 0.315 |
*P < 0.001 for all estimates of α; **4-point rating scale (ET = essential tremor); ***5-point Fahn–Tolosa–Marín rating scale.
Standard error for the estimates of α
| Laboratory . | Method . | . | log T = α · TRS + β . | |||
|---|---|---|---|---|---|---|
| . | . | . | . | |||
| . | . | . | α . | Standard error of α* . | Ratio T2 : T1 . | |
| . | . | . | . | . | . | |
| . | . | . | . | . | ΔTRS = 1 . | ΔTRS = −1 . |
| Columbia | Accelerometry | Kinetic hand tremor | 0.306 | 0.027 | 2.023 | 0.494 |
| Columbia | Digitizing tablet Spiral | Hand tremor | 0.355 | 0.033 | 2.265 | 0.442 |
| Mayo | Linkage device | Postural finger tremor | 0.577 | 0.045 | 3.776 | 0.265 |
| SIU | Digitizing tablet Cursive e | Hand tremor | 0.414 | 0.057 | 2.594 | 0.385 |
| SIU | Digitizing tablet Cursive l | Hand tremor | 0.425 | 0.060 | 2.661 | 0.376 |
| SIU | Digitizing tablet Spiral | Hand tremor | 0.574 | 0.063 | 3.750 | 0.267 |
| SIU | Accelerometry | Postural hand tremor | 0.545 | 0.061 | 3.508 | 0.285 |
| Kiel | Accelerometry | Rest tremor (ET)** | 0.588 | 0.056 | 3.873 | 0.258 |
| Kiel | Accelerometry | Postural tremor (ET)** | 0.355 | 0.054 | 2.265 | 0.442 |
| Kiel | Accelerometry | Rest tremor (non-ET)** | 0.651 | 0.039 | 4.477 | 0.223 |
| Kiel | Accelerometry | Postural tremor (non-ET)** | 0.419 | 0.039 | 2.624 | 0.381 |
| Kiel | Accelerometry | Postural hand tremor (ET)*** | 0.417 | 0.124 | 2.612 | 0.383 |
| TRG | Digitizing tablet multiple tasks) | Hand tremor | 0.502 | 0.074 | 3.177 | 0.315 |
| Laboratory . | Method . | . | log T = α · TRS + β . | |||
|---|---|---|---|---|---|---|
| . | . | . | . | |||
| . | . | . | α . | Standard error of α* . | Ratio T2 : T1 . | |
| . | . | . | . | . | . | |
| . | . | . | . | . | ΔTRS = 1 . | ΔTRS = −1 . |
| Columbia | Accelerometry | Kinetic hand tremor | 0.306 | 0.027 | 2.023 | 0.494 |
| Columbia | Digitizing tablet Spiral | Hand tremor | 0.355 | 0.033 | 2.265 | 0.442 |
| Mayo | Linkage device | Postural finger tremor | 0.577 | 0.045 | 3.776 | 0.265 |
| SIU | Digitizing tablet Cursive e | Hand tremor | 0.414 | 0.057 | 2.594 | 0.385 |
| SIU | Digitizing tablet Cursive l | Hand tremor | 0.425 | 0.060 | 2.661 | 0.376 |
| SIU | Digitizing tablet Spiral | Hand tremor | 0.574 | 0.063 | 3.750 | 0.267 |
| SIU | Accelerometry | Postural hand tremor | 0.545 | 0.061 | 3.508 | 0.285 |
| Kiel | Accelerometry | Rest tremor (ET)** | 0.588 | 0.056 | 3.873 | 0.258 |
| Kiel | Accelerometry | Postural tremor (ET)** | 0.355 | 0.054 | 2.265 | 0.442 |
| Kiel | Accelerometry | Rest tremor (non-ET)** | 0.651 | 0.039 | 4.477 | 0.223 |
| Kiel | Accelerometry | Postural tremor (non-ET)** | 0.419 | 0.039 | 2.624 | 0.381 |
| Kiel | Accelerometry | Postural hand tremor (ET)*** | 0.417 | 0.124 | 2.612 | 0.383 |
| TRG | Digitizing tablet multiple tasks) | Hand tremor | 0.502 | 0.074 | 3.177 | 0.315 |
*P < 0.001 for all estimates of α; **4-point rating scale (ET = essential tremor); ***5-point Fahn–Tolosa–Marín rating scale.
After converting the Kiel α-values to a 5-point scale, we computed the correlations between α and β in Table 1, and between α and the computed value of logT at TRS = 4, to see if α was influenced by the clinicians' definitions of normal and most severe tremor (i.e. TRS = 0 and 4). The correlations were −0.186 (P = 1.0) and 0.371 (P = 0.547).
