-
PDF
- Split View
-
Views
-
Cite
Cite
Anne Ducros, Monique Boukobza, Raphaël Porcher, Mariana Sarov, Dominique Valade, Marie-Germaine Bousser, The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients, Brain, Volume 130, Issue 12, December 2007, Pages 3091–3101, https://doi.org/10.1093/brain/awm256
Close - Share Icon Share
Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) is characterized by the association of severe headaches with or without additional neurological symptoms and a ‘string and beads’ appearance on cerebral arteries, which resolves spontaneously in 1–3 months. We present the clinical, neuroimaging and outcome data of 67 consecutive patients prospectively diagnosed over 3 years in our institution with an angiographically confirmed RCVS. There were 43 females and 24 males with a mean age of 42 years (19–70). RCVS was spontaneous in 37% of patients and secondary in the 63% others, to postpartum in 5 and to exposure to various vasoactive substances in 37, mainly cannabis, selective serotonin-recapture inhibitors and nasal decongestants. The main pattern of presentation (94% of patients) was multiple thunderclap headaches recurring over a mean period of 1 week. In 51 patients (76%), headaches resumed the clinical presentation. Various complications were observed, with different time courses. Cortical subarachnoid haemorrhage (cSAH) (22%), intracerebral haemorrhage (6%), seizures (3%) and reversible posterior leukoencephalopathy (9%) were early complications, occurring mainly within the first week. Ischaemic events, including TIAs (16%) and cerebral infarction (4%), occurred significantly later than haemorrhagic events, mainly during the second week. Significant sex differences were observed: women were older, had more frequent single-drug exposure and a higher rate of stroke and cSAH. Sixty-one patients were treated by nimodipine: 36% had recurrent headaches, 7% TIAs and one multiple infarcts. The different time courses of thunderclap headaches, vasoconstriction and strokes suggest that the responsible vasospastic disorder starts distally and progresses towards medium sized and large arteries. No relapse was observed during the 16 ± 12.4 months of follow-up. Our data suggest that RCVS is more frequent than previously thought, is more often secondary particularly to vasoactive substances, and should be considered in patients with recurrent thunderclap headaches, cSAH or cryptogenic strokes with severe headaches.
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) is a unifying term (Call et al., 1988; Calabrese et al., 2007) used to describe a group of disorders sharing cardinal angiographic and clinical features, namely, a reversible segmental and multifocal vasoconstriction of cerebral arteries, and severe headaches with or without focal neurological deficits or seizures. Patients with these features were previously reported under many appellations (Table 1). The most common clinical feature of RCVS is a severe acute headache, often qualifying as thunderclap headache (Dodick et al., 1999, 2003; Singhal and Bernstein, 2005), a sudden excruciating headache that peaks in less than 1 min, like a ‘clap of thunder’, mimicking that of a ruptured aneurysm (Day and Raskin, 1986; Dodick, 2002). The major complication of RCVS is stroke, -ischaemic or haemorrhagic-, eventually leading to permanent sequelae and even death (Singhal et al., 2002; Calabrese et al., 2007; Williams et al., 2007). In the absence of large prospective series, the exact incidence of stroke is unknown, but has been estimated to range from 7% (Chen et al., 2006a) up to 54% (Calabrese et al., 2007). Non-aneurismal cortical subarachnoid haemorrhage (cSAH) has been less-frequently reported (Singhal, 2004b; Sengoku et al., 2005). Although the pathophysiology of RCVS remains unknown, the prevailing hypothesis involves a transient disturbance in the control of cerebral vascular tone (Schwedt et al., 2006). RCVS has a female preponderance (Chen et al., 2006a; Calabrese et al., 2007). It may occur spontaneously or be provoked by various precipitating factors, the most common being postpartum (Singhal and Bernstein, 2005) and exposure to various vasoactive substances (Table 2) (Bousser et al., 2001; Calabrese et al., 2007). In early case reports, conventional cerebral angiography was crucial to the diagnosis, typically showing diffuse, multifocal, segmental narrowings involving large and medium-sized arteries in the anterior and posterior circulations, with occasional dilated segments, like ‘strings and beads’ or ‘sausage strings’ (Bousser et al., 2001). Angiography had to be repeated after a few weeks or months to demonstrate the normalization of cerebral arteries. More recently, non-invasive investigations such as magnetic resonance angiography (MRA) and transcranial Doppler (TCD) have been largely used for the assessment and follow-up of vasoconstriction (Bogousslavsky et al., 1989; Gomez et al., 1991; Zunker et al., 2002; Krasnianski et al., 2004). The growing interest in RCVS is reflected by the recent publication of numerous case-reports, retrospective small series and reviews (Calabrese et al., 2007). However, only two studies including more than 10 patients have so far been published: a retrospective series of 16 patients of whom 10 had a repeat angiography to assess reversibility of vasoconstriction (Hajj-Ali et al., 2002) and a large prospective series of 56 patients with recurrent thunderclap headaches of whom only 22 had a proven vasoconstriction (Chen et al., 2006a).
Appellations used to describe RCVS in the literature
| Appellations . | References . |
|---|---|
| Isolated benign cerebral vasculitis | (Snyder and McClelland, 1978) |
| Benign acute cerebral angiopathy | (Michel et al., 1985) |
| Call-Fleming syndrome | (Call et al., 1988) |
| CNS pseudovasculitis | (Razavi et al., 1999) |
| Benign angiopathy of the central nervous system | (Bousser et al., 2001; Hajj-Ali et al., 2002) |
| Postpartum angiopathy | (Bogousslavsky et al., 1989) |
| Migrainous vasospasm | (Serdaru et al., 1984; Solomon et al., 1990) |
| Migraine angiitis | (Jackson et al., 1993) |
| Thunderclap headache with reversible vasospam | (Dodick et al., 1999) |
| Idiopathic thunderclap headache | (Liao et al., 2003) |
| Primary thunderclap headache | (Chen et al., 2006b) |
| Drug-induced cerebral vasculopathy | (Mateo et al., 2005) |
| Drug-induced cerebral angiopathy | (Martin et al., 1995) |
| Appellations . | References . |
|---|---|
| Isolated benign cerebral vasculitis | (Snyder and McClelland, 1978) |
| Benign acute cerebral angiopathy | (Michel et al., 1985) |
| Call-Fleming syndrome | (Call et al., 1988) |
| CNS pseudovasculitis | (Razavi et al., 1999) |
| Benign angiopathy of the central nervous system | (Bousser et al., 2001; Hajj-Ali et al., 2002) |
