-
PDF
- Split View
-
Views
-
Cite
Cite
Dalia Dimitri, Olivier Benveniste, Odile Dubourg, Thierry Maisonobe, Bruno Eymard, Zahir Amoura, Laetitia Jean, Kiet Tiev, Jean-Charles Piette, David Klatzmann, Serge Herson, Olivier Boyer, Shared blood and muscle CD8+ T-cell expansions in inclusion body myositis, Brain, Volume 129, Issue 4, April 2006, Pages 986–995, https://doi.org/10.1093/brain/awl020
Close - Share Icon Share
Abstract
Inclusion body myositis (IBM) is the most frequent inflammatory myopathy over the age of fifty. Pathological findings suggest that two processes may contribute to IBM pathogenesis: a primary degenerative process affecting muscle fibre and/or an autoimmune process mediated by major histocompatibility complex (MHC) class-I-restricted cytotoxic CD8+ T cells. Previous studies have demonstrated that muscle-infiltrating CD8+ T cells in IBM display restricted expression of T-cell receptor (TCR)-BV families or evidenced oligoclonal T-cell expansions. This study was performed to investigate whether blood T cells similarly exhibit clonal expansions due to the recirculation of muscle-infiltrating T cells in the periphery. For this, we studied the T-cell repertoire of 17 IBM patients by complementarity-determining-region (CDR) 3 length distribution (immunoscope) analysis of TCR-B transcripts. Mean age was 68 years (range 53–88) and mean duration of the disease was 6.5 years (2–20). Oligoclonal T-cell expansions were observed in the blood of IBM patients. The quantitative average perturbation D index was significantly increased in IBM patients [D = 13.7% ± 1.2%, mean ± standard error of measurement (SEM)] as compared with 17 age-matched controls suffering from connective tissue diseases not associated with T-cell repertoire perturbation, that is, dermatomyositis (DM) and systemic sclerosis (9.3 ± 0.6%, P < 0.005). Nevertheless, there was no correlation between the level of blood perturbation and muscle inflammation. Sorting experiments showed that these perturbations were due to oligoclonal expansions of CD8+ T cells. In the three IBM patients analysed, we could relate the blood expansions to T-cell clones also found in muscle. The clonally expanded blood T cells dramatically responded to interleukin-2 (IL-2) in vitro, suggesting that they had been primed in vivo, presumably in response to yet unknown muscle auto-antigens. Together, our results indicate that clonally expanded muscle-infiltrating CD8+ T cells re-circulate in the blood and support the concept of a CD8+ T-cell-mediated autoimmune component in IBM, similarly to what is observed in polymyositis (PM).
Introduction
Inflammatory myopathies (or myositis) can be classified into three main categories on the basis of clinical and histological features (Griggs et al., 1995; Dalakas, 2004; Hoogendijk et al., 2004): dermatomyositis (DM), a systemic microvascular disorder, polymyositis (PM) and inclusion body myositis (IBM). IBM is the most common muscle inflammatory disease affecting individuals over 50 years of age. It is a chronic, slowly progressive inflammatory myopathy, generally resistant to immunosuppressive therapy. Two important pathological features of IBM are invasion of muscle fibres by T cells and presence of rimmed vacuoles with congophilic inclusions containing accumulation of various degenerative proteins (Askanas and Engel, 2002). It has been reported that muscle fibres invaded by T cells in IBM are not vacuolated, whereas vacuolated fibres are not surrounded by T cells (Pruitt et al., 1996), suggesting that two parallel processes might be implied in this disease—one immunopathological and another degenerative. The immunopathological features of IBM are similar to that of PM in many points. Notably, these two myositides are associated with major histocompatibility complex (MHC) class-I upregulation and infiltration by cytotoxic CD8+ T cells, that is, diffuse MHC-I expression is consistently observed on fibres, endomysial T cells are cytotoxic to autologous myotube (Hohlfeld and Engel, 1994), co-stimulatory molecules are upregulated (Murata and Dalakas, 1999b) and auto-invasive CD8+ T cells release perforin and granzyme A (Orimo et al., 1994). Restricted usage of T-cell receptor (TCR) gene families or T-cell clonal expansions have both been evidenced in muscle biopsies of PM and IBM patients (Mantegazza et al., 1993; O'Hanlon et al., 1994a, b; Fyhr et al., 1997; Bender et al., 1998; Amemiya et al., 2000; Benveniste et al., 2001; Nishio et al., 2001; van der Meulen et al., 2002; Hofbauer et al., 2003; Muntzing et al., 2003). Nevertheless, data concerning the presence and function of expanded T cells in peripheral blood of IBM patients are sparse and contradictory (Lindberg et al., 1994; Fyhr et al., 1996, 1998).
We and others have shown that muscle-infiltrating T-cell clonal expansions can be detected in the blood of PM patients by T-cell repertoire immunoscope, that is, analysis of TCR-B complementarity-determining-region (CDR) 3 length distribution (Benveniste et al., 2001; Nishio et al., 2001). Furthermore, we have shown that these CD8+ clonally expanded T cells persist over time—even during remission of treated PM patients—display a memory phenotype, produce intracellular perforin as described previously (Goebels et al., 1996) and dramatically respond to interleukin-2 (IL-2), revealing a potential to be re-activated upon appropriate conditions (Benveniste et al., 2004). To get further understanding of the role of T cells in IBM pathogenesis, we have studied the T-cell repertoire of 17 IBM patients using immunoscopy and compared it with that of 17 age-matched patients with connective tissue diseases that have been shown not to be associated with blood T-cell repertoire perturbation, that is, DM (Benveniste et al., 2001) and established systemic sclerosis (Tiev et al., 2005).
