-
PDF
- Split View
-
Views
-
Cite
Cite
Marija Djukic, Alexander Mildner, Hauke Schmidt, Dirk Czesnik, Wolfgang Brück, Josef Priller, Roland Nau, Marco Prinz, Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice, Brain, Volume 129, Issue 9, September 2006, Pages 2394–2403, https://doi.org/10.1093/brain/awl206
Close - Share Icon Share
Abstract
Previous studies have demonstrated a potential role of brain endogenous microglia and meningeal macrophages in inflammation and brain injury during bacterial meningitis. However, the contribution of previously engrafted monocytes and microglia to this process is still unknown. We therefore used genetically labelled bone marrow-derived cells from transgenic mice expressing the green fluorescent protein (GFP) under the chicken β-actin promoter to deliver fluorescently labelled monocytes to the diseased brain. Approximately 24 hours after Streptococcus pneumoniae infection, GFP-expressing parenchymal microglia changed their morphology to an activated phenotype and upregulated major histocompatibility complex class II molecules. Bacterial meningitis increased the engraftment of GFP+ monocytes and their differentiation to microglia during the post-inflammatory period, but not during acute meningitis. Importantly, these newly recruited monocytes became an integral part of the pool of parenchymal microglia and contributed to the clearance of damaged tissue by increased lysosomal activity and close location to apoptotic cells. Thus, circulating cells entering the brain such as monocytes/macrophages might provide a potential cellular target for the treatment of the tissue damage following meningitis via peripheral cell therapy.
Abbreviations
- BMC
bone marrow-derived cells
- GFP
green fluorescent protein
- MHC
major histocompatibility complex
Introduction
Microglia are representatives of the resident mononuclear phagocyte population within the central nervous system (CNS). These cells share many phenotypical and functional characteristics with other tissue macrophages as well as peripheral blood monocytes, suggesting that microglia participate in innate immune reactions of the brain. They are rapidly activated in response to any pathology within the CNS, where they have a key role in the defence of the brain parenchyma against infection, in inflammation, ischaemia, trauma, brain tumours and neurodegeneration (Kreutzberg, 1996). In addition to their beneficial effects, microglia—when chronically and/or pathologically activated—have been implicated in contributing to tissue damage (Benveniste, 1997; Combs et al., 2001; Iliev et al., 2004; Perry, 2004).
Despite a likely role in the pathogenesis of CNS diseases, microgliosis is a rather poorly understood process (Streit et al., 2004). One difficulty is to determine the extent to which circulating monocyte derivatives contribute to the microglial response. Monocyte-derived and resident CNS parenchymal microglia are virtually indistinguishable on the basis of known immunophenotypic markers, but could be functionally heterogeneous (Guillemin and Brew, 2004). We and others had previously shown that cells derived from bone marrow enter the brain early during postnatal development to differentiate into microglia (Hickey et al., 1992; Hickey, 1999; Priller et al., 2001a) but not into astrocytes or neurons (Wehner et al., 2003).
Bacterial meningitis caused by Streptococcus pneumoniae is a major challenge in industrialized countries with a mortality rate of ∼25% in adults and many survivors suffering from post-infectious disabilities, in particular, deafness, epileptic seizures, motor dysfunction and neuropsychological deficits (Bohr et al., 1984; Schmidt et al., 2006). Morphologically, tissue injury is most frequent in the dentate gyrus of the hippocampal formation. Dentate granule cells predominantly die by apoptosis (Nau and Bruck, 2002; Nau et al., 2004). In other regions of the brain, damage during meningitis typically occurs as micronecrosis as a consequence of vasculitis. Brain oedema and occlusion of large vessels less frequently cause territorial infarctions (Nau and Bruck, 2002; Nau et al., 2004). Delineating the relative contribution of bone marrow-derived cells (BMCs) to the microglial reaction during neuroinflammatory conditions may be important for the design of therapies that selectively target deleterious and/or beneficial effects of the glial response. In the present study, we therefore aimed at elucidating the relationship between BMCs and the brain during S. pneumoniae meningitis.
We used allogenic transplantation of gene-marked BMCs to characterize the spatial and temporal kinetics of macrophage/microglia engraftment into the CNS following S. pneumoniae meningitis. Our experiments revealed that BMCs predominantly of the myeloid lineage were recruited to the meninges in response to acute S. pneumoniae infection. In the following weeks, meningeal inflammation led to an increased migration of BMC cells into the brain parenchyma. There they properly integrated into the pool of brain parenchymal microglia where they became an increasing component of disease-associated pathology and participated in disease-associated processes including the clearance of cell debris.
