-
PDF
- Split View
-
Views
-
Cite
Cite
Marilyn F. Kraus, Teresa Susmaras, Benjamin P. Caughlin, Corey J. Walker, John A. Sweeney, Deborah M. Little, White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study, Brain, Volume 130, Issue 10, October 2007, Pages 2508–2519, https://doi.org/10.1093/brain/awm216
Close - Share Icon Share
Abstract
Traumatic brain injury (TBI) is a serious public health problem. Even injuries classified as mild, the most common, can result in persistent neurobehavioural impairment. Diffuse axonal injury is a common finding after TBI, and is presumed to contribute to outcomes, but may not always be apparent using standard neuroimaging. Diffusion tensor imaging (DTI) is a more recent method of assessing axonal integrity in vivo. The primary objective of the current investigation was to characterize white matter integrity utilizing DTI across the spectrum of chronic TBI of all severities. A secondary objective was to examine the relationship between white matter integrity and cognition. Twenty mild, 17 moderate to severe TBI and 18 controls underwent DTI and neuropsychological testing. Fractional anisotropy, axial diffusivity and radial diffusivity were calculated from the DTI data. Fractional anisotropy was the primary measure of white matter integrity. Region of interest analysis included anterior and posterior corona radiata, cortico-spinal tracts, cingulum fibre bundles, external capsule, forceps minor and major, genu, body and splenium of the corpus callosum, inferior fronto-occipital fasciculus, superior longitudinal fasciculus and sagittal stratum. Cognitive domain scores were calculated from executive, attention and memory testing. Decreased fractional anisotropy was found in all 13 regions of interest for the moderate to severe TBI group, but only in the cortico-spinal tract, sagittal stratum and superior longitudinal fasciculus for the mild TBI group. White Matter Load (a measure of the total number of regions with reduced FA) was negatively correlated with all cognitive domains. Analysis of radial and axial diffusivity values suggested that all severities of TBI can result in a degree of axonal damage, while irreversible myelin damage was only apparent for moderate to severe TBI. The present data emphasize that white matter changes exist on a spectrum, including mild TBI. An index of global white matter neuropathology (White Matter Load) was related to cognitive function, such that greater white matter pathology predicted greater cognitive deficits. Mechanistically, mild TBI white matter changes may be primarily due to axonal damage as opposed to myelin damage. The more severe injuries impact both. DTI provides an objective means for determining the relationship of cognitive deficits to TBI, even in cases where the injury was sustained years prior to the evaluation.
Introduction
Traumatic brain injury (TBI) of all severities is a significant public health problem with an incidence between 180 and 500 per 100 000 population per year (Bruns and Hauser, 2001, 2003; Bazarian et al., 2005). Recently the numbers of soldiers returning from military conflicts with TBI has created a clinical crisis for the United States Veterans Administration Hospitals (Taber et al., 2006). In addition, greater public attention is finally being paid to the problems of athletes with persistent problems secondary to TBI (Guskiewicz et al., 2000; Pellman et al., 2004). Taken together, the burden on healthcare systems for both civilian and military TBI is large.
TBI is clinically rated as mild, moderate or severe based on acute TBI variables that include duration of loss of consciousness (LOC), Glasgow Coma Score (GCS) and post-traumatic amnesia (PTA) (Levin et al., 1979). Mild TBI (MTBI) is the most common severity, with a recent WHO task force reporting that 70–90% of all treated TBI fell into this category (Holm et al., 2005).
Neurobehavioural deficits, especially in cognition, are often the cause of significant disability after TBI (CDC, 2003). Observed cognitive changes that follow TBI can include decreased mental flexibility, trouble shifting sets, impaired attention, poor planning, lack of organization, problems with sequencing, impaired judgment, deficits in verbal fluency, problems with working memory, as well as increased impulsivity (Levin and Kraus, 1994; Miller, 2000; Godefroy, 2003). Determining the extent of clinically relevant neuropathology (defined as neuropathology associated with persistent neurobehavioural deficits) associated with TBI, particularly in the milder spectrum, is problematic. As such, there is a need for objective and quantifiable measures of neuropathology that can be applied to all severities of TBI for the purpose of determining the relationship between trauma and persistent disability. This methodology would provide the foundation for more accurate injury severity grading, prognosis and treatment planning without having to rely on often incomplete or inaccurate historical data that has been used as predictors of outcomes including LOC, PTA and GCS.
Pathophysiology of TBI
There are several significant pathophysiologic sequelae of TBI that are likely important to neurobehavioural outcome, including the location and severity of the injury, diffuse effects and secondary mechanisms of injury. Primary neurologic injury due to TBI can be direct and/or indirect. Contusions are common following TBI, and can directly disrupt function in both cortical and sub-cortical regions. Certain brain regions may be more vulnerable to contusion following trauma, such as the frontal and anterior temporal cortices, due to their position within the skull (Adams et al., 1980; Levin et al., 1992). Disruption of function can also result from more diffuse damage to white matter tracts that are particularly susceptible to the shearing forces that often occur with TBI (Graham et al., 2002). Such diffuse axonal injury (DAI) can disrupt critical cortical-subcortical pathways and lead to widespread cognitive dysfunction (Gennarelli et al., 1982; Povlishok, 1992). DAI can result directly from the trauma, or secondary due to ischaemia. Brain oedema and shift can compromise blood supply and lead to secondary infarction in the corpus callosum and deep grey matter, and elevated intracranial pressure (ICP) can cause damage to the brainstem in TBI (Graham et al., 1987). And although the diagnosis of DAI can only be clearly confirmed by microscopic examination, it may be inferred from specific neuroimaging findings such as haemorrhages in the corpus callosum or areas of rostral brainstem (Geddes, 1997; Geddes et al., 1997).
DAI may be the only significant pathology found in certain cases of TBI, and has been identified via direct pathological studies as well as neuroimaging in mild TBI (Povlishock et al., 1983; Graham et al., 1989; Blumbergs et al., 1994; Goodman, 1994; Mittl et al., 1994; Aihara et al., 1995; Blumbergs et al., 1995; Gennarelli, 1996; Inglese et al., 2005b). Changes in white matter, observed as hyperintense T2 signal, have been observed in mild TBI (Inglese et al., 2005a, b). These lesions have been reported primarily in the corpus callosum, internal capsule, and centrum semiovale (Inglese et al., 2005b). Another issue is the specificity of lesion type and the clinical relevance of these lesions found in mild TBI. Kurca and colleagues reported that mild TBI subjects with defined traumatic lesions (including both gray and white matter) showed significantly greater impairment on neuropsychological evaluations and subjective reports of symptoms consistent with postconcussion syndrome (Kurca et al., 2006).
As would be expected, as injury severity increases, the pathophysiology identified on MRI also increases. For example, chronic moderate to severe TBI has been related to atrophy in the corpus callosum. The degree of atrophy in the corpus callosum did appear to be related to behavioural measures of reaction time (although not significantly) (Mathias et al., 2004). In chronic (at least 3 months post injury) severe TBI, increased atrophy was reported in the corpus callosum, fornix, anterior limb of the internal capsule, superior frontal gyrus, para-hippocampal gyrus, optic radiations and optic chiasma (Tomaiulo et al., 2005). There were only modest correlations between atrophy of the corpus callosum and memory function (Tomaiulo et al., 2005).
Although there is some evidence to suggest that standard T1- or T2-weighted anatomic MR imaging shows promise for quantifying pathophysiology in TBI, it may not be as sensitive to the neuropathology of milder injuries (Hughes et al., 2004). The limitation of standard imaging is highlighted by modest relationships between cognitive function and standard anatomic imaging findings. Diffusion tensor imaging is a very promising methodology in this regard.
Diffusion tensor imaging
Diffusion tensor imaging (DTI) is a relatively recent tool developed using MRI technology. DTI allows for the specific examination of the integrity of white matter tracts, tracts which are especially vulnerable to the mechanical trauma of TBI. DTI is a modification of diffusion-weighted imaging. Standard MRI structural imaging itself is not sensitive enough in identifying impairment in mild injury (Hughes et al., 2004). Because DTI is more sensitive to changes in the microstructure of white matter, it shows considerable promise in the assessment of TBI.
DTI is based upon the diffusivity of water molecules, which is variably restricted in different tissues. In white matter, it is more limited in the directions of diffusion. In healthy tracts, the anisotropy (limited directionality of diffusion) is higher than in less-organized gray matter. This difference allows for the calculation of fractional anisotropy (FA) values for tissue, and the generation of white matter fibre maps. The values for FA range from 0 to 1 where 0 represents isotropic diffusion, or lack of directional organization, and 1 represents anisotropic diffusion, or organized tissues such as in white matter tracts [see Le Bihan et al. (2001)]. Recently, there has been an increase in applications of DTI, with previous research demonstrating its potential utility in qualifying and quantifying neuropathology in TBI, in which diffuse axonal injury is common (Huismana et al., 2004). Although the specifics are still not well understood, FA is believed to reflect many factors including the degree of myelination and axonal density and/or integrity (Arfanakis et al., 2002; Song et al., 2002b, 2003; Harsan et al., 2006). More discrete analysis of the axial (λ∥) and radial diffusivity (λ⊥) also provide potential measures of the mechanisms that underlie changes in white matter following injury (Pierpaoli et al., 2001; Song et al., 2002a). λ∥ reflects diffusivity parallel to axonal fibres. Increases in λ∥ are thought to reflect pathology of the axon itself, such as from trauma. λ⊥ reflects diffusivity perpendicular to axonal fibres and appears to be more strongly correlated with myelin abnormalities, either dysmyelination or demyelination. Although there is some preliminary evidence that these measures might be useful in vivo in trauma (Rugg-Gunn et al., 2001) it is not yet entirely clear whether λ∥ and λ⊥ are differentially affected by trauma, and this may be a function of severity as well as acuity.
