-
PDF
- Split View
-
Views
-
Cite
Cite
Christian R. A. Mondadori, Andreas Buchmann, Henrietta Mustovic, Conny F. Schmidt, Peter Boesiger, Roger M. Nitsch, Christoph Hock, Johannes Streffer, Katharina Henke, Enhanced brain activity may precede the diagnosis of Alzheimer's disease by 30 years, Brain, Volume 129, Issue 11, November 2006, Pages 2908–2922, https://doi.org/10.1093/brain/awl266
Close - Share Icon Share
Abstract
Presenilin 1 (PSEN1) mutations cause autosomal dominant familial Alzheimer's disease (FAD). PSEN1 mutation carriers undergo the course of cognitive deterioration, which is typical for sporadic Alzheimer's disease but disease onset is earlier and disease progression is faster. Here, we sought to detect signs of FAD in presymptomatic carriers of the PSEN1 mutation (C410Y) by use of a neuropsychological examination, functional MRI during learning and memory tasks and MRI volumetry. We examined five non-demented members of a FAD family and 21 non-related controls. Two of the five family members were carrying the mutation; one was 20 years old and the other 45 years old. The age of clinical manifestation of FAD in the family studied here is ∼48 years. Neuropsychological assessments suggested subtle problems with episodic memory in the 20-year-old mutation carrier. The middle-aged mutation carrier fulfilled criteria for amnestic mild cognitive impairment. The 20-year-old mutation carrier exhibited increased, while the middle-aged mutation carrier exhibited decreased brain activity compared to controls within memory-related neural networks during episodic learning and retrieval, but not during a working-memory task. The increased memory-related brain activity in the young mutation carrier might reflect a compensatory effort to overcome preclinical neural dysfunction caused by first pathological changes. The activity reductions in the middle-aged mutation carrier might reflect gross neural dysfunction in a more advanced stage of neuropathology. These data suggest that functional neuroimaging along with tasks that challenge specifically those brain areas which are initial targets of Alzheimer's disease pathology may reveal activity alterations on a single-subject level decades before the clinical manifestation of Alzheimer's disease.
Abbreviations
- APOE4
apolipoprotein E ɛ4
- CERAD-NAB
Consortium to Establish a Registry for Alzheimer's Disease—Neuropsychological Assessment Battery
- FAD
familial Alzheimer's disease
- fMRI
functional MRI
- HAWIE-R
Hamburg Wechsler Intelligence Scale Revised
- MTL
medial temporal lobe
- PSEN1
presenilin 1
- ROI
region of interest
- SAD
sporadic Alzheimer's disease
- WMS-R
Wechsler Memory Scale—Revised
Introduction
The first clinical signs of Alzheimer's disease are usually difficulties with episodic memory. Episodic memory is critically dependent on the intact functioning of the medial temporal lobe (MTL) (Rempel-Clower et al., 1996; Vargha-Khadem et al., 1997). The MTL is the brain area initially affected by intracellular neurofibrillary tangle formation in the course of Alzheimer's disease (Braak and Braak, 1996). Neurofibrillary tangle formation spreads from the MTL to the temporal neocortex and on to regions of the parietal, frontal and finally the occipital lobes during the course of Alzheimer's disease. Extracellular amyloid beta 42, on the other hand, is broadly and diffusely deposited within cortex with no clear neuroanatomical pattern of progression (Price et al., 1991; Braak and Braak, 1996; Delacourte et al., 1999).
Compared to the common sporadic forms of Alzheimer's disease (SAD), the less frequent forms of familial Alzheimer's disease (FAD) are associated with a younger age of first clinical symptoms, a faster disease progression and more abundant tissue histopathology (Gomez-Isla et al., 1997; Lippa et al., 2000; Sakamoto et al., 2002). FAD is a fully-penetrant autosomal dominant trait. It has been associated with mutations in the amyloid precursor protein gene on chromosome 21 (Goate et al., 1991), the presenilin 1 (PSEN1) gene on chromosome 14 (Alzheimer's Disease Collaborative Group, 1995; Sherrington et al., 1995) and the presenilin 2 gene on chromosome 1 (Levy-Lahad et al., 1995; Rogaev et al., 1995). The presenilins play not only essential roles in the neural development by acting on the notch signalling pathway, but also in synaptic plasticity, long-term memory and neuronal survival (for reviews seeCzech et al., 2000; Wines-Samuelson and Shen, 2005). Of all FAD cases ∼55% are caused by mutations in PSEN1 (Cruts and van Broeckhoven, 1998). To date, >128 mutations in PSEN1 have been detected (Author Webpage), with the majority of them being missense mutations that give rise to a single amino acid substitution. These mutations are associated with ages of Alzheimer's disease diagnosis ranging from 25 to 64 years (Tandon et al., 2000; Rogaeva, 2002). On the cellular level, pathogenic PSEN1 mutations modify amyloid precursor protein processing, thereby leading to an enhanced amyloid beta 42 secretion (Scheuner et al., 1996). Alzheimer's disease patients carrying PSEN1 mutations exhibit significant increases in plasma amyloid beta 42 levels and massive deposition of amyloid beta 42 in the brain (Lemere et al., 1996; Iwatsubo, 1998), which precede overt neuronal loss (Lippa et al., 1998). A role of PSEN1 mutations in tau pathology has also been suggested by the finding of tau hyperphosphorylation in PSEN1 transgenic mice (Boutajangout, 2002).
To our knowledge, there are no neuroimaging studies in the literature on the effects of PSEN1 mutations on brain activity in young mutation carriers. Examinations with fluorodeoxyglucose positron emission tomography (PET) in cognitively intact carriers of the apolipoprotein E ɛ4 allele (APOE4), the major genetic risk factor for sporadic Alzheimer's disease (SAD; Small et al., 2000; de Leon et al., 2001; Reiman et al., 2004), revealed a reduced glucose metabolism in the resting state. These cognitively intact subjects exhibited abnormally low rates of glucose metabolism in the same brain regions as patients with probable Alzheimer's disease, namely the posterior cingulate gyrus, parietal, temporal and prefrontal regions.
We hypothesize that a handicapped system—in the case of Alzheimer's disease the MTL memory system—should reveal its dysfunction more readily under challenge than at rest. In this study, we therefore challenged the hippocampus and the rhinal cortex, areas initially affected by neurofibrillary tangle formation, by memory tasks tailored to the computational functions of these regions in order to uncover earliest functional alterations in presymptomatic PSEN1 mutation carriers. In this vein, Bookheimer et al. (2000) and Bondi et al. (2005) found a greater magnitude and extent of brain activity in elderly, but cognitively intact APOE4 carriers compared to non-carriers during memory-activation tasks. These authors suggested that APOE4 carriers had increased their memory-related brain activity in an effort to compensate for preclinical dysfunction caused by neuropathology. Here we explore whether such memory-related, compensatory activity increases might be found even in young subjects at risk for Alzheimer's disease and on a single-subject level instead of group statistics. Young carriers of a gene mutation associated with FAD are ideal candidates to investigate these questions because their risk of developing FAD is ∼100%. Because neurofibrillary tangle formation within the MTL can be found as early as 30 years prior to the diagnosis of SAD (Price et al., 1991; Ohm et al., 1995; Braak and Braak, 1996; Ghebremedhin et al., 1998; Delacourte et al., 1999), we assume that tangle formation would also occur early in FAD and that it might reflect in subtle functional disturbances that can be picked up with functional imaging.
Here, we report a family with FAD caused by the published PSEN1 C410Y mutation (Campion et al., 1995). Memory-related brain activity was measured in five non-demented members of this family and in 21 healthy non-related controls using functional MRI (fMRI). We sought to detect functional changes related to memory formation and retrieval in a mutation carrier several decades before the clinical manifestation of Alzheimer's disease, which is ∼48 years in this family. Two of the five family members were carrying the PSEN1 mutation, a 20-year-old and a 45-year-old. On grounds of the ‘compensatory hypothesis’ (Bookheimer et al., 2000; Bondi et al., 2005), we anticipated enhanced brain activity during learning and memory in the 20-year-old mutation carrier compared with non-carrying relatives and controls. Because reductions in hippocampal, entorhinal and perirhinal volumes have been found years before the clinical manifestation of Alzheimer's disease (Fox et al., 1996a, b; Kaye et al., 1997; Reiman et al., 1998), we also examined the brain morphological variability in our subjects. We measured the volumes of the hippocampus, parahippocampal gyrus, total grey and total white matter. Moreover, all members of our FAD family underwent an extensive neuropsychological assessment to reveal early preclinical cognitive dysfunctions.
Material and methods
Subjects
The five members of our FAD family were three young and two middle-aged individuals. Of these, a 20-year-old and a 45-year-old subject carried the PSEN1 C410Y mutation first described by Campion et al. (1995). To conceal the identity of the family members, the family pedigree, the familial degrees of relationship, and the subjects' gender are not reported. The control group consisted of 21 healthy subjects who were matched to the three young family members' ages, years of education and APOE genotypes (seeTable 1). All controls and family members denied any past or current psychiatric and neurological conditions and the consumption of illegal drugs. Their anatomical T1-weighted MRI scans showed normal brain morphology. All subjects gave written informed consent to participate in the study after the nature and possible consequences of the study had been explained. None of the family members wished to be informed of their genetic status after genetic counselling. The experimenters were blind to the genotype of the subjects during data collection. The study was approved by the local Ethics Committee.
Demographics
| . | Y1 C410Y . | Y2 . | Y3 . | M1 C410Y . | M2 . | Controls (n = 21) . |
|---|---|---|---|---|---|---|
| Age | 20 | 20 | 23 | 45 | 52 | 22.2 ± 1.75* |
| HAWIE-R total IQ | 133 | 131 | 102 | 111 | 101 | 122.5 ± 10.18* |
| HAWIE-R performance IQ | 134 | 116 | 108 | 100 | 110 | 119.7 ± 11.78* |
| HAWIE-R verbal IQ | 126 | 136 | 96 | 118 | 94 | 121.1 ± 10.35* |
| Handedness | R | R | R | R | L | R (n = 19), L (n = 2) |
| Years of education | 13 | 12 | 15 | 13 | 9 | 14.13 ± 1.39* |
| APOE | ɛ2/ɛ3 | ɛ2/ɛ3 | ɛ2/ɛ4 | ɛ2/ɛ3 | ɛ2/ɛ3 | ɛ2/ɛ3 (n = 11), ɛ3/ɛ3 (n = 10) |
| . | Y1 C410Y . | Y2 . | Y3 . | M1 C410Y . | M2 . | Controls (n = 21) . |
|---|---|---|---|---|---|---|
| Age | 20 | 20 | 23 | 45 | 52 | 22.2 ± 1.75* |
| HAWIE-R total IQ | 133 | 131 | 102 | 111 | 101 | 122.5 ± 10.18* |
| HAWIE-R performance IQ | 134 | 116 | 108 | 100 | 110 | 119.7 ± 11.78* |
| HAWIE-R verbal IQ | 126 | 136 | 96 | 118 | 94 | 121.1 ± 10.35* |
| Handedness | R | R | R | R | L | R (n = 19), L (n = 2) |
| Years of education | 13 | 12 | 15 | 13 | 9 | 14.13 ± 1.39* |
| APOE | ɛ2/ɛ3 | ɛ2/ɛ3 | ɛ2/ɛ4 | ɛ2/ɛ3 | ɛ2/ɛ3 | ɛ2/ɛ3 (n = 11), ɛ3/ɛ3 (n = 10) |
Y, young; M, middle-aged; C410Y, mutation in the PSEN1 gene; APOE, apolipoprotein E; R, right; L, left.