Patients and methods
Patients
Seventeen consecutive patients with a diagnosis of IBM (male : female = 10 : 7, mean age = 68 years, range: 53–88 years) (Table 1) were included in the study between December 1998 and June 2003. The diagnostic criteria for IBM was progressive muscle weakness (affecting proximal and distal muscles of four limbs with at least one of the following features: finger flexor weakness, wrist flexor > wrist extensor weakness, quadriceps weakness) combined with mononuclear cell invasion of non-necrotic fibres and rimmed vacuoles [probable sporadic-IBM according to Griggs et al. (1995)]. At inclusion, patients had no treatment (12 out of 17) or received low-dose corticosteroids (<10 mg/day, in combination with azathioprine 1/5, or intravenous immunoglobulin 1/5, Table 1). Because physiological accumulation of CD8+ T cells may occur with age, seventeen age-matched untreated patients (male : female = 7 : 10; mean age = 65, range: 56–87) with other connective tissue diseases (six DM, eight limited systemic sclerosis and three diffuse systemic sclerosis) were included as controls. The institutional review board approved the study protocol, and all patients were enrolled after written, informed consent was obtained.
Clinical and pathological data of the 17 IBM patients
| Patients . | Age (years)/sex . | Disease duration (years) . | Previous treatment . | Current treatment . | CK (UI/l) . |
|---|---|---|---|---|---|
| IBM-01 | 67/M | 5 | Pred, Aza, Mtx, CsA | 0 | ND |
| IBM-02 | 59/M | 12 | 0 | Pred 5 mg/d | 377 |
| IBM-03 | 76/F | 3 | 0 | 0 | 348 |
| IBM-04 | 61/M | 2 | 0 | 0 | 715 |
| IBM-05 | 72/F | 11 | Pred, IVIg, Mtx, Aza | Pred 10 mg/d, IVIg | 166 |
| IBM-06 | 77/M | 4 | 0 | 0 | 262 |
| IBM-07 | 81/M | 9 | Pred, IVIg, MM | 0 | 122 |
| IBM-08 | 56/M | 2 | 0 | 0 | 414 |
| IBM-09 | 75/M | 4 | 0 | 0 | 838 |
| IBM-10 | 65/F | 9 | 0 | 0 | 607 |
| IBM-11 | 66/F | 5 | 0 | Pred 5 mg/d, Aza | ND |
| IBM-12 | 68/F | 7 | 0 | 0 | 336 |
| IBM-13 | 66/F | 4 | 0 | 0 | 138 |
| IBM-14 | 66/M | 20 | Pred, IVIg | Pred 7 mg/d | 166 |
| IBM-15 | 68/F | 3 | 0 | 0 | 472 |
| IBM-16 | 53/M | 7 | 0 | 0 | 293 |
| IBM-17 | 88/M | 5 | 0 | 0 | 147 |
| Patients . | Age (years)/sex . | Disease duration (years) . | Previous treatment . | Current treatment . | CK (UI/l) . |
|---|---|---|---|---|---|
| IBM-01 | 67/M | 5 | Pred, Aza, Mtx, CsA | 0 | ND |
| IBM-02 | 59/M | 12 | 0 | Pred 5 mg/d | 377 |
| IBM-03 | 76/F | 3 | 0 | 0 | 348 |
| IBM-04 | 61/M | 2 | 0 | 0 | 715 |
| IBM-05 | 72/F | 11 | Pred, IVIg, Mtx, Aza | Pred 10 mg/d, IVIg | 166 |
| IBM-06 | 77/M | 4 | 0 | 0 | 262 |
| IBM-07 | 81/M | 9 | Pred, IVIg, MM | 0 | 122 |
| IBM-08 | 56/M | 2 | 0 | 0 | 414 |
| IBM-09 | 75/M | 4 | 0 | 0 | 838 |
| IBM-10 | 65/F | 9 | 0 | 0 | 607 |
| IBM-11 | 66/F | 5 | 0 | Pred 5 mg/d, Aza | ND |
| IBM-12 | 68/F | 7 | 0 | 0 | 336 |
| IBM-13 | 66/F | 4 | 0 | 0 | 138 |
| IBM-14 | 66/M | 20 | Pred, IVIg | Pred 7 mg/d | 166 |
| IBM-15 | 68/F | 3 | 0 | 0 | 472 |
| IBM-16 | 53/M | 7 | 0 | 0 | 293 |
| IBM-17 | 88/M | 5 | 0 | 0 | 147 |
Pred, prednisone; Aza, azathioprine; Mtx, methotrexate; IVIg, intravenous immunoglobulins; CsA, cyclosporin A; MM, mycophenolate mofetil; CK, creatine kinase (normal ≤ 160 UI/l).
