-
PDF
- Split View
-
Views
-
Cite
Cite
Jörg Spiegel, Dirk Hellwig, Marc-Oliver Möllers, Stefanie Behnke, Wolfgang Jost, Klaus Fassbender, Samuel Samnick, Ulrich Dillmann, Georg Becker, Carl-Martin Kirsch, Transcranial sonography and [123I]FP-CIT SPECT disclose complementary aspects of Parkinson's disease, Brain, Volume 129, Issue 5, May 2006, Pages 1188–1193, https://doi.org/10.1093/brain/awl042
Close - Share Icon Share
Abstract
Hyperechogenic signal of substantia nigra (SN) in transcranial sonography (TCS) and reduced striatal uptake in FP-CIT SPECT are common findings in idiopathic Parkinson's disease (PD). But so far it is unknown whether the extent of SN hyperechogenicity represents a correlate for the degeneration of presynaptic dopaminergic neurons in PD. We performed TCS and 123I-labelled N-(3-fluoropropyl)-2ß-carbomethoxy-3ß-(4-iodophenyl)nortropane ([123I]FP-CIT) SPECT in 53 patients with PD. Striatal FP-CIT uptake was quantified by measuring the striatal/posterior lobe binding of [123I]FP-CIT. SN echogenicity was quantified by planimetric measurement of the maximum extension of hyperechogenic signals. We found no correlation between striatal FP-CIT uptake and echogenicity of the SN, neither contralateral to the clinically more affected body side (r = +0.08, P = 0.57; Pearson's correlation) nor ipsilateral (r = +0.01; P = 0.92). Our data show that the extent of SN hyperechogenicity does not correlate with the degeneration of presynaptic dopaminergic nerve terminals. Obviously SN hyperechogenicity and degeneration of presynaptic dopaminergic nerve terminals exist independently from each other and may be based on different pathomechanisms.
Introduction
Transcranial sonography (TCS) of the substantia nigra (SN) represents a new imaging technique in the diagnosis of idiopathic Parkinson's disease (PD; Berg and Becker, 2002). More than 90% of PD patients reveal a hyperechogenic SN (Berg et al., 2001a; Walter et al., 2002), whereas only 9% of healthy adults show a hyperechogenic SN (Berg et al., 1999a, 2002). The reason for SN hyperechogenicity in PD patients is unknown. Animal and postmortem studies (Berg et al., 1999b, 2002) point to a relation between SN echogenicity and tissue iron content. It is assumed that binding of an increased amount of iron to iron metabolizing proteins or structural alteration of iron binding proteins might lead to differences in the reflection of the ultrasound beam, displayed as hyperechogenic signals (Behnke et al., 2005). This hypothesis is supported by Zecca et al. (2005), who found a significant correlation between the echogenic area of the SN and the concentration of iron, H- and L-ferritin in postmortem brains. But so far, it is unknown how this pathomorphological finding of hyperechogenic SN in TCS reflects the functional relevant changes in PD, which are mainly caused by degeneration of presynaptic dopaminergic nerve terminals. Accordingly, it is unknown whether the extent of SN hyperechogenicity correlates with the degeneration of presynaptic dopaminergic neurons. The latter can be measured by 123I-labelled N-(3-fluoropropyl)-2ß-carbomethoxy-3ß-(4-iodophenyl)nortropane ([123I]FP-CIT) SPECT. The [123I]FP-CIT SPECT measures concentration of striatal dopamine reuptake transporters (Winogrodzka et al., 2003). Previous studies (Benamer et al., 2000a; Booij et al., 2001) reported a high sensitivity of [123I]FP-CIT SPECT concerning the diagnosis of PD. Furthermore, the striatal uptake of [123I]FP-CIT correlates significantly with the motor symptoms (Benamer et al., 2000b; Booij et al., 2001) underlining the pathophysiological relevance of FP-CIT SPECT. In the present paper, we examine whether the pathomorphologically relevant parameters in PD—echogenicity of SN in TCS—correlate with functional parameters—striatal uptake of [123I]FP-CIT.
Methods
Patients
Our investigations involved 53 patients, 20 females and 33 males, with idiopathic PD [age 28–78 years, 59 ± 11 years (mean ± SD), Table 1]. Thirty-two patients were graded as Hoehn and Yahr Stage 1, 9 patients as Hoehn and Yahr Stage 2, 10 patients as Hoehn and Yahr Stage 3 and 2 patients as Hoehn and Yahr Stage 4 (Hoehn and Yahr, 1967). Idiopathic PD was diagnosed according to the criteria of the UK Parkinson's Disease Society Brain Bank (Hughes et al., 1992). Two different neurologists examined the patients. There was no evidence of gaze palsy, cerebellar deficit or pyramidal signs. None of the patients was found to have a history of neuroleptic intake or other identifiable possible causes of secondary parkinsonism. Cerebral magnetic resonance imaging (MRI) findings were normal in all patients, particularly regarding the absence of either white matter lesions or ventricular widening. The motor part (Part III) of the Unified Parkinson's Disease Rating Scale (UPDRS) was used to assess the severity of the disease. The scale was rated during an ‘off’ phase (12 h off drug).