| Postpartum angiopathy | (Bogousslavsky et al., 1989) |
| Migrainous vasospasm | (Serdaru et al., 1984; Solomon et al., 1990) |
| Migraine angiitis | (Jackson et al., 1993) |
| Thunderclap headache with reversible vasospam | (Dodick et al., 1999) |
| Idiopathic thunderclap headache | (Liao et al., 2003) |
| Primary thunderclap headache | (Chen et al., 2006b) |
| Drug-induced cerebral vasculopathy | (Mateo et al., 2005) |
| Drug-induced cerebral angiopathy | (Martin et al., 1995) |
Appellations used to describe RCVS in the literature
| Appellations . | References . |
|---|---|
| Isolated benign cerebral vasculitis | (Snyder and McClelland, 1978) |
| Benign acute cerebral angiopathy | (Michel et al., 1985) |
| Call-Fleming syndrome | (Call et al., 1988) |
| CNS pseudovasculitis | (Razavi et al., 1999) |
| Benign angiopathy of the central nervous system | (Bousser et al., 2001; Hajj-Ali et al., 2002) |
| Postpartum angiopathy | (Bogousslavsky et al., 1989) |
| Migrainous vasospasm | (Serdaru et al., 1984; Solomon et al., 1990) |
| Migraine angiitis | (Jackson et al., 1993) |
| Thunderclap headache with reversible vasospam | (Dodick et al., 1999) |
| Idiopathic thunderclap headache | (Liao et al., 2003) |
| Primary thunderclap headache | (Chen et al., 2006b) |
| Drug-induced cerebral vasculopathy | (Mateo et al., 2005) |
| Drug-induced cerebral angiopathy | (Martin et al., 1995) |
| Appellations . | References . |
|---|---|
| Isolated benign cerebral vasculitis | (Snyder and McClelland, 1978) |
| Benign acute cerebral angiopathy | (Michel et al., 1985) |
| Call-Fleming syndrome | (Call et al., 1988) |
| CNS pseudovasculitis | (Razavi et al., 1999) |
| Benign angiopathy of the central nervous system | (Bousser et al., 2001; Hajj-Ali et al., 2002) |
| Postpartum angiopathy | (Bogousslavsky et al., 1989) |
| Migrainous vasospasm | (Serdaru et al., 1984; Solomon et al., 1990) |
| Migraine angiitis | (Jackson et al., 1993) |
| Thunderclap headache with reversible vasospam | (Dodick et al., 1999) |
| Idiopathic thunderclap headache | (Liao et al., 2003) |
| Primary thunderclap headache | (Chen et al., 2006b) |
| Drug-induced cerebral vasculopathy | (Mateo et al., 2005) |
| Drug-induced cerebral angiopathy | (Martin et al., 1995) |
Precipitating factors and conditions associated with RCVS
| Precipitating factors . |
|---|
| Postpartum |
| Postpartum alonea, postpartum + exposure to drugsa, eclampsia, preeclampsia |
| Exposure to drugs, alcohol, medications and blood products |
| Cannabisa, cocainea, ecstasy, amphetamine derivates, lysergic acid diethylamine |
| Binge alcohol drinkingb |
| Selective serotonin reuptake inhibitorsa |
| Nasal decongestantsa, phenylpropanolaminea, pseudoephedrinea, ephedrinea |
| Ergotamine tartrate, methergine, bromocriptinea, lisuride, sumatriptan, isometheptine |
| Tacrolimus (FK-506), cyclophosphamide, erythropoietin, intravenous immune globulins, red blood cell |
| transfusion, interferon alphab |
| Nicotine patchesa |
| Catecholamine-secreting tumour |
| Pheochromocytoma, bronchial carcinoid tumour |
| Miscellaneous |
| Hypercalcemia, porphyria, head trauma, spinal subdural hematoma, postcarotid endarterectomy, neurosurgical procedures |
| Precipitating factors . |
|---|
| Postpartum |
| Postpartum alonea, postpartum + exposure to drugsa, eclampsia, preeclampsia |
| Exposure to drugs, alcohol, medications and blood products |
| Cannabisa, cocainea, ecstasy, amphetamine derivates, lysergic acid diethylamine |
| Binge alcohol drinkingb |
| Selective serotonin reuptake inhibitorsa |
| Nasal decongestantsa, phenylpropanolaminea, pseudoephedrinea, ephedrinea |
| Ergotamine tartrate, methergine, bromocriptinea, lisuride, sumatriptan, isometheptine |
| Tacrolimus (FK-506), cyclophosphamide, erythropoietin, intravenous immune globulins, red blood cell |
| transfusion, interferon alphab |
| Nicotine patchesa |
| Catecholamine-secreting tumour |
| Pheochromocytoma, bronchial carcinoid tumour |
| Miscellaneous |
| Hypercalcemia, porphyria, head trauma, spinal subdural hematoma, postcarotid endarterectomy, neurosurgical procedures |
| Associated conditions . |
|---|
| Large artery lesions |
| Cervical artery dissectiona, Unruptured saccular cerebral aneurysma, cerebral arterial dysplasiaa |
| Associated conditions . |
|---|
| Large artery lesions |
| Cervical artery dissectiona, Unruptured saccular cerebral aneurysma, cerebral arterial dysplasiaa |
Precipitating factors and conditions associated with RCVS
| Precipitating factors . |
|---|
| Postpartum |
| Postpartum alonea, postpartum + exposure to drugsa, eclampsia, preeclampsia |
| Exposure to drugs, alcohol, medications and blood products |
| Cannabisa, cocainea, ecstasy, amphetamine derivates, lysergic acid diethylamine |
| Binge alcohol drinkingb |
| Selective serotonin reuptake inhibitorsa |
| Nasal decongestantsa, phenylpropanolaminea, pseudoephedrinea, ephedrinea |
| Ergotamine tartrate, methergine, bromocriptinea, lisuride, sumatriptan, isometheptine |
| Tacrolimus (FK-506), cyclophosphamide, erythropoietin, intravenous immune globulins, red blood cell |
| transfusion, interferon alphab |
| Nicotine patchesa |
| Catecholamine-secreting tumour |
| Pheochromocytoma, bronchial carcinoid tumour |
| Miscellaneous |
| Hypercalcemia, porphyria, head trauma, spinal subdural hematoma, postcarotid endarterectomy, neurosurgical procedures |
| Precipitating factors . |
|---|
| Postpartum |
| Postpartum alonea, postpartum + exposure to drugsa, eclampsia, preeclampsia |
| Exposure to drugs, alcohol, medications and blood products |
| Cannabisa, cocainea, ecstasy, amphetamine derivates, lysergic acid diethylamine |
| Binge alcohol drinkingb |
| Selective serotonin reuptake inhibitorsa |
| Nasal decongestantsa, phenylpropanolaminea, pseudoephedrinea, ephedrinea |
| Ergotamine tartrate, methergine, bromocriptinea, lisuride, sumatriptan, isometheptine |
| Tacrolimus (FK-506), cyclophosphamide, erythropoietin, intravenous immune globulins, red blood cell |
| transfusion, interferon alphab |
| Nicotine patchesa |
| Catecholamine-secreting tumour |
| Pheochromocytoma, bronchial carcinoid tumour |
| Miscellaneous |
| Hypercalcemia, porphyria, head trauma, spinal subdural hematoma, postcarotid endarterectomy, neurosurgical procedures |
| Associated conditions . |
|---|
| Large artery lesions |
| Cervical artery dissectiona, Unruptured saccular cerebral aneurysma, cerebral arterial dysplasiaa |
| Associated conditions . |
|---|
| Large artery lesions |
| Cervical artery dissectiona, Unruptured saccular cerebral aneurysma, cerebral arterial dysplasiaa |
We present herein the first large prospective study of RCVS, based on the 67 consecutive patients with angiographically proven reversible vasoconstriction who were prospectively diagnosed in our institution in the last 3 years.