Clinical and pathological data of the 17 IBM patients
| Patients . | Age (years)/sex . | Disease duration (years) . | Previous treatment . | Current treatment . | CK (UI/l) . |
|---|---|---|---|---|---|
| IBM-01 | 67/M | 5 | Pred, Aza, Mtx, CsA | 0 | ND |
| IBM-02 | 59/M | 12 | 0 | Pred 5 mg/d | 377 |
| IBM-03 | 76/F | 3 | 0 | 0 | 348 |
| IBM-04 | 61/M | 2 | 0 | 0 | 715 |
| IBM-05 | 72/F | 11 | Pred, IVIg, Mtx, Aza | Pred 10 mg/d, IVIg | 166 |
| IBM-06 | 77/M | 4 | 0 | 0 | 262 |
| IBM-07 | 81/M | 9 | Pred, IVIg, MM | 0 | 122 |
| IBM-08 | 56/M | 2 | 0 | 0 | 414 |
| IBM-09 | 75/M | 4 | 0 | 0 | 838 |
| IBM-10 | 65/F | 9 | 0 | 0 | 607 |
| IBM-11 | 66/F | 5 | 0 | Pred 5 mg/d, Aza | ND |
| IBM-12 | 68/F | 7 | 0 | 0 | 336 |
| IBM-13 | 66/F | 4 | 0 | 0 | 138 |
| IBM-14 | 66/M | 20 | Pred, IVIg | Pred 7 mg/d | 166 |
| IBM-15 | 68/F | 3 | 0 | 0 | 472 |
| IBM-16 | 53/M | 7 | 0 | 0 | 293 |
| IBM-17 | 88/M | 5 | 0 | 0 | 147 |
| Patients . | Age (years)/sex . | Disease duration (years) . | Previous treatment . | Current treatment . | CK (UI/l) . |
|---|---|---|---|---|---|
| IBM-01 | 67/M | 5 | Pred, Aza, Mtx, CsA | 0 | ND |
| IBM-02 | 59/M | 12 | 0 | Pred 5 mg/d | 377 |
| IBM-03 | 76/F | 3 | 0 | 0 | 348 |
| IBM-04 | 61/M | 2 | 0 | 0 | 715 |
| IBM-05 | 72/F | 11 | Pred, IVIg, Mtx, Aza | Pred 10 mg/d, IVIg | 166 |
| IBM-06 | 77/M | 4 | 0 | 0 | 262 |
| IBM-07 | 81/M | 9 | Pred, IVIg, MM | 0 | 122 |
| IBM-08 | 56/M | 2 | 0 | 0 | 414 |
| IBM-09 | 75/M | 4 | 0 | 0 | 838 |
| IBM-10 | 65/F | 9 | 0 | 0 | 607 |
| IBM-11 | 66/F | 5 | 0 | Pred 5 mg/d, Aza | ND |
| IBM-12 | 68/F | 7 | 0 | 0 | 336 |
| IBM-13 | 66/F | 4 | 0 | 0 | 138 |
| IBM-14 | 66/M | 20 | Pred, IVIg | Pred 7 mg/d | 166 |
| IBM-15 | 68/F | 3 | 0 | 0 | 472 |
| IBM-16 | 53/M | 7 | 0 | 0 | 293 |
| IBM-17 | 88/M | 5 | 0 | 0 | 147 |
Pred, prednisone; Aza, azathioprine; Mtx, methotrexate; IVIg, intravenous immunoglobulins; CsA, cyclosporin A; MM, mycophenolate mofetil; CK, creatine kinase (normal ≤ 160 UI/l).
Pathological score of inflammation and immunohistochemistry
The degree of inflammation was evaluated on transverse frozen sections of muscle stained with haematoxylin and eosin. It was graded independently by two pathologists into three categories, depending on the number of mononuclear cells per square millimetre: grade 1, <25 cells per square millimetre; grade 2, 25–50 cells per square millimetre; grade 3, >50 cells per square millimetre.
Frozen muscle sections of 10 μm thickness were incubated with an anti-BV1 monoclonal antibody (Beckman Coulter), revealed with enhanced horseradish peroxidase-conjugated streptavidin and a substrate chromogen.
Biological samples and cell culture
Peripheral blood mononuclear cells (PBMC) were separated on a Ficoll–Hypaque gradient, washed in RPMI medium (Invitrogen, Paisley, UK) and re-suspended in sterile phosphate-buffered saline (PBS). A minimum of 7 × 106 PBMC was used for RNA extraction and further repertoire analysis. Alternatively, T cells were sorted using MACS CD4 or CD8 microbeads (Miltenyi, Bergisch Gladbach, Germany) and CD4+ or CD8+ T cells were further submitted to RNA extraction.
For some patients, 2 × 106 PBMC were grown in six-well tissue culture plates at a concentration of 106 cells/ml in RPMI medium supplemented with 10% heat-inactivated pooled human AB serum, 1% glutamine, 100 U/ml penicillin G and 100 μg/ml streptomycin. Recombinant human IL-2 (Chiron, Emeryville, CA, USA) was added at a final concentration of 600 U/ml. Fresh medium was added at Day 3. Cells were harvested at Day 7 and washed in PBS before RNA extraction and further repertoire analysis.