Patients with idiopathic PD
| . | Hoehn and Yahr . | . | . | |||
|---|---|---|---|---|---|---|
. | Stage 1 . | Stage 2 . | Stages 3 and 4 . | |||
| Patients | ||||||
| N | n = 32 | n = 9 | n = 12 | |||
| Females | n = 10 | n = 3 | n = 7 | |||
| Males | n = 22 | n = 6 | n = 5 | |||
| Age (mean ± SD years) | 57.0 ± 9.7 | 57.9 ± 12.4 | 63.9 ± 12.5 | |||
| Disease duration (mean ± SD years) | 2.1 ± 1.9 | 3.7 ± 1.7 | 6.5 ± 2.2 | |||
| UPDRS (mean ± SD) | 11.6 ± 4.9 | 23.2 ± 6.2 | 28.5 ± 9.3 | |||
| Clinical subtypes | ||||||
| Akinetic-rigid type | n = 11 | n = 3 | n = 9 | |||
| Mixed type | n = 11 | n = 3 | n = 2 | |||
| Tremor-dominance type | n = 10 | n = 3 | n = 1 | |||
| TCS | ||||||
| Contralat. TCS (mean ± SD) | 0.24 ± 0.06 | 0.19 ± 0.05 | 0.23 ± 0.05 | |||
| Ipsilat. TCS (mean ± SD) | 0.20 ± 0.05 | 0.15 ± 0.05 | 0.19 ± 0.05 | |||
| Contralat. TCS normal | n = 3 | n = 4 | n = 2 | |||
| Contralat. TCS pathological | n = 28 | n = 5 | n = 10 | |||
| Insuffic. contr. bone window | n = 1 | n = 0 | n = 0 | |||
| FP-CIT uptake ratios | ||||||
| Contral. FP-CIT (mean ± SD) | 2.22 ± 0.56 | 2.07 ± 0.37 | 1.63 ± 0.22 | |||
| Ipsilat. FP-CIT (mean ± SD) | 2.37 ± 0.53 | 2.27 ± 0.37 | 1.81 ± 0.24 | |||
| Contralat. FP-CIT normal | n = 7 | n = 0 | n = 0 | |||
| Contralat. FP-CIT pathological | n = 25 | n = 9 | n = 12 | |||
| . | Hoehn and Yahr . | . | . | |||
|---|---|---|---|---|---|---|
. | Stage 1 . | Stage 2 . | Stages 3 and 4 . | |||
| Patients | ||||||
| N | n = 32 | n = 9 | n = 12 | |||
| Females | n = 10 | n = 3 | n = 7 | |||
| Males | n = 22 | n = 6 | n = 5 | |||
| Age (mean ± SD years) | 57.0 ± 9.7 | 57.9 ± 12.4 | 63.9 ± 12.5 | |||
| Disease duration (mean ± SD years) | 2.1 ± 1.9 | 3.7 ± 1.7 | 6.5 ± 2.2 | |||
| UPDRS (mean ± SD) | 11.6 ± 4.9 | 23.2 ± 6.2 | 28.5 ± 9.3 | |||
| Clinical subtypes | ||||||
| Akinetic-rigid type | n = 11 | n = 3 | n = 9 | |||
| Mixed type | n = 11 | n = 3 | n = 2 | |||
| Tremor-dominance type | n = 10 | n = 3 | n = 1 | |||
| TCS | ||||||
| Contralat. TCS (mean ± SD) | 0.24 ± 0.06 | 0.19 ± 0.05 | 0.23 ± 0.05 | |||
| Ipsilat. TCS (mean ± SD) | 0.20 ± 0.05 | 0.15 ± 0.05 | 0.19 ± 0.05 | |||
| Contralat. TCS normal | n = 3 | n = 4 | n = 2 | |||
| Contralat. TCS pathological | n = 28 | n = 5 | n = 10 | |||
| Insuffic. contr. bone window | n = 1 | n = 0 | n = 0 | |||
| FP-CIT uptake ratios | ||||||
| Contral. FP-CIT (mean ± SD) | 2.22 ± 0.56 | 2.07 ± 0.37 | 1.63 ± 0.22 | |||
| Ipsilat. FP-CIT (mean ± SD) | 2.37 ± 0.53 | 2.27 ± 0.37 | 1.81 ± 0.24 | |||
| Contralat. FP-CIT normal | n = 7 | n = 0 | n = 0 | |||
| Contralat. FP-CIT pathological | n = 25 | n = 9 | n = 12 | |||
Disease duration = duration of parkinsonian symptoms, UPDRS = part III (motor part) of UPDRS. Age and disease duration are given in years. Due to the small case number we combined the patients at Hoehn and Yahr Stage 3 (n = 10) and Hoehn and Yahr Stage 4 (n = 2) to one group. TCS = transcranial sonography. The values of the transcranial sonography are given in cm2. Contralat. TCS = TCS contralateral to the clinically more affected body side. Ipsilat. TCS = TCS ipsilateral to the clinically more affected body side. Contralat. TCS normal: TCS was considered normal, if SN echogenicity was ≤0.19 cm2 (mean + 1 SD, see Methods). Contralat. TCS pathological = TCS was considered pathological, when SN echogenicity was >0.19 cm2. Insuffic. contr. bone window = insufficient temporal bone window contralateral to the clinically more affected body side. Contral. FP-CIT = FP-CIT SPECT contralateral to the clinically more affected body side. Ipsilat. FP-CIT = FP-CIT SPECT ipsilateral to the clinically more affected body side. The values of FP-CIT SPECT are given as the quotient (striatal binding of [123I]FP-CIT/posterior lobe binding of [123I]FP-CIT; without a unit). Contralat. FP-CIT normal = number of patients with normal contralateral FP-CIT uptake. Contralat. FP-CIT pathological = FP-CIT was considered pathological, when (i) the absolute tracer accumulation was <2.56 (mean − 2 SD) or (ii) the side-to-side difference was >Δ0.15 (mean + 2 SD).