The literature involving the application of DTI in chronic TBI is limited but shows promise. In chronic moderate to severe TBI, reduced FA has been reported (Nakayama et al., 2006; Tisserand et al., 2006; Xu et al., 2007), even in the absence of observable lesions in standard structural MRI (Nakayama et al., 2006). Despite general acceptance of this finding of abnormal FA, the relationship of white matter integrity to cognitive function in TBI is not yet clear, and the few studies to assess this in TBI have varied in outcomes. For example, in a group of chronic severe TBI subjects with cognitive impairment there was no relationship between reduced FA in the corpus callosum and neuropsychological measures of memory or executive function, though there was a relationship with performance on the Mini-Mental State Exam (Nakayama et al., 2006). However, Salmond and colleagues reported a relationship between reduced FA and measures of learning and memory (Salmond et al., 2006) in moderate and severe TBI. One problem is that the existing studies differ in methodology, including placement of regions of interest, variability in patient populations (such as severity and acuity/chronicity of TBI subjects), and in the specific neuropsychological testing used to assess cognition.
Hence, given the potential importance of white matter pathology to outcome in TBI, and the sensitivity of DTI in determining the integrity of white matter, further studies are warranted. A more standardized methodology is needed that can be used to assess the spectrum of white matter abnormalities in TBI, at any point after injury, that would also allow for correlation with clinically relevant issues such as cognitive function. The current investigation was designed with these issues in mind.
In this study, a group of chronic TBI subjects of all severities and a group of demographically matched healthy controls underwent MRI (anatomical and diffusion tensor imaging), neuropsychological testing and a neurobehavioural examination.
The primary objective of the current investigation was to test the hypothesis that white matter integrity is reduced across the spectrum of TBI severity in chronic subjects. The secondary objective was to examine the relationship between white matter integrity and cognition assessed with standard neuropsychological testing across the domains of executive, attention and memory function.
Methods
Subjects
A total of 39 subjects with a history of TBI, closed head type, participated in this study (Table 1). Twenty-two subjects (13 females, 9 males) had a history of MTBI and 17 (9 females, 8 males) had a history of moderate to severe TBI (M/STBI). Of these, two subjects with a history of MTBI were excluded for excessive head motion. The final sample included 20 MTBI subjects (12 females, 8 males) and 17 (9 females, 8 males) had a history of M/STBI. All were at least 6 months out from injury; with the average time out from injury being 107 months for all TBI subjects. Subjects were recruited from the University of Illinois Medical Center and via advertisements. Eighteen healthy controls (11 females, 7 males) were recruited from the community. Experimental procedures complied with the code of ethics of the World Medical Association and the standards of the University of Illinois Institutional Review Board. All subjects provided written informed consent consistent with the Declaration of Helsinki.
Demographic information for traumatic brain injury and control subjects
| . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Age | 32.83 | 2.51 | 35.85 | 2.10 | 34.88 | 2.82 | 0.673 | 0.360 | 0.590 | 0.781 |
| Number of years of education | 16.76 | 0.44 | 16.55 | 0.53 | 15.47 | 0.77 | 0.276 | 0.763 | 0.154 | 0.244 |
| WTAR Pre-morbid | 113.24 | 1.80 | 112.65 | 2.43 | 106.59 | 2.60 | 0.100 | 0.852 | 0.043* | 0.098 |
| IQ estimate | ||||||||||
| Time from injury (in months) | 92.55 | 18.61 | 124.35 | 23.12 | 0.286 | 0.286 | ||||
| Age at time of injury (years) | 29.00 | 2.37 | 24.50 | 2.51 | 0.199 | 0.199 | ||||
| Length of LOC (h) | 0.11 | 0.05 | 237.00 | 111.50 | 0.042* | 0.042* | ||||
| . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Age | 32.83 | 2.51 | 35.85 | 2.10 | 34.88 | 2.82 | 0.673 | 0.360 | 0.590 | 0.781 |
| Number of years of education | 16.76 | 0.44 | 16.55 | 0.53 | 15.47 | 0.77 | 0.276 | 0.763 | 0.154 | 0.244 |
| WTAR Pre-morbid | 113.24 | 1.80 | 112.65 | 2.43 | 106.59 | 2.60 | 0.100 | 0.852 | 0.043* | 0.098 |
| IQ estimate | ||||||||||
| Time from injury (in months) | 92.55 | 18.61 | 124.35 | 23.12 | 0.286 | 0.286 | ||||
| Age at time of injury (years) | 29.00 | 2.37 | 24.50 | 2.51 | 0.199 | 0.199 | ||||
| Length of LOC (h) | 0.11 | 0.05 | 237.00 | 111.50 | 0.042* | 0.042* | ||||
P-values are listed under each contrast and asterisks indicate significant differences between groups (*P < 0.05). SEM = standard error of the mean; WTAR = Wechsler test of adult reading; MTBI = mild traumatic brain injury; M/STBI = moderate to severe traumatic brain injury; LOC = loss of consciousness.
Demographic information for traumatic brain injury and control subjects
| . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Age | 32.83 | 2.51 | 35.85 | 2.10 | 34.88 | 2.82 | 0.673 | 0.360 | 0.590 | 0.781 |
| Number of years of education | 16.76 | 0.44 | 16.55 | 0.53 | 15.47 | 0.77 | 0.276 | 0.763 | 0.154 | 0.244 |
| WTAR Pre-morbid | 113.24 | 1.80 | 112.65 | 2.43 | 106.59 | 2.60 | 0.100 | 0.852 | 0.043* | 0.098 |
| IQ estimate | ||||||||||
| Time from injury (in months) | 92.55 | 18.61 | 124.35 | 23.12 | 0.286 | 0.286 | ||||
| Age at time of injury (years) | 29.00 | 2.37 | 24.50 | 2.51 | 0.199 | 0.199 | ||||
| Length of LOC (h) | 0.11 | 0.05 | 237.00 | 111.50 | 0.042* | 0.042* | ||||
| . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Age | 32.83 | 2.51 | 35.85 | 2.10 | 34.88 | 2.82 | 0.673 | 0.360 | 0.590 | 0.781 |
| Number of years of education | 16.76 | 0.44 | 16.55 | 0.53 | 15.47 | 0.77 | 0.276 | 0.763 | 0.154 | 0.244 |
| WTAR Pre-morbid | 113.24 | 1.80 | 112.65 | 2.43 | 106.59 | 2.60 | 0.100 | 0.852 | 0.043* | 0.098 |
| IQ estimate | ||||||||||
| Time from injury (in months) | 92.55 | 18.61 | 124.35 | 23.12 | 0.286 | 0.286 | ||||
| Age at time of injury (years) | 29.00 | 2.37 | 24.50 | 2.51 | 0.199 | 0.199 | ||||
| Length of LOC (h) | 0.11 | 0.05 | 237.00 | 111.50 | 0.042* | 0.042* | ||||
P-values are listed under each contrast and asterisks indicate significant differences between groups (*P < 0.05). SEM = standard error of the mean; WTAR = Wechsler test of adult reading; MTBI = mild traumatic brain injury; M/STBI = moderate to severe traumatic brain injury; LOC = loss of consciousness.
Subjects were excluded if they had a history of psychiatric disorder before the TBI, substance abuse, current pending litigation or any other neurological or medical condition that could result in cognitive changes (e.g. severe hypertension, diabetes). Subjects were not receiving any treatments for cognitive deficits at the time of the study, pharmacological or otherwise. The criteria used for defining MTBI, set forth by the American Congress of Rehabilitation Medicine (Medicine, 1993), are as follows: MTBI is diagnosed when at least one of the following criteria is met (1) any period of loss of consciousness; (2) any loss of memory for events immediately before or after the accident; (3) any alteration in mental state at the time of the accident (e.g. feeling dazed, disoriented or confused) and (4) focal neurological deficit(s) that may or may not be transient (Medicine, 1993; Cassidy et al., 2004), For this study, subjects were categorized as moderate or greater severity TBI if the LOC was greater than 30 min and/or the GCS was less than 13 (Levin et al., 1992; Medicine, 1993; Cassidy et al., 2004; Tagliaferri et al., 2006). These criteria allowed the separation of MTBI from moderate to severe TBI for the purposes of the present study. For the MTBI group, the average reported LOC was 0.1 h (range = 0–0.50 h), for the M/STBI group average LOC was 213.5 h (range = 0.25–1440 h). Data on acute TBI variables such as LOC were collected by medical record when available and by subject and family report. For the MTBI cases, all except one (who met criteria for mild TBI by history with positive LOC but did not seek immediate attention) were seen and diagnosed acutely at an ER or outpatient setting.