Patients and Methods
Patients who satisfied the three following diagnostic criteria for RCVS were included: (i) unusual, recent, severe headaches of progressive or sudden onset, with or without focal neurological deficit and/or seizures; (ii) cerebral vasoconstriction assessed by MRA or conventional angiography, with at least two narrowings per artery on two different cerebral arteries; (iii) disappearance of arterial abnormalities in less than 3 months. From January 2004 to January 2007, 67 consecutive patients satisfying these criteria were prospectively recruited in our institution, 51 from the emergency headache centre, and 16 from the neurology department. All patients but one were hospitalized in the neurology department for further investigations (see below). The remaining patient was managed as an outpatient.
All patients were directly interviewed by the same neurologist (A.D.) using a semi-structured questionnaire. They were informed that they were included in a descriptive study and agreed to participate. The following data were collected: sex, age, history of primary headache disorders, history of arterial hypertension, recent pregnancy and delivery, medications and drugs taken within 15 days before the onset of the disorder, and detailed characteristics of present headaches, blood-pressure, focal neurological deficits and seizures. Headache intensity was evaluated by using a verbal scale ranging from 0 (no pain) to 10 (the maximum pain that you can ever imagine). Headache reaching a maximum intensity above 7/10 in less than 1 min were qualified as ‘thunderclap headache’ and in 1–5 min as ‘acute severe headache’. Diagnosis of primary headache disorders was made according to the 2004 criteria of the International Headache Society (IHS, 2004). RCVS was qualified as secondary in the presence of a potential precipitating factor and spontaneous when none could be identified.
Neuroimaging investigations were performed within 48 h after arrival in our institution. They included cerebral computed-tomography (CT) scan (n = 65); cerebral 1.5 Tesla MRI (n = 67) with diffusion weighted-images (WI), fluid-attenuated inversion recovery (FLAIR) images, T1WI, and gradient-echo T2* WI; cervical T1 Fat Sat MRI (n = 20); two-dimensional time-of-flight cerebral MRA (n = 67); cervical and transcranial Doppler ultrasonography (TCD) (n = 64) and conventional transfemoral angiography (n = 45).
Biological analysis included blood analysis (n = 67) (blood-count, serum electrolytes, liver and renal function tests, C-reactive-protein level, erythrocyte sedimentation rate, rheumatoid factor, antinuclear and antineutrophilcytoplasmic antibody tests), cerebrospinal fluid (CSF) analysis (n = 62), urine analysis for cannabinoids, cocaine and amphetamine (n = 51) and for vanillylmandelic acid and 5-hydroxyindoleacetic acid levels in all patients with past or present elevated blood-pressure.
No standard treatment protocol was used. Nimodipine was recommended. Steroids were avoided. Symptomatic analgesic treatment was used in all patients.
A first clinical follow-up visit was performed in all patients within 3–6 weeks after discharge. In all 67 patients, the reversibility of arterial abnormalities was established within 1–3 months after disease onset, by MRA (n = 54), TCD (n = 30) or conventional angiography (n = 13). Further follow-up clinical visits were performed 6 and 12 months after disease onset, then every year. The mean duration of clinical follow-up was 16 ± 12.4 months (range 2–38). For the purpose of the study, all patients were assessed again by telephone interview in March 2007.
Statistics
Data are presented as mean and SD or as frequency and percent. Categorical variables were compared across groups using Fisher's exact tests or Chi-square tests for trend for ordered categories. Continuous variables were compared across groups using Student t-tests. Gender-related differences concerning vasoactive medications were studied comparing non-postpartum women and men. A sex ratio was estimated with its 95% confidence interval (95%CI). Mean delays from headache onset to haemorrhagic (intracerebral haemorrhage or cortical subarachnoid haemorrhage) or ischaemic (transient ischaemic attacks and cerebral infarction) events were compared using linear mixed models with random subject effects, to account for a possible correlation between delays observed on a same patient. Cumulative incidence curves of these events (event probability over time) were estimated using Kaplan–Meier method, as no competing event occurred (no deaths during the first month of follow-up). All tests were two-sided. All analyses were carried out using R 2.4.0 statistical software (The R Foundation for Statistical Computing, Vienna, Austria).
Results
The demographic data of the 67 patients are indicated in Table 3. There was a female preponderance (female : male ratio 1.8 : 1). The mean age was significantly higher in women than in men. Five patients (7%) had a history of hypertension, treated in 2. Other treated chronic medical conditions included depression (n = 12), anxiety disorders (n = 9), gastritis (n = 5), hypercholesterolemia (n = 3), hypothyroidism (n = 2) and epilepsy (n = 1). Half the patients reported a history of primary headache disorder, episodic tension-type headache being the most common (36%), followed by migraine without aura (19%), migraine with aura (3%), episodic cluster headache (1.5%) and chronic tension type headache (1.5%). In addition, five patients (7%) reported a history of previous sexual headaches.
Demographics and clinical data in 67 patients with RCVS, comparison of females and males
| . | Total (n = 67) . | Females (n = 43) . | Males (n = 24) . | P . |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 42.5 ± 11.8 | 46.9 ± 11.5 | 34.7 ± 7.7 | <0.0001 |
| Range | 19–70 | 22–70 | 19–56 | |
| Baseline characteristics | ||||
| History of migraine | 13 (19) | 10 (23) | 3 (12.5) | 0.35 |
| Current smoking | 28 (42) | 13 (30) | 15 (62.5) | 0.019 |
| History of hypertension | 5 (7) | 4 (9) | 1 (4) | 0.65 |
| Precipitating factor | ||||
| None | 25 (37) | 18 (42) | 7 (29) | 0.19a |
| Postpartum | 5 (8) | 5 (12) | – | |