Patients with idiopathic PD
| . | Hoehn and Yahr . | . | . | |||
|---|---|---|---|---|---|---|
. | Stage 1 . | Stage 2 . | Stages 3 and 4 . | |||
| Patients | ||||||
| N | n = 32 | n = 9 | n = 12 | |||
| Females | n = 10 | n = 3 | n = 7 | |||
| Males | n = 22 | n = 6 | n = 5 | |||
| Age (mean ± SD years) | 57.0 ± 9.7 | 57.9 ± 12.4 | 63.9 ± 12.5 | |||
| Disease duration (mean ± SD years) | 2.1 ± 1.9 | 3.7 ± 1.7 | 6.5 ± 2.2 | |||
| UPDRS (mean ± SD) | 11.6 ± 4.9 | 23.2 ± 6.2 | 28.5 ± 9.3 | |||
| Clinical subtypes | ||||||
| Akinetic-rigid type | n = 11 | n = 3 | n = 9 | |||
| Mixed type | n = 11 | n = 3 | n = 2 | |||
| Tremor-dominance type | n = 10 | n = 3 | n = 1 | |||
| TCS | ||||||
| Contralat. TCS (mean ± SD) | 0.24 ± 0.06 | 0.19 ± 0.05 | 0.23 ± 0.05 | |||
| Ipsilat. TCS (mean ± SD) | 0.20 ± 0.05 | 0.15 ± 0.05 | 0.19 ± 0.05 | |||
| Contralat. TCS normal | n = 3 | n = 4 | n = 2 | |||
| Contralat. TCS pathological | n = 28 | n = 5 | n = 10 | |||
| Insuffic. contr. bone window | n = 1 | n = 0 | n = 0 | |||
| FP-CIT uptake ratios | ||||||
| Contral. FP-CIT (mean ± SD) | 2.22 ± 0.56 | 2.07 ± 0.37 | 1.63 ± 0.22 | |||
| Ipsilat. FP-CIT (mean ± SD) | 2.37 ± 0.53 | 2.27 ± 0.37 | 1.81 ± 0.24 | |||
| Contralat. FP-CIT normal | n = 7 | n = 0 | n = 0 | |||
| Contralat. FP-CIT pathological | n = 25 | n = 9 | n = 12 | |||
| . | Hoehn and Yahr . | . | . | |||
|---|---|---|---|---|---|---|
. | Stage 1 . | Stage 2 . | Stages 3 and 4 . | |||
| Patients | ||||||
| N | n = 32 | n = 9 | n = 12 | |||
| Females | n = 10 | n = 3 | n = 7 | |||
| Males | n = 22 | n = 6 | n = 5 | |||
| Age (mean ± SD years) | 57.0 ± 9.7 | 57.9 ± 12.4 | 63.9 ± 12.5 | |||
| Disease duration (mean ± SD years) | 2.1 ± 1.9 | 3.7 ± 1.7 | 6.5 ± 2.2 | |||
| UPDRS (mean ± SD) | 11.6 ± 4.9 | 23.2 ± 6.2 | 28.5 ± 9.3 | |||
| Clinical subtypes | ||||||
| Akinetic-rigid type | n = 11 | n = 3 | n = 9 | |||
| Mixed type | n = 11 | n = 3 | n = 2 | |||
| Tremor-dominance type | n = 10 | n = 3 | n = 1 | |||
| TCS | ||||||
| Contralat. TCS (mean ± SD) | 0.24 ± 0.06 | 0.19 ± 0.05 | 0.23 ± 0.05 | |||
| Ipsilat. TCS (mean ± SD) | 0.20 ± 0.05 | 0.15 ± 0.05 | 0.19 ± 0.05 | |||
| Contralat. TCS normal | n = 3 | n = 4 | n = 2 | |||
| Contralat. TCS pathological | n = 28 | n = 5 | n = 10 | |||
| Insuffic. contr. bone window | n = 1 | n = 0 | n = 0 | |||
| FP-CIT uptake ratios | ||||||
| Contral. FP-CIT (mean ± SD) | 2.22 ± 0.56 | 2.07 ± 0.37 | 1.63 ± 0.22 | |||
| Ipsilat. FP-CIT (mean ± SD) | 2.37 ± 0.53 | 2.27 ± 0.37 | 1.81 ± 0.24 | |||
| Contralat. FP-CIT normal | n = 7 | n = 0 | n = 0 | |||
| Contralat. FP-CIT pathological | n = 25 | n = 9 | n = 12 | |||
Disease duration = duration of parkinsonian symptoms, UPDRS = part III (motor part) of UPDRS. Age and disease duration are given in years. Due to the small case number we combined the patients at Hoehn and Yahr Stage 3 (n = 10) and Hoehn and Yahr Stage 4 (n = 2) to one group. TCS = transcranial sonography. The values of the transcranial sonography are given in cm2. Contralat. TCS = TCS contralateral to the clinically more affected body side. Ipsilat. TCS = TCS ipsilateral to the clinically more affected body side. Contralat. TCS normal: TCS was considered normal, if SN echogenicity was ≤0.19 cm2 (mean + 1 SD, see Methods). Contralat. TCS pathological = TCS was considered pathological, when SN echogenicity was >0.19 cm2. Insuffic. contr. bone window = insufficient temporal bone window contralateral to the clinically more affected body side. Contral. FP-CIT = FP-CIT SPECT contralateral to the clinically more affected body side. Ipsilat. FP-CIT = FP-CIT SPECT ipsilateral to the clinically more affected body side. The values of FP-CIT SPECT are given as the quotient (striatal binding of [123I]FP-CIT/posterior lobe binding of [123I]FP-CIT; without a unit). Contralat. FP-CIT normal = number of patients with normal contralateral FP-CIT uptake. Contralat. FP-CIT pathological = FP-CIT was considered pathological, when (i) the absolute tracer accumulation was <2.56 (mean − 2 SD) or (ii) the side-to-side difference was >Δ0.15 (mean + 2 SD).
Patients with a clinical history of cerebrovascular events, psychiatric disorders or any other disease that might affect the uptake of FP-CIT SPECT were excluded. No patient was treated by tricyclic antidepressant drugs or other serotonin reuptake inhibitors, reserpine, clonidine, amphetamines or phenylpropanolamine. Therapy with selegiline was discontinued at least 18 h before FP-CIT application to avoid interaction of its metabolites with FP-CIT at the dopamine transporter (Laruelle et al., 1993). Further antiparkinsonian medication was continued during the SPECT examination: because of the problem facing FP-CIT SPECT in patients treated with antiparkinsonian drugs, the present data (Laruelle et al., 1993; Innis et al., 1999; Guttman et al., 2001; The Parkinson Study Group, 2002) do not allow a clear conclusion concerning the effect of antiparkinsonian drugs on dopamine transporter binding. Informed consent was obtained from all patients prior to examination. The protocol was approved by the local ethical committee (Ethical Committee of General Medical Council, Saarland, Germany).
[123I]FP-CIT SPECT
Cerebral SPECT imaging was performed with iodine-123 FP-CIT (DaTscan®, Amersham Cygne, Eindhoven, The Netherlands). Four hours following thyroid gland blocking and intravenous injection of the radioactive substance (185 MBq) cerebral SPECT images were obtained. A triple-head gamma camera (MultiSPECT III, Siemens, Erlangen, Germany) equipped with low-energy high-resolution collimators was used. Data was acquired in a 128 × 128 matrix covering 120° per head, 50 s per view. A total of 120 views at 3° steps was acquired. Energy discrimination was centred on 158 keV with a 15% window. Image processing was performed with a HERMES workstation (Nuclear Diagnostics, Stockholm, Sweden). Transaxial images were reconstructed using an iterative algorithm (OSEM, six subsets, four iterations) including contour-based attenuation correction (μ = 0.1/cm) and post-reconstruction filtering (Butterworth 4th order, cut-off 1.0/cm). The SPECT images were evaluated with the BRASS program package (Nuclear Diagnostics, Stockholm, Sweden), modified for this purpose (van Laere et al., 2002). The cerebral striatal/posterior lobe binding of [123I]FP-CIT was assessed semiquantitatively using a region of interest technique after registration of the patient's SPECT images with a template of the mean activity distribution obtained from a control group. This control group consisted of 19 age-matched healthy volunteers without any present or previous neurological disease (age 38–76 years, 59 ± 11 years, mean ± SD). The striatal/posterior lobe binding in these volunteers amounted to a ratio of 3.44 ± 0.44. The cerebral binding of [123I]FP-CIT was considered pathological when (i) the absolute tracer accumulation was <2.56 (mean − 2 SD) or (ii) the side-to-side difference was >Δ0.15 (mean + 2 SD).