*Means ± SD.
Demographics
| . | Y1 C410Y . | Y2 . | Y3 . | M1 C410Y . | M2 . | Controls (n = 21) . |
|---|---|---|---|---|---|---|
| Age | 20 | 20 | 23 | 45 | 52 | 22.2 ± 1.75* |
| HAWIE-R total IQ | 133 | 131 | 102 | 111 | 101 | 122.5 ± 10.18* |
| HAWIE-R performance IQ | 134 | 116 | 108 | 100 | 110 | 119.7 ± 11.78* |
| HAWIE-R verbal IQ | 126 | 136 | 96 | 118 | 94 | 121.1 ± 10.35* |
| Handedness | R | R | R | R | L | R (n = 19), L (n = 2) |
| Years of education | 13 | 12 | 15 | 13 | 9 | 14.13 ± 1.39* |
| APOE | ɛ2/ɛ3 | ɛ2/ɛ3 | ɛ2/ɛ4 | ɛ2/ɛ3 | ɛ2/ɛ3 | ɛ2/ɛ3 (n = 11), ɛ3/ɛ3 (n = 10) |
| . | Y1 C410Y . | Y2 . | Y3 . | M1 C410Y . | M2 . | Controls (n = 21) . |
|---|---|---|---|---|---|---|
| Age | 20 | 20 | 23 | 45 | 52 | 22.2 ± 1.75* |
| HAWIE-R total IQ | 133 | 131 | 102 | 111 | 101 | 122.5 ± 10.18* |
| HAWIE-R performance IQ | 134 | 116 | 108 | 100 | 110 | 119.7 ± 11.78* |
| HAWIE-R verbal IQ | 126 | 136 | 96 | 118 | 94 | 121.1 ± 10.35* |
| Handedness | R | R | R | R | L | R (n = 19), L (n = 2) |
| Years of education | 13 | 12 | 15 | 13 | 9 | 14.13 ± 1.39* |
| APOE | ɛ2/ɛ3 | ɛ2/ɛ3 | ɛ2/ɛ4 | ɛ2/ɛ3 | ɛ2/ɛ3 | ɛ2/ɛ3 (n = 11), ɛ3/ɛ3 (n = 10) |
Y, young; M, middle-aged; C410Y, mutation in the PSEN1 gene; APOE, apolipoprotein E; R, right; L, left.
*Means ± SD.
Genotyping
Genomic DNA was extracted from whole blood. Mutation screening was performed by direct sequencing of both strands of the PCR-amplified coding exons of PSEN1 (exons 2–12), presenilin 2 (exons 3–12), and amyloid precursor protein (exons 16 and 17). Amplification was done by a universal ‘touch down’ protocol, using primers as described earlier (Finckh et al., 2000). Purified PCR products were sequenced by cycle sequencing using fluorescent dye dideoxy terminators (ABI PRISM® BigDye(tm) Terminators v 3.0 Cycle Sequencing Kit) and analysis was performed on an ABI PRISM® 310 Genetic Analyzer. APOE genotypes were assessed using the LightCycler™ instrument (Roche Diagnostics Corporation) (Bernard et al., 1999).
Functional MRI procedure
All subjects underwent two fMRI experiments, one on episodic memory and the other on working memory, as well as structural MRI and a neuropsychological examination. Trials in the fMRI experiments were blocked. The same stimuli were used for all subjects to avoid stimulus-generated variance. The sequence of condition blocks within fMRI time-series was counterbalanced across subjects. Instruction slides announced each task block to subjects. Responses were collected with a response box that subjects held in their dominant hand. Subjects practised all fMRI tasks prior to scanning (with different stimuli) until they felt comfortable with tasks.
Encoding
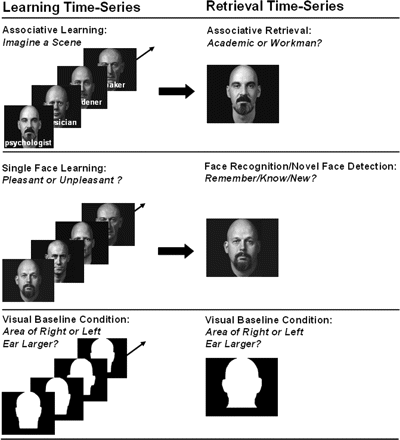
We presented 16 face–profession pairs in the associative learning condition, 16 faces in the single face learning condition and 24 head contours (without physiognomy) in the visual baseline condition of a first encoding run which constituted one fMRI time-series. This fMRI time-series (Fig. 1) was repeated twice adding to three encoding runs or three fMRI time-series. The repetition of learning runs had the purpose to allow for a progressively deeper encoding of the learning material. For associative learning of the face–profession pairs, subjects were instructed to imagine each presented person acting in a scene of the written profession and to indicate by button press whether it was easy or hard to imagine a scene. This imagination task automatically induces the establishment of semantic person-occupation associations and activates the hippocampus and associated cortices (Degonda et al., 2005). Importantly, subjects were requested to imagine the same scene for a given face–profession pair during encoding Runs 2 and 3 as during encoding Run 1. This adds the retrieval of the scenes imagined in previous runs to the encoding processes in Runs 2 and 3. The instruction for the learning of single faces was to decide whether a face was pleasant or unpleasant. This instruction induces a deep (semantic) encoding of the presented faces. The instruction given for the visual baseline task was to indicate by button press whether the area of the left or right ear of a head contour was larger. Learning conditions consisted of four 24 s blocks with four trials of 6 s per block. The visual baseline condition consisted also of four 24 s blocks, with six trials of 4 s per block.
FMRI tasks. The left side shows example stimuli used for associative learning, single face learning and the visual baseline condition. The right side shows example stimuli used for associative retrieval, face recognition, novel face detection, and the visual baseline condition. Alex Kayser granted us permission to use and reproduce faces from his book ‘Heads’, 1985, New York: Abbeville Press.
Retrieval
There was a single fMRI time-series for retrieval, which included the associative retrieval condition (face–profession associations), the face recognition condition (studied faces), a novel faces detection condition (unstudied faces) and the same visual baseline condition that had been included in the encoding time-series. For associative retrieval, the previously presented faces were given as cues. Subjects were instructed to remember the associated occupation and to indicate its superordinate category (academic versus workman) by button press. For face recognition/novel face detection, studied and unstudied faces were presented in separate blocks with the request to indicate by button press whether a face was remembered (recollection; episodic memory) or known (familiarity; semantic memory) or judged new (Tulving, 1985; Gardiner, 1988). Retrieval conditions consisted of four 24s blocks with four trials of 6 s per block. The visual baseline condition consisted also of four 24 s blocks with six trials of 4 s per block.
Working memory
There was a single fMRI time-series with a 2-back task assessing working memory and a baseline task (‘x-target’ task) measuring concentration. The 2-back task required subjects to respond to a letter repeat with one intervening letter (e.g. S – f – s – g …). The ‘x-target’ task required subjects to respond to the occurrence of the letter ‘x’ in a sequence of letters (e.g. N – l – X – g …). We presented 50 upper- or lowercase letters typed in black on white background. There were five blocks of 26 s per condition. Each block comprised 13 upper- or lowercase letters, which were presented for 2 s each.
MRI data acquisition
MR measurements were performed on a 3T Philips Intera whole body MR scanner equipped with an eight-channel Philips SENSE head coil. Functional data were obtained from 32 transverse slices parallel to the AC–PC plane covering the whole brain with a measured spatial resolution of 2.8 × 2.8 × 4 mm3 (acquisition matrix 80 × 80) and a reconstructed resolution of 1.7 × 1.7 × 4 mm3. Data were acquired using a parallel imaging technique, SENSE-sshEPI, with an acceleration factor of R = 2.0. Other scan parameters were TE = 35 ms, TR = 3000 ms, θ = 82°. We have previously tested this protocol against other SENSE protocols and against coventional ssh-EPI on this 3T Philips scanner (Schmidt et al., 2005) with the associative learning task used in the present study. This protocol provided excellent image quality particularly in the MTL, but also in neocortex, by markedly reducing susceptibility related distortions and saving signal that can be compared across conditions (for raw images and SNR in MTL and neocortex using this protocol seeSchmidt et al., 2005).
A standard 3D T1-weighted scan was obtained for anatomical reference with a measured spatial resolution of 1 × 1 × 1.5 mm3 (acquisition matrix 224 × 224), a reconstructed resolution of 0.9 × 0.9 × 0.8 mm3, TE = 2.3 ms, TR = 20 ms and θ = 20°, no interslice gaps. A 2D T1-weighted inversion-recovery anatomical scan, oriented perpendicularly to the long axis of the hippocampus, was obtained for hippocampal and parahippocampal volumetry over 33–39 slices with a measured spatial resolution of 0.5 × 0.6 × 1.5 mm3 (acquisition matrix 400 × 320) and a reconstructed spatial resolution of 0.4 × 0.4 × 1.5 mm3, TE = 15 ms, TR = 4200 ms, θ = 20°, IR delay 400 ms, no interslice gaps.
Analysis of fMRI data
Image pre- and post-processing and the statistical analyses were performed with SPM2 (Author Webpage) using standard preprocessing procedures (Friston et al., 1995a).
For the whole-brain SPM analyses, data were realigned, spatially normalized and spatially smoothed to a full width of 8 mm at half-maximal resolution using a Gaussian filter.
For the region-of-interest (ROI) analyses within the subjects' native brain spaces, data were realigned and smoothed to a full width of only 4 mm at half-maximal resolution and no spatial normalization was performed
At the single-subject level, data were analysed according to the fixed effects model of SPM2. The six head movement parameters were included in the model as confounding factors. Data were high-pass filtered with a filter-value tailored to each fMRI time-series according to 2 × SOA (stimulus onset asynchrony) × TR (repetition time). Contrasts were computed for each subject comparing the learning (Run 1), the retrieval and the novelty detection conditions to the visual baseline condition and the 2-back task to the x-target task. At the second level, within-subject contrasts were entered into random effects analyses (Two sample t-tests, SPM2; Friston et al., 1995b). Each family member was compared to the control group. SPM takes the control group's variance as an estimate for the single subject's variance when calculating two sample t-tests with only one subject in a group. To determine whether the family members' activity alterations might be attributable to normal inter-subject variability, we randomly selected five young control subjects (R1, R2, R3, R4 and R5) from our control group of 21 subjects and compared their associative learning and associative retrieval contrasts (each versus visual baseline) to the contrasts of the remaining 20 controls. Height thresholds were set at P = 0.001 (uncorrected for multiple comparisons) and extent thresholds were 15 voxels in neocortex and 1 voxel in hippocampus and rhinal cortex. If activity differences in the hippocampus and rhinal cortex reached significance only at the more liberal height threshold of P = 0.005, these results are also indicated in the results tables and marked as such.
The ROI analyses were performed in native space for the hippocampus using the MarsBar toolbox for SPM2 (Brett et al., 2002). Each subject's hippocampi were manually delineated on the anatomical 3D-T1-weighted coronal MRI slices and divided into an anterior, a middle and a posterior section. Mean contrast values per subject and condition (versus the visual baseline task) were extracted for each ROI. The mean contrast values of each family member and of each of the five randomly selected controls (from the larger control group) were then compared with the 7th (P7) and 93rd (P93) percentile of the control group's mean contrast values.