| Vasoactive substance | 37 (55) | 20 (46) | 17 (71) | |
| Headaches | 67 (100) | 43 (100) | 24 (100) | 1.00 |
| Recurrent thunderclap | 63 (94) | 40 (93) | 23 (96) | |
| Single thunderclap | 3 (4.5) | 2 (5) | 1 (4) | |
| Recurrent severe peaking in > 1 min | 1 (1.5) | 1 (2) | 0 | |
| Focal neurological deficits | 14 (21) | 12 (28) | 2 (8) | 0.069 |
| Transient (minutes to 4 h) | 11 (16) | 10 (23) | 1 (4) | 0.082 |
| Lasting >24 h | 5 (7) | 4 (9) | 1 (4) | 0.65 |
| Seizures | 2 (3) | 1 (2) | 1 (4) | 1.00 |
| SBP ≥ 160 and/or DBP ≥ 90 mmHg | 22 (33) | 14 (33) | 8 (33) | 1.00 |
| Cervical artery dissection | 4 (6) | 3 (7) | 1 (4) | 1.00 |
| Abnormal brain MRI | 19 (28) | 18 (42) | 1 (4) | 0.0007 |
| At least one cSAH or stroke | 17 (25) | 16 (37) | 1 (4) | 0.003 |
| cSAH | 15 (22) | 15 (35) | 0 | |
| At least one stroke | 6 (9) | 5 (12) | 1 (4) | |
| Symptomatic intracerebral haemorrhage | 4 (6) | 3 (7) | 1 (4) | |
| Symptomatic cerebral infarct | 2 (3) | 2 (3) | 0 | |
| Silent infarct | 1 (1) | 1 (2) | 0 | |
| RPLS | 6 (9) | 6 (14) | 0 | 0.081 |
| . | Total (n = 67) . | Females (n = 43) . | Males (n = 24) . | P . |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 42.5 ± 11.8 | 46.9 ± 11.5 | 34.7 ± 7.7 | <0.0001 |
| Range | 19–70 | 22–70 | 19–56 | |
| Baseline characteristics | ||||
| History of migraine | 13 (19) | 10 (23) | 3 (12.5) | 0.35 |
| Current smoking | 28 (42) | 13 (30) | 15 (62.5) | 0.019 |
| History of hypertension | 5 (7) | 4 (9) | 1 (4) | 0.65 |
| Precipitating factor | ||||
| None | 25 (37) | 18 (42) | 7 (29) | 0.19a |
| Postpartum | 5 (8) | 5 (12) | – | |
| Vasoactive substance | 37 (55) | 20 (46) | 17 (71) | |
| Headaches | 67 (100) | 43 (100) | 24 (100) | 1.00 |
| Recurrent thunderclap | 63 (94) | 40 (93) | 23 (96) | |
| Single thunderclap | 3 (4.5) | 2 (5) | 1 (4) | |
| Recurrent severe peaking in > 1 min | 1 (1.5) | 1 (2) | 0 | |
| Focal neurological deficits | 14 (21) | 12 (28) | 2 (8) | 0.069 |
| Transient (minutes to 4 h) | 11 (16) | 10 (23) | 1 (4) | 0.082 |
| Lasting >24 h | 5 (7) | 4 (9) | 1 (4) | 0.65 |
| Seizures | 2 (3) | 1 (2) | 1 (4) | 1.00 |
| SBP ≥ 160 and/or DBP ≥ 90 mmHg | 22 (33) | 14 (33) | 8 (33) | 1.00 |
| Cervical artery dissection | 4 (6) | 3 (7) | 1 (4) | 1.00 |
| Abnormal brain MRI | 19 (28) | 18 (42) | 1 (4) | 0.0007 |
| At least one cSAH or stroke | 17 (25) | 16 (37) | 1 (4) | 0.003 |
| cSAH | 15 (22) | 15 (35) | 0 | |
| At least one stroke | 6 (9) | 5 (12) | 1 (4) | |
| Symptomatic intracerebral haemorrhage | 4 (6) | 3 (7) | 1 (4) | |
| Symptomatic cerebral infarct | 2 (3) | 2 (3) | 0 | |
| Silent infarct | 1 (1) | 1 (2) | 0 | |
| RPLS | 6 (9) | 6 (14) | 0 | 0.081 |
aSpontaneous RCVS versus vasoactive substance precipitated RCVS.
SBP = systolic blood pressure; DBP = diastolic blood pressure; cSAH = cortical subarachnoid haemorrhage; RPLS = reversible posterior leukoencephalopathy syndrome.
Demographics and clinical data in 67 patients with RCVS, comparison of females and males
| . | Total (n = 67) . | Females (n = 43) . | Males (n = 24) . | P . |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 42.5 ± 11.8 | 46.9 ± 11.5 | 34.7 ± 7.7 | <0.0001 |
| Range | 19–70 | 22–70 | 19–56 | |
| Baseline characteristics | ||||
| History of migraine | 13 (19) | 10 (23) | 3 (12.5) | 0.35 |
| Current smoking | 28 (42) | 13 (30) | 15 (62.5) | 0.019 |
| History of hypertension | 5 (7) | 4 (9) | 1 (4) | 0.65 |
| Precipitating factor | ||||
| None | 25 (37) | 18 (42) | 7 (29) | 0.19a |
| Postpartum | 5 (8) | 5 (12) | – | |
| Vasoactive substance | 37 (55) | 20 (46) | 17 (71) | |
| Headaches | 67 (100) | 43 (100) | 24 (100) | 1.00 |
| Recurrent thunderclap | 63 (94) | 40 (93) | 23 (96) | |
| Single thunderclap | 3 (4.5) | 2 (5) | 1 (4) | |
| Recurrent severe peaking in > 1 min | 1 (1.5) | 1 (2) | 0 | |
| Focal neurological deficits | 14 (21) | 12 (28) | 2 (8) | 0.069 |
| Transient (minutes to 4 h) | 11 (16) | 10 (23) | 1 (4) | 0.082 |
| Lasting >24 h | 5 (7) | 4 (9) | 1 (4) | 0.65 |
| Seizures | 2 (3) | 1 (2) | 1 (4) | 1.00 |
| SBP ≥ 160 and/or DBP ≥ 90 mmHg | 22 (33) | 14 (33) | 8 (33) | 1.00 |
| Cervical artery dissection | 4 (6) | 3 (7) | 1 (4) | 1.00 |
| Abnormal brain MRI | 19 (28) | 18 (42) | 1 (4) | 0.0007 |
| At least one cSAH or stroke | 17 (25) | 16 (37) | 1 (4) | 0.003 |
| cSAH | 15 (22) | 15 (35) | 0 | |
| At least one stroke | 6 (9) | 5 (12) | 1 (4) | |
| Symptomatic intracerebral haemorrhage | 4 (6) | 3 (7) | 1 (4) | |
| Symptomatic cerebral infarct | 2 (3) | 2 (3) | 0 | |
| Silent infarct | 1 (1) | 1 (2) | 0 | |
| RPLS | 6 (9) | 6 (14) | 0 | 0.081 |
| . | Total (n = 67) . | Females (n = 43) . | Males (n = 24) . | P . |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 42.5 ± 11.8 | 46.9 ± 11.5 | 34.7 ± 7.7 | <0.0001 |
| Range | 19–70 | 22–70 | 19–56 | |
| Baseline characteristics | ||||
| History of migraine | 13 (19) | 10 (23) | 3 (12.5) | 0.35 |
| Current smoking | 28 (42) | 13 (30) | 15 (62.5) | 0.019 |
| History of hypertension | 5 (7) | 4 (9) | 1 (4) | 0.65 |
| Precipitating factor | ||||
| None | 25 (37) | 18 (42) | 7 (29) | 0.19a |
| Postpartum | 5 (8) | 5 (12) | – | |
| Vasoactive substance | 37 (55) | 20 (46) | 17 (71) | |
| Headaches | 67 (100) | 43 (100) | 24 (100) | 1.00 |
| Recurrent thunderclap | 63 (94) | 40 (93) | 23 (96) | |
| Single thunderclap | 3 (4.5) | 2 (5) | 1 (4) | |
| Recurrent severe peaking in > 1 min | 1 (1.5) | 1 (2) | 0 | |
| Focal neurological deficits | 14 (21) | 12 (28) | 2 (8) | 0.069 |
| Transient (minutes to 4 h) | 11 (16) | 10 (23) | 1 (4) | 0.082 |
| Lasting >24 h | 5 (7) | 4 (9) | 1 (4) | 0.65 |
| Seizures | 2 (3) | 1 (2) | 1 (4) | 1.00 |
| SBP ≥ 160 and/or DBP ≥ 90 mmHg | 22 (33) | 14 (33) | 8 (33) | 1.00 |
| Cervical artery dissection | 4 (6) | 3 (7) | 1 (4) | 1.00 |
| Abnormal brain MRI | 19 (28) | 18 (42) | 1 (4) | 0.0007 |
| At least one cSAH or stroke | 17 (25) | 16 (37) | 1 (4) | 0.003 |
| cSAH | 15 (22) | 15 (35) | 0 | |
| At least one stroke | 6 (9) | 5 (12) | 1 (4) | |
| Symptomatic intracerebral haemorrhage | 4 (6) | 3 (7) | 1 (4) | |
| Symptomatic cerebral infarct | 2 (3) | 2 (3) | 0 | |
| Silent infarct | 1 (1) | 1 (2) | 0 | |
| RPLS | 6 (9) | 6 (14) | 0 | 0.081 |
aSpontaneous RCVS versus vasoactive substance precipitated RCVS.