Material and methods
Generation of BM chimeric mice
Bone marrow chimeric mice were generated as described recently (Priller et al., 2001a; Prinz et al., 2003; Prinz et al., 2006). In brief, 6- to 8-week-old C57BL/6 recipient mice were reconstituted with BMCs derived from tibias and femurs from adult C57BL/6 (ACTβ-EGFP) mice (Okabe et al., 1997). BMCs (5 × 106 cells) were injected into the tail vein of recipients conditioned by whole-body irradiation (1100 cGy) 24 h earlier. Six to eight weeks after grafting, reconstitution was assessed by fluorescence-activated cell sorter (FACS) analysis of peripheral blood. Blood samples were prepared at 4°C in buffer solution [phosphate-buffered saline (PBS) containing 2% FCS and 0.2% NaN3] and stained with CD11b, Gr-1, B220 or CD3 (BD Pharmingen, Heidelberg, Germany). After lysis of erythrocytes with FACS lysis solution (Becton Dickinson, San Jose, CA, USA) and washing, cell suspensions were analysed on a FACSCalibur (Becton Dickinson). Data were acquired with CellQuest software (Becton Dickinson).
Induction of meningitis
A strain of S. pneumoniae serotype 3 (gift of Prof M. G. Täuber, Department of Clinical Microbiology, University of Bern, Switzerland) was used. Bacteria were grown on blood agar plates overnight and harvested with 0.9% saline, washed and re-suspended in 0.9% saline, aliquoted and stored frozen at −70°C. Frozen aliquots were used for the experiments and adjusted with saline to the required concentration of 4 log10 colony-forming units (CFU) in 25 μl.
Meningitis experiments were conducted in chimeric mice 6 to 8 weeks after reconstitution using a modification of a previously published model of meningitis based on injection of bacteria into the lumbar cerebrospinal fluid (Tang et al., 1996; Angstwurm et al., 2004). In brief, mice were anaesthetized by intraperitoneal injection of ketamine 100 mg/kg and xylazine 20 mg/kg. A skin incision was made exposing the lumbar spine. Using a 29-gauge needle, 25 μl of a suspension containing 4 log10 CFU S. pneumoniae or an equal amount of saline was slowly injected into the spinal canal at the level of L4 or L5. All animals resumed their normal behaviour after awaking from anaesthesia. To characterize the acute phase of infection, mice were not treated with antibiotics and killed 48 h after infection or saline injection. Mice for long-term engraftment (4 weeks after meningitis) received 200 mg/kg ceftriaxone (Rocephin®, Hoffmann-LaRoche, Grenzach-Wyhlen, Germany) or saline subcutaneously beginning 30 h after infection and then every 12 h for 4 days (n = 8 each). Mice for both acute phase and 4 weeks after meningitis were always transcardially perfused with 4% paraformaldehyde (PFA).
In order to confirm sufficient bacterial infection, some indicator mice were not perfused with PFA, and bacterial titres in blood were determined by plating serial 10-fold dilutions in 0.9% saline on sheep blood agar plates.
All experiments were performed at the Central Animal Care Facility of the University Hospital Göttingen. The protocol was approved by the Braunschweig district legislation for animal experiments, Germany.
Analysis of donor cell engraftment in the CNS
After transcardial perfusion with 4% PFA, brains were removed, post-fixed in 4% PFA for 2–4 h and cryoprotected in 30% sucrose overnight at 4°C. After tissue freezing in OCT compound (Sakura, Zoeterwoude, Netherlands) on dry ice, 20 μm cryosections were obtained from the brains. Sections were incubated in PBS containing 5% normal goat serum. Primary antibodies were added overnight at a dilution of 1 : 100 for Iba-1 (WACO, Japan), 1 : 50 for F4/80 (Serotec, Oxford, UK) immunohistochemistry. Cy3 conjugated secondary antibodies (Dianova, Hamburg, Germany) were added at a dilution of 1 : 100 for Iba-1 and 1 : 600 for F4/80 for 1 h.
Quantification of regional microglia and haematopoietic engraftment
Two independent investigators counted green fluorescent protein (GFP) expressing ramified cells and Iba-1+ or F4/80+ macrophages/microglia in two sections each of 26 animals. Inter-individual variability between both investigators was small since counted cell numbers differed only by <4% in individual counts. Finally, the means of all counts were used. Engraftment following meningitis was quantified by counting all GFP-expressing cells in sections from the temporal lobe including the hippocampus (coordinates: −1.22 to −2.80 mm from the bregma) and cerebellar (coordinates: −5.80 to −7.20 mm from the bregma) brain region of untreated mice and mice with meningitis. The number of infiltrating GFP+ cells within the brain parenchyma was examined microscopically using a 400-fold microscopical magnification using a conventional fluorescence microscope equipped with a colour scanner (Zeiss, Germany) and a confocal laser scanning microscope (Zeiss LSM 510 attached to an Axiovert 100M). Serial optical sections were imaged and saved as three-dimensional stacks. For illustrations, series of optical sections were projected into one image with greater focal depth. For DAPI stainings a Zeiss Axiovert 200M (Zeiss, Germany) epifluorescence microscope was used.