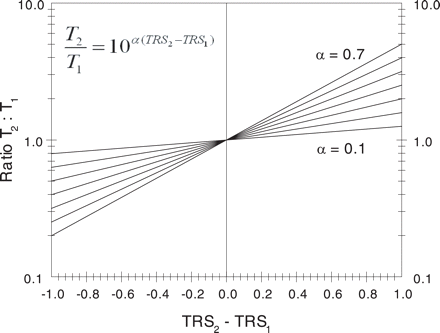
The ratio of final (T2) to initial (T1) tremor amplitude is plotted versus the change in TRS scores, using the equation shown and values of α equal to 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 and 0.7.
Discussion
Psychophysics is the scientific study of relationships between physical stimuli and the sensations or perceptions evoked by these stimuli (Gescheider, 1997). In the case of tremor, a clinician's sensation or perception is expressed as a TRS score, and the physical stimulus is tremor amplitude. We found that the Weber–Fechner law of psychophysics (Equation 2) is as good as Stevens' power law (Equation 4) for describing the relationship between a 4- or 5-point TRS and precision measurements of tremor. The values of α in Equation 2 ranged from 0.266 to 0.577, after converting the Kiel values to a 5-point rating scale.
We searched the literature for additional published TRS and tremor amplitude data in order to confirm our range of α, and we found only the study by Morgan et al. (1975), in which hand tremor was assessed in 30 patients with various aetiologies of intention tremor. Tremor was measured with an accelerometer during horizontal movement of the pronated limb from the shoulder to a target at arm's length in front of the patient. We computed α for their data and obtained a value of 0.282 (standard error = 0.021, r = 0.866, P < 0.001). Morgan et al. did not report the tremor frequency for each patient, so we could not compute α for tremor displacement versus TRS, as was done with our data. The value of α for Morgan's data would be higher if tremor frequency was lower in the patients with greater tremor because tremor displacement is approximately equal to acceleration divided by the squared frequency in radians per second (Elble and Koller, 1990). Dividing the larger tremors by lower squared frequencies would make the logT versus TRS curves steeper (i.e. α greater).
Our data were derived from five independent studies involving multiple raters and vastly different 4- and 5-point TRSs. Many of our patients had ET, but diagnosis per se had no apparent influence on α (Tables 1 and 2). Consequently, we suspect that the range of α in this report is representative of most published studies in which hand tremor was assessed during rest, posture, drawing, writing or finger–nose–finger testing. Therefore, we recommend a value of α in the range of 0.3–0.6 when Equations 5 or 6 are used to estimate the change in tremor amplitude corresponding to a change in TRS.
A logarithmic relationship between TRS and tremor amplitude was previously reported by two of us (Elble et al., 1996; Matsumoto et al., 1999). Owing to this relationship, the estimated linear relationship between TRS and amplitude will be predictably poor, and this fact probably explains the poor linear correlations between rating scales and transducer measurements, reported by others (Bain et al., 1993). We disagree with the notion that transducer measures of tremor lack clinical validity because they correlate poorly with various clinical rating scales. We found excellent correlations between TRS and transducer measures when a logarithmic or exponential relationship was considered.
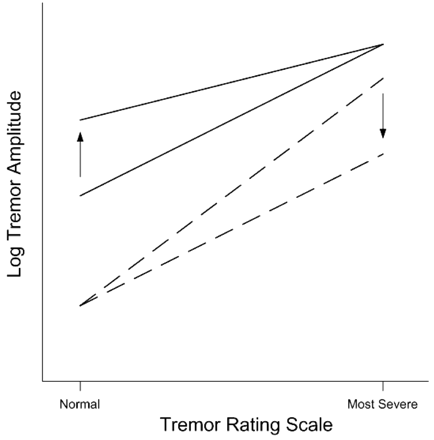
The items of the rating scales used in this study measured different properties of tremor, including hand displacement, volume of water spilled and even the social handicap of rest tremor. The question arises as to why the TRS versus tremor amplitude relationship is so similar for so many different rating scales? The mean values of most severe and normal tremor amplitudes define a straight line between the highest and lowest possible TRS values if tremor amplitude is plotted on a log scale (Fig. 3). Therefore, given the linear relationship between logT and TRS, consistent estimates of the most severe and normal tremor amplitudes will produce consistent values of α. Underestimating the range (mean) of tremor amplitudes corresponding to most severe tremor (TRS = 4) and overestimating the range (mean) of tremor amplitudes corresponding to normal tremor (TRS = 0) will result in lower values of α (Fig. 3). We hypothesized that variations in the estimates of normal and most severe tremor account for some of the variation in α, but this hypothesis was not supported by the insignificant correlations between α and β and between α and the computed value of logT at TRS = 4. Nevertheless, these theoretical considerations suggest that a clinician's concept (definition) of normal and most severe may have a significant effect on the value of α.
These hypothetical Weber–Fechner curves illustrate the effect on slope (α) of differing estimates of normal (TRS = 0) and most severe (TRS = 4) tremor. The solid lines show the hypothetical reduction in slope (α) that would result from an increase (arrow) in the mean tremor amplitude deemed to be normal. The broken lines illustrate the hypothetical reduction in α that would result from a decrease (arrow) in the mean tremor amplitude deemed most severe.