SBP = systolic blood pressure; DBP = diastolic blood pressure; cSAH = cortical subarachnoid haemorrhage; RPLS = reversible posterior leukoencephalopathy syndrome.
Precipitating factors
RCVS qualified as spontaneous in 37% of patients and as secondary in the others. Symptoms started during delivery or early postpartum in 5 women (12%). In two, symptoms started concomitantly with the peridural injection of anaesthetics associated with epinephrine. In the remaining three women, there was a mean delay of 6.3 days between delivery and the onset of symptoms (range: 4–11 days). Only one received bromocriptine during 48 h after delivery to inhibit lactation (Table 3).
Among the 62 other cases (38 females, 24 males), 60% used various vasoactive substances (Tables 3 and 4). The three most common were cannabis (32%), selective serotonin-reuptake inhibitors (SSRI) (21%) and over-the-counter nasal decongestants (13%). The various SSRI were paroxetine (n = 5), citalopram (n = 4), fluoxetine (n = 3) and sertraline (n = 1). The overall frequency of substance-intake was not significantly different in women and men but exposure to multiple substances was significantly more frequent in men (P = 0.008). Moreover, cannabis was more commonly used in men (71 versus 8%) whereas SSRI intake was more common in women (29 versus 8%). The majority of patients (n = 26) had been using vasoactive substances on a regular basis for more than three months. But 11 had used nasal decongestants (n = 8), SSRI (n = 2) or nicotine patches (n = 1) for less than 20 days. In addition, 7 patients (11%), who were all regular cannabis smokers, reported a binge drinking the night before the onset of symptoms.
Exposure to vasoactive substances (with or without alcohol), comparison of females not in postpartum and males
| . | Total (n = 62) . | Females (no postpartum) (n = 38) . | Males (n = 24) . |
|---|---|---|---|
| Use of any vasoactive substancea | 37 (60) | 20 (53) | 17 (71) |
| Cannabis | 20 (32) | 3 (8) | 17 (71) |
| Serotonin recapture inhibitors (SRI) | 13 (21) | 11 (29) | 2 (8) |
| Nasal decongestant | 8 (13) | 8 (21) | 0 |
| Cocaine | 3 (5) | 0 | 3 (12.5) |
| Interferon | 2 (3) | 1 (3) | 1 (4) |
| Nicotine patches | 1 (2) | 1 (3) | 0 |
| Binge-drinking (OH)b | 7 (11) | 0 | 7 (29) |
| Use of a single substancec | 24 (39) | 16 (42) | 8 (33) |
| Cannabis | 9 (14.5) | 1 (3) | 8 (33) |
| SRI | 9 (14.5) | 9 (24) | 0 |
| Nasal decongestant | 5 (8) | 5 (13) | 0 |
| Interferon | 1 (2) | 1 (3) | 0 |
| Use of two vasoactive substances or one + OHc | 9 (14.5) | 4 (10.5) | 5 (21) |
| Cannabis + OH | 4 (6) | 0 | 4 (17) |
| Cannabis + nasal decongestant | 2 (3) | 2 (5) | 0 |
| Cannabis + cocaine | 1 (2) | 0 | 1 (4) |
| SRI + nasal decongestant | 1 (2) | 1 (3) | 0 |
| SRI + nicotine patches | 1 (2) | 1 (3) | 0 |
| Use of three vasoactive substances or two + OHc | 4 (6) | 0 | 4 (17) |
| Cannabis + cocaine + OH | 2 (3) | 0 | 2 (8) |
| Cannabis + SRI + OH | 1 (2) | 0 | 1 (4) |
| Cannabis + SRI + interferon | 1 (2) | 0 | 1 (4) |
| No vasoactive substancec | 25 (40) | 18 (47) | 7 (29) |
| . | Total (n = 62) . | Females (no postpartum) (n = 38) . | Males (n = 24) . |
|---|---|---|---|
| Use of any vasoactive substancea | 37 (60) | 20 (53) | 17 (71) |
| Cannabis | 20 (32) | 3 (8) | 17 (71) |
| Serotonin recapture inhibitors (SRI) | 13 (21) | 11 (29) | 2 (8) |
| Nasal decongestant | 8 (13) | 8 (21) | 0 |
| Cocaine | 3 (5) | 0 | 3 (12.5) |
| Interferon | 2 (3) | 1 (3) | 1 (4) |
| Nicotine patches | 1 (2) | 1 (3) | 0 |
| Binge-drinking (OH)b | 7 (11) | 0 | 7 (29) |
| Use of a single substancec | 24 (39) | 16 (42) | 8 (33) |
| Cannabis | 9 (14.5) | 1 (3) | 8 (33) |
| SRI | 9 (14.5) | 9 (24) | 0 |
| Nasal decongestant | 5 (8) | 5 (13) | 0 |
| Interferon | 1 (2) | 1 (3) | 0 |
| Use of two vasoactive substances or one + OHc | 9 (14.5) | 4 (10.5) | 5 (21) |
| Cannabis + OH | 4 (6) | 0 | 4 (17) |
| Cannabis + nasal decongestant | 2 (3) | 2 (5) | 0 |
| Cannabis + cocaine | 1 (2) | 0 | 1 (4) |
| SRI + nasal decongestant | 1 (2) | 1 (3) | 0 |
| SRI + nicotine patches | 1 (2) | 1 (3) | 0 |
| Use of three vasoactive substances or two + OHc | 4 (6) | 0 | 4 (17) |
| Cannabis + cocaine + OH | 2 (3) | 0 | 2 (8) |
| Cannabis + SRI + OH | 1 (2) | 0 | 1 (4) |
| Cannabis + SRI + interferon | 1 (2) | 0 | 1 (4) |
| No vasoactive substancec | 25 (40) | 18 (47) | 7 (29) |
aP = 0.19. bAlways found in combination with at least one vasoactive substance. cChi-square trend test for comparison of no substance/one substance/two substances or one + OH/three substances or 2 + OH: P = 0.008.