Results
Successful delivery of GFP+ haematopoietic cells by autologous bone marrow reconstitution
In order to distinguish invading BMCs from brain endogenous microglia during bacterial meningitis, bone marrow chimeras were created as described previously (Priller et al., 2001a; Prinz et al., 2003).
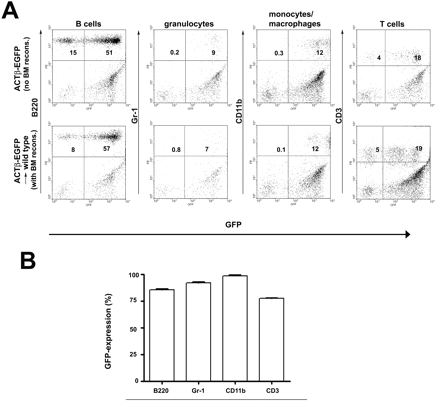
We utilized bone marrow from transgenic C57BL/6-Tg (ACTβ-EGFP) mice that express GFP ubiquitously in their body. Importantly, not all CD3+ T cells (∼78%) and B220+ B cells (∼81%) but the vast majority of myeloid cells [monocytes/macrophages/granulocytes (CD11b) and monocytes/granulocytes (Gr-1) in ∼98%] expressed the GFP transgene in non-irradiated C57BL/6-Tg (ACTβ-EGFP) mice (Fig. 1A, upper row). Donor BMCs were transplanted into lethally irradiated C57BL/6 mice. Six weeks after transplantation GFP-marked peripheral blood cells were analysed by FACS analysis. The rate of GFP-marked cells in chimeras was ∼85% for B cells (B220), 92% for granulocytes/monocytes (Gr-1), 99% for monocytes/macrophages/granulocytes (CD11b) and ∼77% for all T cells (CD3, Fig. 1A and B). Hence, chimeras reached similar levels of gene expression in haematopoietic cells as non-transplanted C57BL/6-Tg (ACTβ-EGFP) mice. These data are in line with earlier reports (Priller et al., 2001a). GFP expression was stable over the entire observation period of up to 5 months, suggesting that bone marrow reconstitution led to stable haematopoietic engraftment.
High reconstitution of haematopoiesis with GFP-expressing peripheral blood cells in chimeric mice. (A) Flow cytometry of GFP+ mononuclear and polymorphonuclear cells in peripheral blood 6 weeks after transplantation with C57BL/6-Tg (ACTβ-EGFP) BM cells into lethally irradiated mice. GFP fluorescence was measured in B cells (B220), granulocytes/monocytes (Gr-1), monocytes/macrophages/granulocytes (CD11b) and T cells (CD3). (B) Percentage of GFP-expressing cells in the peripheral blood of chimeric mice compared with the control C57BL/6-Tg (ACTβ-EGFP) mice. Data are expressed as mean ± standard deviation. At least four mice were examined for each cell type.
GFP+ bone marrow cells gave rise to microglia in the non-diseased brain
In order to assess the ability of cells derived from bone marrow to differentiate into distinct cell types within the non-diseased brain, recipient mice were analysed histologically 6 to 8 weeks after bone marrow transplantation (Fig. 2).
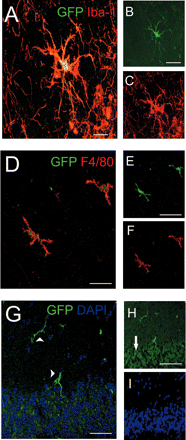
Morphological features of engrafted GFP+ BMCs in the brains of uninfected recipient mice 8 weeks after transplantation. (A–C) Confocal image of an engrafted cell in the hippocampus with typical microglial shape and distinct arborization pattern expressing both GFP (B, green) and the macrophage/microglia-specific marker Iba-1 (C, red). The overlay of confocal microscopic images (A) shows that the fluorophores are in the same cell. (D–F) Donor-derived hippocampal microglia also expressed the pan-macrophage marker F4/80. (D) Overlay, (E) GFP (green), (F) F4/80 (red). (G–I) In the cerebellum GFP-labelled microglia were predominantly visible in the molecular layer (arrowhead), whereas the granule cell layer (arrow) remained largely free of engrafted cells. (G) Overlay, (H) GFP (green). Note the autofluorescence of neurons in the granule cell layer (arrow). (I) DAPI staining (blue) of nuclei. Scale bars = 20 μm (A) or 10 μm (B, C, D and G) or 5 μm (E, F, H and I).