We attach no special significance to our finding that the Weber–Fechner and Stevens relationships were virtually equal in their approximation of our TRS versus tremor amplitude data. We examined both because they are widely discussed in the literature of psychophysics. Both predict that the relationship between TRS and tremor amplitude is non-linear. The purpose of our study was not to show which relationship was better; rather, our purpose was to derive a useful empirical relationship between TRS and tremor amplitude so that clinicians may compare the results of clinical studies using a TRS with those using a linear motion transducer. To do this, one need only assume a value of α and relate the change in TRS with the tremor amplitude ratio or fractional change in amplitude, using Equations 5 and 6.
The non-linear relationships demonstrated in this paper are highly useful when interpreting the clinical significance of changes in TRS. For example, many clinical trials used a TRS to quantify ET while others used accelerometry (Zesiewicz et al., 2005). Comparing changes in TRS with those in accelerometry is impossible without considering the relationships reported in this paper. For example, a 0.5-point reduction in TRS may not seem very impressive or significant, but for α = 0.5, the final tremor amplitude would be approximately half (0.562) the original tremor amplitude (Fig. 2).
The results of this study are limited by the limitations of accelerometry and digitizing tablets in quantifying tremor. Uniaxial accelerometers cannot capture the complex three-dimensional translational and rotational motion that occurs in tremor (Elble, 2005). However, we mounted our accelerometers so that the axis of sensitivity was in the principal direction of motion. Furthermore, the value of α obtained with the three-dimensional mechanical-linkage device (Mayo Laboratory) was comparable with the values obtained with accelerometry. Digitizing tablets capture the two-dimensional motion of the pen point but cannot record the complex motion of the upper limb, which may influence a rater's tremor score. These limitations of accelerometry and digitizing tablets could explain some of the variability in α.
Another limitation of our study is that spectral (Fourier) analysis gives the mean amplitude, averaged over the period of recording. It is not known whether clinical raters perceive the average tremor amplitude or whether they are more influenced by other characteristics of tremor, such as the largest tremor excursions, tremor frequency, anatomical distribution and kinematic complexity (i.e. the extent of three-dimensional translation and rotation). Moreover, raters may differ in the way they perceive or value these characteristics of tremor in the same motor task and among different tasks (e.g. posture and finger–nose testing). These concerns are important topics for future study.
A third limitation of our study was that the tremor ratings and quantitative measurements were not always performed simultaneously. The Tremor Research Group's digitizing tablet measurements were performed simultaneously with the tremor ratings, and rating assessments were performed immediately before transducer recording at Mayo and within 5 min before or after transducer recording at Kiel. The ratings and transducer recordings were performed within 4 weeks at Columbia and SIU. Consequently, spontaneous fluctuations in tremor amplitude may have contributed to some of the unexplained variance in the curve fitting analyses, particularly at Columbia and SIU. Nevertheless, the values of α computed from the Columbia and SIU data were comparable with those from Mayo, Kiel and the Tremor Research Group (Tables 1 and 2). Thus, delays of a few weeks between TRS and transducer recordings do not appear to have a significant impact on α.
The crude sensitivity of 5-point TRSs is a good reason for supplementing these scales with precision (transducer) measures when possible. Bain et al. (1993) concluded that spiral drawing and volumetric assessment of holding a cup of water are two good tests for assessing upper limb function in patients with tremor, and the motor function subscale of the Fahn–Tolosa–Marín TRS consists of writing, drawing (spirals and straight lines) and pouring assessments. Writing and drawing are easily quantified with commercially available digitizing tablets, like those used in this study, and quantitative volumetric assessment of holding or pouring a cup of water requires no equipment other than measuring cups or a precision balance for measuring mass of the cup and water (Bain et al., 1993).
Portions of this work were supported by the Spastic Paralysis Research Foundation of Kiwanis International, Illinois—Eastern Iowa District (R.J.E.), the National Institutes of Health (R01 NS20973, R.J.E.), a Parkinson's Disease Foundation Research Grant (S.L.P.) and the Deutsche Forschungsgemeinschaft and the Bundesministerium für Forschung und Bildung (G.D. and J.R.). Tremor Research Group: Rodger Elble (Southern Illinois University School of Medicine), Ray Watts (University of Alabama Birmingham), Kapil Sethi (Medical College of Georgia), Kelly Lyons (University of Kansas), Cynthia Comella (Rush Medical School, Chicago), Stanley Fahn (Columbia University in New York), Joseph Jankovic (Baylor College of Medicine, Houston, TX), Jorge Juncos (Emory University in Atlanta, GA), William Ondo (Baylor College of Medicine), Rajesh Pahwa (University of Kansas) and Ron Tintner (Methodist Neuroscience Research Institute, Houston, TX).