MRI volumetry
Volumes of the total grey and white matter were computed with SPM2 on the 3D-T1-weighted structural whole-brain MRI scans. Images were normalized into the MNI T1 template by use of the standard bounding box. Next, they were segmented into grey matter, white matter and CSF. The multiplication of the standardized grey and white matter volumes by the determinant of the linear transformation matrix yielded grey and white matter volumes in cm3. In addition, two independent raters manually delineated the hippocampal formation (CA regions, dentate gyrus and subiculum, excluding the fimbria) and the parahippocampal gyrus (Henke et al., 1999) on the 2D-T1-weighted high-resolution structural MRI scans using the software Pmod (Author Webpage). The parahippocampal gyrus was delineated over a length that corresponded to the length of the hippocampus. Raters relied on descriptions of anatomical landmarks and subdivisions of the MTL as described by Insausti et al. (1998) and Duvernoy (1998). Inter-rater reliabilities ranged between r = 0.8 and 0.98. Each family member's volumes were compared with the 7th (P7) and 93rd (P93) percentile of the control group's volumes. Male subjects were compared with a male control group (n = 9; age: mean = 21.1, SD = 1.7), female subjects with a female control group (n = 13; age: mean = 22, SD = 1.8).
Neuropsychology
Family members and controls (controls: n = 20, because one control subject was not available for neuropsychological testing) underwent a comprehensive neuropsychological examination. Memory functions were assessed with the Wechsler Memory Scale—Revised (WMS-R), intelligence with the Hamburg Wechsler Intelligence Scale Revised (HAWIE-R), spatial cognition with the Luria Mental Rotation Test, fluency with a verbal (S-words) and a non-verbal (5-point) production task, concept finding/switching with the Kramer Card Sorting Test, and the control of interference with the Stroop test. The two middle-aged family members took also the CERAD-NAB test, which includes the Mini-Mental State Examination (MMSE). Scores obtained by each family member were compared with the 7th (P7) and 93rd (P93) percentile of the control group's scores and with the normative values provided by the WMS-R, HAWIE-R and CERAD-NAB.
Results
Neuropsychological performance
Young family members
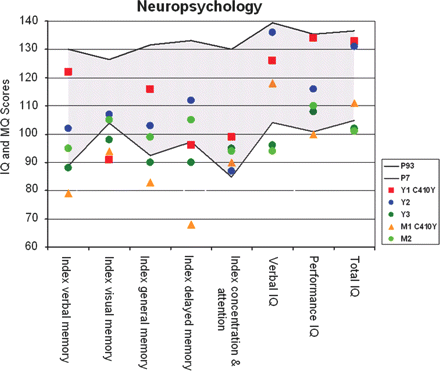
Y1 C410Y (Y1, young no. 1, carrying the C410Y mutation) was 20 years old at the time of this examination. This subject fell below the young control group's 7th percentile in the WMS-R indices for visual memory and delayed memory but was within mean (M) ± 1 SD of the age-matched test norms (Fig. 2, Supplementary Table S1). It was the performance in the visual paired associates (VPA) immediate cued recall (VPA1) and the delayed cued recall (VPA2) subtests that lowered these two indices. VPA1 and VPA2 fell below the young control group's M − 2 SD (VPA1: Y1 C410Y = 10, controls M = 17.4, SD = 0.88; VPA2: Y1 C410Y = 4, controls M = 5.95, SD = 0.22), but just within M ± 1.5 SD of the age-matched test norms (VPA1: Y1 C410Y = 10, norm group M = 14.1, SD = 3.14; VPA2: Y1 C410Y = 4, norm group M = 5.48, SD = 0.87). Y1 C410Y performed within the young control group's P7/P93 range with respect to all other neuropsychological variables (Supplementary Table S1).
Neuropsychology. The figure shows the performance scores of the five family members in the indices of the WMS-R and the HAWIE-R. Family members are represented by colours (see legend). The grey area spans the control group's performance range between the 7th and the 93rd percentile. IQ, intelligence quotient; MQ, memory quotient.
Subject Y2 (Y2, young no. 2, no mutation carrier) was 20 years old at the time of this examination. Y2 performed within the young control group's P7/P93 range of scores in all neuropsychological tasks (Fig. 2, Supplementary Table S1).
Subject Y3 (Y3, young no. 3, no mutation carrier) was 23 years old at the time of this examination. Y3 exhibited rather weak cognitive abilities across cognitive domains relative to the other young family members. The WMS-R indices for verbal memory, visual memory, general memory and delayed memory as well as the total IQ score and the verbal IQ score were ranging below the 7th percentile of the young control group, but were within M ± 1 SD of age-matched test norms (Fig. 2, Supplementary Table S1).
Middle-aged family members
M1 C410Y (M1, middle-aged no. 1, carrying the C410Y mutation) was 45 years old at the time of this examination. This subject fell below the young control group's 7th percentile in all WMS-R memory indices (Fig. 2). The WMS-R delayed memory index and the MMSE score also ranged below M − 1.5 SD of the age-matched test norms. General intelligence, spatial cognition, and executive functions were within norms and even within the young control group's P7/P93 range (Fig. 2, Supplementary Table S1). M1 C410Y's cognitive profile therefore fulfilled the criteria for amnestic mild cognitive impairment by Petersen et al. (2001).
Subject M2 (M2, middle-aged no. 2, no mutation carrier) was 52 years old at the time of this examination. The total IQ index and the verbal IQ index fell below the young control group's P7/P93 range (Fig. 2, Supplementary Table S1), but were within M ± 1 SD of the age-matched test norms. All other performance measures were within M ± 1.5 SD of the age-matched test norms.
Behavioural performance in fMRI experiments
Young family members
The three young family members performed within the young control group's P7/P93 performance range in all behavioural measures collected during the fMRI experiments (Table 2).
Performance fMRI tasks
| . | Y1 C410Y . | Y2 . | Y3 . | M1 C410Y . | M2 . | Controls . | |
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |
| . | . | . | . | . | . | (M ± SD) . | (P7/P93) . |
| Associative learning | |||||||
| No. easy (run 1) | 9 | 9 | 9 | 9 | 11 | 8.76 ± 2.28 | 5/12.46 |
| No. easy (run 2) | 10 | 12 | 9 | 9 | 10 | 9.57 ± 2.64 | 4.54/13.46 |
| No. easy (run 3) | 13 | 10 | 10 | 7 | 10 | 10.67 ± 2.35 | 6.62/14.46 |
| No. easy (runs 1–3) | −1 | −4 | −1 | 2 | 1 | −1.9 ± 2.07 | −5.92/1 |
| Single face learning | |||||||
| No. pleasant (run 1) | 8 | 8 | 5 | 7 | 6 | 7 ± 2.21 | 3.08/11 |
| No. pleasant (run 2) | 8 | 8 | 4 | 8 | 6 | 7 ± 2.72 | 2.08/12 |
| No. pleasant (run 3) | 9 | 9 | 5 | 9 | 7 | 7.29 ± 2.49 | 2.62/11.46 |
| No. pleasant (runs 1–3) | −1 | −1 | 0 | −2 | −1 | −0.29 ± 1.49 | −3/2 |
| Associative retrieval | |||||||
| No. correct | 11 | 11 | 9 | 11 | 11 | 11.9 ± 2.2 | 7.54/14.46 |
| Face recognition | |||||||
| No. correct remember (hits - false alarms) | 14 | 11 | 7 | 2 | 2 | 10.25 ± 3.75 | 3.41/15.53 |
| No. correct know (hits - false alarms) | 1 | 3 | 5 | 1 | 6 | 2.95 ± 3.55 | −1.06/10.53 |
| Novel face detection | |||||||
| No. correct new (correct rejections – misses) | 15 | 14 | 12 | 4 | 9 | 13.29 ± 2.19 | 9.54/16 |
| Working memory | |||||||
| 2-back No. correct (hits - false alarms) | 11 | 10 | 10 | 8 | 3 | 8.85 ± 2.89 | 3.47/12 |
| Attention | |||||||
| x-target No. correct (hits - false alarms) | 13 | 13 | 13 | 13 | 12 | 12.95 ± 0.22 | 12.47/13 |
| . | Y1 C410Y . | Y2 . | Y3 . | M1 C410Y . | M2 . | Controls . | |
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |
| . | . | . | . | . | . | (M ± SD) . | (P7/P93) . |
| Associative learning | |||||||
| No. easy (run 1) | 9 | 9 | 9 | 9 | 11 | 8.76 ± 2.28 | 5/12.46 |
| No. easy (run 2) | 10 | 12 | 9 | 9 | 10 | 9.57 ± 2.64 | 4.54/13.46 |
| No. easy (run 3) | 13 | 10 | 10 | 7 | 10 | 10.67 ± 2.35 | 6.62/14.46 |
| No. easy (runs 1–3) | −1 | −4 | −1 | 2 | 1 | −1.9 ± 2.07 | −5.92/1 |
| Single face learning | |||||||
| No. pleasant (run 1) | 8 | 8 | 5 | 7 | 6 | 7 ± 2.21 | 3.08/11 |
| No. pleasant (run 2) | 8 | 8 | 4 | 8 | 6 | 7 ± 2.72 | 2.08/12 |
| No. pleasant (run 3) | 9 | 9 | 5 | 9 | 7 | 7.29 ± 2.49 | 2.62/11.46 |
| No. pleasant (runs 1–3) | −1 | −1 | 0 | −2 | −1 | −0.29 ± 1.49 | −3/2 |
| Associative retrieval | |||||||
| No. correct | 11 | 11 | 9 | 11 | 11 | 11.9 ± 2.2 | 7.54/14.46 |
| Face recognition | |||||||
| No. correct remember (hits - false alarms) | 14 | 11 | 7 | 2 | 2 | 10.25 ± 3.75 | 3.41/15.53 |
| No. correct know (hits - false alarms) | 1 | 3 | 5 | 1 | 6 | 2.95 ± 3.55 | −1.06/10.53 |
| Novel face detection | |||||||
| No. correct new (correct rejections – misses) | 15 | 14 | 12 | 4 | 9 | 13.29 ± 2.19 | 9.54/16 |
| Working memory | |||||||
| 2-back No. correct (hits - false alarms) | 11 | 10 | 10 | 8 | 3 | 8.85 ± 2.89 | 3.47/12 |
| Attention | |||||||
| x-target No. correct (hits - false alarms) | 13 | 13 | 13 | 13 | 12 | 12.95 ± 0.22 | 12.47/13 |
Performance fMRI tasks
| . | Y1 C410Y . | Y2 . | Y3 . | M1 C410Y . | M2 . | Controls . | |