In terms of clinical details concerning the index traumatic event, for many of the cases the TBI was the primary diagnosis at the time of their injury. Five MTBI and five M/STBI cases had associated injuries (traumatic injuries other than the TBI). Of these, most were fractures of the clavicle or an extremity. The most common mechanisms of injury were motor vehicle accidents (17 subjects). The remainder included bicycle accidents, blunt head trauma and falls. On the neurological exam (exclusive of cognitive testing) done at the time of evaluation, only eight TBI subjects (two MTBI, six M/STBI) showed abnormalities, which were primarily soft signs such as mildy unsteady tandem gait. Of the MTBI group, all but two were employed or in school at the time of evaluation; all but three of the M/STBI group were either employed or in school at the time of evaluation.
Healthy controls were excluded if they had any history of psychiatric illness or TBI, substance abuse/dependency or a history of significant medical or neurological illness that would be associated with significant changes in the brain, such as diabetes, seizures or stroke. The healthy control group was not significantly different from the TBI groups in age or years of education (Table 1). The controls and MTBI groups were not significantly different in estimates of premorbid IQ (Table 1). The M/STBI did differ from the controls in terms of premorbid IQ estimates. The M/STBI group did not differ from MTBI in age at the time of injury.
DTI data acquisition
Studies were acquired on a 3.0-Tesla whole body scanner (Signa VHi, General Electric Medical Systems, Waukesha, WI) using a customized DTI pulse sequence with a quadrature head coil. The sequence is based on a single-shot EPI pulse sequence with the capability of compensating eddy currents induced by the diffusion gradients via dynamically modifying the imaging gradient waveforms. The diffusion-weighting orientations are designed based on the electrostatic repulsion model proposed by Jones et al. (1999) (TR = 5200 ms, TE = minimum (81 ms), b-values = 0, 750 s/mm2, diffusion gradient directions = 27, FOV = 22 cm, Matrix = 132 × 132 (reconstructed to 256 × 256, slice thickness = 5 mm, gap = 1.5 mm, ramp-sampling = on, NEX = 2, total acquisition time = 5:46).
An additional 3D high-resolution anatomical scan was also acquired to allow coregistration with the DTI data and normalization to the Montreal Neurological Institute template (MNI) (3D inversion recovery fast spoiled gradient recalled (3D IRfSPGR), plane = axial, TR = 9 ms, TE = 2.0 ms, flip angle = 25°, NEX = 1, bandwidth = 15.6 kHz, acquisition matrix = 256 × 256, FOV = 22 × 16.5 cm2, slice thickness/gap = 1.5/0 mm/mm, slices = 124).
Neuropsychological assessment
Subjects completed a test battery that was assembled to assess executive function, attention and memory. Since TBI commonly affects frontal lobe function, the battery was weighed more heavily on executive measures to heighten sensitivity to deficits in this area of cognition. Tests included the Tower of London (Shallice, 1982; Culbertson and Zilmer, 2001), Stroop Colour–Word Test (Stroop, 1935; Jensen and Rohwer, 1966; Golden and Freshwater, 2002), Paced Auditory Serial Addition Test (PASAT) (Gronwall and Sampson, 1974; Gronwall, 1977), Trail Making Test (Reitan, 1958), Conners’ Continuous Performance Test (Conners and Staff, 2000), Controlled Oral Word Association Test (COWAT) (Benton and Hamsher, 1976; Benton and Hamsher, 1989), Ruff Figural Fluency Test (Ruff, 1988), Wechsler Test of Adult Reading (WTAR) (Psychological, 2001), California Verbal Learning Test – Second Edition (CVLT-II) (Delis et al., 2000), Brief Visual Spatial Memory Test – Revised (BVMT-R) (Benedict, 1997), Digit Span and Spatial Span from the Wechsler Memory Scales – Third Edition (Wechsler, 1997) and the Grooved Pegboard (Klove, 1964; Matthews and Klove, 1964). In addition, subjects had to pass tests for malingering and effort, including the Test of Memory Malingering (TOMM) and Dot Counting to ensure that only subjects who performed testing effortfully were included (Rey, 1941; Tombaugh, 1996, 1997).
Z-scores were calculated for all subjects, with the mean and SD of data from healthy subjects used to define z-scores for all subject groups. Negative scores indicate performance below the mean of healthy subjects. Domain scores for measures of executive function, attention and memory were generated by averaging the standardized data from tests assessing these cognitive domains as presented in Table 2.
Neuropsychological test results and domain scores for all groups
| . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Executive measures executive domain | 0.00 | 0.15 | −0.37 | 0.14 | −0.87 | 0.14 | <0.001** | 0.075 | <0.001** | 0.016** |
| Tower of London (total moves) | 101.89 | 4.03 | 98.20 | 3.49 | 99.65 | 3.24 | 0.764 | 0.491 | 0.670 | 0.766 |
| Stroop color-word [age-corrected (s)] | 52.22 | 2.74 | 45.30 | 2.28 | 38.12 | 2.98 | 0.002** | 0.059 | 0.001** | 0.060 |
| PASAT total | 133.00 | 11.87 | 125.40 | 7.42 | 109.31 | 10.67 | 0.263 | 0.583 | 0.151 | 0.212 |
| Trails B (s) | 50.17 | 3.88 | 58.10 | 6.27 | 77.53 | 7.52 | 0.009** | 0.302 | 0.002** | 0.053 |
| CPT number of errors of commission | 8.06 | 1.43 | 14.15 | 1.46 | 13.76 | 1.95 | 0.016* | 0.005** | 0.023* | 0.873 |
| COWAT total | 44.44 | 2.49 | 40.35 | 2.23 | 36.24 | 2.90 | 0.090 | 0.227 | 0.038* | 0.261 |
| RUFF unique designs | 48.88 | 2.99 | 46.49 | 1.56 | 37.21 | 1.38 | <.001** | 0.470 | 0.001** | <0.001** |
| Digit span backward scaled score | 8.00 | 0.54 | 7.85 | 0.63 | 6.71 | 0.47 | 0.228 | 0.859 | 0.079 | 0.168 |
| Spatial Span Backward scaled score | 8.83 | 0.36 | 8.50 | 0.53 | 7.18 | 0.46 | 0.039* | 0.613 | 0.007** | 0.070 |
| Attention measures attention domain | 0.00 | 0.15 | −0.93 | 0.46 | −1.83 | 0.60 | 0.022* | 0.075 | 0.005** | 0.237 |
| Digit span forward scaled score | 10.61 | 0.61 | 11.60 | 0.44 | 11.00 | 0.68 | 0.461 | 0.190 | 0.673 | 0.450 |
| Spatial span forward scaled score | 9.89 | 0.46 | 9.10 | 0.45 | 8.59 | 0.41 | 0.134 | 0.229 | 0.043* | 0.415 |
| Trails A (s) | 21.33 | 1.96 | 23.75 | 1.64 | 33.06 | 3.88 | 0.007** | 0.347 | 0.010** | 0.026* |
| CPT number of errors of omission | 0.67 | 0.16 | 3.00 | 1.11 | 4.35 | 1.31 | 0.042* | 0.056 | 0.007** | 0.434 |
| Memory measures memory domain | 0.00 | 0.21 | −0.15 | 0.17 | −1.04 | 0.31 | 0.006** | 0.586 | 0.008** | 0.013* |
| CVLT total trials 1–5 | 58.50 | 1.96 | 55.95 | 2.27 | 46.76 | 2.75 | 0.003** | 0.406 | 0.001** | 0.014* |
| CVLT long-free recall | 12.56 | 0.58 | 12.60 | 0.57 | 10.06 | 1.09 | 0.037* | 0.957 | 0.049* | 0.039* |
| BVMT trials 1–3 | 27.22 | 1.41 | 25.65 | 0.99 | 21.47 | 1.82 | 0.019* | 0.360 | 0.017* | 0.043* |
| BVMT delay recall | 10.06 | 0.45 | 10.00 | 0.42 | 8.59 | 0.68 | 0.090 | 0.928 | 0.076 | 0.075 |
| Other measures | ||||||||||
| CPT Hit reaction Time (ms) | 405.83 | 16.77 | 368.99 | 10.90 | 400.77 | 22.00 | 0.230 | 0.068 | 0.855 | 0.184 |
| Grooved pegboard [dominant hand (s)] | 62.17 | 2.57 | 64.75 | 1.93 | 76.71 | 3.93 | 0.002** | 0.421 | 0.004** | 0.007** |
| . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Executive measures executive domain | 0.00 | 0.15 | −0.37 | 0.14 | −0.87 | 0.14 | <0.001** | 0.075 | <0.001** | 0.016** |
| Tower of London (total moves) | 101.89 | 4.03 | 98.20 | 3.49 | 99.65 | 3.24 | 0.764 | 0.491 | 0.670 | 0.766 |
| Stroop color-word [age-corrected (s)] | 52.22 | 2.74 | 45.30 | 2.28 | 38.12 | 2.98 | 0.002** | 0.059 | 0.001** | 0.060 |