Sonographic examination of the substantia nigra
For TCS, we used a commercial ultrasound device (Elegra, Siemens, Erlangen, Germany) with a 2.5 MHz phased array transducer. This transducer provides an axial resolution of 0.7 mm. The lateral resolution, which depends on the width of ultrasonic beam and varying depth of the object, can be approximated to be ∼3 mm (Berg and Becker, 2002). Ultrasound system parameters were chosen as described previously (Berg and Becker, 2002). TCS examination started with the examination of mesencephalic brainstem in an axial scanning plane through preauricular temporal acoustic bone window with a penetration depth of 16 cm and a dynamic range of 45 dB. The SN was identified within the butterfly-shaped structure of the mesencephalic brainstem: within the low echogenic mesencephalic peduncle, the SN regularly reveals a low to slightly increased echogenic signal. The SN was scanned from both temporal bone windows. SN echogenicity was quantified by a planimetric measurement of the maximum extension of hyperechogenic signals. The mean value of SN echogenicity in our ultrasound laboratory is 0.12 ± 0.07 cm2 (mean ± SD), based on the examination of 500 healthy volunteers (254 females, 246 males) without any present or previous neurological or psychiatric disease [age 50–65 years, 57 ± 4 years (mean ± SD)]. According to Berg et al. (2001a) we used the mean + 1 SD (= 0.19 cm2) as upper norm value. It was observed by Berg et al. (2001a) that a cut-off value of mean + 1 SD is the better marker to distinguish between parkinsonian patients and healthy volunteers than a cut-off value of mean + 2 SD. Clinical examination including clinical scores, FP-CIT SPECT examination and TCS examination were performed independently by physicians, who were blinded to the results of the other examinations.
Statistical analysis
All data were tested for normal distribution. In case of normally distributed data, correlations were calculated using the Pearson's correlation coefficient, the Student's paired t-test was chosen for comparison of paired data and the unpaired t-test for unpaired data.
In case of not normally distributed data, correlations were calculated using the Spearman's correlation coefficient, the Wilcoxon matched pairs test was chosen for comparison of paired data and the Mann–Whitney test for unpaired data.
Results
FP-CIT SPECT
In all 53 PD patients [123I]FP-CIT SPECT was measured. There was a significantly lower FP-CIT uptake in the striatum contralateral to the clinically more affected body side [= contralateral striatum; n = 53, 2.06 ± 0.52 (mean ± SD), Fig. 1, Table 1] than in the striatum ipsilateral to the clinically more affected body side [= ipsilateral striatum; n = 53, 2.23 ± 0.51, P < 0.0001, Student's paired t-test). There was a significant inverse correlation between contralateral FP-CIT uptake and motor part of UPDRS (r = −0.50, P < 0.0001, Pearson's correlation), Hoehn and Yahr stage (r = −0.43, P < 0.01, Spearman's correlation) and disease duration (r = −0.33, P < 0.02, Spearman's correlation). The contralateral FP-CIT SPECT was pathological in 46 out of 53 patients resulting in a sensitivity of 0.87. All seven patients with a normal contralateral FP-CIT uptake belonged to Hoehn und Yahr Stage 1.
Pathological [123I]FP-CIT SPECT in a 65-year-old patient with akinetic-rigid type of PD, idiopathic PD, Hoehn and Yahr Stage 1. [123I]FP-CIT SPECT was pathological in this patient due to a symmetrically impaired striatal binding. The transaxial SPECT image shows bilateral loss of FP-CIT uptake in the putamina and severe reduction in the caudate nuclei. The 40% isocontour of the normal brain template is shown as an overlaid line to illustrate the normal shape of FP-CIT uptake in the basal ganglia.
Transcranial sonography
TCS was performed in all 53 patients. There was a sufficient temporal bone window contralateral to the clinically more affected body side (= contralateral SN) in 52 out of 53 patients. We found a sufficient temporal bone window ipsilateral to the clinically more affected body side (= ipsilateral SN) in 47 out of 53 patients. The contralateral SN showed a significantly more echogenic signal (0.23 ± 0.06 cm2, n = 52, Fig. 2) than the ipsilateral SN (0.19 ± 0.05 cm2, n = 47, P < 0.0001, Student's paired t-test with 46 pairs of values). Echogenicity of contralateral SN did not correlate with motor part of UPDRS (r = +0.02, P = 0.86, Pearson's correlation), Hoehn and Yahr stage (r = −0.08, P = 0.58, Spearman's correlation) or disease duration (r = −0.04, P = 0.80, Spearman's correlation).
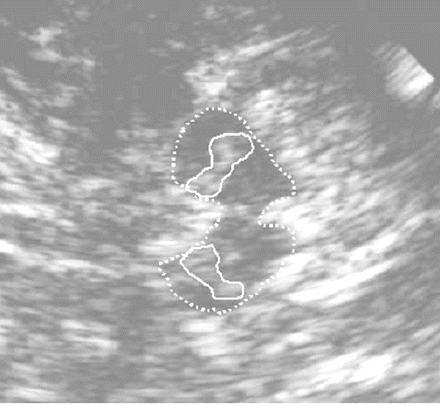
Hyperechogenic SN on both sides in TCS in the same patient as in Fig. 1. The mesencephalic brainstem (encircled with dotted lines) presents with relatively homogeneously low echogenicity and is surrounded by the hyperechogenic basal cisterns. The SN on both sides (each encircled with continuous lines) is hyperechogenic. The upper part of the picture is nearer to the ultrasound probe.