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |
| . | . | . | . | . | . | (M ± SD) . | (P7/P93) . |
| Associative learning | |||||||
| No. easy (run 1) | 9 | 9 | 9 | 9 | 11 | 8.76 ± 2.28 | 5/12.46 |
| No. easy (run 2) | 10 | 12 | 9 | 9 | 10 | 9.57 ± 2.64 | 4.54/13.46 |
| No. easy (run 3) | 13 | 10 | 10 | 7 | 10 | 10.67 ± 2.35 | 6.62/14.46 |
| No. easy (runs 1–3) | −1 | −4 | −1 | 2 | 1 | −1.9 ± 2.07 | −5.92/1 |
| Single face learning | |||||||
| No. pleasant (run 1) | 8 | 8 | 5 | 7 | 6 | 7 ± 2.21 | 3.08/11 |
| No. pleasant (run 2) | 8 | 8 | 4 | 8 | 6 | 7 ± 2.72 | 2.08/12 |
| No. pleasant (run 3) | 9 | 9 | 5 | 9 | 7 | 7.29 ± 2.49 | 2.62/11.46 |
| No. pleasant (runs 1–3) | −1 | −1 | 0 | −2 | −1 | −0.29 ± 1.49 | −3/2 |
| Associative retrieval | |||||||
| No. correct | 11 | 11 | 9 | 11 | 11 | 11.9 ± 2.2 | 7.54/14.46 |
| Face recognition | |||||||
| No. correct remember (hits - false alarms) | 14 | 11 | 7 | 2 | 2 | 10.25 ± 3.75 | 3.41/15.53 |
| No. correct know (hits - false alarms) | 1 | 3 | 5 | 1 | 6 | 2.95 ± 3.55 | −1.06/10.53 |
| Novel face detection | |||||||
| No. correct new (correct rejections – misses) | 15 | 14 | 12 | 4 | 9 | 13.29 ± 2.19 | 9.54/16 |
| Working memory | |||||||
| 2-back No. correct (hits - false alarms) | 11 | 10 | 10 | 8 | 3 | 8.85 ± 2.89 | 3.47/12 |
| Attention | |||||||
| x-target No. correct (hits - false alarms) | 13 | 13 | 13 | 13 | 12 | 12.95 ± 0.22 | 12.47/13 |
| . | Y1 C410Y . | Y2 . | Y3 . | M1 C410Y . | M2 . | Controls . | |
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |
| . | . | . | . | . | . | (M ± SD) . | (P7/P93) . |
| Associative learning | |||||||
| No. easy (run 1) | 9 | 9 | 9 | 9 | 11 | 8.76 ± 2.28 | 5/12.46 |
| No. easy (run 2) | 10 | 12 | 9 | 9 | 10 | 9.57 ± 2.64 | 4.54/13.46 |
| No. easy (run 3) | 13 | 10 | 10 | 7 | 10 | 10.67 ± 2.35 | 6.62/14.46 |
| No. easy (runs 1–3) | −1 | −4 | −1 | 2 | 1 | −1.9 ± 2.07 | −5.92/1 |
| Single face learning | |||||||
| No. pleasant (run 1) | 8 | 8 | 5 | 7 | 6 | 7 ± 2.21 | 3.08/11 |
| No. pleasant (run 2) | 8 | 8 | 4 | 8 | 6 | 7 ± 2.72 | 2.08/12 |
| No. pleasant (run 3) | 9 | 9 | 5 | 9 | 7 | 7.29 ± 2.49 | 2.62/11.46 |
| No. pleasant (runs 1–3) | −1 | −1 | 0 | −2 | −1 | −0.29 ± 1.49 | −3/2 |
| Associative retrieval | |||||||
| No. correct | 11 | 11 | 9 | 11 | 11 | 11.9 ± 2.2 | 7.54/14.46 |
| Face recognition | |||||||
| No. correct remember (hits - false alarms) | 14 | 11 | 7 | 2 | 2 | 10.25 ± 3.75 | 3.41/15.53 |
| No. correct know (hits - false alarms) | 1 | 3 | 5 | 1 | 6 | 2.95 ± 3.55 | −1.06/10.53 |
| Novel face detection | |||||||
| No. correct new (correct rejections – misses) | 15 | 14 | 12 | 4 | 9 | 13.29 ± 2.19 | 9.54/16 |
| Working memory | |||||||
| 2-back No. correct (hits - false alarms) | 11 | 10 | 10 | 8 | 3 | 8.85 ± 2.89 | 3.47/12 |
| Attention | |||||||
| x-target No. correct (hits - false alarms) | 13 | 13 | 13 | 13 | 12 | 12.95 ± 0.22 | 12.47/13 |
Middle-aged family members
Both middle-aged family members fell below the young control group's P7/P93 range with their remember (Tulving, 1985; Gardiner, 1988) and new answers to single faces (Table 2). Notably, the middle-aged mutation carrier M1 C410Y yielded the smallest number of correct remember and know (Tulving, 1985; Gardiner, 1988) answers to faces compared with the young control group and with the other family members (Σ remember and know; Y1 C410Y : 15, Y2: 14, Y3: 12, M1 C410Y : 3, M2: 8, Table 2). M2 appeared to compensate the low number of correct remember answers (reflecting episodic memory) with a larger number of correct know answers (reflecting semantic memory). On the other hand, the two middle-aged family members' numbers of correctly recalled face–profession associations were within the P7/P93 performance range of the young control group (Table 2). M2 exhibited difficulties with the 2-back task relative to the other family members and scored below the P7/P93 performance range of the young control group.
Functional imaging results
The full results of SPM contrasts between each family member and the controls are given in Supplementary Tables 2–6 and the full results of the ROI analyses in Supplementary Table S7.
Episodic memory
Young family members
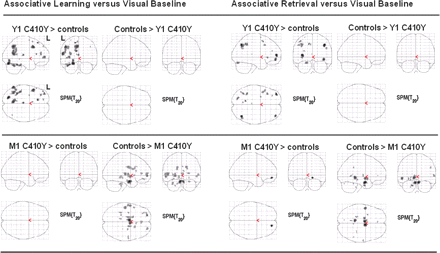
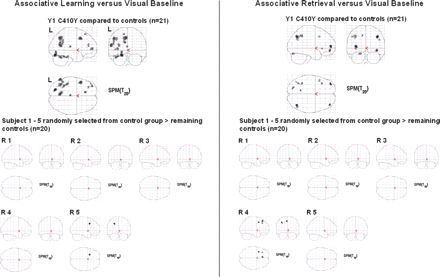
Y1 C410Y showed enhanced brain activity during learning, retrieval, and novelty detection relative to the young controls (Supplementary Table S2), while the young controls did not exhibit a single spot of activity enhancement over Y1 C410Y. Almost all of Y1 C410Y's activity enhancements were situated in left frontal, left temporal, and left parietal neocortices, and the left hippocampus (Tables 3 and 4, Supplementary Table S2, Fig. 3). To determine whether Y1 C410Y's activity enhancements might be attributable to causes of inter-subject variability other than the mutation, we randomly selected five young control subjects (R1, R2, R3, R4 and R5) and compared their associative learning and associative retrieval contrasts (each versus visual baseline) to the contrasts of the remaining 20 controls. There were only minor deviations of activity levels in the selected subjects. R4 (retrieval) and R5 (learning) showed some left frontal activity enhancements, but these were discrete relative to Y1 C410Y's general activity overshoot (Fig. 4). R1, R2, and R3 did not show any activity deviations. The ROI analysis confirmed Y1 C410Y's activity enhancement within the left hippocampus during associative learning: Y1 C410Y's activity in the middle portion of the left hippocampus exceeded the 93rd percentile of the young controls' activity (Supplementary Table S7). For single face learning, both retrieval conditions, and novelty detection, Y1 C410Y's hippocampal activity was well within the controls' P7/P93 limits.
Brain activity differences between each PSEN1 C410Y mutation carrier and the controls for the contrast Run 1 of associative learning versus visual baseline and the contrast associative retrieval versus visual baseline. Upper panel: The young mutation carrier exhibited areas of enhanced activity. Lower panel: The middle-aged mutation carrier exhibited areas of reduced activity.
Enhanced brain activity in the young mutation carrier. Upper panel: Displayed are activity enhancements in the young mutation carrier versus the controls for the contrast Run 1 of associative learning versus visual baseline and the contrast associative retrieval versus visual baseline. Lower panel: Five subjects randomly drawn from the control group (n = 21) are each compared with the remaining controls (n = 20). Their learning- and retrieval-related brain activity was within the controls' range.
Brain activation differences between each family member and the control group during associative learning
| Brain region . | Left/right . | MNI coordinates (mm) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | . | |||||
| . | . | x . | y . | z . | BA . | kE . | t . |
| Two sample t-test | |||||||
| Associative learning versus visual baseline | |||||||
| Y1 C410Y > controls | |||||||
| Hippocampus | L | −38 | −34 | −6 | 7 | 3.44* | |
| Fusiform gyrus | L | −22 | −78 | −18 | 19 | 147 | 7.31 |
| Fusiform gyrus | L | −34 | −46 | −22 | 37 | 210 | 6.02 |
| Superior frontal gyrus | L | −12 | 30 | 58 | 8 | 20 | 6.3 |
| Middle frontal gyrus | L | −50 | 16 | 42 | 8 | 112 | 5.92 |
| Middle frontal gyrus | L | −34 | 56 | 4 | 10 | 61 | 5.02 |
| Middle frontal gyrus | L | −30 | 42 | 16 | 46 | 47 | 4.68 |
| Middle temporal gyrus | L | −60 | −58 | 16 | 37 | 210 | 5.78 |
| Supramarginal gyrus | L | −38 | −60 | 38 | 40 | 57 | 4.44 |
| Precuneus | L | −6 | −60 | 44 | 7 | 265 | 5.98 |
| Y2 > controls | |||||||
| Superior temporal gyrus | R | 52 | 16 | −12 | 38 | 20 | 3.8 |
| Insula | R | 40 | −14 | 2 | 16 | 4.12 | |
| Y3 > controls | |||||||
| Fusiform gyrus | R | 40 | −50 | −20 | 37 | 19 | 4.69 |
| Fusiform gyrus | L | −46 | −48 | −30 | 37 | 19 | 4.66 |
| M1 C410Y > controls | |||||||
| No significant differences | |||||||
| M2 > controls | |||||||
| Superior frontal gyrus | L | −18 | −14 | 60 | 6 | 33 | 4.69 |
| Superior temporal sulcus | R | 46 | −28 | 4 | 44 | 5.36 | |
| Fusiform gyrus | L | −20 | −74 | −8 | 19 | 32 | 5.43 |
| Associative learning versus visual baseline | |||||||
| Controls > Y1 C410Y | |||||||
| No significant differences | |||||||
| Controls > Y2 | |||||||
| No significant differences | |||||||
| Controls > Y3 | |||||||
| Superior temporal gyrus | L | −58 | −24 | 10 | 22 | 18 | 4.13 |
| Controls > M1 C410Y | |||||||
| Hippocampus | R | 26 | −10 | −22 | 17 | 4.56 | |
| Hippocampus | R | 26 | −30 | −6 | 5 | 3.98 | |
| Amygdala | L | −32 | −8 | −26 | 11 | 4.74 | |
| Middle frontal gyrus | L | −24 | 60 | −2 | 10 | 72 | 7.45 |