As expected, we could easily find some elongated GFP+ cells in the meninges of uninfected control animals. They were closely vessel-associated, resembling blood-derived meningeal macrophages and perivascular cells, respectively (data not shown). Our main focus, however, was parench-ymal cells expressing the marker GFP. Microscopical investigations of the temporal lobe and the cerebellum revealed some parenchymal cells with typical microglial morphology such as round to spindle-shaped soma and a distinct arborization pattern. These cells were positive for the microglia marker Iba-1 (Fig. 2A–C) as well as for the pan-macrophage marker F4/80 (Fig. 2D–F). GFP+ microglia in the cerebellum were mostly detectable in the molecular layer, whereas the granular cell layer exhibited only few engrafted cells (Fig. 2G–I). The relative number of engrafted GFP-expressing microglia compared with the overall amount of microglia 8 weeks after bone marrow reconstitution was estimated to be <5% in the temporal lobe and in the cerebellum.
Moreover, we never observed GFP+ cell types in the brain with a neuronal or macroglial morphology. Indeed, all GFP+ parenchymal cells were found to be positive for the microglia marker Iba-1. Given the relatively short period after bone marrow reconstitution addition, no GFP+ Purkinje cells (Priller et al., 2001b) were found in the cerebellum.
Green fluorescent bone marrow-derived microglia expressed MHC class II molecules and changed their morphology during acute S. pneumoniae meningitis
After having shown that engrafted monocytes differentiated into GFP+ microglia and were phenotypically indistinguishable from endogenous microglia under non-diseased conditions, we wanted to know their behaviour during acute bacterial meningitis. For this purpose, chimeras were challenged with 104 CFU of S. pneumoniae injected into the lumbar cerebrospinal fluid, and morphological changes of the previously engrafted microglia were investigated (Fig. 3). Only brains with inflammatory infiltrates restricted to the meninges without any obvious migration of granulocytes or bacteria into the brain parenchyma were chosen for histological examinations.
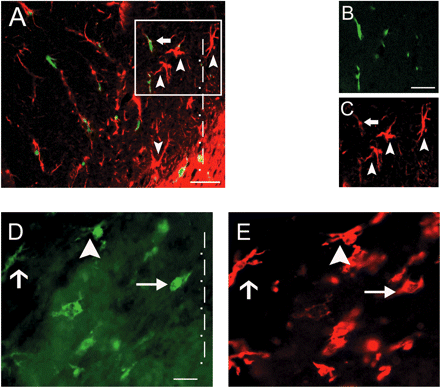
MHC class II upregulation and morphological transformation of engrafted monocytes differentiated into macrophages/microglia during S. pneumoniae meningitis. (A–C) MHC class II expression on endogenous cells (arrowheads) and recently engrafted macrophages/microglia (arrow) in the temporal lobe 24 h after disease induction. (A) Overlay, (B) GFP (green), (C) MHC II (red). (D and E) Morphological stages of macrophages/microglia activation in vivo. Hippocampal microglia changed their morphology upon bacterial challenge (24 h post-infection). Figure shows resting microglia with typical morphology with many processes (short arrow, left), transitional, already stimulated cells (arrowhead, middle) and fully activated macrophages/microglia with round soma and largely retracted processes (long arrow, right). GFP (D) and Iba-1 staining (E) are shown. The tailed arrow shows an Iba-1+ endogenous microglial cell. The dotted line indicates region nearest to the meninges (A and D). Scale bars = 20 μm (A and D) and 10 μm (B and C).
As early as 24 h after infection, endogenous GFP− as well as GFP+ macrophages/microglia showed morphological signs of activation, namely retraction of processes and rounding of cell somata (Fig. 3A–D). These morphological changes were accompanied by an upregulation of major histocompatibility complex (MHC) class II molecules as a sign of activation (Fig. 3A–C).