T-cell receptor CDR3 size analysis and sequencing
The generation of TCR-B chain diversity occurs during differentiation of T-cell precursors in the thymus by rearrangement of germline variable (V), diversity (D) and joining (J) gene segments of the TCRB locus. As a result of this somatic recombination process, a complete TCR-B molecule is composed of TCR-BV, BD and BJ segments, followed by a TCR-BC constant region. Analyses were performed as described (Benveniste et al., 2001, 2004). Briefly, cDNA was amplified by polymerase chain reaction (PCR) using one primer specific for each of the 14 BV studied and a common BC primer. Each BV-BC PCR product was subjected to run-off cycles primed with a nested fluorophore-labelled BC or with one primer specific for each of the 13 BJ segments, and analysed on an ABI377 automated sequencer (Applied Biosystems). Raw data were analysed with Immunoscope 3.01b software (Pannetier et al., 1993).
The level of repertoire perturbation was quantified by calculating a perturbation index, as described (Benveniste et al., 2001). Briefly, the mean of individual Dk perturbations for all 14 BVk families yields the average perturbation D = ∑kDk/14. Our previous analysis revealed a threshold value Dthres = 11%, under which the D values of healthy controls were distributed.
After reverse transcription-polymerase chain reaction (RT–PCR) using BV1 and BC primers, amplified products were gel-purified using the QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA, USA) before being ligated into the pT-Adv vector (Clontech, Paolo Alto, CA, USA) and transformed into bacteria. Plasmid DNA from 11–23 randomly picked colonies were isolated using the NucleoSpin Plasmid Kit (Machery-Nagel, Düren, Germany) and sequenced using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster, CA, USA) using the universal forward-sequencing primer M13. The sequences were run on an ABI PRISM 3100 GA-V1 capillary sequencer (Applied Biosystems).
Statistical analyses
Statistical analyses were performed using Statview software (SAS Inc.). The Mann–Whitney test was used to compare data.
Results
Qualitative and quantitative analysis of the blood T-cell repertoire in IBM
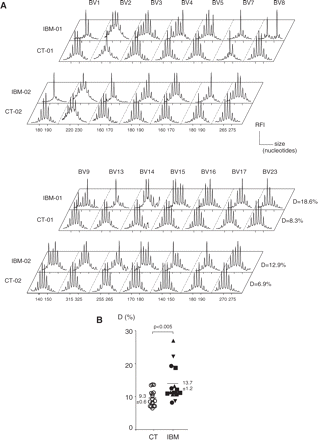
Using the immunoscope method, the distribution of TCR-B CDR3 lengths is visualized as a series of peaks separated by a distance of three nucleotides corresponding to in-frame transcripts. A physiologically diverse repertoire yields a Gaussian-like profile, whereas the presence of T-cell clonal expansions manifests as a distribution skewed by the accumulation of larger peaks or, ultimately, as a single peak. We studied the blood T-cell repertoire of 17 IBM and 17 control patients suffering from connective tissue diseases that were previously shown not to be associated with blood T-cell repertoire perturbation, that is, DM (Benveniste et al., 2001) and established systemic sclerosis (Tiev et al., 2005). Qualitative immunoscope analysis revealed perturbed T-cell repertoires in IBM patients. These T-cell expansions appeared to be oligoclonal and, for a given patient, one or more discrete peaks accumulated within several BV families. For instance, single or double dominant peaks were found in the BV1, BV7, BV8 or BV13 families of patient IBM-01 and BV1, BV3 or BV7 families of IBM-02 (Fig. 1A). From one patient to another, these expansions did not concern the same BV and no recurrence of a particular peak was found. In contrast, the majority of control patients presented a Gaussian-like distribution in most of the BV families (Fig. 1A).
Immunoscope analysis of the T-cell repertoire in IBM patients and age-matched controls. The T-cell repertoire was studied in 17 IBM patients and 17 age-matched controls by analysing TCR-B CDR3 length distributions using immunoscope in 14 BV families. (A) Example of immunoscope profiles in IBM-01 and IBM-02 patients and two age-matched controls. T-cell clonal expansions manifest as a distribution skewed by the presence of larger peaks that accumulate above the Gaussian-like background of polyclonal T cells. (B) Quantitative analysis of T-cell repertoire perturbations. Average perturbation (D values) that reflect the level of repertoire perturbation are given for each IBM patient (black symbols) and controls (open symbols). There was no correlation between D value of IBM patients and their level of inflammation in the muscle biopsy (up triangle, grade 1; square, grade 2; down triangle, grade 3; circle, not done). Numbers are mean ± SEM for each group.
In order to allow statistical analysis, we quantified the extent of perturbation of the T-cell repertoire by computing an index (D) varying from 0% (no perturbation) to 100% (theoretical maximum), normal values from healthy subjects being distributed below Dthres = 11% in our experience. Among the 17 IBM patients, 13 had D values superior to 11% and only two of them had D values below 10%. The average D index for the 17 IBM patients was 13.7% ± 1.2% [(mean ± standard error of measurement (SEM)], which was significantly higher than that for the 17 control patients, that is, 9.3 ± 0.6%, (P < 0.005, Fig. 1B). There was no correlation between D values and age, duration of disease nor creatine kinase (CK) levels. Similarly, no correlation was evidenced between the D values of blood repertoire perturbations and the extent of the inflammatory infiltrates in muscular biopsy evaluated blind by two independent pathologists. For instance, IBM-4 has the highest D value (27%) despite a moderate inflammation in muscle (pathological grade 1). We conclude that IBM patients display significantly perturbed blood T-cell repertoires as compared with controls.