| Middle frontal gyrus | R | 24 | 60 | 8 | 10 | 31 | 4.92 |
| Superior temporal gyrus | L | −56 | −2 | −4 | 22 | 101 | 6.16 |
| Superior temporal gyrus | L | −50 | −20 | 4 | 22 | 92 | 6.1 |
| Superior temporal gyrus | R | 52 | −8 | −4 | 22 | 38 | 4.29 |
| Cingulate gyrus | R | 2 | 38 | 6 | 24 | 35 | 3.58 |
| Fusiform gyrus | L | −32 | −36 | −30 | 36 | 26 | 4.65 |
| Controls > M2 | |||||||
| Hippocampus | R | 36 | −28 | −14 | 48 | 5.8 | |
| Hippocampus | R | 22 | −28 | −12 | 5 | 4.33 | |
| Parahippocampal gyrus | L | −14 | −44 | −4 | 30 | 624 | 7.77 |
| Superior temporal sulcus | R | 44 | 2 | −24 | 23 | 5.2 | |
| Brain region . | Left/right . | MNI coordinates (mm) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | . | |||||
| . | . | x . | y . | z . | BA . | kE . | t . |
| Two sample t-test | |||||||
| Associative learning versus visual baseline | |||||||
| Y1 C410Y > controls | |||||||
| Hippocampus | L | −38 | −34 | −6 | 7 | 3.44* | |
| Fusiform gyrus | L | −22 | −78 | −18 | 19 | 147 | 7.31 |
| Fusiform gyrus | L | −34 | −46 | −22 | 37 | 210 | 6.02 |
| Superior frontal gyrus | L | −12 | 30 | 58 | 8 | 20 | 6.3 |
| Middle frontal gyrus | L | −50 | 16 | 42 | 8 | 112 | 5.92 |
| Middle frontal gyrus | L | −34 | 56 | 4 | 10 | 61 | 5.02 |
| Middle frontal gyrus | L | −30 | 42 | 16 | 46 | 47 | 4.68 |
| Middle temporal gyrus | L | −60 | −58 | 16 | 37 | 210 | 5.78 |
| Supramarginal gyrus | L | −38 | −60 | 38 | 40 | 57 | 4.44 |
| Precuneus | L | −6 | −60 | 44 | 7 | 265 | 5.98 |
| Y2 > controls | |||||||
| Superior temporal gyrus | R | 52 | 16 | −12 | 38 | 20 | 3.8 |
| Insula | R | 40 | −14 | 2 | 16 | 4.12 | |
| Y3 > controls | |||||||
| Fusiform gyrus | R | 40 | −50 | −20 | 37 | 19 | 4.69 |
| Fusiform gyrus | L | −46 | −48 | −30 | 37 | 19 | 4.66 |
| M1 C410Y > controls | |||||||
| No significant differences | |||||||
| M2 > controls | |||||||
| Superior frontal gyrus | L | −18 | −14 | 60 | 6 | 33 | 4.69 |
| Superior temporal sulcus | R | 46 | −28 | 4 | 44 | 5.36 | |
| Fusiform gyrus | L | −20 | −74 | −8 | 19 | 32 | 5.43 |
| Associative learning versus visual baseline | |||||||
| Controls > Y1 C410Y | |||||||
| No significant differences | |||||||
| Controls > Y2 | |||||||
| No significant differences | |||||||
| Controls > Y3 | |||||||
| Superior temporal gyrus | L | −58 | −24 | 10 | 22 | 18 | 4.13 |
| Controls > M1 C410Y | |||||||
| Hippocampus | R | 26 | −10 | −22 | 17 | 4.56 | |
| Hippocampus | R | 26 | −30 | −6 | 5 | 3.98 | |
| Amygdala | L | −32 | −8 | −26 | 11 | 4.74 | |
| Middle frontal gyrus | L | −24 | 60 | −2 | 10 | 72 | 7.45 |
| Middle frontal gyrus | R | 24 | 60 | 8 | 10 | 31 | 4.92 |
| Superior temporal gyrus | L | −56 | −2 | −4 | 22 | 101 | 6.16 |
| Superior temporal gyrus | L | −50 | −20 | 4 | 22 | 92 | 6.1 |
| Superior temporal gyrus | R | 52 | −8 | −4 | 22 | 38 | 4.29 |
| Cingulate gyrus | R | 2 | 38 | 6 | 24 | 35 | 3.58 |
| Fusiform gyrus | L | −32 | −36 | −30 | 36 | 26 | 4.65 |
| Controls > M2 | |||||||
| Hippocampus | R | 36 | −28 | −14 | 48 | 5.8 | |
| Hippocampus | R | 22 | −28 | −12 | 5 | 4.33 | |
| Parahippocampal gyrus | L | −14 | −44 | −4 | 30 | 624 | 7.77 |
| Superior temporal sulcus | R | 44 | 2 | −24 | 23 | 5.2 | |
t = value of peak within significantly activated cluster of voxels; kE = cluster size (in voxels); BA = Brodmann area; L = left; R = right.
*P < 0.005.
Brain activation differences between each family member and the control group during associative learning
| Brain region . | Left/right . | MNI coordinates (mm) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | . | |||||
| . | . | x . | y . | z . | BA . | kE . | t . |
| Two sample t-test | |||||||
| Associative learning versus visual baseline | |||||||
| Y1 C410Y > controls | |||||||
| Hippocampus | L | −38 | −34 | −6 | 7 | 3.44* | |
| Fusiform gyrus | L | −22 | −78 | −18 | 19 | 147 | 7.31 |
| Fusiform gyrus | L | −34 | −46 | −22 | 37 | 210 | 6.02 |
| Superior frontal gyrus | L | −12 | 30 | 58 | 8 | 20 | 6.3 |
| Middle frontal gyrus | L | −50 | 16 | 42 | 8 | 112 | 5.92 |
| Middle frontal gyrus | L | −34 | 56 | 4 | 10 | 61 | 5.02 |
| Middle frontal gyrus | L | −30 | 42 | 16 | 46 | 47 | 4.68 |
| Middle temporal gyrus | L | −60 | −58 | 16 | 37 | 210 | 5.78 |
| Supramarginal gyrus | L | −38 | −60 | 38 | 40 | 57 | 4.44 |
| Precuneus | L | −6 | −60 | 44 | 7 | 265 | 5.98 |
| Y2 > controls | |||||||
| Superior temporal gyrus | R | 52 | 16 | −12 | 38 | 20 | 3.8 |
| Insula | R | 40 | −14 | 2 | 16 | 4.12 | |
| Y3 > controls | |||||||
| Fusiform gyrus | R | 40 | −50 | −20 | 37 | 19 | 4.69 |
| Fusiform gyrus | L | −46 | −48 | −30 | 37 | 19 | 4.66 |
| M1 C410Y > controls | |||||||
| No significant differences | |||||||
| M2 > controls | |||||||
| Superior frontal gyrus | L | −18 | −14 | 60 | 6 | 33 | 4.69 |
| Superior temporal sulcus | R | 46 | −28 | 4 | 44 | 5.36 | |
| Fusiform gyrus | L | −20 | −74 | −8 | 19 | 32 | 5.43 |
| Associative learning versus visual baseline | |||||||
| Controls > Y1 C410Y | |||||||
| No significant differences | |||||||
| Controls > Y2 | |||||||
| No significant differences | |||||||
| Controls > Y3 | |||||||
| Superior temporal gyrus | L | −58 | −24 | 10 | 22 | 18 | 4.13 |
| Controls > M1 C410Y | |||||||
| Hippocampus | R | 26 | −10 | −22 | 17 | 4.56 | |
| Hippocampus | R | 26 | −30 | −6 | 5 | 3.98 | |
| Amygdala | L | −32 | −8 | −26 | 11 | 4.74 | |
| Middle frontal gyrus | L | −24 | 60 | −2 | 10 | 72 | 7.45 |
| Middle frontal gyrus | R | 24 | 60 | 8 | 10 | 31 | 4.92 |
| Superior temporal gyrus | L | −56 | −2 | −4 | 22 | 101 | 6.16 |
| Superior temporal gyrus | L | −50 | −20 | 4 | 22 | 92 | 6.1 |
| Superior temporal gyrus | R | 52 | −8 | −4 | 22 | 38 | 4.29 |
| Cingulate gyrus | R | 2 | 38 | 6 | 24 | 35 | 3.58 |
| Fusiform gyrus | L | −32 | −36 | −30 | 36 | 26 | 4.65 |
| Controls > M2 | |||||||
| Hippocampus | R | 36 | −28 | −14 | 48 | 5.8 | |
| Hippocampus | R | 22 | −28 | −12 | 5 | 4.33 | |
| Parahippocampal gyrus | L | −14 | −44 | −4 | 30 | 624 | 7.77 |
| Superior temporal sulcus | R | 44 | 2 | −24 | 23 | 5.2 | |
| Brain region . | Left/right . | MNI coordinates (mm) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | . | |||||
| . | . | x . | y . | z . | BA . | kE . | t . |
| Two sample t-test | |||||||
| Associative learning versus visual baseline | |||||||
| Y1 C410Y > controls | |||||||
| Hippocampus | L | −38 | −34 | −6 | 7 | 3.44* | |
| Fusiform gyrus | L | −22 | −78 | −18 | 19 | 147 | 7.31 |
| Fusiform gyrus | L | −34 | −46 | −22 | 37 | 210 | 6.02 |
| Superior frontal gyrus | L | −12 | 30 | 58 | 8 | 20 | 6.3 |
| Middle frontal gyrus | L | −50 | 16 | 42 | 8 | 112 | 5.92 |
| Middle frontal gyrus | L | −34 | 56 | 4 | 10 | 61 | 5.02 |
| Middle frontal gyrus | L | −30 | 42 | 16 | 46 | 47 | 4.68 |
| Middle temporal gyrus | L | −60 | −58 | 16 | 37 | 210 | 5.78 |
| Supramarginal gyrus | L | −38 | −60 | 38 | 40 | 57 | 4.44 |
| Precuneus | L | −6 | −60 | 44 | 7 | 265 | 5.98 |
| Y2 > controls | |||||||
| Superior temporal gyrus | R | 52 | 16 | −12 | 38 | 20 | 3.8 |
| Insula | R | 40 | −14 | 2 | 16 | 4.12 | |
| Y3 > controls | |||||||
| Fusiform gyrus | R | 40 | −50 | −20 | 37 | 19 | 4.69 |
| Fusiform gyrus | L | −46 | −48 | −30 | 37 | 19 | 4.66 |
| M1 C410Y > controls | |||||||
| No significant differences | |||||||
| M2 > controls | |||||||
| Superior frontal gyrus | L | −18 | −14 | 60 | 6 | 33 | 4.69 |
| Superior temporal sulcus | R | 46 | −28 | 4 | 44 | 5.36 | |
| Fusiform gyrus | L | −20 | −74 | −8 | 19 | 32 | 5.43 |
| Associative learning versus visual baseline | |||||||
| Controls > Y1 C410Y | |||||||
| No significant differences | |||||||
| Controls > Y2 | |||||||
| No significant differences | |||||||
| Controls > Y3 | |||||||
| Superior temporal gyrus | L | −58 | −24 | 10 | 22 | 18 | 4.13 |
| Controls > M1 C410Y | |||||||
| Hippocampus | R | 26 | −10 | −22 | 17 | 4.56 | |
| Hippocampus | R | 26 | −30 | −6 | 5 | 3.98 | |
| Amygdala | L | −32 | −8 | −26 | 11 | 4.74 | |
| Middle frontal gyrus | L | −24 | 60 | −2 | 10 | 72 | 7.45 |
| Middle frontal gyrus | R | 24 | 60 | 8 | 10 | 31 | 4.92 |
| Superior temporal gyrus | L | −56 | −2 | −4 | 22 | 101 | 6.16 |
| Superior temporal gyrus | L | −50 | −20 | 4 | 22 | 92 | 6.1 |
| Superior temporal gyrus | R | 52 | −8 | −4 | 22 | 38 | 4.29 |
| Cingulate gyrus | R | 2 | 38 | 6 | 24 | 35 | 3.58 |
| Fusiform gyrus | L | −32 | −36 | −30 | 36 | 26 | 4.65 |
| Controls > M2 | |||||||
| Hippocampus | R | 36 | −28 | −14 | 48 | 5.8 | |
| Hippocampus | R | 22 | −28 | −12 | 5 | 4.33 | |
| Parahippocampal gyrus | L | −14 | −44 | −4 | 30 | 624 | 7.77 |
| Superior temporal sulcus | R | 44 | 2 | −24 | 23 | 5.2 | |
t = value of peak within significantly activated cluster of voxels; kE = cluster size (in voxels); BA = Brodmann area; L = left; R = right.