Contralateral TCS was pathological in 43 out of 52 patients, the corresponding sensitivity was 0.83. In nine patients TCS was normal. These nine patients with normal contralateral SN belonged to different Hoehn and Yahr stages and were significantly younger (51 ± 13 years) than patients with a hyperechogenic contralateral SN (60 ± 10 years, P < 0.05, unpaired t-test). No significant differences between these subgroups (normal versus hyperechogenic contralateral SN) were found concerning Hoehn and Yahr stage, disease duration (both P > 0.05, Mann–Whitney test) or motor part of UPDRS (P > 0.05, unpaired t-test).
Correlation between FP-CIT SPECT and TCS
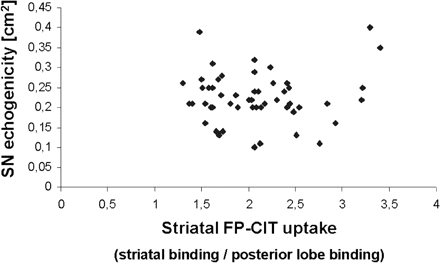
We found no significant correlation between striatal FP-CIT uptake and echogenicity of the SN in TCS, neither contralateral to the clinically more affected body side (r = +0.08; P = 0.57; n = 52, Pearson's correlation; Fig. 3) nor ipsilateral (r = +0.01; P = 0.92; n = 47, Pearson's correlation). Even the pooling of ipsilateral and contralateral findings revealed no significant correlation between FP-CIT uptake and SN echogenicity (r = +0.02; P = 0.87; n = 99, Pearson's correlation). The lack of any correlation between FP-CIT uptake and SN echogenicity is illustrated by two details: first, six of seven patients with a normal contralateral FP-CIT uptake revealed an increased contralateral SN echogenicity; secondly, all nine patients with a normal contralateral TCS exhibited a reduced contralateral striatal FP-CIT uptake. When we combined both methods FP-CIT SPECT and TCS, 52 out of 53 patients showed a reduced contralateral FP-CIT uptake and/or a hyperechogenic contralateral SN. Thus, the combined use of FP-CIT SPECT and TCS results in a sensitivity of 0.98 concerning the diagnosis of PD.
Correlation between FP-CIT uptake and SN echogenicity in the contralateral striatum. Each patient is represented by one point. There is no correlation between striatal FP-CIT uptake contralateral to the clinically more affected body side on the one hand and contralateral SN hyperechogenicity on the other hand.
In a further step, we were interested whether FP-CIT uptake and SN echogenicity correlated in patients in whom both FP-CIT SPECT and TCS delivered pathological results in the striatum/SN contralateral to the clinically more affected body side. For this reason we excluded all patients with normal contralateral FP-CIT uptake or normal contralateral SN. Thirty-eight patients revealed reduced contralateral FP-CIT uptake and hyperechogenic contralateral SN. In these 38 patients there was no correlation between contralateral FP-CIT uptake and area of contralateral SN echogenicity (r = −0.07; P = 0.66; n = 38, Pearson's correlation).
As explained in the Methods section, we used an upper norm value with mean + 1 SD (= 0.19 cm2) for area of SN echogenicity, because a cut-off value of mean + 1 SD distinguishes better between parkinsonian patients and healthy volunteers than a cut-off value of mean + 2 SD (Berg et al., 2001a). By using an upper norm value with mean + 2 SD (= 0.26 cm2), the SN contralateral to the clinically more affected body side was considered normal in 40 out of 52 patients and pathological in 12 of 52 patients with a sufficient temporal bone window. The resulting sensitivity of TCS concerning the diagnosis of PD was 0.23. FP-CIT SPECT was pathological in 10 and normal in 2 out of these 12 patients with SN echogenicity >0.26 cm2. In these 12 patients there was no correlation between contralateral FP-CIT uptake and contralateral SN hyperechogenicity (r = +0.08; P = 0.71; n = 12, Pearson's correlation).
Discussion
In our patients, SN echogenicity did not correlate with striatal FP-CIT uptake reflecting the actual state of the neurodegenerative process of dopaminergic neurons at the SN. The FP-CIT uptake correlated significantly with the motor part of UPDRS score, the Hoehn and Yahr stage and the disease duration. In contrast to FP-CIT uptake, SN echogenicity did not correlate with the motor part of UPDRS score, Hoehn and Yahr stage, or disease duration. The same size of SN echogenicity could be found in early as well as late stages of PD. Our data are in accordance with previous studies showing that SN echogenicity does not correlate with clinical severity of disease or disease duration (Berg et al., 2001a) and that SN echogenicity remains constant in PD patients during a time period of 5 years, whereas parkinsonian symptoms clearly progress during this time period (Berg et al., 2005). These previous studies suggest that SN echogenicity is no quantitative marker for SN degeneration. In addition, the SN echogenicity seems to represent an intraindividually stable marker. Our data now strongly support the assumption that SN hyperechogenicity—shown by TCS—and degeneration of presynaptic dopaminergic nerve terminals—quantified by FP-CIT SPECT—exist independently from each other and are based on different pathomechanisms.