T-cell repertoire in muscle infiltrates of IBM patients
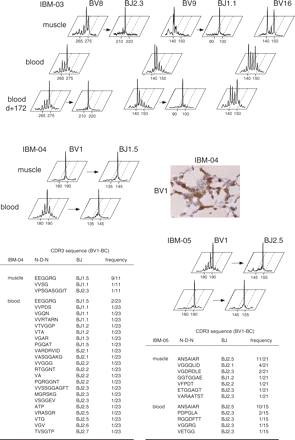
To demonstrate that blood T-cell expansions in IBM are clonally related to muscle-infiltrating T cells, we further analysed the repertoire of muscle T cells in three IBM patients for whom a frozen biopsy was available for RNA extraction, and compared it with their blood repertoire. Two different clones using the same BV gene segment can fortuitously share the same CDR3 length when analysed at the BV-BC level, which would therefore yield indistinguishable peaks. Since one given BV can associate with 1 out of 13 BJ gene families, analyses were further performed at the BV-BJ resolution, and finally TCRs were sequenced for two patients so as to definitively ascertain clonal identity (Fig. 2). In the case of IBM-03, the BV8 expansion in both muscle and blood corresponded to one major clone using a BJ2.3 gene segment. Similarly, the BV9 expansion in this patient corresponded to one clone using a BJ1.1 gene segment. These two clones could still be found in the blood after 172 days of follow-up. In the case of IBM-04, the BV1-BJ1.5 TCR expansion corresponded to a major T-cell clone, as confirmed by a defined TCR sequence (EEGGRG) found in muscle but also detected in the blood among a series of non-recurrent TCRs using various TCR-BJ. In support of a pathogenic role, immunochemistry revealed that muscle fibres were indeed surrounded and invaded by BV1-positive T cells (Fig. 2). For IBM-05, the same clonotypic BV1-BJ2.5 TCR sequence (ANSAIAR) was found in both muscle (11 out of 21) and blood (10 out of 15). For IBM-03, IBM-04 and IBM-05, muscle biopsies were performed, respectively, 1 year, forty days and 4 years earlier than the blood T-cell repertoire analysis. Together, these results indicate that oligoclonal expansions observed in the blood of IBM patients are clonally related to those present in the muscle infiltrates and suggest a long-term persistence of repertoire anomalies in IBM.
Comparative analysis of blood and muscle T-cell repertoire in three IBM patients. The blood T-cell repertoire of patients IBM-03, IBM-04 and IBM-05 was compared with that of the muscle infiltrate. To better ascertain the clonal identity of the peaks observed in both blood and muscle at the BV-BC level, immunoscope analysis was further performed at the BV-BJ resolution. Blood samples were collected at 1 year, forty days and 4 years after muscle biopsy for IBM-03, IBM-04 and IBM-05, respectively. For IBM-03, an additional blood analysis was performed 172 days after the initial blood sampling. For IBM-04 muscle, the result of anti-BV1 immunohistochemical T-cell staining is shown. The frequency of CDR3 clonotypes found after cloning and sequencing of BV1-BC RT–PCR products from patient IBM-04 and IBM-05 is given.
Repertoire analysis of blood CD4+ and CD8+ T-cell subsets
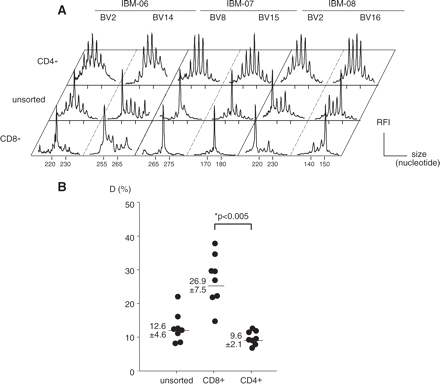
To better characterize the origin of IBM-associated T-cell expansions, we sorted the CD4+ and CD8+ subsets in eight patients. Since muscle infiltrates in IBM are mostly represented by CD8+ T cells, the abnormal distributions of CDR3 lengths in the blood should be found within this sub-population but not within the CD4+ subset. As shown in Fig. 3A, the peaks identified by immunoscope analysis of unsorted PBMC corresponded to CD8+ expansions, whereas the distributions in CD4+ T cells were mostly Gaussian. Hence, in the CD8+ subset, the average perturbation D reached the high value of 26.9% ± 7.5% (Fig. 3B), whereas D values remained in the normal range for the CD4+ subset (9.6% ± 2.1%, P < 0.005).
Immunoscope analysis of blood CD4+ and CD8+ T-cell repertoires. CD4+ and CD8+ blood T cell were purified by immunomagnetic sorting. (A) Comparison of immunoscope profiles in two representative BV families where T-cell expansions were observed in the unsorted T-cell population. (B) Quantification of T-cell repertoire perturbations. Numbers are mean ± SEM. The difference between average perturbation D (%) of CD4+ and CD8+ subsets was statistically significant.