*P < 0.005.
Brain activation differences between each family member and the control group during associative retrieval
| Brain region . | Left/right . | MNI coordinates (mm) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | . | |||||
| . | . | x . | y . | z . | BA . | kE . | t . |
| Two sample t-test | |||||||
| Associative retrieval versus visual baseline | |||||||
| Y1 C410Y > controls | |||||||
| Middle frontal gyrus | L | −38 | 56 | 8 | 10 | 49 | 6.08 |
| Middle frontal gyrus | R | 40 | 52 | −10 | 10 | 47 | 5.23 |
| Inferior frontal gyrus | L | −50 | 16 | 42 | 9 | 23 | 3.95 |
| Inferior frontal gyrus | L | −38 | 24 | −2 | 47 | 19 | 4.22 |
| Fusiform gyrus | L | −22 | −78 | −20 | 19 | 35 | 4.41 |
| Supramarginal gyrus | R | 40 | −64 | 40 | 40 | 61 | 6.08 |
| Inferior parietal lobule | L | −38 | −46 | 44 | 40 | 36 | 4.34 |
| Inferior parietal lobule | L | −38 | −60 | 40 | 40 | 41 | 4.24 |
| Supramarginal gyrus | R | 50 | −64 | 32 | 40 | 18 | 3.91 |
| Y2 > controls | |||||||
| Inferior frontal gyrus | R | 44 | 34 | −6 | 47 | 44 | 5.6 |
| Y3 > controls | |||||||
| Fusiform gyrus | R | 40 | −52 | −24 | 37 | 76 | 7.11 |
| Fusiform gyrus | R | 34 | −70 | −20 | 19 | 36 | 4.28 |
| Fusiform gyrus | L | −44 | −48 | −26 | 37 | 17 | 4.72 |
| M1 C410Y > controls | |||||||
| Orbital gyrus | R | 18 | 54 | −10 | 11 | 22 | 6.07 |
| M2 > controls | |||||||
| No significant differences | |||||||
| Associative retrieval versus visual baseline | |||||||
| Controls > Y1 C410Y | |||||||
| No significant differences | |||||||
| Controls > Y2 | |||||||
| Superior frontal gyrus | L | −2 | 64 | 16 | 9 | 33 | 4.62 |
| Cingulate gyrus | L | −8 | −58 | 26 | 31 | 37 | 4.6 |
| Cingulate gyrus | L | −6 | 46 | 6 | 32 | 43 | 4.49 |
| Controls > Y3 | |||||||
| Parahippocampal gyrus | L | −12 | −36 | −2 | 15 | 4.21 | |
| Rhinal cortex | L | −22 | −6 | −32 | 15 | 5.02 | |
| Medial frontal gyrus | R | 2 | 58 | −10 | 10 | 15 | 5.02 |
| Retrosplenial cortex | L/R | −8 | −48 | 10 | 29 | 147 | 5.11 |
| Controls > M1 C410Y | |||||||
| Hippocampus | L | −22 | −30 | −10 | 10 | 4.07 | |
| Hippocampus | R | 24 | −10 | −20 | 2 | 3.68 | |
| Parahippocampal gyrus | R | 30 | −32 | −14 | 6 | 3.81 | |
| Perirhinal cortex | L | −16 | −4 | −32 | 19 | 5.43 | |
| Superior temporal gyrus | L | −52 | −2 | −6 | 22 | 51 | 5.38 |
| Superior temporal gyrus | R | 62 | −8 | 4 | 22 | 18 | 4.36 |
| Controls > M2 | |||||||
| Hippocampus | R | 34 | −30 | −12 | 95 | 5.17 | |
| Hippocampus | L | −26 | −34 | −8 | 14 | 4.24 | |
| Middle frontal gyrus | L | −40 | 10 | 54 | 6 | 71 | 4.93 |
| Superior temporal gyrus | R | 48 | 10 | −26 | 38 | 74 | 5.27 |
| Retrosplenial cortex | L | −6 | −46 | 14 | 29 | 17 | 5.03 |
| Brain region . | Left/right . | MNI coordinates (mm) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | . | |||||
| . | . | x . | y . | z . | BA . | kE . | t . |
| Two sample t-test | |||||||
| Associative retrieval versus visual baseline | |||||||
| Y1 C410Y > controls | |||||||
| Middle frontal gyrus | L | −38 | 56 | 8 | 10 | 49 | 6.08 |
| Middle frontal gyrus | R | 40 | 52 | −10 | 10 | 47 | 5.23 |
| Inferior frontal gyrus | L | −50 | 16 | 42 | 9 | 23 | 3.95 |
| Inferior frontal gyrus | L | −38 | 24 | −2 | 47 | 19 | 4.22 |
| Fusiform gyrus | L | −22 | −78 | −20 | 19 | 35 | 4.41 |
| Supramarginal gyrus | R | 40 | −64 | 40 | 40 | 61 | 6.08 |
| Inferior parietal lobule | L | −38 | −46 | 44 | 40 | 36 | 4.34 |
| Inferior parietal lobule | L | −38 | −60 | 40 | 40 | 41 | 4.24 |
| Supramarginal gyrus | R | 50 | −64 | 32 | 40 | 18 | 3.91 |
| Y2 > controls | |||||||
| Inferior frontal gyrus | R | 44 | 34 | −6 | 47 | 44 | 5.6 |
| Y3 > controls | |||||||
| Fusiform gyrus | R | 40 | −52 | −24 | 37 | 76 | 7.11 |
| Fusiform gyrus | R | 34 | −70 | −20 | 19 | 36 | 4.28 |
| Fusiform gyrus | L | −44 | −48 | −26 | 37 | 17 | 4.72 |
| M1 C410Y > controls | |||||||
| Orbital gyrus | R | 18 | 54 | −10 | 11 | 22 | 6.07 |
| M2 > controls | |||||||
| No significant differences | |||||||
| Associative retrieval versus visual baseline | |||||||
| Controls > Y1 C410Y | |||||||
| No significant differences | |||||||
| Controls > Y2 | |||||||
| Superior frontal gyrus | L | −2 | 64 | 16 | 9 | 33 | 4.62 |
| Cingulate gyrus | L | −8 | −58 | 26 | 31 | 37 | 4.6 |
| Cingulate gyrus | L | −6 | 46 | 6 | 32 | 43 | 4.49 |
| Controls > Y3 | |||||||
| Parahippocampal gyrus | L | −12 | −36 | −2 | 15 | 4.21 | |
| Rhinal cortex | L | −22 | −6 | −32 | 15 | 5.02 | |
| Medial frontal gyrus | R | 2 | 58 | −10 | 10 | 15 | 5.02 |
| Retrosplenial cortex | L/R | −8 | −48 | 10 | 29 | 147 | 5.11 |
| Controls > M1 C410Y | |||||||
| Hippocampus | L | −22 | −30 | −10 | 10 | 4.07 | |
| Hippocampus | R | 24 | −10 | −20 | 2 | 3.68 | |
| Parahippocampal gyrus | R | 30 | −32 | −14 | 6 | 3.81 | |
| Perirhinal cortex | L | −16 | −4 | −32 | 19 | 5.43 | |
| Superior temporal gyrus | L | −52 | −2 | −6 | 22 | 51 | 5.38 |
| Superior temporal gyrus | R | 62 | −8 | 4 | 22 | 18 | 4.36 |
| Controls > M2 | |||||||
| Hippocampus | R | 34 | −30 | −12 | 95 | 5.17 | |
| Hippocampus | L | −26 | −34 | −8 | 14 | 4.24 | |
| Middle frontal gyrus | L | −40 | 10 | 54 | 6 | 71 | 4.93 |
| Superior temporal gyrus | R | 48 | 10 | −26 | 38 | 74 | 5.27 |
| Retrosplenial cortex | L | −6 | −46 | 14 | 29 | 17 | 5.03 |
t = value of peak within significantly activated cluster of voxels; kE = cluster size (in voxels); BA = Brodmann area; L, left; R, right.
*P < 0.005.
Brain activation differences between each family member and the control group during associative retrieval
| Brain region . | Left/right . | MNI coordinates (mm) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | . | |||||
| . | . | x . | y . | z . | BA . | kE . | t . |
| Two sample t-test | |||||||
| Associative retrieval versus visual baseline | |||||||
| Y1 C410Y > controls | |||||||
| Middle frontal gyrus | L | −38 | 56 | 8 | 10 | 49 | 6.08 |
| Middle frontal gyrus | R | 40 | 52 | −10 | 10 | 47 | 5.23 |
| Inferior frontal gyrus | L | −50 | 16 | 42 | 9 | 23 | 3.95 |
| Inferior frontal gyrus | L | −38 | 24 | −2 | 47 | 19 | 4.22 |
| Fusiform gyrus | L | −22 | −78 | −20 | 19 | 35 | 4.41 |
| Supramarginal gyrus | R | 40 | −64 | 40 | 40 | 61 | 6.08 |
| Inferior parietal lobule | L | −38 | −46 | 44 | 40 | 36 | 4.34 |
| Inferior parietal lobule | L | −38 | −60 | 40 | 40 | 41 | 4.24 |
| Supramarginal gyrus | R | 50 | −64 | 32 | 40 | 18 | 3.91 |
| Y2 > controls | |||||||
| Inferior frontal gyrus | R | 44 | 34 | −6 | 47 | 44 | 5.6 |
| Y3 > controls | |||||||
| Fusiform gyrus | R | 40 | −52 | −24 | 37 | 76 | 7.11 |
| Fusiform gyrus | R | 34 | −70 | −20 | 19 | 36 | 4.28 |
| Fusiform gyrus | L | −44 | −48 | −26 | 37 | 17 | 4.72 |
| M1 C410Y > controls | |||||||
| Orbital gyrus | R | 18 | 54 | −10 | 11 | 22 | 6.07 |
| M2 > controls | |||||||
| No significant differences | |||||||
| Associative retrieval versus visual baseline | |||||||
| Controls > Y1 C410Y | |||||||
| No significant differences | |||||||
| Controls > Y2 | |||||||
| Superior frontal gyrus | L | −2 | 64 | 16 | 9 | 33 | 4.62 |
| Cingulate gyrus | L | −8 | −58 | 26 | 31 | 37 | 4.6 |
| Cingulate gyrus | L | −6 | 46 | 6 | 32 | 43 | 4.49 |
| Controls > Y3 | |||||||
| Parahippocampal gyrus | L | −12 | −36 | −2 | 15 | 4.21 | |
| Rhinal cortex | L | −22 | −6 | −32 | 15 | 5.02 | |
| Medial frontal gyrus | R | 2 | 58 | −10 | 10 | 15 | 5.02 |
| Retrosplenial cortex | L/R | −8 | −48 | 10 | 29 | 147 | 5.11 |
| Controls > M1 C410Y | |||||||
| Hippocampus | L | −22 | −30 | −10 | 10 | 4.07 | |
| Hippocampus | R | 24 | −10 | −20 | 2 | 3.68 | |
| Parahippocampal gyrus | R | 30 | −32 | −14 | 6 | 3.81 | |
| Perirhinal cortex | L | −16 | −4 | −32 | 19 | 5.43 | |
| Superior temporal gyrus | L | −52 | −2 | −6 | 22 | 51 | 5.38 |
| Superior temporal gyrus | R | 62 | −8 | 4 | 22 | 18 | 4.36 |
| Controls > M2 | |||||||
| Hippocampus | R | 34 | −30 | −12 | 95 | 5.17 | |
| Hippocampus | L | −26 | −34 | −8 | 14 | 4.24 | |
| Middle frontal gyrus | L | −40 | 10 | 54 | 6 | 71 | 4.93 |
| Superior temporal gyrus | R | 48 | 10 | −26 | 38 | 74 | 5.27 |
| Retrosplenial cortex | L | −6 | −46 | 14 | 29 | 17 | 5.03 |
| Brain region . | Left/right . | MNI coordinates (mm) . | |||||
|---|---|---|---|---|---|---|---|
| . | . | . | |||||
| . | . | x . | y . | z . | BA . | kE . | t . |
| Two sample t-test | |||||||
| Associative retrieval versus visual baseline | |||||||
| Y1 C410Y > controls | |||||||
| Middle frontal gyrus | L | −38 | 56 | 8 | 10 | 49 | 6.08 |
| Middle frontal gyrus | R | 40 | 52 | −10 | 10 | 47 | 5.23 |
| Inferior frontal gyrus | L | −50 | 16 | 42 | 9 | 23 | 3.95 |
| Inferior frontal gyrus | L | −38 | 24 | −2 | 47 | 19 | 4.22 |
| Fusiform gyrus | L | −22 | −78 | −20 | 19 | 35 | 4.41 |
| Supramarginal gyrus | R | 40 | −64 | 40 | 40 | 61 | 6.08 |
| Inferior parietal lobule | L | −38 | −46 | 44 | 40 | 36 | 4.34 |
| Inferior parietal lobule | L | −38 | −60 | 40 | 40 | 41 | 4.24 |
| Supramarginal gyrus | R | 50 | −64 | 32 | 40 | 18 | 3.91 |
| Y2 > controls | |||||||
| Inferior frontal gyrus | R | 44 | 34 | −6 | 47 | 44 | 5.6 |