Exposure to vasoactive substances (with or without alcohol), comparison of females not in postpartum and males
| . | Total (n = 62) . | Females (no postpartum) (n = 38) . | Males (n = 24) . |
|---|---|---|---|
| Use of any vasoactive substancea | 37 (60) | 20 (53) | 17 (71) |
| Cannabis | 20 (32) | 3 (8) | 17 (71) |
| Serotonin recapture inhibitors (SRI) | 13 (21) | 11 (29) | 2 (8) |
| Nasal decongestant | 8 (13) | 8 (21) | 0 |
| Cocaine | 3 (5) | 0 | 3 (12.5) |
| Interferon | 2 (3) | 1 (3) | 1 (4) |
| Nicotine patches | 1 (2) | 1 (3) | 0 |
| Binge-drinking (OH)b | 7 (11) | 0 | 7 (29) |
| Use of a single substancec | 24 (39) | 16 (42) | 8 (33) |
| Cannabis | 9 (14.5) | 1 (3) | 8 (33) |
| SRI | 9 (14.5) | 9 (24) | 0 |
| Nasal decongestant | 5 (8) | 5 (13) | 0 |
| Interferon | 1 (2) | 1 (3) | 0 |
| Use of two vasoactive substances or one + OHc | 9 (14.5) | 4 (10.5) | 5 (21) |
| Cannabis + OH | 4 (6) | 0 | 4 (17) |
| Cannabis + nasal decongestant | 2 (3) | 2 (5) | 0 |
| Cannabis + cocaine | 1 (2) | 0 | 1 (4) |
| SRI + nasal decongestant | 1 (2) | 1 (3) | 0 |
| SRI + nicotine patches | 1 (2) | 1 (3) | 0 |
| Use of three vasoactive substances or two + OHc | 4 (6) | 0 | 4 (17) |
| Cannabis + cocaine + OH | 2 (3) | 0 | 2 (8) |
| Cannabis + SRI + OH | 1 (2) | 0 | 1 (4) |
| Cannabis + SRI + interferon | 1 (2) | 0 | 1 (4) |
| No vasoactive substancec | 25 (40) | 18 (47) | 7 (29) |
| . | Total (n = 62) . | Females (no postpartum) (n = 38) . | Males (n = 24) . |
|---|---|---|---|
| Use of any vasoactive substancea | 37 (60) | 20 (53) | 17 (71) |
| Cannabis | 20 (32) | 3 (8) | 17 (71) |
| Serotonin recapture inhibitors (SRI) | 13 (21) | 11 (29) | 2 (8) |
| Nasal decongestant | 8 (13) | 8 (21) | 0 |
| Cocaine | 3 (5) | 0 | 3 (12.5) |
| Interferon | 2 (3) | 1 (3) | 1 (4) |
| Nicotine patches | 1 (2) | 1 (3) | 0 |
| Binge-drinking (OH)b | 7 (11) | 0 | 7 (29) |
| Use of a single substancec | 24 (39) | 16 (42) | 8 (33) |
| Cannabis | 9 (14.5) | 1 (3) | 8 (33) |
| SRI | 9 (14.5) | 9 (24) | 0 |
| Nasal decongestant | 5 (8) | 5 (13) | 0 |
| Interferon | 1 (2) | 1 (3) | 0 |
| Use of two vasoactive substances or one + OHc | 9 (14.5) | 4 (10.5) | 5 (21) |
| Cannabis + OH | 4 (6) | 0 | 4 (17) |
| Cannabis + nasal decongestant | 2 (3) | 2 (5) | 0 |
| Cannabis + cocaine | 1 (2) | 0 | 1 (4) |
| SRI + nasal decongestant | 1 (2) | 1 (3) | 0 |
| SRI + nicotine patches | 1 (2) | 1 (3) | 0 |
| Use of three vasoactive substances or two + OHc | 4 (6) | 0 | 4 (17) |
| Cannabis + cocaine + OH | 2 (3) | 0 | 2 (8) |
| Cannabis + SRI + OH | 1 (2) | 0 | 1 (4) |
| Cannabis + SRI + interferon | 1 (2) | 0 | 1 (4) |
| No vasoactive substancec | 25 (40) | 18 (47) | 7 (29) |
aP = 0.19. bAlways found in combination with at least one vasoactive substance. cChi-square trend test for comparison of no substance/one substance/two substances or one + OH/three substances or 2 + OH: P = 0.008.
Headaches
Recent severe headache was by definition the presenting symptom in all cases. Severe headache was the only symptom of RCVS in 51 cases (76%). In the remaining 16 patients (24%), it was associated with other neurological symptoms. There was a mean delay of 7.3 ± 5.3 days (range 0–28) between headache onset and the first examination in our institution. Among the 67 patients, 63 had multiple thunderclap headaches (94%) and 4 had a different headache pattern (Table 3).
In 63 patients, multiple thunderclap headaches (mean number = 4.5; range 2–18) recurred over a mean period of 7.4 ± 5.6 days (range: 1–26). Most patients described an excruciating pain peaking in less than 10 s: ‘I thought my head was going to blow up’. The maximal pain intensity was graded as a mean of 9.5 on the verbal scale. Pain was often so severe that patients were agitated, crying or shouting and unable to speak more than a few words. Duration of the peak pain ranged from 5 min to 36 h (mean: 5 h). Ten patients reported short-lasting thunderclap headaches of less than 1 h (mean duration 26 min, range 5–50 min). Fifty patients (79%) reported at least one trigger: sexual intercourse (29%), defecation (21%), sudden emotion (19%), physical exertion (16%), urination without effort (11%), cough (11%), sneezing (11%), bathing or showering (10%) and sudden head movement (9%). In 13 patients (21%) all thunderclap headaches started while they were at rest. Pain location was bilateral in all but three patients. Nausea was the most frequent associated symptom (57%), followed by vomiting (38%), agitation (32%) and photophobia (30%). All the patients who had a history of migraine (n = 13) or cluster headache (n = 1) stated that their headaches were completely different from that of their usual attacks. Most patients had between their thunderclap headaches, other varieties of headache including persistent lingering headaches of variable severity (73%), headaches waking them up from sleep (17%) or attacks of ‘acute severe headaches’ peaking in 2–3 min (5%).
Four subjects (6%) did not exhibit this pattern of recurrent thunderclap headaches: three had a single thunderclap headache, which lasted 12–72 h before slowly fading over several days (mean 9.7; range 8–12). The last patient had four attacks of ‘acute severe headache’ with nausea, each peaking to 10/10 in 2–3 min, and lasting 1–6 h.
Other neurological symptoms
Sixteen patients (24%) had other neurological symptoms, focal deficits in 14 and seizures in 2 (Table 3). Among the 14 patients with focal deficits, 9 had only transient symptoms, 3 had a persistent deficit and 2 had both transient symptoms and a persistent deficit (Tables 3 and 5).