| PASAT total | 133.00 | 11.87 | 125.40 | 7.42 | 109.31 | 10.67 | 0.263 | 0.583 | 0.151 | 0.212 |
| Trails B (s) | 50.17 | 3.88 | 58.10 | 6.27 | 77.53 | 7.52 | 0.009** | 0.302 | 0.002** | 0.053 |
| CPT number of errors of commission | 8.06 | 1.43 | 14.15 | 1.46 | 13.76 | 1.95 | 0.016* | 0.005** | 0.023* | 0.873 |
| COWAT total | 44.44 | 2.49 | 40.35 | 2.23 | 36.24 | 2.90 | 0.090 | 0.227 | 0.038* | 0.261 |
| RUFF unique designs | 48.88 | 2.99 | 46.49 | 1.56 | 37.21 | 1.38 | <.001** | 0.470 | 0.001** | <0.001** |
| Digit span backward scaled score | 8.00 | 0.54 | 7.85 | 0.63 | 6.71 | 0.47 | 0.228 | 0.859 | 0.079 | 0.168 |
| Spatial Span Backward scaled score | 8.83 | 0.36 | 8.50 | 0.53 | 7.18 | 0.46 | 0.039* | 0.613 | 0.007** | 0.070 |
| Attention measures attention domain | 0.00 | 0.15 | −0.93 | 0.46 | −1.83 | 0.60 | 0.022* | 0.075 | 0.005** | 0.237 |
| Digit span forward scaled score | 10.61 | 0.61 | 11.60 | 0.44 | 11.00 | 0.68 | 0.461 | 0.190 | 0.673 | 0.450 |
| Spatial span forward scaled score | 9.89 | 0.46 | 9.10 | 0.45 | 8.59 | 0.41 | 0.134 | 0.229 | 0.043* | 0.415 |
| Trails A (s) | 21.33 | 1.96 | 23.75 | 1.64 | 33.06 | 3.88 | 0.007** | 0.347 | 0.010** | 0.026* |
| CPT number of errors of omission | 0.67 | 0.16 | 3.00 | 1.11 | 4.35 | 1.31 | 0.042* | 0.056 | 0.007** | 0.434 |
| Memory measures memory domain | 0.00 | 0.21 | −0.15 | 0.17 | −1.04 | 0.31 | 0.006** | 0.586 | 0.008** | 0.013* |
| CVLT total trials 1–5 | 58.50 | 1.96 | 55.95 | 2.27 | 46.76 | 2.75 | 0.003** | 0.406 | 0.001** | 0.014* |
| CVLT long-free recall | 12.56 | 0.58 | 12.60 | 0.57 | 10.06 | 1.09 | 0.037* | 0.957 | 0.049* | 0.039* |
| BVMT trials 1–3 | 27.22 | 1.41 | 25.65 | 0.99 | 21.47 | 1.82 | 0.019* | 0.360 | 0.017* | 0.043* |
| BVMT delay recall | 10.06 | 0.45 | 10.00 | 0.42 | 8.59 | 0.68 | 0.090 | 0.928 | 0.076 | 0.075 |
| Other measures | ||||||||||
| CPT Hit reaction Time (ms) | 405.83 | 16.77 | 368.99 | 10.90 | 400.77 | 22.00 | 0.230 | 0.068 | 0.855 | 0.184 |
| Grooved pegboard [dominant hand (s)] | 62.17 | 2.57 | 64.75 | 1.93 | 76.71 | 3.93 | 0.002** | 0.421 | 0.004** | 0.007** |
P-values are listed under each contrast and asterisks indicate significant differences between groups after correction for multiple comparisons (*P < 0.05; **P < 0.01). PASAT = paced auditory serial addition test; Trails = trail making test; CPT = Conners’ continuous performance test; COWAT = controlled oral word association test; RUFF = Ruff figural fluency test; CVLT = California verbal learning test; BVMT = brief visual spatial memory test.
Neuropsychological test results and domain scores for all groups
| . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Executive measures executive domain | 0.00 | 0.15 | −0.37 | 0.14 | −0.87 | 0.14 | <0.001** | 0.075 | <0.001** | 0.016** |
| Tower of London (total moves) | 101.89 | 4.03 | 98.20 | 3.49 | 99.65 | 3.24 | 0.764 | 0.491 | 0.670 | 0.766 |
| Stroop color-word [age-corrected (s)] | 52.22 | 2.74 | 45.30 | 2.28 | 38.12 | 2.98 | 0.002** | 0.059 | 0.001** | 0.060 |
| PASAT total | 133.00 | 11.87 | 125.40 | 7.42 | 109.31 | 10.67 | 0.263 | 0.583 | 0.151 | 0.212 |
| Trails B (s) | 50.17 | 3.88 | 58.10 | 6.27 | 77.53 | 7.52 | 0.009** | 0.302 | 0.002** | 0.053 |
| CPT number of errors of commission | 8.06 | 1.43 | 14.15 | 1.46 | 13.76 | 1.95 | 0.016* | 0.005** | 0.023* | 0.873 |
| COWAT total | 44.44 | 2.49 | 40.35 | 2.23 | 36.24 | 2.90 | 0.090 | 0.227 | 0.038* | 0.261 |
| RUFF unique designs | 48.88 | 2.99 | 46.49 | 1.56 | 37.21 | 1.38 | <.001** | 0.470 | 0.001** | <0.001** |
| Digit span backward scaled score | 8.00 | 0.54 | 7.85 | 0.63 | 6.71 | 0.47 | 0.228 | 0.859 | 0.079 | 0.168 |
| Spatial Span Backward scaled score | 8.83 | 0.36 | 8.50 | 0.53 | 7.18 | 0.46 | 0.039* | 0.613 | 0.007** | 0.070 |
| Attention measures attention domain | 0.00 | 0.15 | −0.93 | 0.46 | −1.83 | 0.60 | 0.022* | 0.075 | 0.005** | 0.237 |
| Digit span forward scaled score | 10.61 | 0.61 | 11.60 | 0.44 | 11.00 | 0.68 | 0.461 | 0.190 | 0.673 | 0.450 |
| Spatial span forward scaled score | 9.89 | 0.46 | 9.10 | 0.45 | 8.59 | 0.41 | 0.134 | 0.229 | 0.043* | 0.415 |
| Trails A (s) | 21.33 | 1.96 | 23.75 | 1.64 | 33.06 | 3.88 | 0.007** | 0.347 | 0.010** | 0.026* |
| CPT number of errors of omission | 0.67 | 0.16 | 3.00 | 1.11 | 4.35 | 1.31 | 0.042* | 0.056 | 0.007** | 0.434 |
| Memory measures memory domain | 0.00 | 0.21 | −0.15 | 0.17 | −1.04 | 0.31 | 0.006** | 0.586 | 0.008** | 0.013* |
| CVLT total trials 1–5 | 58.50 | 1.96 | 55.95 | 2.27 | 46.76 | 2.75 | 0.003** | 0.406 | 0.001** | 0.014* |
| CVLT long-free recall | 12.56 | 0.58 | 12.60 | 0.57 | 10.06 | 1.09 | 0.037* | 0.957 | 0.049* | 0.039* |
| BVMT trials 1–3 | 27.22 | 1.41 | 25.65 | 0.99 | 21.47 | 1.82 | 0.019* | 0.360 | 0.017* | 0.043* |
| BVMT delay recall | 10.06 | 0.45 | 10.00 | 0.42 | 8.59 | 0.68 | 0.090 | 0.928 | 0.076 | 0.075 |
| Other measures | ||||||||||
| CPT Hit reaction Time (ms) | 405.83 | 16.77 | 368.99 | 10.90 | 400.77 | 22.00 | 0.230 | 0.068 | 0.855 | 0.184 |
| Grooved pegboard [dominant hand (s)] | 62.17 | 2.57 | 64.75 | 1.93 | 76.71 | 3.93 | 0.002** | 0.421 | 0.004** | 0.007** |
| . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Executive measures executive domain | 0.00 | 0.15 | −0.37 | 0.14 | −0.87 | 0.14 | <0.001** | 0.075 | <0.001** | 0.016** |
| Tower of London (total moves) | 101.89 | 4.03 | 98.20 | 3.49 | 99.65 | 3.24 | 0.764 | 0.491 | 0.670 | 0.766 |
| Stroop color-word [age-corrected (s)] | 52.22 | 2.74 | 45.30 | 2.28 | 38.12 | 2.98 | 0.002** | 0.059 | 0.001** | 0.060 |
| PASAT total | 133.00 | 11.87 | 125.40 | 7.42 | 109.31 | 10.67 | 0.263 | 0.583 | 0.151 | 0.212 |
| Trails B (s) | 50.17 | 3.88 | 58.10 | 6.27 | 77.53 | 7.52 | 0.009** | 0.302 | 0.002** | 0.053 |
| CPT number of errors of commission | 8.06 | 1.43 | 14.15 | 1.46 | 13.76 | 1.95 | 0.016* | 0.005** | 0.023* | 0.873 |
| COWAT total | 44.44 | 2.49 | 40.35 | 2.23 | 36.24 | 2.90 | 0.090 | 0.227 | 0.038* | 0.261 |
| RUFF unique designs | 48.88 | 2.99 | 46.49 | 1.56 | 37.21 | 1.38 | <.001** | 0.470 | 0.001** | <0.001** |
| Digit span backward scaled score | 8.00 | 0.54 | 7.85 | 0.63 | 6.71 | 0.47 | 0.228 | 0.859 | 0.079 | 0.168 |
| Spatial Span Backward scaled score | 8.83 | 0.36 | 8.50 | 0.53 | 7.18 | 0.46 | 0.039* | 0.613 | 0.007** | 0.070 |
| Attention measures attention domain | 0.00 | 0.15 | −0.93 | 0.46 | −1.83 | 0.60 | 0.022* | 0.075 | 0.005** | 0.237 |
| Digit span forward scaled score | 10.61 | 0.61 | 11.60 | 0.44 | 11.00 | 0.68 | 0.461 | 0.190 | 0.673 | 0.450 |
| Spatial span forward scaled score | 9.89 | 0.46 | 9.10 | 0.45 | 8.59 | 0.41 | 0.134 | 0.229 | 0.043* | 0.415 |
| Trails A (s) | 21.33 | 1.96 | 23.75 | 1.64 | 33.06 | 3.88 | 0.007** | 0.347 | 0.010** | 0.026* |
| CPT number of errors of omission | 0.67 | 0.16 | 3.00 | 1.11 | 4.35 | 1.31 | 0.042* | 0.056 | 0.007** | 0.434 |
| Memory measures memory domain | 0.00 | 0.21 | −0.15 | 0.17 | −1.04 | 0.31 | 0.006** | 0.586 | 0.008** | 0.013* |
| CVLT total trials 1–5 | 58.50 | 1.96 | 55.95 | 2.27 | 46.76 | 2.75 | 0.003** | 0.406 | 0.001** | 0.014* |
| CVLT long-free recall | 12.56 | 0.58 | 12.60 | 0.57 | 10.06 | 1.09 | 0.037* | 0.957 | 0.049* | 0.039* |
| BVMT trials 1–3 | 27.22 | 1.41 | 25.65 | 0.99 | 21.47 | 1.82 | 0.019* | 0.360 | 0.017* | 0.043* |
| BVMT delay recall | 10.06 | 0.45 | 10.00 | 0.42 | 8.59 | 0.68 | 0.090 | 0.928 | 0.076 | 0.075 |
| Other measures | ||||||||||
| CPT Hit reaction Time (ms) | 405.83 | 16.77 | 368.99 | 10.90 | 400.77 | 22.00 | 0.230 | 0.068 | 0.855 | 0.184 |
| Grooved pegboard [dominant hand (s)] | 62.17 | 2.57 | 64.75 | 1.93 | 76.71 | 3.93 | 0.002** | 0.421 | 0.004** | 0.007** |