IL-2 responsiveness of blood T-cell expansions in IBM
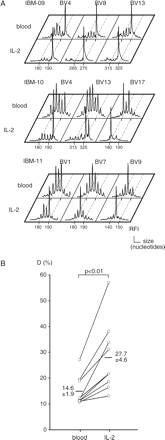
To get some insight into the functional properties of expanded T cells in IBM, we investigated whether they could be activated in vitro. For this, PBMC of 9 out of the 17 IBM patients were grown for 7 days in the presence of IL-2 without any TCR-mediated activation. In these conditions, only in vivo primed T cells can survive and expand, whereas naïve unprimed T cells die during the culture. The sole presence of this cytokine was sufficient to provoke a dramatic augmentation of perturbation indices that reached the high value of 27.7% ± 4.6% (Fig. 4A). This increase was due to the expansion of T-cell clones that were readily detectable before culture. This was manifested by a quasi-monoclonal aspect within several BV families, together with a strong reduction of Gaussian-distributed peaks, the latter representing polyclonal, presumably unprimed naïve T cells (Fig. 4B). These results suggest that the T-cell clones that accumulate in the blood of IBM patients correspond to CD8+ T cells that have been primed in vivo, possibly in response to yet unknown muscle auto-antigens.
T-cell repertoire analysis after in vitro culture of PBMC in presence of IL-2. PMBC of nine IBM patients were cultured for 7 days in the presence of IL-2. (A) Comparison of post-culture (IL-2) and pre-culture (blood) immunoscope profiles in IBM-09, IBM-10 and IBM-11 patients. (B) Quantification of average T-cell repertoire perturbations before and after IL-2 culture. The difference between average perturbation D (%) (mean ± SEM) between post-IL-2 (27.7% ± 4.6%) and pre-culture (14.6% ± 1.9%) was statistically significant.
Discussion
Different strategies for detecting and quantifying antigen-specific T cells in autoimmune diseases are available (Hohlfeld, 2003). Among these, immunoscopy appears as a sensitive method for the identification of T-cell clonal expansions in healthy and diseased humans (Pannetier et al., 1995). Although restricted usage of TCR-BV families or clonal expansions of T cells has already been reported within the muscle of IBM patients using immunohistochemical (Lindberg et al., 1994) or molecular biology methods (O'Hanlon et al., 1994a; Fyhr et al., 1997; Bender et al., 1998; Amemiya et al., 2000; van der Meulen et al., 2002; Muntzing et al., 2003), few data are available regarding the blood T-cell repertoire [only from three studies of two to four patients (Lindberg et al., 1994; Fyhr et al., 1996, 1998)]. Here, we demonstrate that IBM is associated with oligoclonal expansions of blood CD8+ T cells. Because some level of CD8+ T-cell expansion can occur in physiological situations, specially in the elderly (Posnett et al., 1994; Schwab et al., 1997), we compared our patients with age-matched patients with DM or established systemic sclerosis, two connective tissue diseases that were previously shown not to be associated with blood T-cell repertoire perturbation (Benveniste et al., 2001; Tiev et al., 2005). Repertoire perturbations in most IBM patients were higher than the threshold level of Dthres = 11%, under which healthy subjects are distributed in our experimental conditions. They were also significantly higher than those in the control population. We further showed that the blood T-cell expansions were clonally related to muscle-infiltrating clones in the three IBM patients analysed. Hence, clonal expansion in the blood presumably reflects the recirculation of muscle-infiltrating T cells. Nevertheless, another possibility might be that the same antigen, for example a virus, can independently induce the same expansion in the blood and in the muscle biopsies, if this antigen is present at both sites. Also, the repertoire might change over a long period of time; it is striking that these expansions persist even late in the course of IBM, as the mean duration of the disease at time of inclusion was 6.5 years (2–20 years) in this series of patients. This is in agreement with the observations made from repeated muscle biopsies that also evidenced long-term persistence of infiltrating T-cell clones (Amemiya et al., 2000; Muntzing et al., 2003). The long-term duration of these T-cell abnormalities over time supports the hypothesis of a sustained antigen-driven immune response directed towards a limited number of auto-antigens presented by MHC-I-expressing muscle cells. In addition, and similarly to PM, IBM-associated T-cell clones were highly responsive to IL-2, as evidenced by a striking dominance after culture of the very peaks that were readily detectable before culture. Together, these results indicate that the clonal expansions found in IBM correspond to T cells that had been primed in vivo and suggest that they could exert a pathogenic role against muscle cells in IBM. The latter hypothesis is reinforced by our immunochemistry data.
Unexpectedly, we did not find correlation between the level of muscle inflammation and the degree of blood repertoire perturbation. Several hypotheses could explain this finding. First, while the level of muscle inflammation may vary considerably between IBM patients—from sparse or absent signs of inflammation to florid—no correlation has been found between this parameter and disease duration or severity (Pruitt et al., 1996). Secondly, the inflammatory infiltrates may not only be heterogeneous but are also generally multi-focal within a single patient. For instance, different proportions of clonally expanded endomysial T cells, on the one hand, and clonally diverse interstitial T cells, on the other hand, may be observed (Lindberg et al., 1994; Bender et al., 1998). This may result in a sampling problem if the biopsy is performed in a poorly inflammatory region, for instance. In this line, upregulation of MHC class I is consistently found in IBM muscle, but is irrespective of the absence or presence of inflammatory cell infiltration (Dahlbom et al., 2002). Finally, it can not be formally excluded that in some patients with severe inflammation, the most aggressive T-cell clones do not re-circulate in the blood because they find high-affinity ligands in the muscle.