The morphological transformation to the fully activated macrophage-like type of microglia was more pronounced in the superficial cortical layers close to the meninges and correlated inversely with the distance from meningeal inflammation. Therefore, several morphologically distinct forms of macrophages/microglia were found within a distance of ∼150 μm from the meninges in the temporal lobe (Fig. 3D). This strong cellular reaction was accompanied by an enhanced number of Iba-1+ endogenous microglia leading to a marked microgliosis. As we have shown previously, brain regions with the highest microglial turnover were the cerebellum and the temporal lobe including the hippocampus (Priller et al., 2001a). We therefore focused our analysis on these two brain regions. High numbers of Iba-1+ macrophages/microglia were found in the temporal lobe including the hippocampus at 48 h [110.9 ± 30.9 cells/mm2 (mean ± standard deviation, n = 7 mice) after infection compared with 82.1 ± 14.1 cells/mm2 after saline treatment (P = 0.038, n = 5 mice)] and in the cerebellum [93.9 ± 31.8 cells/mm2 after infection versus 78.3 ± 6.9 cells/mm2 (P = 0.38) after saline treatment]. Iba-1+ cells were often found in clusters within areas of gliosis. The GFP+ donor-derived macrophages/microglia, however, were more diffusely distributed during the acute phase of meningitis and did not change their numbers during the acute disease stage.
Enhanced microglial engraftment 1 month after S. pneumoniae meningitis
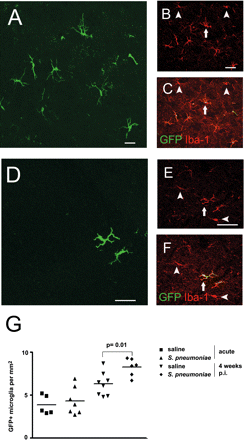
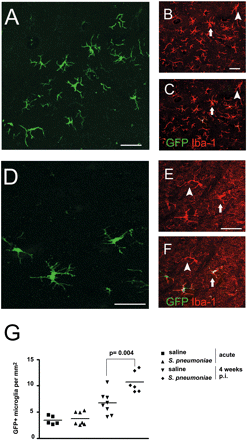
Twelve weeks after BM reconstitution, the number of engrafted monocytes that had differentiated into GFP+ microglia rose to a maximum of 5–8 cells/mm2 in the cerebellum (Fig. 4) and in the temporal lobe (Fig. 5) of saline-injected mice. In order to determine whether the long-term engraftment of microglia from haematopoietic cells was changed following bacterial meningitis we examined the number of GFP+ monocyte-derived microglia 4 weeks after infection compared with saline-infected controls (Figs 4 and 5). Again, GFP-expressing cells were observed throughout the meninges, in close association with blood vessels (data not shown) and within the brain parenchyma in saline and S. pneumoniae-treated mice. A distinct increase of GFP-labelled cells in the cerebellum (Fig. 4A–F) and temporal lobe (Fig. 5A–F) was observed in S. pneumoniae-challenged mice compared with control animals. They exhibited an activated morphology including some amoeboid cells (Fig. 4A and D). Detailed quantification of the GFP-expressing microglia revealed 8.3 ± 1.1 cells/mm2 in the cerebellum of mice after meningitis (n = 6 animals) compared with 6.3 ± 1.4 cells/mm2 in saline-injected mice (P = 0.01, n = 8 animals) (Fig. 4G) and 10.7 ± 1.9 cells/mm2 in the temporal lobe compared with 6.7 ± 2.2 cells/mm2 in saline-injected mice (P = 0.004), respectively (Fig. 5G). In order to determine the relevance of this increase in cell number, we compared the amount of GFP+ microglia with the number of all Iba-1+ microglia 1 month after infection. Importantly, the percentage of engrafted GFP+ Iba-1+ microglia significantly rose from 6.4 ± 1.8% (temporal lobe) and 6.1 ± 1.7% (cerebellum) in saline-treated animals to 13.5 ± 2.7% (temporal lobe, P = 0.02) and 11.0 ± 2.2% (cerebellum, P = 0.03) in infected mice. These data clearly indicate that this relatively minor component of the CNS parenchyma is strongly increased during inflammatory disease by recruitment of blood-derived macrophages/microglia.
Increased number of donor-derived microglia in the cerebellum 1 month after bacterial meningitis. (A–F) Histological sections from the cerebellum revealed enhanced microglial engraftment in the cerebellum 4 weeks after meningitis (A–C) compared with mice treated with saline only (D–F). Some Iba-1+ microglia were brain endogenous cells (arrowheads), whereas others were newly recruited (arrows). (A and D) GFP (green), (B and E) Iba-1 (red), (C and F) overlay (yellow). (G) Quantification of cellular engraftment in the cerebellum during acute meningitis and 4 weeks after infection compared with saline-treated animals. Each symbol represents one individual animal. Scale bars = 20 μm (A), 10 μm (B, C, E and F) and 25 μm (D).