P-values are listed under each contrast and asterisks indicate significant differences between groups after correction for multiple comparisons (*P < 0.05; **P < 0.01). PASAT = paced auditory serial addition test; Trails = trail making test; CPT = Conners’ continuous performance test; COWAT = controlled oral word association test; RUFF = Ruff figural fluency test; CVLT = California verbal learning test; BVMT = brief visual spatial memory test.
DTI data analysis
The 28 diffusion directions, along with the B0 image, were used to calculate FA as the primary indicator of white matter integrity. The images were reconstructed and FA, λ1, λ2 and λ3 were calculated using the program from Johns Hopkins, DTI Studio (Wakana et al., 2004). The 28 diffusion-weighted image sets were examined for image quality and head movement. Head movement was required to be within one voxel across the image acquisition. Because noise can introduce bias in estimates of the eigenvalues and because noise decreases the signal-to-noise ratio we applied a background noise level to all subjects prior to calculation of pixel-wise FA and the eigenvalues (λ1, λ2, λ3) (background noise = 125). It is important to note that the application of this criterion and the noise itself can influence calculation of anisotropy. However, because the analyses focus on differences between groups the bias introduced by this noise floor should not influence group differences. The FA, λ1, λ2 and λ3 were then converted to ANALYSE format and read into Statistical Parametric Mapping software for analysis (SPM2, Wellcome Department of Imaging Neuroscience, London, UK). DTI data from each subject was co-registered with their corresponding T1-weighted anatomic image set (after skull stripping) using a normalized mutual information cost function and trilinear interpolation. Normalization parameters were determined based upon the high-resolution T1 image relative to the Montreal Neurological Institute (MNI) template. These normalization parameters were then applied to the FA and eigenvalue images. Each image was visually checked for accuracy after both the co-registration and normalization steps. From these eigenvalue maps, axial (λ∥ = λ1) and radial [λ⊥ = (λ2 + λ3)/2] diffusivity were calculated. Although no additional smoothing was applied to the data the magnitude of spatial filtering which occurs during normalization to standardized space can potentially affect the DTI data (see Jones et al., 2005; Smith et al., 2006). In some cases, large smoothing kernels can potentially reduce group differences (Jones et al., 2005).
Region-of-interest analysis
All ROI analyses were carried out on data from each individual subject and hand-drawn in standardized space. ROIs were drawn individually on the FA maps with respect to the T2 FSE and colour-coded FA maps.
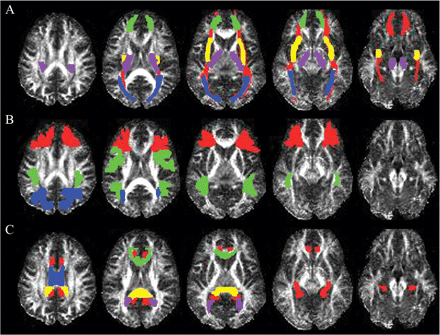
The specific ROIs included: anterior and posterior corona radiata (respectively, ACR and PCR), cortico-spinal tracts (CST) which included parts of the cortico-pontine tract and parts of the superior thalamic radiation, cingulum (CG) fibres, forceps minor (fMin), forceps major (fMaj), the body, genu and splenium of the corpus callosum (bCC, gCC and sCC), the inferior fronto-occipital (IFO) fasciculus, the superior longitudinal fasciculus (SLF), external capsule (ExCap) and the sagittal stratum including the optic radiations (SS). A description of the identification of these ROIs follows. A representative subject's FA map with superimposed ROIs is presented in Fig. 1.
Example region of interest masks for a single representative subject: (A) forceps minor (green), cortico-spinal tract (purple), inferior frontal-occipital fasciculus (red), external capsule (yellow), sagittal stratum (blue); (B) anterior corona radiata (green), superior longitudinal fasciculus (red), posterior corona radiata (blue); (C) cingulum (red), corpus callosum body (blue), splenium (yellow), and genu (green) and forceps major (purple).
The cingulum was defined firstly as the long association fibre that is located internal to the cingulate gyrus and running along its entire length continuing into the parahippocampal gyrus. It was defined dorsally by the corpus callosum continuing into the temporal lobe along the ventral/medial wall of the hippocampal gyri. Some of the cingulum fibres intersect with fibres of the superior longitudinal fasciculus, inferior longitudinal fasciculus, superior fronto-occipital fasciculus, inferior fronto-occipital fasciculus and uncinate fasciculus. The anterior and posterior corona radiata are the fibres which run throughout the internal capsule. The anterior corona radiata was defined as those fibres which run through limb of the internal capsule and contain nerve tracts running to and from the anterior areas of the cortex. The posterior corona radiata was defined by the posterior limb of the internal capsule. However, the cortico-spinal tract is a large part of the corona radiata. However, because we wanted to examine the cortico-spinal tract individually we have excluded these fibres from our definitions of anterior and posterior corona radiata. The external capsule contains cortico–cortico association fibres. The superior longitudinal fasciculus (fibres running from frontal to parietal to occipital and vice versa), inferior fronto-occipital fasciculus and the uncinate fasciculus (fibres running from ventral frontal lobe to pole of temporal lobe) run through the external capsule. The external capsule was defined as the white matter tracts located lateral to the lentiform nucleus, most specifically the putamen of the basal ganglia, and lateral to the extreme capsule is the claustrum. The external capsule, claustrum and extreme capsule are very closely associated. We are unable to discriminate between these tracts. In order to examine the external capsule separately from the SLF and IFO we excluded any fibre defined as external capsule from the SLF or IFO. The IFO runs from the frontal lobe to the occipital and temporal lobes ipsilaterally. It is deep within the cerebral hemisphere and runs laterally to the caudate nucleus. The SLF connects the anterior part of the frontal lobe to the occipital and temporal lobes. This tract has extensive branching in the frontal, parietal and temporal lobes. We excluded fibres associated with the IFO from these masks. Although the corpus callosum contains fibres which run anterior to posterior we wanted to investigate differential loss of the genu, splenium, and body of the corpus callosum as well as in forceps major and minor. The corpus callosum was first defined as a whole and then subdivided. The forceps minor were characterized as those fibres located inferior to the IFO and medially to the anterior portion of the corona radiata. Forceps major was defined as those fibres posterior to the posterior corona radiata and medial to the sagittal stratum. The corticospinal tract was identified by following the fibre bundle from the brainstem into the cortex. We refer herein to the corticospinal tract but also include the cortico-bulbar and cortico-pontine tract in this ROI. Although we define these regions there is considerable overlap between many of these tracts. Because of this we inspected each ROI relative to every other ROI to ensure that the same voxel was not included in more than one ROI. To ensure that FA was only calculated from white matter tissue, a threshold of 0.20 was applied prior to extraction of individual subjects’ FA maps.