The reasons for SN hyperechogenicity are not yet clear: associations between development of PD and particularly of SN hyperechogenicity on the one hand and an altered cerebral iron metabolism on the other hand are discussed (Dexter et al., 1991; Deplazes et al., 2004). SN echogenicity correlates significantly with the tissue iron content in animal studies (Berg et al., 1999b) and postmortem studies (Berg et al., 2002; Zecca et al., 2005). Furthermore, the hyperechogenic area of SN correlates significantly with the concentration of H- and L-ferritin in postmortem brains (Zecca et al., 2005). The binding of increased amount of iron to iron metabolizing proteins or structural alteration of iron binding proteins is assumed to induce an altered reflection of the ultrasound beam, which is displayed as a hyperechogenic signal (Behnke et al., 2005). A previous study (Krick et al., 2004) found a significant inverse correlation between SN echogenicity, measured by TCS, and T2 relaxation times of SN, measured by MRI. Measurement of T2 relaxation times represents an MRI method to measure regional iron concentration (Mondino et al., 2002). These data of Krick et al. (2004) give further evidence that SN hyperechogenicity is associated with nigral iron deposition.
Contralateral SN was normal (≤0.19 cm2) in 9 of our 52 patients with a sufficient temporal bone window; these 9 patients were significantly younger than PD patients with hyperechogenic contralateral SN. Already Berg et al. (2001a) found a bilaterally normal SN in 9 out of 103 PD patients with a sufficient temporal bone window. These findings show that SN echogenicity may be normal in a small subgroup of PD patients. Possibly this subgroup with normal SN echogenicity represents a disease entity, in which the iron metabolism plays a minor pathophysiological role. In patients with normal SN, additional factors must play an important aetiological role for development of PD. It can be speculated that an increased iron deposition is not the only reason for a functional loss of nigral neurons. Other processes such as inflammatory, ischaemic or toxic processes may also be able to damage nigral neurons. An association between environmental and occupational factors on the one hand and development of PD on the other hand was reported (Seidler et al., 1996).
TCS reveals a high sensitivity and specificity concerning the diagnosis of PD (Berg et al., 2001a; Walter et al., 2002, 2003; Behnke et al., 2005). This high sensitivity and specificity indicate that SN hyperechogenicity discloses a predisposing factor for PD. But, on the other hand, we found no correlation between SN hyperechogenicity and relevant clinical parameters such as Hoehn and Yahr stage, motor part of the UPDRS or disease duration. Our results correspond to previous findings: the area of SN echogenicity in healthy volunteers with a hyperechogenic SN is in a similar size range as in patients with clinical PD (Berg et al., 1999a, 2002). Moreover, there is no change of SN echogenic size over a time period of 5 years in PD patients, whereas parkinsonian symptoms clearly progress during this time period (Berg et al., 2005). Finally, psychiatric patients with severe parkinsonism after neuroleptic treatment show a significantly larger area of SN echogenicity than patients with no or minimal parkinsonian symptoms after neuroleptic treatment (Berg et al., 2001b). These data suggest that SN hyperechogenicity reflects structural SN changes, which increase the vulnerability of nigrostriatal dopaminergic transmission and thus increase the risk to develop PD. But further factors, apart from a hyperechogenic SN, are necessary to ‘cause’ PD in the end. These factors are unknown and might consist of environmental or genetic factors.
Since SN hyperechogenicity—visualized by TCS—and degeneration of presynaptic dopaminergic nerve terminals—quantified by FP-CIT SPECT—exist independently from each other, the combined use of both methods TCS and FP-CIT SPECT seems to be useful in clinical practice. Applying both methods together we reach a sensitivity of 0.98 concerning the diagnosis of PD. Furthermore, it allows to differentiate between PD and atypical parkinsonian syndromes (Walter et al., 2003; Behnke et al., 2005). Therefore the combined use of TCS as a non-invasive bedside test and FP-CIT SPECT represents a good diagnostic tool in diagnosis of PD, particularly in diagnostically unclear patients.
In summary, our data show that the extent of SN hyperechogenicity does not correlate with the degeneration of presynaptic dopaminergic nerve terminals. Obviously, SN hyperechogenicity and degeneration of presynaptic dopaminergic nerve terminals exist independently from each other and may be based on different pathomechanisms.
We thank Viola Günther for her technical assistance.
References
Behnke S, Berg D, Naumann M, Becker G. Differentiation of Parkinson's disease and atypical parkinsonian syndromes by transcranial ultrasound.
Benamer HTS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E et al. Accurate differentiation of parkinsonian and essential tremor using visual assessment of [123I]-FP-CIT-SPECT imaging: the [123I]-FP-CIT study group.