Enhanced recruitment of GFP+ microglia in the temporal cortex during the resolution of inflammation. (A–F) Confocal images illustrating morphology and distribution of GFP-expressing microglia derived from blood monocytes in the temporal region either 4 weeks after meningitis (A–C) or without bacterial challenge (D–F). Some cells express Iba-1 only (arrowheads) but others co-express GFP and Iba-1 (arrows). (A and D) GFP (green), (B and E) Iba-1 (red), (C and F) overlay (red). (G) Quantitative assessment of GFP+ microglia per square millimetre. Individual mice and the mean per group are shown. Scale bars = 30 μm (A and D) and 10 μm (B, C, E and F).
Notably, these GFP-labelled cells were frequently found in the superficial cortical layers of the temporal lobe and in the molecular cell layer of the cerebellum. This observation suggested a gradient of monocyte/microglia recruitment to the site of inflammation. In order to test this hypothesis we examined the distribution of recruited GFP+ cells in superficial and deep cortical layers of both the cerebellum and the temporal cortex (Table 1). We could observe that the overall increase of GFP+ macrophages/microglia in the brains during disease is due to elevated numbers mainly on the superficial cortical layers, suggesting a gradient of inflammatory factors. In conclusion we believe that acute bacterial inflammation by S. pneumoniae is an important trigger for enhanced monocyte influx in the brain, which leads to an orchestrated and directed recruitment of differentiated macrophages/microglia during the resolution of infection.
Preferential recruitment of engrafted microglia to the superficial cortical layers in S. pneumoniae-challenged mice
| Brain region . | Cortical region . | Non-infected GFP+ cells . | Infected GFP+ cells . |
|---|---|---|---|
| Cerebellum | Molecular layer | 67.4 ± 16.9 | 98.2 ± 12.9* |
| Granule cell layer | 20.3 ± 3.6 | 25.5 ± 5.9 | |
| Temporal cortex | Superficial layers | 6.0 ± 1.8 | 32.5 ± 6.8* |
| Deep layers | 15.9 ± 7.6 | 20.2 ± 3.1 |
| Brain region . | Cortical region . | Non-infected GFP+ cells . | Infected GFP+ cells . |
|---|---|---|---|
| Cerebellum | Molecular layer | 67.4 ± 16.9 | 98.2 ± 12.9* |
| Granule cell layer | 20.3 ± 3.6 | 25.5 ± 5.9 | |
| Temporal cortex | Superficial layers | 6.0 ± 1.8 | 32.5 ± 6.8* |
| Deep layers | 15.9 ± 7.6 | 20.2 ± 3.1 |
Absolute number of GFP-expressing cells in the superficial versus deep cortical layers were compared in non-infected and infected mice 1 month after meningitis. Superficial cortical layers in the temporal cortex were defined as all layers within a distance of 80 μm from the cortical surface, whereas all underlying layers until the white matter were considered to be deep layers. Data are expressed as mean ± standard deviation, n ≥ 6. An unpaired Student's t-test was performed for statistical comparison of infected and non-infected mice. *P < 0.05.
Preferential recruitment of engrafted microglia to the superficial cortical layers in S. pneumoniae-challenged mice
| Brain region . | Cortical region . | Non-infected GFP+ cells . | Infected GFP+ cells . |
|---|---|---|---|
| Cerebellum | Molecular layer | 67.4 ± 16.9 | 98.2 ± 12.9* |
| Granule cell layer | 20.3 ± 3.6 | 25.5 ± 5.9 | |
| Temporal cortex | Superficial layers | 6.0 ± 1.8 | 32.5 ± 6.8* |
| Deep layers | 15.9 ± 7.6 | 20.2 ± 3.1 |
| Brain region . | Cortical region . | Non-infected GFP+ cells . | Infected GFP+ cells . |
|---|---|---|---|
| Cerebellum | Molecular layer | 67.4 ± 16.9 | 98.2 ± 12.9* |
| Granule cell layer | 20.3 ± 3.6 | 25.5 ± 5.9 | |
| Temporal cortex | Superficial layers | 6.0 ± 1.8 | 32.5 ± 6.8* |
| Deep layers | 15.9 ± 7.6 | 20.2 ± 3.1 |
Absolute number of GFP-expressing cells in the superficial versus deep cortical layers were compared in non-infected and infected mice 1 month after meningitis. Superficial cortical layers in the temporal cortex were defined as all layers within a distance of 80 μm from the cortical surface, whereas all underlying layers until the white matter were considered to be deep layers. Data are expressed as mean ± standard deviation, n ≥ 6. An unpaired Student's t-test was performed for statistical comparison of infected and non-infected mice. *P < 0.05.