White matter load
This was used as an index of global white matter integrity. It was defined as the number of ROIs that showed significantly decreased FA values compared to controls. This measure was used as it may be more sensitive to white matter abnormalities by looking at the actual number of affected areas across the brain independent of individual variability in the specific location of these white matter abnormalities. To measure the White Matter Load, z-scores were calculated for the FA within each ROI. The control group mean and SD were treated as zero. We then calculated the number of ROIs which showed decreased FA for each subject. We used a conservative criterion of 1 SD below the control mean to define decreased integrity. White Matter Load was then calculated as the total number of regions which showed impaired white matter relative to values from controls. The value for White Matter Load can range from 0 to 13 (13 ROIs).
Statistical analyses
Neuropsychological test scores were analysed using a one-way ANOVA with group membership (controls, MTBI, M/STBI) and were corrected for multiple comparisons using the least significant difference post-hoc tests. The primary measures of interest were three scores which were each a composite of those individual test results which loaded preferentially on executive, memory and attention domains, respectively. Because these three domain scores are more stable than individual tests scores they were also used to assess relationships between measures of white matter integrity and cognition using bivariate Pearson correlations.
The primary analyses carried out on the dependent measures extracted from the DTI data was a two-way mixed design ANOVA with cerebral hemisphere (right, left) as the within subjects comparison and group membership (controls, MTBI and M/STBI) as the between subjects comparison. For those regions where areas in both hemispheres were assessed together (corpus callosum and cerebral peduncles) the analysis was a one-way between subjects ANOVA with group membership (controls, MTBI and M/STBI) as the between-subjects comparison. The primary dependent measure was FA. Data were confirmed to have a normal distribution using the Kolmogorov–Smirnov test.
Results
Neuropsychological testing
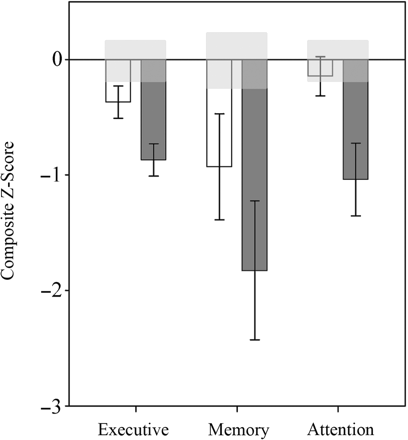
Group means are presented for the each neuropsychological test in Table 2. Of note, the only individual measure which differed significantly between the controls and MTBI was the number of commissions on the CPT [F(1,37) = 8.86, P = 0.005], which is a measure associated strongly with prefrontal function (Miranda et al., in press). Mean cognitive domain scores are also presented in Fig. 2. M/STBI differed from the controls on almost all measures. The trend in means for the individual tests indicate that the controls have the highest performance, followed by MTBI, with M/STI showing the most severe and global impairment. The MTBI group did not differ significantly from controls in any domain scores when compared to the controls (P > 0.050). The M/STBI group performed significantly worse than both the controls and MTBI in the executive [M/STBI versus Controls: F(1,34) = 18.08, P < 0.001; versus MTBI: F(1,35) = 6.39, P = 0.016] and memory domains [M/STBI versus Controls: F(1,34) = 7.83, P = 0.008; versus MTBI: F(1,35) = 6.79, P = 0.013]. M/STBI performed considerably worse than the controls on the attention domain [F(1,34) = 9.14, P = 0.005] but did not differ from MTBI [F(1,34) = 3.194, P = 0.083].
Mean domain scores (normalized z-scores) for the MTBI (white) and M/STBI (dark gray). Note that the light gray box around zero indicates 1 SEM around the control mean.
Fractional anisotropy: symmetry
Although there was a main effect of symmetry across the CST (P = 0.038) and ACR (P = 0.043) with FA in the right hemisphere being higher than the left there were no differential symmetry effects across the three groups. As such, the remaining analyses are presented collapsed across hemispheres.
Fractional anisotropy
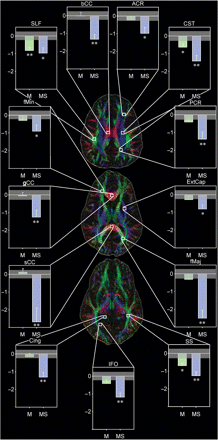
Overall, there was a main effect of group membership on whole brain FA [F(2,54) = 4.52, P = 0.015] relative to controls. Post-hoc testing demonstrated that the M/STBI had reduced FA relative to both controls [F(1,34) = 6.47, P = 0.016] and MTBI [F(1,36) = 5.36, P = 0.027]. In the ROI analyses, with the exception of fMin [F(2,54) = 2.71, P = 0.076] and ExCap [F(2,54) = 3.06, P = 0.055], significant main effects of group membership were observed for all other ROIs. As the primary contrast of interest was comparison between controls and both TBI subject groups, z-scores were calculated with the controls set to zero. As can be seen in Fig. 3, MTBI showed reduced FA along the CST [F(1,37) = 4.99, P = 0.032], SLF [F(1,37) = 9.08, P = 0.005] and SS [F(1,37) = 6.84, P = 0.013]. FA values for all ROIs in the M/STBI group were decreased compared to controls (P < 0.05; see Table 3).
Mean normalized FA for each ROI for the MTBI (green) and M/STBI (blue). Single asterisks indicate P < 0.05, double asterisks indicate P < 0.001 for the control group compared to either MTBI (M) or M/STBI (MS). Note that the light gray box around zero indicates 1SEM around the control mean. Abbreviations: ACR, PCR: anterior and posterior corona radiata, CST: corticospinal tracts, Cing: cingulum, fMin, fMaj: forceps minor and major, bCC, sCC, gCC: body, genu and splenium of the corpus callosum, IFO: inferior fronto-occipital fasciculus, SLF: superior longitudinal fasciculus, SS: sagittal stratum, ExtCap: external capsule. Note that white boxes on the colour-coded direction map indicate the target fibres but do not indicate the entire region of interest.
Mean FA for all three groups for each ROI.
| Region of interest (ROI) . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Whole brain | 0.35 | 0.002 | 0.35 | 0.001 | 0.34 | 0.003 | 0.015* | 0.375 | 0.016* | 0.027* |
| Cingulum (Cing) | 0.38 | 0.005 | 0.38 | 0.004 | 0.35 | 0.005 | <0.001** | 0.599 | 0.001** | 0.001** |
| External capsule (ExCap) | 0.36 | 0.003 | 0.36 | 0.003 | 0.35 | 0.004 | 0.055 | 0.370 | 0.027* | 0.106 |
| Cortico-spinal tract (CST) | 0.48 | 0.004 | 0.46 | 0.003 | 0.45 | 0.006 | 0.001** | 0.032* | 0.001** | 0.028* |
| Inf. frontal-occipital (IFO) | 0.40 | 0.005 | 0.39 | 0.006 | 0.37 | 0.005 | 0.007** | 0.256 | 0.001** | 0.042* |
| Anterior corona radiata (ACR) | 0.35 | 0.004 | 0.34 | 0.003 | 0.33 | 0.006 | 0.033* | 0.475 | 0.023* | 0.060 |
| Posterior corona radiata (PCR) | 0.40 | 0.003 | 0.39 | 0.003 | 0.38 | 0.006 | 0.006** | 0.222 | 0.005** | 0.035* |
| Forceps major (fMaj) | 0.39 | 0.006 | 0.38 | 0.005 | 0.37 | 0.009 | 0.076 | 0.367 | 0.044* | 0.145 |
| Forceps minor (fMin) | 0.50 | 0.007 | 0.49 | 0.008 | 0.42 | 0.014 | <0.001** | 0.393 | <0.001** | <0.001** |
| Sagittal stratum (SS) | 0.47 | 0.008 | 0.45 | 0.004 | 0.43 | 0.007 | <0.001** | 0.013* | <0.001** | 0.028* |
| Sup. longitudinal (SLF) | 0.41 | 0.005 | 0.39 | 0.003 | 0.39 | 0.006 | 0.009** | 0.005** | 0.015* | 0.655 |
| Corpus callosum | ||||||||||
| Body (bCC) | 0.42 | 0.012 | 0.42 | 0.010 | 0.36 | 0.013 | <0.001** | 0.967 | 0.001** | <0.001** |
| Genu (gCC) | 0.50 | 0.009 | 0.50 | 0.007 | 0.45 | 0.015 | 0.003 | 0.854 | 0.009** | 0.006** |
| Splenium (sCC) | 0.56 | 0.006 | 0.57 | 0.005 | 0.49 | 0.020 | <0.001** | 0.054 | 0.002** | <0.001** |
| Region of interest (ROI) . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Whole brain | 0.35 | 0.002 | 0.35 | 0.001 | 0.34 | 0.003 | 0.015* | 0.375 | 0.016* | 0.027* |
| Cingulum (Cing) | 0.38 | 0.005 | 0.38 | 0.004 | 0.35 | 0.005 | <0.001** | 0.599 | 0.001** | 0.001** |
| External capsule (ExCap) | 0.36 | 0.003 | 0.36 | 0.003 | 0.35 | 0.004 | 0.055 | 0.370 | 0.027* | 0.106 |
| Cortico-spinal tract (CST) | 0.48 | 0.004 | 0.46 | 0.003 | 0.45 | 0.006 | 0.001** | 0.032* | 0.001** | 0.028* |
| Inf. frontal-occipital (IFO) | 0.40 | 0.005 | 0.39 | 0.006 | 0.37 | 0.005 | 0.007** | 0.256 | 0.001** | 0.042* |
| Anterior corona radiata (ACR) | 0.35 | 0.004 | 0.34 | 0.003 | 0.33 | 0.006 | 0.033* | 0.475 | 0.023* | 0.060 |
| Posterior corona radiata (PCR) | 0.40 | 0.003 | 0.39 | 0.003 | 0.38 | 0.006 | 0.006** | 0.222 | 0.005** | 0.035* |
| Forceps major (fMaj) | 0.39 | 0.006 | 0.38 | 0.005 | 0.37 | 0.009 | 0.076 | 0.367 | 0.044* | 0.145 |
| Forceps minor (fMin) | 0.50 | 0.007 | 0.49 | 0.008 | 0.42 | 0.014 | <0.001** | 0.393 | <0.001** | <0.001** |
| Sagittal stratum (SS) | 0.47 | 0.008 | 0.45 | 0.004 | 0.43 | 0.007 | <0.001** | 0.013* | <0.001** | 0.028* |
| Sup. longitudinal (SLF) | 0.41 | 0.005 | 0.39 | 0.003 | 0.39 | 0.006 | 0.009** | 0.005** | 0.015* | 0.655 |
| Corpus callosum | ||||||||||
| Body (bCC) | 0.42 | 0.012 | 0.42 | 0.010 | 0.36 | 0.013 | <0.001** | 0.967 | 0.001** | <0.001** |
| Genu (gCC) | 0.50 | 0.009 | 0.50 | 0.007 | 0.45 | 0.015 | 0.003 | 0.854 | 0.009** | 0.006** |
| Splenium (sCC) | 0.56 | 0.006 | 0.57 | 0.005 | 0.49 | 0.020 | <0.001** | 0.054 | 0.002** | <0.001** |
Standard errors of the mean (SEM) are presented in parentheses. P-values are listed under each contrast and asterisks indicate significant differences between groups (*P < 0.05; **P < 0.01). Inf = Inferior, Sup = Superior.