| Y3 > controls | |||||||
| Fusiform gyrus | R | 40 | −52 | −24 | 37 | 76 | 7.11 |
| Fusiform gyrus | R | 34 | −70 | −20 | 19 | 36 | 4.28 |
| Fusiform gyrus | L | −44 | −48 | −26 | 37 | 17 | 4.72 |
| M1 C410Y > controls | |||||||
| Orbital gyrus | R | 18 | 54 | −10 | 11 | 22 | 6.07 |
| M2 > controls | |||||||
| No significant differences | |||||||
| Associative retrieval versus visual baseline | |||||||
| Controls > Y1 C410Y | |||||||
| No significant differences | |||||||
| Controls > Y2 | |||||||
| Superior frontal gyrus | L | −2 | 64 | 16 | 9 | 33 | 4.62 |
| Cingulate gyrus | L | −8 | −58 | 26 | 31 | 37 | 4.6 |
| Cingulate gyrus | L | −6 | 46 | 6 | 32 | 43 | 4.49 |
| Controls > Y3 | |||||||
| Parahippocampal gyrus | L | −12 | −36 | −2 | 15 | 4.21 | |
| Rhinal cortex | L | −22 | −6 | −32 | 15 | 5.02 | |
| Medial frontal gyrus | R | 2 | 58 | −10 | 10 | 15 | 5.02 |
| Retrosplenial cortex | L/R | −8 | −48 | 10 | 29 | 147 | 5.11 |
| Controls > M1 C410Y | |||||||
| Hippocampus | L | −22 | −30 | −10 | 10 | 4.07 | |
| Hippocampus | R | 24 | −10 | −20 | 2 | 3.68 | |
| Parahippocampal gyrus | R | 30 | −32 | −14 | 6 | 3.81 | |
| Perirhinal cortex | L | −16 | −4 | −32 | 19 | 5.43 | |
| Superior temporal gyrus | L | −52 | −2 | −6 | 22 | 51 | 5.38 |
| Superior temporal gyrus | R | 62 | −8 | 4 | 22 | 18 | 4.36 |
| Controls > M2 | |||||||
| Hippocampus | R | 34 | −30 | −12 | 95 | 5.17 | |
| Hippocampus | L | −26 | −34 | −8 | 14 | 4.24 | |
| Middle frontal gyrus | L | −40 | 10 | 54 | 6 | 71 | 4.93 |
| Superior temporal gyrus | R | 48 | 10 | −26 | 38 | 74 | 5.27 |
| Retrosplenial cortex | L | −6 | −46 | 14 | 29 | 17 | 5.03 |
t = value of peak within significantly activated cluster of voxels; kE = cluster size (in voxels); BA = Brodmann area; L, left; R, right.
*P < 0.005.
Y2 exhibited learning-, retrieval- and novelty-related activity levels comparable to those in the young controls. There were very few areas of activity differences, which went in both directions, Y2 over the controls and the controls over Y2 (Tables 3 and 4, Supplementary Table S3). Bidirectional activity differences were also found in the ROI analysis: Y2 showed left anterior hippocampal activity that exceeded the controls' P93 during face recognition and left posterior hippocampal activity that fell below the controls' P7 during associative retrieval (Supplementary Table S7).
Y3 showed a few learning-, retrieval- and novelty-related activity increases mainly in the fusiform gyrus relative to controls (Tables 3 and 4, Supplementary Table S4). The controls, on the other hand, exhibited activity increases relative to Y3 in the parahippocampal gyrus, rhinal cortex, medial frontal gyrus, superior temporal gyrus and retrosplenial cortex during associative retrieval, face recognition and novel face detection. A rather weak MTL activity in Y3 became again apparent in the ROI analysis: Y3's mean activity fell below the controls' P7 within many segments of the left hippocampus for single face learning, associative retrieval, and face recognition, and within the right anterior hippocampus for face recognition (Supplementary Table S7).
Middle-aged family members
M1 C410Y exhibited virtually no brain area where task-related activity exceeded activity levels of the young controls (Tables 3 and 4, Fig. 3, Supplementary Table S5). But the young controls exhibited increased activity levels compared to M1 C410Y throughout the MTL, the prefrontal cortex and superior temporal gyrus during learning, retrieval and novelty detection. These activity differences were most pronounced during associative learning and retrieval (Tables 3 and 4). The hippocampal ROI analysis confirmed these results: M1 C410Y's activity levels were generally low and fell below the control group's 7th percentile in many left and right hippocampal segments for associative learning and retrieval, single face learning, face recognition and novel face detection (Supplementary Table S7).
M2 also exhibited reduced task-related brain activity levels relative to the young controls, but these were restricted to fewer brain areas than M1 C410Y's. M2's weak activity levels (relative to the young controls') were focused on the MTL; some areas of weak activity were also found in prefrontal, temporal and parietal areas. These weak (relative to the controls') task-related activity increases appeared chiefly during associative learning and associative retrieval (Tables 3 and 4) (Supplementary Table S6). The ROI analysis indicated that M2's left posterior hippocampal activity was consistently ranging below the controls' P7 during all tasks (Supplementary Table S7).
Working memory
All family members
The family members showed good working memory and concentration skills, except for M2 who performed <M − 2 SD in the 2-back task relative to the young controls. All family members exhibited brain activity enhancements relative to the young controls in the 2-back task (versus the x-target task) within prefrontal, parietal, temporal and cerebellar areas (Supplementary Tables S2–S6).
MRI volumetry results
The manual measurements of the hippocampus and parahippocampal gyrus revealed comparable volumes between the family members and their sex-matched control groups (data not shown in order to conceal the subjects' gender which is reflected in the sizes). Automated whole-brain grey and white matter segmentation showed smaller total grey matter volumes in subjects Y1 C410Y and Y3 (below the control group's 7th percentile) but just sufficient white matter volumes in both subjects. The head transformation matrices of Y1 C410Y and Y3, which resulted from the spatial normalization in SPM2, suggest that the small grey matter values were probably due to small head sizes.
Discussion
We examined five non-demented members of a family with FAD due to the PSEN1 C410Y mutation (Campion et al., 1995). The age of clinical manifestation of Alzheimer's disease in this family is ∼48 years. Our aim was to uncover possible preclinical abnormalities in cognitive functions, memory-related brain activity and brain volumes in those family members who carry the mutation. Of the five family members, a 20-year-old and a 45-year-old subject carried the mutation.
The 20-year-old mutation carrier's cognitive performance in the neuropsychological assessment was normal relative to the published norms of the applied inventories. However, this subject's performance in the visual paired-associates learning and recall test (WMS-R subtests) fell below the matched controls' M − 2 SD range. Although this mutation carrier was well within the range of the matched controls with respect to all other test scores, this subject lost 13 points from the immediate recall of the Wechsler stories (Logical Memory I) to the delayed recall (Logical Memory II). The controls lost between 0 and 8 points (mean 2.4 points). Moreover, Y1's performance on the visual paired-associates learning/recall test and the loss of information in the stories test appear disproportionately low when Y1's high IQ scores (total IQ: 133; performance IQ: 134; verbal IQ: 126) are taken into account (Rentz et al., 2004). Therefore, we consider this young mutation carrier as memory-impaired even though he does not formally fulfil the criteria for amnestic mild cognitive impairment by Petersen et al. (2001). The 45-year-old-mutation carrier, on the other hand, who is roughly 3 years separated from a probable Alzheimer's disease diagnosis, exhibited deficits in the retrieval from episodic memory in both the verbal and non-verbal domain. This subject's WMS-R delayed memory index fell below M − 1.5 SD of test norms and therefore fulfilled the criteria for amnestic mild cognitive impairment by Petersen et al. (2001). The other three family members, two subjects ∼20 years (Y2 and Y3) and one subject of 52 years (M2) were not carrying the mutation. They served as within-family controls. Y2's cognitive performance was completely normal compared with the matched controls' and with test norms. Y3 performed at or below the matched controls' 7th percentile in all neuropsychological tests, but within published test norms. M2's performance measures were also within test norms. Therefore, we rated these family members cognitively intact.
Retrieval performance was more even among family members in the associative memory-fMRI task than in the neuropsychological examination. A likely reason for this discrepancy might be the fact that the face–profession combinations given for learning during fMRI had to be learned over three learning runs and with an imagination task that required subjects to imagine the same scene for a given person on repeated learning runs. The latter adds a retrieval component to the re-encoding process in Runs 2 and 3. This effective encoding procedure is probably the reason why the two middle-aged family members retrieved an equal amount of correct associations as their young relatives and the young controls during the fMRI experiment.