Summary of clinical and MRI data in 5 patients with symptomatic strokes
| Case– Gender/Age . | Thunderclap headaches . | Transient focal deficit . | Persistent focal deficits (lasting > 24 h) . | Brain lesions on CT/MRI scans . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Last at daya . | n . | At day . | Side/type/duration in minutes . | At day . | Side/type . | cSAH . | Infarct . | Intracerebral haemorrhage . |
| 1–F/57 | 10 | 12 | 3 | 15 | R/paresthesia/10 | 15 | R/hemianopia | + | L/occipital | − |
| 2–F/44 | 4 | 4 | 3 | 5–7 | L/paresthesia; numbness/5 | 4 | R/sensory loss, pain | − | − | L/deep/thalamic |
| 3–M/42 | 1 | 0 | 0 | – | – | 0 | L/hemianopia | − | − | R/lobar/occipital |
| 4–F/27 | 1 | 0 | 0 | – | – | 0 | L/hemiplegia | + | - | R/deep/capsular |
| 5–F/48 | 5 | 11 | 0 | – | – | 12 | R/hemiplegia; aphasia; B/cortical blindness | + | L/frontoparietal B/occipital | R/lobar/frontal |
| Case– Gender/Age . | Thunderclap headaches . | Transient focal deficit . | Persistent focal deficits (lasting > 24 h) . | Brain lesions on CT/MRI scans . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Last at daya . | n . | At day . | Side/type/duration in minutes . | At day . | Side/type . | cSAH . | Infarct . | Intracerebral haemorrhage . |
| 1–F/57 | 10 | 12 | 3 | 15 | R/paresthesia/10 | 15 | R/hemianopia | + | L/occipital | − |
| 2–F/44 | 4 | 4 | 3 | 5–7 | L/paresthesia; numbness/5 | 4 | R/sensory loss, pain | − | − | L/deep/thalamic |
| 3–M/42 | 1 | 0 | 0 | – | – | 0 | L/hemianopia | − | − | R/lobar/occipital |
| 4–F/27 | 1 | 0 | 0 | – | – | 0 | L/hemiplegia | + | - | R/deep/capsular |
| 5–F/48 | 5 | 11 | 0 | – | – | 12 | R/hemiplegia; aphasia; B/cortical blindness | + | L/frontoparietal B/occipital | R/lobar/frontal |
aDay 0 = day of onset of the first acute headache; day n = n days after the first acute headache; R = right; L = left; B = bilateral; cSAH = cortical subarachnoid haemorrhage.
Summary of clinical and MRI data in 5 patients with symptomatic strokes
| Case– Gender/Age . | Thunderclap headaches . | Transient focal deficit . | Persistent focal deficits (lasting > 24 h) . | Brain lesions on CT/MRI scans . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Last at daya . | n . | At day . | Side/type/duration in minutes . | At day . | Side/type . | cSAH . | Infarct . | Intracerebral haemorrhage . |
| 1–F/57 | 10 | 12 | 3 | 15 | R/paresthesia/10 | 15 | R/hemianopia | + | L/occipital | − |
| 2–F/44 | 4 | 4 | 3 | 5–7 | L/paresthesia; numbness/5 | 4 | R/sensory loss, pain | − | − | L/deep/thalamic |
| 3–M/42 | 1 | 0 | 0 | – | – | 0 | L/hemianopia | − | − | R/lobar/occipital |
| 4–F/27 | 1 | 0 | 0 | – | – | 0 | L/hemiplegia | + | - | R/deep/capsular |
| 5–F/48 | 5 | 11 | 0 | – | – | 12 | R/hemiplegia; aphasia; B/cortical blindness | + | L/frontoparietal B/occipital | R/lobar/frontal |
| Case– Gender/Age . | Thunderclap headaches . | Transient focal deficit . | Persistent focal deficits (lasting > 24 h) . | Brain lesions on CT/MRI scans . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Last at daya . | n . | At day . | Side/type/duration in minutes . | At day . | Side/type . | cSAH . | Infarct . | Intracerebral haemorrhage . |
| 1–F/57 | 10 | 12 | 3 | 15 | R/paresthesia/10 | 15 | R/hemianopia | + | L/occipital | − |
| 2–F/44 | 4 | 4 | 3 | 5–7 | L/paresthesia; numbness/5 | 4 | R/sensory loss, pain | − | − | L/deep/thalamic |
| 3–M/42 | 1 | 0 | 0 | – | – | 0 | L/hemianopia | − | − | R/lobar/occipital |
| 4–F/27 | 1 | 0 | 0 | – | – | 0 | L/hemiplegia | + | - | R/deep/capsular |
| 5–F/48 | 5 | 11 | 0 | – | – | 12 | R/hemiplegia; aphasia; B/cortical blindness | + | L/frontoparietal B/occipital | R/lobar/frontal |
aDay 0 = day of onset of the first acute headache; day n = n days after the first acute headache; R = right; L = left; B = bilateral; cSAH = cortical subarachnoid haemorrhage.
Eleven subjects (16%) had a total of 26 episodes of transient symptoms, lasting 1 min to 4 h. Visual symptoms were the most frequent, followed by unilateral sensory symptoms, aphasia and hemiparesis. Twenty-three episodes (in 9 patients) were typical TIAs, whereas three mimicked a migraine aura with positive symptoms progressing over a few minutes. Nine of the 11 subjects had a normal initial and follow-up brain MRI including MR diffusion. In contrast, the other two subjects developed a brain infarction.
Five patients (7%) had persistent deficits due to stroke (Table 5). One patient had an ischaemic stroke (case 1). This woman had 10 thunderclap headaches from onset to day 12, before she developed on day 15 a left occipital infarct with persisting right hemianopia and three sensory TIAs. Three patients (cases 2–4) had an intracerebral haemorrhage (ICH). A woman (case 2) had three thunderclap headaches from onset to day 3, followed on day 4, by another thunderclap headache with a right-sided sensory loss that revealed a left thalamic ICH. Furthermore, between days 5 and 7, she had three sensory TIAs on the left side without new stroke. Two patients (cases 3 and 4) had a single thunderclap headache concomitant to a persistent focal deficit that revealed an ICH. The last patient (case 5) had both an ICH and multiple cerebral infarcts. This woman had a normal CT after her first two thunderclap headaches, but another CT performed after a third thunderclap headache on day 3 showed a cSAH and a right frontal ICH. Two other thunderclap headaches occurred on day 9 and 11. On day 12, she had a right hemiplegia with aphasia and cortical blindness due to a left fronto-parietal and bilateral occipital infarcts (Fig. 2D).
Generalized tonic–clonic seizures occurred in two patients (3%) without previous history of epilepsy. A woman in postpartum had multiple seizures requiring admission in intensive care unit. Her MRI showed FLAIR occipital white-matter hypersignals consistent with reversible posterior leukoencephalopathy syndrome (RPLS). A man had a single generalized seizure with no lesion on brain imaging.
Blood pressure
Twenty-two subjects (33%) had a systolic blood pressure ≥160 mmHg and/or a diastolic blood pressure ≥90 mmHg during their acute headaches. No other general, cardiac or renal sign or symptom was observed. Two had a previous history of untreated hypertension. None had persistently elevated blood pressure or clinical features of hypertensive encephalopathy, but three had MRI FLAIR white-matter hypersignals consistent with RPLS. Two women were in postpartum, one had a RPLS, but none had proteinuria or generalized oedema.
Vascular imaging
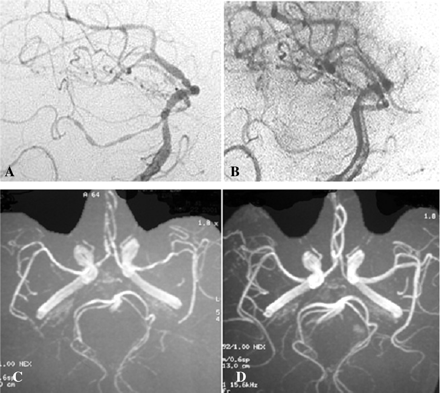
Magnetic resonance angiography (MRA) showed diffuse segmental arterial constriction in 59 patients (88%) (Fig. 1). It was detectable on the first MRA performed at a mean of 7.9 days after headache onset in 53 patients, whereas in six patients, it was visible only on a second MRA performed at a mean of 13.6 days after headache onset. Among the 64 patients who had at least one TCD, 44 (69%) had increased arterial velocities with a mean of 163 ± 27 cm/s on middle cerebral arteries and 148 ± 20 cm/s on carotid siphons. Six of these 44 patients had a normal MRA. Forty-five patients had a conventional 4-vessel angiography that showed multifocal segmental arterial constriction in all (Fig. 1), including those with normal MRA, of whom six also had a normal TCD. Within 1 h after angiography, four patients (9%) had a transient neurological deficit associated with a new thunderclap headache in one.