Donor-derived microglia integrated into the pool of parenchymal microglia and participated in the clearance and repair of tissue debris
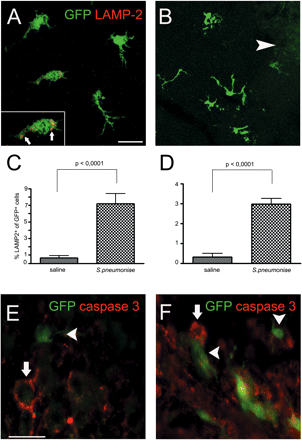
Despite higher numbers of GFP+ microglia 1 month after meningeal inflammation it remained unclear whether these newly engrafted cells were really functional and took part in the resolution of inflammation and the repair of tissue damage. In order to test this hypothesis, we examined lysosomal activity in engrafted microglia as a marker for their phagocytotic rate (Fig. 6A–D). We found strong lysosomal LAMP-2 activity in GFP-expressing microglia of infected (Fig. 6A) but not in saline-treated (Fig. 6B) cerebellum as well as in the temporal lobe (data not shown). Interestingly, the percentage of LAMP-2+GFP+ microglia significantly increased 1 month after infection to 7.2 ± 3.0% compared with 0.6 ± 0.2% in the non-infected cerebellum (Fig. 6C, P < 0.001 ) and to 3.0 ± 0.6% compared with 0.3 ± 0.1% in the temporal lobe (Fig. 6D, P < 0.001).
Newly recruited GFP+ macrophages/microglia participate in the clearance and repair of damaged tissue. (A and B) Increased lysosomal activity indicating high phagocytotic rate in microglia previously derived from blood monocytes 4 weeks after infection. Confocal image of LAMP-2 immunoreactivity (red) in GFP-expressing cells (green) in infected (A) or non-infected (B) cerebellum. Arrowheads refer to engrafted cells. Insert demonstrates lysosomal staining of LAMP-2 (arrows). (C and D) Quantification of LAMP-2+ GFP+ cells after S. pneumoniae meningitis in the cerebellum (C) and temporal lobe (D). (E and F) GFP+ cells with typical macrophage/microglia morphology were in close proximity to caspase 3+ cells in the cerebellum (E) and hippocampus (F). Arrows refer to caspase 3+ cells; arrowheads refer to engrafted cells. Caspase 3 (red) and GFP (green). Scale bars = 10 μm (A and B) and 20 μm (E and F).
Since cells frequently die during meningitis owing to apoptosis, we wanted to know whether macrophages/microglia differentiated from previously attracted monocytes were also recruited to dying cells. Despite the fact that we found only exceptional apoptotic cells with condensed nuclei in brain tissue 4 weeks after infection, some GFP+ microglia were found in close proximity to caspase 3+ apoptotic cells in the infected cerebellum (Fig. 6E) and temporal lobe (Fig. 6F), suggesting a role of engrafted cells in the clearance of dead cells (Fig. 6).
Discussion
Here, we provide experimental evidence that during bacterial infection of the CNS haematopoietic cells are an important source for parenchymal microglia even when the brain does not show gross signs of inflammation such as microabscess development. Notably, conversion of blood monocytes/macrophages into microglia was not increased during the acute phase of meningitis, but it significantly rose during the post-infectious period crucial for the recovery of tissue homeostastis. Most importantly, these newly engrafted cells participated actively in the resolution of tissue damage.
Brain-specific macrophages, the microglia, play a crucial role during CNS infections (Kreutzberg, 1996). These cells are continuously surveying the brain parenchyma in order to sense any changes of brain homeostasis. Upon activation, for example, through interaction with bacterial products or bacteria, they initiate an appropriate adaptive immune response (Kreutzberg, 1996). Previously considered to be a stable cell population within the brain, recent reports identified microglia as versatile cells that underlie a significant exchange with circulating cells under healthy conditions (Priller et al., 2001a; Nimmerjahn et al., 2005). The turnover, however, is significantly increased in several neurodegenerative and vascular diseases such as stroke, facial nerve axotomy, hippocampal lesions and a mouse model of Alzheimer's disease (Priller et al., 2001a; Bechmann et al., 2005; Stalder et al., 2005).