Benamer HTS, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson's disease severity and duration with [123I]-FP-CIT-SPECT striatal uptake.
Berg D, Becker G, Zeiler B, Tucha O, Hofmann E, Preier M et al. Vulnerability of the nigrostriatal system as detected by transcranial ultrasound.
Berg D, Grote C, Rausch WD, Maurer M, Wesemann W, Riederer P et al. Iron accumulation in the substantia nigra in rats visualized by ultrasound.
Berg D, Siefker C, Becker G. Echogenicity of the substantia nigra in Parkinson's disease and its relation to clinical findings.
Berg D, Jabs B, Merschdorf U, Beckmann H, Becker G. Echogenicity of substantia nigra determined by transcranial ultrasound correlates with severity of parkinsonian symptoms induced by neuroleptic therapy.
Berg D, Roggendorf W, Schroder U, Klein R, Tatschner T, Benz P et al. Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury.
Berg D, Merz B, Reiners K, Naumann M, Becker G. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson's disease.
Booij J, Bergmans P, Winogrodzka A, Speelman JD, Wolters EC. Imaging of dopamine transporters with [123I]FP-CIT does not suggest a significant effect on age on the symptomatic threshold of disease in Parkinson's disease.
Deplazes J, Schobel K, Hochstrasser H, Bauer P, Walter U, Behnke S et al. Screening for mutations of the IRP2 gene in Parkinson's disease patients with hyperechogenicity of the substantia nigra.
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia.
Guttman M, Stewart D, Hussey D, Wilson A, Houle S, Kish S. Influence of L-dopa and pramipexole on striatal dopamine transporter in early PD.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases.
Innis RB, Marek KL, Sheff K, Zoghbi S, Castronuovo J, Feigin A et al. Effect of treatment with L-dopa / carbidopa or L-selegiline on striatal dopamine transporter SPECT imaging with [123I]ß-CIT.
Krick C, Fuss G, Schröder U, Behnke S, Dillmann U, Schreckenberger M et al. Screening for early stages of nigrostriatal alteration (I): MR-T2-relaxation and transcranial sonography in healthy subjects with increased echogenicity.
Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, al-Tikriti MS et al. SPECT imaging of dopamine and serotonin transporters with [123I]ß-CIT: pharmacological characterization of brain uptake in non-human primates.
Mondino F, Filippi P, Magliola U, Duca S. Magnetic resonance relaxometry in Parkinson's disease.
The Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson's disease progression.
Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J et al. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case-control study in Germany.
Van Laere KJ, Warwick J, Versijpt J, Goethals I, Audenaert K, van Heerden B et al. Analysis of clinical brain SPECT data based on anatomic standardization and reference to normal data: an ROC-based comparison of visual, semiquantitative, and voxel-based methods.
Walter U, Wittstock M, Benecke R, Dressler D. Substantia nigra echogenicity is normal in non-extrapyramidal cerebral disorders but increased in Parkinson's disease.
Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D. Brain parenchyma sonography discriminates Parkinson's disease and atypical parkinsonian syndromes.
Winogrodzka A, Bergmans P, Booij J, van Royen EA, Stoof JC, Wolters EC. [(123)I]beta-CIT SPECT is a useful method for monitoring dopaminergic degeneration in early-stage Parkinson's disease.
Author notes
Departments of 1Neurology and 2Nuclear Medicine, Saarland University, Homburg/Saar and 3Department of Neurology, Deutsche Klinik fur Diagnostik, Wiesbaden, Germany

![Pathological [123I]FP-CIT SPECT in a 65-year-old patient with akinetic-rigid type of PD, idiopathic PD, Hoehn and Yahr Stage 1. [123I]FP-CIT SPECT was pathological in this patient due to a symmetrically impaired striatal binding. The transaxial SPECT image shows bilateral loss of FP-CIT uptake in the putamina and severe reduction in the caudate nuclei. The 40% isocontour of the normal brain template is shown as an overlaid line to illustrate the normal shape of FP-CIT uptake in the basal ganglia.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/129/5/10.1093/brain/awl042/2/m_awl042f1.gif?Expires=1716565408&Signature=ExVoqmIm1o4Ud0-ZiXA0A8HqPFbbr0ThM6pK6~qo23O7lywi7ZLtiPq4qNy-vWh0C776WjyCuoz~LnoC115CSTAQMUGWhPWbfRzdOdLzp-~0aoVJXAzl8TcXoAIDVrEVBzNFkgW7ZXDpEWI43D~P-xtfhBt8B3pB-yMdcEgKpH7S~evisNW5G3dwjImq3vJpz5NzT~GkZ-MdCuzjgmFfkjRN1EGnHylzOiQnUKtYMXa4A5uuPGmx1EGCIypRCtXon7PrYYGbCgA1c2b3jfXI2HwnbZawtivhMPVyQR634zGbFYpSv8wLKfVb-2CETcgOrE8LjQwN5fX3o8eo8IDtRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)