The fMRI results revealed that the young and the middle-aged mutation carriers exhibited significantly altered patterns of memory-related brain activity compared with controls and the family members without the mutation. The young mutation carrier exhibited an overshoot in learning- and retrieval-related brain activity compared with controls. The middle-aged mutation carrier, on the other hand, exhibited very weak brain activity increases when challenged with learning and retrieval tasks. Crucially, the enhanced brain activity in the young mutation carrier appeared in spite of comparable performance measures in the fMRI tasks compared with those of the other young family members and those of the controls. Therefore, differences in brain activity between the young mutation carrier and the controls cannot be attributed to differences in retrieval success (McDermott et al., 2000; Meltzer and Constable, 2005) but rather to the presence and the consequences of the PSEN1 mutation. In particular, the young mutation carrier displayed enhanced brain activity in left frontal, temporal, and parietal neocortices during learning, retrieval, and novelty detection relative to the young controls, while the young controls never increased their brain activity over the young mutation carrier's. Furthermore, activity levels in randomly selected subjects did not clearly deviate from the controls' suggesting that the enhancement of learning- and retrieval-related activity in the young mutation carrier does not correspond to normal inter-subject variability but might be related to the PSEN1 mutation. We focused our fMRI analysis on the MTL because neurofibrillary tangle formation in the course of Alzheimer's disease can usually be first detected in the entorhinal-hippocampal area (Braak and Braak, 1996). Indeed, the young mutation carrier exhibited increased levels of left hippocampal activity relative to controls during the face–profession learning task as a result of both the whole-brain SPM analysis and the hippocampal ROI analysis performed on the non-normalized fMRI images. This activity overshoot might reflect additional cognitive work to accomplish the same task and, at the neural level, a compensatory recruitment of well functioning neural populations to balance dysfunction in neural populations already affected by Alzheimer's disease-related neuropathology. Such compensatory activity increases during learning and retrieval have been found in the MTL and neocortex of presymptomatic individuals at risk for SAD due to the presence of the APOE4 allele (Bookheimer et al., 2000; Bondi et al., 2005) and in individuals with mild cognitive impairment (Dickerson et al., 2004; Rosano et al., 2005). The degree of activity enhancement in these individuals was predictive of further cognitive decline (Bookheimer et al., 2000; Dickerson et al., 2004). Such compensatory activity increases may be related to MTL dysfunction secondary to the formation of neurofibrillary tangles. The entorhinal cortex has been found affected by intracellular neurofibrillary tangles 30–50 years prior to Alzheimer's disease diagnosis (Price et al., 1991; Ohm et al., 1995; Braak and Braak, 1996; Ghebremedhin et al., 1998; Delacourte et al., 1999). Specifically, PSEN1 mutations have been associated with large densities of neurofibrillary tangles in the CA regions of the hippocampus in post-mortem brains (Sudo et al., 2005). There may be both direct effects of the PSEN1 protein on tau phosphorylation and indirect effects on tau pathology (Takashima et al., 1998; Boutajangout et al., 2002). Furthermore, PSEN1 mutations have been associated with significant increases of plasma amyloid beta 42 levels and with a massive deposition of amyloid beta 42 in the whole brain (Lemere et al., 1996; Iwatsubo, 1998). Amyloid beta accumulation appears to be an early and initiating event that triggers a series of downstream processes including the misprocessing of the tau protein (St George-Hyslop and Petit, 2005). Because the genetic defect in FAD is present since birth, it is conceivable that this cascade of Alzheimer's disease-related neuropathological processes takes its course already in childhood or early adulthood. To our knowledge, there are no neuropathological studies on children or young adults with FAD in the literature, but studies in Down's syndrome with Alzheimer's disease showed that deposition of amyloid beta 1–42 could already be observed in the third decade of life (Teller et al., 1996; Stoltzner et al., 2000).
The two young family members, Y2 and Y3, who were not carrying the PSEN1 mutation, exhibited a rather even pattern of brain activity changes during learning and retrieval in the SPM contrasts. They exhibited areas of both enhanced and decreased learning- and retrieval-related brain activity relative to controls. In the ROI analyses, Y2 showed one area of lower hippocampal activity in one task and one area of higher hippocampal activity in another task. Since these deviations were again bidirectional, they probably reflect normal inter-subject variability. Also one of the five randomly selected controls, which were compared to the remaining 20 controls, showed two loci of lowered hippocampal activity. The other four randomly selected subjects ranged within the remaining controls' span. Y3, on the other hand, exhibited hippocampal hypoactivation during three tasks in the ROI analyses plus significantly lowered medial temporal activities during two tasks in the SPM contrasts. In light of Y3's rather low—compared with controls, but not test norms—memory, intelligence and executive function scores, these data might reflect a naturally modest cognitive status unrelated to Alzheimer's disease.
A factor that may have contributed to the enhancement of the fMRI signal in the young mutation carrier versus controls is this individual's high total IQ score of 133 (Table 1, Fig. 2, Supplementary Table S1). To assess this possibility, we also compared the young mutation carrier with those subjects from the control group who reached a total IQ between 130 and 137 (n = 7). The young mutation carrier was ranging within the p7/p93 span of these intelligent controls with regard to all neuropsychological variables and all behavioural variables of the fMRI tasks, but still displayed a learning- and retrieval-related activity enhancement within similar locations as reported for the comparison to the whole control group (data not shown). It should also be noted that Y2's total IQ score was nearly as high as the young mutation carrier's, and Y2's verbal IQ score was even 10 points higher than the young mutation carrier's. Nevertheless, Y2 did not exhibit a learning- and retrieval-related brain activity enhancement relative to controls (Tables 3 and 4; Supplementary Table S3).
The middle-aged PSEN1 mutation carrier, M1, exhibited significantly weaker MTL activity in the ROI analyses and SPM contrasts as well as many areas of weaker neocortical activity relative to the young controls. These differences might not only reflect ageing but also gross neural dysfunction associated with a more advanced stage of Alzheimer's disease-related neuropathology as this family member fulfilled the criteria for amnestic mild cognitive impairment (Petersen et al., 2001). This result is reminiscent of observations in patients in the early stages of Alzheimer's disease who showed decreased task-related brain activity levels (Small et al., 1999; Buckner et al., 2000; Kato et al., 2001). The more aggressive nature of Alzheimer's disease pathology in FAD relative to SAD (Gomez-Isla et al., 1997; Lippa et al., 2000; Sakamoto et al., 2002) might have led to this pattern of results in our case at age 45. The other middle-aged family member, M2, who was not carrying the mutation, exhibited also reduced learning- and retrieval-related MTL activity in the ROI analyses and SPM contrasts as well as few regions of neocortical activity alterations when compared with the young controls. Unfortunately, a middle-aged control group is missing in this study, which limits the interpretation of the two middle-aged individuals' data, because ageing is known to both increase and decrease learning- and retrieval-related brain activity, even when behavioural performance is matched across age groups (e.g. Daselaar et al., 2003, 2006; Maguire and Frith, 2003; Buckner, 2004; Cabeza et al., 2004; Hedden and Gabrieli, 2004). We assume that M2's activity alterations are attributable to the process of normal ageing, because they were restricted to few brain areas and focused on the MTL, particularly the hippocampus (whole-brain contrasts, ROI analyses). Altered hippocampal activity in healthy older subjects is a common finding in memory studies of normal ageing and most probably relate to an encoding deficit (Daselaar et al., 2003, 2006; Maguire and Frith, 2003; Cabeza et al., 2004). Interestingly, M2 showed no hippocampal differences to the controls during face encoding and face recognition, which are tasks that do not rely as much on hippocampal processing as relational memory tasks (Henke et al., 1997). Daselaar and colleagues (2006) reported that recollection-related hippocampal activity was reduced by ageing, while familiarity-related rhinal cortex activity was increased by ageing suggesting that older persons compensate for their recollection deficits (associated with hippocampal dysfunction) by relying more on familiarity judgements (associated with rhinal cortex functions). In line with these results, M2 showed a remember (recollection) score as low as M1's, but achieved a much better know (familiarity) score than M1 (Table 2) in the face recognition task given during fMRI. Our fMRI block design and the small number of trials did not allow for a separate analysis of recollection and familiarity responses. But M2's remember/know dissociation, the normal neuropsychology scores (relative to test norms), and the relatively focal alterations in brain activity speak in favour of normal, rather than pathological, processes of ageing.
It should be noted that the manual measurements of the hippocampus and parahippocampal gyrus revealed comparable volumes between family members and the sex-matched controls suggesting that neither the young nor the middle-aged family members were suffering defined atrophy in the MTL. Thus, partial volume effects due to MTL atrophy cannot account for our imaging results. We would also like to point out that there might be subject differences with respect to the visual baseline condition with which we compared the learning and retrieval conditions. These might have added to the activity differences observed between family members and controls.
The hyperactivation in the young mutation carrier and the hypoactivation in the middle-aged mutation carrier found during episodic memory tasks did not generalize to working memory. Actually, all family members—irrespective of genotype—displayed enhanced working memory-related frontal and parietal activity relative to controls. This result suggests that episodic memory tasks must be used to provoke brain activity alterations which are indicative of preclinical phases of Alzheimer's disease—a result which is in line with previous evidence (Bookheimer et al., 2000; Burggren et al., 2000). We can only speculate about the origin of the increased working memory-related signal in frontal-parietal circuits observed in the five family members. Behaviourally, M2 scored at the low end of the young controls' range in the working-memory task, while M1 and the young family members scored comparable to controls. Each family member's mean reaction latencies for correct and for false answers in the working-memory task ranged within mean ±1 SD of controls. Therefore, there is no behavioural evidence of an increased subjective task difficulty in the young family members. Nevertheless, even in the absence of behavioural differences, it is still possible that the members of this particular family are more challenged than the controls by this 2-back task, perhaps because of less experience with this kind of cognitive challenge or because of less favourable genetic resources, and therefore exerted a greater cognitive effort which reflected in a larger neural recruitment. Increased brain activity during working-memory performance has been found within the working-memory network independently of performance levels within subjects with little compared to more practice on task (Landau et al., 2004; Sayala et al., 2006), in older compared with younger subjects (Mattay et al., 2006), and in carriers of the less favourable polymorphisms of dopamine regulation genes (Bertolino et al., 2006).
Our finding of amplified memory-related brain activity in a young, asymptomatic PSEN1 mutation carrier is also in line with the results obtained in 95 asymptomatic offspring (50–70 years of age) of late-onset Alzheimer's disease cases (Bassett et al., 2006). The group statistics revealed more intense and extensive memory-related fMRI activity in the frontal and temporal lobes including the hippocampus in these at-risk subjects. While it remained uncertain whether the 95 individuals of this study will eventually be diagnosed with Alzheimer's disease, studies in PSEN1 mutation carriers have the advantage of a nearly certain Alzheimer's disease diagnosis. Also, for the initiation of a preclinical treatment of Alzheimer's disease, a preclinical test ideally uncovers first signs of Alzheimer's disease on an individual subject basis.
The aim of our study was to test our memory-fMRI paradigm as a potential preclinical diagnostic tool in cognitively intact individuals who will later manifest Alzheimer's disease with a high probability. Because individuals with the PSEN1 C410Y mutation will develop Alzheimer's disease, they are ideal candidates for testing new diagnostic tools. Although our imaging protocol is not relevant for the early diagnosis of presenilin mutation carriers, because they can be diagnosed by genotyping alone, the present findings validate our diagnostic tool and open the prospect of its application for the early diagnosis of individuals from families with accumulations of sporadic rather than autosomal dominant Alzheimer's disease. A limitation to our study is the inclusion of only one family with FAD. This was due to the rarity of FAD and the circumstance that healthy members from FAD families are not usually inclined to undergo neuropsychological and genetic examinations. Nevertheless, this study shows for the first time that enhanced memory-related brain activity can be identified on a single-subject basis decades before the diagnosis of Alzheimer's disease. Given the likelihood that agents will become available that reliably delay onset and/or slow progression of Alzheimer's disease, it will become essential to detect the disease early in life for best treatment effects.
Supplementary material
Supplementary data are available at Brain Online.
The authors thank the participants for their time and effort, M. Fritsi for laboratory work, and J. Haenggi for help with data analyses. This study was supported by the Swiss National Science Foundation (3100-067114 to KH) and the EU contract LSHM-CT-2003-503330 (APOPIS).
References
- magnetic resonance imaging
- alzheimer's disease
- mutation
- hippocampus
- short-term memory
- middle-aged adult
- neuropsychological tests
- signs and symptoms
- brain
- diagnosis
- memory
- pathology
- cognitive impairment
- episodic memory
- amnestic agents
- functional magnetic resonance imaging
- brain activity
- minimal cognitive impairment
- psen1 gene
- neuropathology
- volumetry