In contrast to PM, degenerative changes are associated with inflammation in IBM. Hence, the concept of IBM as an autoimmune disease has been a matter of debate. The T-cell inflammation may not be related to muscle weakness or disease progression, since immunosuppressive therapy does not prevent disease progression. Indeed, two studies have shown that decreased muscle inflammation after immunosuppressive therapy was not accompanied by a clinical benefit (Barohn et al., 1995; Dalakas et al., 2001). It is also intriguing that the degenerative changes share remarkable similarities with the pathology of Alzheimer's disease (accumulation of amyloid-β precursor protein, phosphorylated tau and other Alzheimer-related proteins) (Askanas and Engel, 2002). Hence, one view is that the CD8+ T-cell-mediated reaction in IBM may be secondary to the over-expression of MHC class I molecules and the subsequent presentation of muscle antigens to these T cells. In this line, T-cell infiltrates may be observed as a secondary process in several human genetic myopathies such as dysferlin deficiency (Confalonieri et al., 2003) or facioscapulohumeral muscular dystrophy (Arahata et al., 1995), for instance. An alternative view is that a primary autoimmune process mediated by antigen-specific T cells promotes the vacuolar degeneration and ectopic expression of the potentially toxic proteins characteristic of IBM. In support of an autoimmune pathogenesis, there is a strong association between IBM and the HLA-B8-DR3 haplotype (Badrising et al., 2004), and IBM may be associated with other autoimmune manifestation (Koffman et al., 1998). Although our study does not definitively rule out a primary degenerative process with a secondary immune component, our results strengthen the concept of a similar pathogenic process between PM and IBM by which a muscle-aggressive autoimmune reaction elicits significant biases in the blood T-cell repertoire. The resemblance between PM and IBM is reinforced by the results of a transcriptome analysis that evidenced a set of immunoglobulin-related genes that were consistently over-expressed in both IBM and PM, relative to DM, whereas a group of interferon-inducible genes exhibited the reverse pattern (Greenberg et al., 2002). Many other characteristics are shared by PM and IBM: (i) CD8+ T cells invade and apparently destroy HLA class I-expressing muscle fibres; (ii) limited expression of TCR genes of selected BV families by the endomysial T cells persist over the course of the disease, which suggests that a limited number of antigens drive the immune response; (iii) auto-invasive T cells and muscle fibres express T-cell co-stimulatory molecules (Behrens et al., 1998; Murata and Dalakas, 1999a); (iv) clonal expansion of CD8+ T cells in blood and muscle suggests recirculation of pathogenic T cells. The two pathogenesis hypotheses are not necessarily mutually exclusive since the two processes might be implied at different stages of the disease. The broad clinical spectrum observed in multiple sclerosis may illustrate this concept: the relapsing-remitting form, which affects young patients, underlies an inflammatory process and is treatable by immunosuppressive or immunomodulatory therapies, whereas the secondary and primary progressive forms, which affect older patients, appear to be predominantly degenerative processes and are resistant to therapy (Noseworthy et al., 2000; Compston and Coles, 2002). Together, our data support the concept of an important and persistent autoimmune component in IBM, similarly to PM. Determining the role of muscle-infiltrating T cells in the pathogenesis of IBM could lead to TCR-based therapy and to the identification of IBM-associated auto-antigens.
These two authors contributed equally to this work
This work was supported by Association Française contre les Myopathies and Faculté de Médecine Pierre et Marie Curie. We acknowledge Marie-Christine Burland, Véronique Bon-Durand and Julien Abriol for technical assistance. We thank Association des Sclérodermiques de France and Assistance Publique-Hôpitaux de Paris.
References
Amemiya K, Granger RP, Dalakas MC. Clonal restriction of T-cell receptor expression by infiltrating lymphocytes in inclusion body myositis persists over time. Studies in repeated muscle biopsies.
Arahata K, Ishihara T, Fukunaga H, Orimo S, Lee JH, Goto K, et al. Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): immunocytochemical and genetic analyses.
Askanas V, Engel WK. Inclusion-body myositis and myopathies: different etiologies, possibly similar pathogenic mechanisms.
Badrising UA, Schreuder GM, Giphart MJ, Geleijns K, Verschuuren JJ, Wintzen AR, et al. Associations with autoimmune disorders and HLA class I and II antigens in inclusion body myositis.
Barohn RJ, Amato AA, Sahenk Z, Kissel JT, Mendell JR. Inclusion body myositis: explanation for poor response to immunosuppressive therapy.
Behrens L, Kerschensteiner M, Misgeld T, Goebels N, Wekerle H, Hohlfeld R. Human muscle cells express a functional costimulatory molecule distinct from B7.1 (CD80) and B7.2 (CD86) in vitro and in inflammatory lesions.