Mean FA for all three groups for each ROI.
| Region of interest (ROI) . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Whole brain | 0.35 | 0.002 | 0.35 | 0.001 | 0.34 | 0.003 | 0.015* | 0.375 | 0.016* | 0.027* |
| Cingulum (Cing) | 0.38 | 0.005 | 0.38 | 0.004 | 0.35 | 0.005 | <0.001** | 0.599 | 0.001** | 0.001** |
| External capsule (ExCap) | 0.36 | 0.003 | 0.36 | 0.003 | 0.35 | 0.004 | 0.055 | 0.370 | 0.027* | 0.106 |
| Cortico-spinal tract (CST) | 0.48 | 0.004 | 0.46 | 0.003 | 0.45 | 0.006 | 0.001** | 0.032* | 0.001** | 0.028* |
| Inf. frontal-occipital (IFO) | 0.40 | 0.005 | 0.39 | 0.006 | 0.37 | 0.005 | 0.007** | 0.256 | 0.001** | 0.042* |
| Anterior corona radiata (ACR) | 0.35 | 0.004 | 0.34 | 0.003 | 0.33 | 0.006 | 0.033* | 0.475 | 0.023* | 0.060 |
| Posterior corona radiata (PCR) | 0.40 | 0.003 | 0.39 | 0.003 | 0.38 | 0.006 | 0.006** | 0.222 | 0.005** | 0.035* |
| Forceps major (fMaj) | 0.39 | 0.006 | 0.38 | 0.005 | 0.37 | 0.009 | 0.076 | 0.367 | 0.044* | 0.145 |
| Forceps minor (fMin) | 0.50 | 0.007 | 0.49 | 0.008 | 0.42 | 0.014 | <0.001** | 0.393 | <0.001** | <0.001** |
| Sagittal stratum (SS) | 0.47 | 0.008 | 0.45 | 0.004 | 0.43 | 0.007 | <0.001** | 0.013* | <0.001** | 0.028* |
| Sup. longitudinal (SLF) | 0.41 | 0.005 | 0.39 | 0.003 | 0.39 | 0.006 | 0.009** | 0.005** | 0.015* | 0.655 |
| Corpus callosum | ||||||||||
| Body (bCC) | 0.42 | 0.012 | 0.42 | 0.010 | 0.36 | 0.013 | <0.001** | 0.967 | 0.001** | <0.001** |
| Genu (gCC) | 0.50 | 0.009 | 0.50 | 0.007 | 0.45 | 0.015 | 0.003 | 0.854 | 0.009** | 0.006** |
| Splenium (sCC) | 0.56 | 0.006 | 0.57 | 0.005 | 0.49 | 0.020 | <0.001** | 0.054 | 0.002** | <0.001** |
| Region of interest (ROI) . | . | . | . | . | . | . | All groups . | Control . | Control . | MTBI . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Control . | MTBI . | M/STBI . | . | vs. . | vs. . | vs. . | |||
| . | M . | SEM . | M . | SEM . | M . | SEM . | . | MTBI . | M/STBI . | M/STBI . |
| Whole brain | 0.35 | 0.002 | 0.35 | 0.001 | 0.34 | 0.003 | 0.015* | 0.375 | 0.016* | 0.027* |
| Cingulum (Cing) | 0.38 | 0.005 | 0.38 | 0.004 | 0.35 | 0.005 | <0.001** | 0.599 | 0.001** | 0.001** |
| External capsule (ExCap) | 0.36 | 0.003 | 0.36 | 0.003 | 0.35 | 0.004 | 0.055 | 0.370 | 0.027* | 0.106 |
| Cortico-spinal tract (CST) | 0.48 | 0.004 | 0.46 | 0.003 | 0.45 | 0.006 | 0.001** | 0.032* | 0.001** | 0.028* |
| Inf. frontal-occipital (IFO) | 0.40 | 0.005 | 0.39 | 0.006 | 0.37 | 0.005 | 0.007** | 0.256 | 0.001** | 0.042* |
| Anterior corona radiata (ACR) | 0.35 | 0.004 | 0.34 | 0.003 | 0.33 | 0.006 | 0.033* | 0.475 | 0.023* | 0.060 |
| Posterior corona radiata (PCR) | 0.40 | 0.003 | 0.39 | 0.003 | 0.38 | 0.006 | 0.006** | 0.222 | 0.005** | 0.035* |
| Forceps major (fMaj) | 0.39 | 0.006 | 0.38 | 0.005 | 0.37 | 0.009 | 0.076 | 0.367 | 0.044* | 0.145 |
| Forceps minor (fMin) | 0.50 | 0.007 | 0.49 | 0.008 | 0.42 | 0.014 | <0.001** | 0.393 | <0.001** | <0.001** |
| Sagittal stratum (SS) | 0.47 | 0.008 | 0.45 | 0.004 | 0.43 | 0.007 | <0.001** | 0.013* | <0.001** | 0.028* |
| Sup. longitudinal (SLF) | 0.41 | 0.005 | 0.39 | 0.003 | 0.39 | 0.006 | 0.009** | 0.005** | 0.015* | 0.655 |
| Corpus callosum | ||||||||||
| Body (bCC) | 0.42 | 0.012 | 0.42 | 0.010 | 0.36 | 0.013 | <0.001** | 0.967 | 0.001** | <0.001** |
| Genu (gCC) | 0.50 | 0.009 | 0.50 | 0.007 | 0.45 | 0.015 | 0.003 | 0.854 | 0.009** | 0.006** |
| Splenium (sCC) | 0.56 | 0.006 | 0.57 | 0.005 | 0.49 | 0.020 | <0.001** | 0.054 | 0.002** | <0.001** |
Standard errors of the mean (SEM) are presented in parentheses. P-values are listed under each contrast and asterisks indicate significant differences between groups (*P < 0.05; **P < 0.01). Inf = Inferior, Sup = Superior.
Comparisons between MTBI and M/STBI showed that the M/STBI had reduced FA in the corpus callosum [gCC: F(1,36) = 8.42, P = 0.006; bCC: F(1,36) = 15.63, P < 0.001; sCC: F(1,36) = 18.76, P < 0.001], Cing [F(1,36) = 12.84, P < 0.001], fMaj [F(1,36) = 18.34, P < 0.001], CST [F(1,36) = 5.27, P = 0.028], IFO [F(1,36) = 4.48, P = 0.042], PCR [F(1,36) = 4.80, P = 0.035] and in the SS [F(1,36) = 5.23, P = 0.028].