We therefore asked whether circulating monocytes might also be rapidly recruited into the lesioned CNS after bacterial inflammation of the meninges and could thus potentially be used as a peripheral target to modify CNS pathology. We observed that, already a few hours after infection with S. pneumoniae, engrafted GFP+ macrophages/microglia as well as resident parenchymal microglia changed their morphology to an activated phenotype, suggesting high cellular activity and demonstrating their ability to sense quickly any changes of their environment. This morphological transformation was still visible 4 weeks post-infection in our bone marrow chimeras. At this time point microglia are supposed to phagocytose debris such as damaged cells. These morphological changes were accompanied by an early MHC class II antigen expression on BM-derived and resident microglia/macrophages during the early phase of meningitis. This compares well with previous observations that during bacterial meningitis microglia rapidly upregulate surface receptors of immune regulation, for example, Fc receptors, complement receptors, CD40, CD86, MHC class I and II molecules (Kielian, 2004).
Our data further indicate that S. pneumoniae meningitis is sufficient to trigger significant recruitment of monocytes into the brain, which subsequently gave rise to macrophages as well as microglia with their typical morphology. This attraction seemed to be orchestrated and focused on the most inflamed superficial regions. It is likely that during meningitis microglia can easily recognize components of S. pneumoniae via TLR2, TLR4 and TLR9. Then they produce in a spatiotemporal manner a broad panel of chemoattractant factors such as CXCL2 and CXCL8 for the initiation of immune cell attraction to the brain (Ostergaard et al., 2000; Zwijnenburg et al., 2001). This interaction of microglia on the one hand and mono- and polymorphonuclear inflammatory cells in the meninges on the other hand might lead to the preferential attraction of monocyte-derived GFP+ macrophages/microglia to the cortical surface.
Most intriguingly, BMC engraftment during S. pneumoniae meningitis occurred particularly during late stages of tissue damage and during tissue repair. Under these conditions, microglia function might include the recovery of tissue homeostastis by producing anti-inflammatory factors such as IL-1Ra, soluble TNFR, IL-18 binding protein and neurotrophic factors such as BDNF and others (Prinz and Hanisch, 1999; Hanisch, 2002). As the major conclusion of our study, we found that monocyte-derived microglia integrated functionally into the pool of brain microglia. They strongly upregulated lysosomal LAMP-2 activity as a sign of increased phagocytosis and they were recruited to the few apoptotic cells present 4 weeks after meningitis within the brain tissue. We could, however, never observe microglia directly digesting dead cells, a process that might already be finished 1 month after meningitis. Thus, their role during this disease stage could be more weighted to tissue repair.
Despite the relative increase of all microglia during chronic infection, engrafted GFP-marked macrophages/microglia became an increasing part of the entire pool of brain microglia by doubling their percentage of all microglia up to 13.5%. It is therefore tempting to speculate that microglia derived from circulating monocytes also participate in the brain's antimicrobial defence and fulfil immunomodulatory functions in the late recovery phase of disease.
How then could BMC, especially haematopoietic stem cells (HSCs), be used to serve as vehicles for gene delivery to the nervous system? First, approaches using either retroviral or lentiviral vectors already successfully demonstrated the utility of gene-engineered HSCs to investigate the nature and fate of BMC under physiological and pathophysiological conditions in the brain (Mezey et al., 2000; Priller et al., 2001a; Wehner et al., 2003). The therapeutic potential of this approach is underscored by the clinical benefit of allogenic bone marrow transplantation in some metabolic storage disorders (Krivit et al., 1999; Peters and Steward, 2003). Although the mechanisms underlying the partial efficacy of bone marrow transplantation to correct brain diseases is not completely understood, it is thought that the gain of functional enzymes by donor-derived cells that have migrated to the CNS and the subsequent uptake by the resident enzyme-deficient CNS cells result in clinical improvement of CNS disorders such as metachromatic leucodystrophies (Krivit et al., 1995; Biffi et al., 2004). Adapted to the experimental model of S. pneumoniae meningitis, CNS delivery of growth and neurotrophic factors such as TGFβ, IGF-2, BDNF and CNTF or others via modified HSC would be applicable.
Taken together, our experiments identified microglia that were derived from blood monocytes as an increasing cell population within the brain parenchyma during meningitis. Our results emphasize the therapeutical potential of blood monocytes in modulating the neuroinflammatory response to CNS infections, in particular during resolution of inflammation and repair of damaged tissue.
We thank Olga Kowatsch and Stefanie Zischkau for excellent technical assistance. Grant acknowledgement: Fritz-Thyssen-Stiftung, CMPB (DFG Research Center for Molecular Physiology of the Brain) and the Gemeinnützige Hertie-Stiftung to M.P., Deutsche Forschungsgemeinschaft to R.N. (Na 165/4-3) and to J.P. (SFB 507 A5). H.S. is a fellow of the Gertrud Reemtsma foundation.
References
Author notes
*These authors have contributed equally to this work.