Bender A, Behrens L, Engel AG, Hohlfeld R. T-cell heterogeneity in muscle lesions of inclusion body myositis.
Benveniste O, Cherin P, Maisonobe T, Merat R, Chosidow O, Mouthon L, et al. Severe perturbations of the blood T cell repertoire in polymyositis, but not dermatomyositis patients.
Benveniste O, Herson S, Salomon B, Dimitri D, Trebeden-Negre H, Jean L, et al. Long-term persistence of clonally expanded T cells in patients with polymyositis.
Confalonieri P, Oliva L, Andreetta F, Lorenzoni R, Dassi P, Mariani E, et al. Muscle inflammation and MHC class I up-regulation in muscular dystrophy with lack of dysferlin: an immunopathological study.
Dahlbom K, Lindberg C, Oldfors A. Inclusion body myositis: morphological clues to correct diagnosis.
Dalakas MC. Inflammatory disorders of muscle: progress in polymyositis, dermatomyositis and inclusion body myositis.
Dalakas MC, Koffman B, Fujii M, Spector S, Sivakumar K, Cupler E. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM.
Fyhr IM, Moslemi AR, Tarkowski A, Lindberg C, Oldfors A. Limited T-cell receptor V gene usage in inclusion body myositis.
Fyhr IM, Moslemi AR, Mosavi AA, Lindberg C, Tarkowski A, Oldfors A. Oligoclonal expansion of muscle infiltrating T cells in inclusion body myositis.
Fyhr IM, Moslemi AR, Lindberg C, Oldfors A. T cell receptor beta-chain repertoire in inclusion body myositis.
Goebels N, Michaelis D, Engelhardt M, Huber S, Bender A, Pongratz D, et al. Differential expression of perforin in muscle-infiltrating T cells in polymyositis and dermatomyositis.
Greenberg SA, Sanoudou D, Haslett JN, Kohane IS, Kunkel LM, Beggs AH, et al. Molecular profiles of inflammatory myopathies.
Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies.
Hofbauer M, Wiesener S, Babbe H, Roers A, Wekerle H, Dornmair K, et al. Clonal tracking of autoaggressive T cells in polymyositis by combining laser microdissection, single-cell PCR, and CDR3-spectratype analysis.
Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands.
Koffman BM, Rugiero M, Dalakas MC. Immune-mediated conditions and antibodies associated with sporadic inclusion body myositis.
Lindberg C, Oldfors A, Tarkowski A. Restricted use of T cell receptor V genes in endomysial infiltrates of patients with inflammatory myopathies.
Mantegazza R, Andreetta F, Bernasconi P, Baggi F, Oksenberg JR, Simoncini O, et al. Analysis of T cell receptor repertoire of muscle-infiltrating T lymphocytes in polymyositis. Restricted V alpha/beta rearrangements may indicate antigen-driven selection.
van der Meulen MF, van Wichen DF, van Blokland WT, van den Berg LH, Wokke JH, Hoogendijk JE, et al. Evidence for heterogeneity of T cell expansion in polymyositis and inclusion body myositis.
Muntzing K, Lindberg C, Moslemi AR, Oldfors A. Inclusion body myositis: clonal expansions of muscle-infiltrating T cells persist over time.
Murata K, Dalakas MC. Expression of the costimulatory molecule BB-1, the ligands CTLA-4 and CD28, and their mRNA in inflammatory myopathies.
Murata K, Dalakas MC. Expression of the costimulatory molecule BB-1, the ligands CTLA-4 and CD28, and their mRNA in inflammatory myopathies.
Nishio J, Suzuki M, Miyasaka N, Kohsaka H. Clonal biases of peripheral CD8 T cell repertoire directly reflect local inflammation in polymyositis.
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis.
O'Hanlon TP, Dalakas MC, Plotz PH, Miller FW. The alpha beta T-cell receptor repertoire in inclusion body myositis: diverse patterns of gene expression by muscle-infiltrating lymphocytes.
O'Hanlon TP, Dalakas MC, Plotz PH, Miller FW. Predominant TCR-alpha beta variable and joining gene expression by muscle-infiltrating lymphocytes in the idiopathic inflammatory myopathies.
Orimo S, Koga R, Goto K, Nakamura K, Arai M, Tamaki M, et al. Immunohistochemical analysis of perforin and granzyme A in inflammatory myopathies.
Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments.
Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples.
Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to ‘benign monoclonal gammapathy’.
Pruitt JNII, Showalter CJ, Engel AG. Sporadic inclusion body myositis: counts of different types of abnormal fibers.
Schwab R, Szabo P, Manavalan JS, Weksler ME, Posnett DN, Pannetier C, et al. Expanded CD4+ and CD8+ T cell clones in elderly humans.
Author notes
1Service de médecine interne 1, 2Laboratoire de neuropathologie, 3Institut de myologie, 4Service de médecine interne 2, Hôpital Pitié-Salpêtrière, 5Service de médecine interne, Hôpital Saint-Antoine, 6CNRS UMR 7087, Hôpital Pitié-Salpêtrière, Paris and 7INSERM U 519, Equipe Avenir ‘Immuno-myologie fondamentale et biothérapies’, Rouen, France