Axial and radial diffusivity
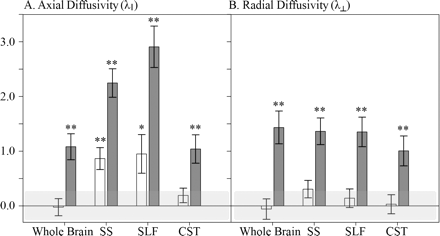
To investigate potential mechanisms for changes in white matter integrity in chronic TBI, both axial and radial diffusivity were extracted from a whole brain white matter mask as well as from the ROIs which showed sensitivity to all severities of head injury (SS, SLF, CST). As with the earlier FA analysis, these values were transformed to z-scores based upon the control group mean. There was an overall main effect of group for both axial (λ∥) and radial (λ⊥) diffusivity in the whole brain (P < 0.004 for all comparisons). However, these results were primarily driven by increased diffusivity in M/STBI. As can be seen in Fig. 4, M/STBI, relative to controls, showed increased λ∥ and λ⊥ in all regions [whole brain λ∥: F(1,34) = 10.40, P = 0.003; whole brain λ⊥: F(1,34) = 14.30, P = 0.001; SS λ∥: F(1,34) = 40.96, P < 0.001; SS λ⊥: F(1,34) = 14.12, P ≤ 0.001; SLF λ∥: F(1,34) = 43.56, P < 0.001; SLF λ⊥: F(1,34) = 14.29, P = 0.001; CST λ∥: F(1,34) = 8.83, P = 0.005; CST λ⊥: F(1,34) = 7.79, P = 0.009). The MTBI showed increased λ∥ relative to controls in the SS [F(1,34) = 4.78, P = 0.008] and SLF [F(1,34) = 4.78, P = 0.035] but not in the whole brain or CST. The MTBI showed no significant increases in radial diffusivity in any region.
Mean normalized axial (λ∥) and radial (λ⊥) diffusivity for the MTBI (white bars) and M/STBI (dark gray bars). Single asterisks indicate P < 0.05, double asterisks indicate P < 0.01 either the MTBI or M/STBI was compared to controls. Note that the light gray box around zero indicates 1 SEM around the control mean. Abbreviations: SS: sagittal stratum, SLF: superior longitudinal fasciculus, CST: corticospinal tract.
White matter load
The White Matter Load was the total number of regions with FA 1SD below the control mean (please see the ‘Methods’ section for a complete description).
Each control, on average, had reduced FA in 3.6 out of 13 ROIs (M = 3.61, SEM = 0.55). The load (or number of regions with reduced FA) increased as the severity of head injury increased. The MTBI had an average load of about six ROIs classified as reduced (M = 5.9, SEM = 0.72), whereas the M/STBI showed reduced FA in 8 out of 14 ROIs (M = 9.06, SEM = 0.89). The controls had significantly lower load than the MTBI [F(1,37) = 6.16, P = 0.018] and M/STBI [F(1,34) = 27.69, P < 0.001]. Finally, the M/STBI did have a larger load than the MTBI [F(1,36) = 7.74, P = 0.009].
Relationship between white matter integrity and neuropsychological function
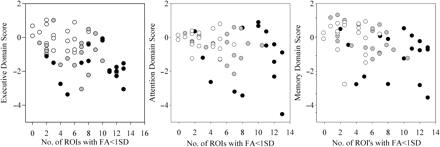
To examine the relationship between both white matter integrity and white matter load with neuropsychological function we conducted a series of correlations for the entire group of TBI subjects. As is depicted in Fig. 5, there was a significant correlation between the executive and memory domains with the composite white matter load [executive: r(54) = −0.41, P = 0.002; attention: r(54) = −0.26, P = 0.058; and memory: r(54) = −0.40, P = 0.000]. Also depicted in Fig. 5 is the overlapping distribution of load and neuropsychological function amongst all the three groups.
Plots indicating the relationship between each normalized domain score (left: executive, middle: attention, right: memory) as a function of the number of ROIs with FA <1 SD from the control mean. Controls are indicated by white dots, MTBI (gray dots), and M/STBI (black dots).
In terms of correlations between FA in specific ROIs with these domain scores there were significant correlations between executive function and bCC (r = −0.368, P = 0.006), sCC (r = −0.348, P = 0.009), CST (r = −0.390, P = 0.003), ExCap (r = −0.265, P = 0.050), fMaj (r = 0.563, P < 0.001), fMin (r = .281, P = 0.038), IFO (r = −0.346, P = 0.009), ACR (r = −0.383, P = 0.004), PCR (r = −0.407, P = 0.002), SLF (r = −0.305, P = 0.023), SS (r = −0.495, P < 0.001) and Cing (r = −0.277, P = 0.041). Only the fMaj (r = −0.310, P = 0.022) and PCR x(r = 0.271, P = 0.046) correlated with the attention domain. The bCC (r = −0.030, P = 0.026), sCC (r = −0.328, P = 0.015), fMaj (r = −0.432, P = 0.001), fMin (r = −0.269, P = 0.047), IFO (r = −0.314, P = 0.019), PCR (r = −0.330, P = 0.014), SS (r = −0.316, P = 0.019) and Cing (r = −0.311, P = 0.021) all corrected with the memory domain. Although we do not have the statistical power to examine these correlations within each subject group the trend is such that these patterns appear consistent within both the MTBI and M/STBI.
Conclusions
In this study, the moderate to severe TBI subjects demonstrated reduced white matter integrity, relative to controls, in all 13 regions of interest. The MTBI showed reduced white matter integrity in the superior longitudinal fasciculus, sagittal stratum and corticospinal tract (Fig. 3). The total number of regions with reduced white matter integrity (White Matter Load) was greatest in the moderate to severe group, and least in the controls (Fig. 5). The MTBI subjects fell between these two groups, being significantly different than controls (Fig. 5).
In M/STBI increased radial and axial diffusivity is observed both in the whole brain and in specific regions of interest (Fig. 4). This finding likely reflects damage to both myelin and to axons. In MTBI, relatively normal radial diffusivity and increased axial diffusivity suggests that irreversible damage to myelin is less common in MTBI as compared to M/STBI but that axonal damage is present even 6 months following injury. It could be that the injury in the MTBI group had less of an effect on myelin due to trauma acutely or that the less severe injury allowed some degree of myelin damage that was reversible. Only three ROIs were assessed in this analysis, and further research is warranted.
M/STBI differed from the controls on almost all measures of cognitive function, being more impaired in each domain than controls or the MTBI group. Although there was a trend in executive and attention function to be more impaired, the MTBI group did not differ significantly from controls in any domain scores.
The moderate to severe TBI subjects showed reduced function across all domains. There was a modest negative correlation between FA in individual regions of interest with cognitive function. However, the relationship between overall white matter load was more strongly related to the domains of executive and memory function than FA in individual ROIs. This suggests that a global measure such as white matter load is a useful index, as it appears to relate more clearly to declines in cognitive functions which rely on widespread cortical and subcortical networks.
While it is not surprising that moderate and severe injuries tend to show evidence of white matter changes and cognitive impairment, acquiring data on all severities in one study allows for the milder injuries to be assessed in the context of a spectrum of injury, from the healthy controls to the more severe injuries. Importantly, the controls were fairly well matched to the TBI groups in terms of age and years of education. None of the subjects were actively involved in litigation. These findings are consistent with TBI existing on a spectrum of neuropathologic severity and resulting disability, placing subjects with a history of mild TBI between controls and more severe injuries. In addition to demonstrating that TBI, regardless of severity, results in chronic changes to the white matter microstructure, the present findings suggest that injury severity may differentially impact axons and myelin. This finding begins to address the issue of mechanism in the differential effects of mild versus more severe TBI on white matter.
In terms of white matter changes, there is some overlap between amount of pathology and the different clinical classifications of TBI severity. This is important in understanding variation in recovery. Certain injuries classified acutely as mild based on acute TBI variables such as loss of consciousness may actually be closer to moderate in the degree of pathology. Conversely, certain individuals with moderate or severe TBI may show more intact white matter than expected based on accepted means of clinical classification of injury severity. The data presented here demonstrate that DTI allows for a more sensitive delineation of severity and mechanism of white matter pathology, and may help to explain apparent discrepancies between clinically diagnosed injury severity and cognitive outcomes across the spectrum of TBI.
Acknowledgements
This work was supported by National Institute of Health [K23 MH068787]. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of either the National Institute of Mental Health or the University of Illinois at Chicago.
References
Abbreviations:
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- TBI
traumatic brain injury
- MTBI
mild traumatic brain injury
- M/STBI
moderate to severe traumatic brain injury
- DAI
diffuse axonal injury
- λ∥
axial diffusivity
- λ⊥
radial diffusivity
- λ
eigenvalues
- ACR
anterior corona radiata
- PCR
posterior corona radiata
- CST
corticospinal tracts
- Cing
cingulum fibres
- fMin
forceps minor
- fMaj
forceps major
- bCC
body of the corpus callosum
- gCC
genu of the corpus callosum
- sCC
splenium of the corpus callosum
- IFO
inferior fronto-occipital fasciculus
- SLF
superior longitudinal fasciculus
- ExCap
external capsule
- SS
sagittal stratum