Other arterial abnormalities were found in seven patients. Four patients, all females, had small unruptured aneurysms (diameter range: 2–3.8 mm), involving, respectively the posterior communicating artery, the middle cerebral artery, the intracavernous carotid artery and the ophthalmic carotid junction. These four patients had no evidence of subarachnoid haemorrhage on CT, MRI FLAIR and T2* sequences, and on CSF analysis. Four patients, three females and one male, had a right-sided vertebral artery dissection disclosed at cervical ultrasound examination (n = 3), angiography (n = 3) and/or MRI Fat Sat T1 (n = 2). All complained of persistent cervical pain in addition to recurrent thunderclap headaches. One woman also had an intracavernous carotid aneurysm. Another woman had during postpartum an association of RCVS, vertebral artery dissection, cSAH and RPLS.
Brain imaging
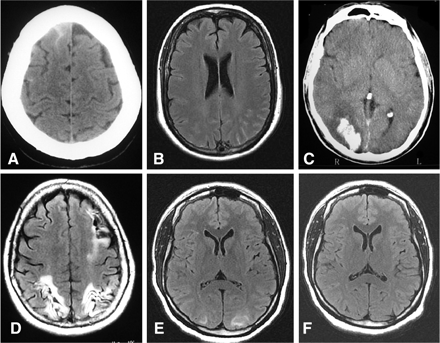
In 65 patients, CT scan was the first investigation. This initial CT, performed at a mean of 4.1 days after headache onset (range 0–20), was abnormal in eight patients (12%) showing a cSAH in five, a parenchymal haemorrhage in two and both types of haemorrhage in one (Fig. 2).
All 67 patients had at least one MRI during the acute phase of the disease. It was abnormal in 28% of cases (Table 3). The most frequent finding (15 patients, 22%) was a small localized unilateral or bilateral cortical subarachnoid haemorrhage (cSAH), visible on both CT scan and MRI in six patients, but only on MRI in the nine others (Fig. 2). All patients with a cSAH were women.
A recent stroke was detected in six patients (9%) of which five had related persistent neurological deficits (Table 5): three had a single symptomatic ICH, two had a single brain infarction that was asymptomatic in one, and the last patient had both an ICH and multiple infarcts. Four of these six patients also had a cSAH. Altogether, cSAH and strokes were significantly more frequent in women (37% versus 4%, P = 0.003).
Furthermore, six patients (9%), all women, had MRI FLAIR hypersignals consistent with RPLS on the first MRI performed at a mean of 4 ± 1.9 days (range 1–6) after headache onset (Fig. 2). Two women had postpartum RCVS, two vasoactive substance-induced RCVS and two idiopathic RCVS. Three women including one in postpartum had blood pressure above 160/90 mmHg during their acute headaches while the other three had no blood-pressure surge. Three patients had an early repeat MRI at a mean of 8.6 days after headache onset that already showed an improvement of RPLS.
Time course of RCVS
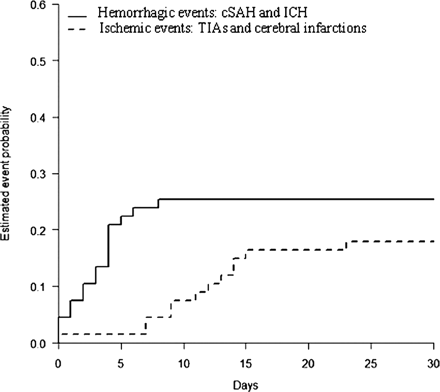
Thunderclap headaches recurred over a mean period of one week and the last attack was observed at a mean of 7.4 ± 5.6 days after onset. ICH and cSAH were early complications: ICH occurred in the first 3 days (1.7 ± 2 days) and cSAH in the first 10 days (5 ± 5) days of headache onset. RPLS and seizures were also early features, occurring during the first week. Ischaemic events occurred significantly later, TIAs at a mean of 11.6 ± 4.9 days after headache onset, ischaemic strokes at a mean of 12 ± 3 days. Overall, ischaemic events occurred 8.2 days (95% CI 4.2–12.2) later than haemorrhagic events (P = 0.005) (Table 6 and Fig. 3).
Mean delay from headache onset to other features of RCVS
| Delay from headache onset to . | Number of cases . | Mean ± SD (days) . | Range (days) . |
|---|---|---|---|
| Diagnosis of intracerebral haemorrhage | 4 | 1.7 ± 2 | 0–4 |
| Diagnosis of cSAH | 15 | 5 ± 5 | 0–20 |
| First seizure | 2 | 3 ± 1.4 | 2–4 |
| RPLS | 6 | 4 ± 1.9 | 1–6 |
| Last recurrent thunderclap headache | 63 | 7.4 ± 5.6 | 0–28 |
| Transient neurological deficit | 11 | 11.6 ± 4.9 | 0–23 |
| Diagnosis of cerebral infarction | 3 | 12 ± 3 | 9–15 |
| Delay from headache onset to . | Number of cases . | Mean ± SD (days) . | Range (days) . |
|---|---|---|---|
| Diagnosis of intracerebral haemorrhage | 4 | 1.7 ± 2 | 0–4 |
| Diagnosis of cSAH | 15 | 5 ± 5 | 0–20 |
| First seizure | 2 | 3 ± 1.4 | 2–4 |
| RPLS | 6 | 4 ± 1.9 | 1–6 |
| Last recurrent thunderclap headache | 63 | 7.4 ± 5.6 | 0–28 |
| Transient neurological deficit | 11 | 11.6 ± 4.9 | 0–23 |
| Diagnosis of cerebral infarction | 3 | 12 ± 3 | 9–15 |
Mean delay from headache onset to other features of RCVS
| Delay from headache onset to . | Number of cases . | Mean ± SD (days) . | Range (days) . |
|---|---|---|---|
| Diagnosis of intracerebral haemorrhage | 4 | 1.7 ± 2 | 0–4 |
| Diagnosis of cSAH | 15 | 5 ± 5 | 0–20 |
| First seizure | 2 | 3 ± 1.4 | 2–4 |
| RPLS | 6 | 4 ± 1.9 | 1–6 |
| Last recurrent thunderclap headache | 63 | 7.4 ± 5.6 | 0–28 |
| Transient neurological deficit | 11 | 11.6 ± 4.9 | 0–23 |
| Diagnosis of cerebral infarction | 3 | 12 ± 3 | 9–15 |
| Delay from headache onset to . | Number of cases . | Mean ± SD (days) . | Range (days) . |
|---|---|---|---|
| Diagnosis of intracerebral haemorrhage | 4 | 1.7 ± 2 | 0–4 |
| Diagnosis of cSAH | 15 | 5 ± 5 | 0–20 |
| First seizure | 2 | 3 ± 1.4 | 2–4 |
| RPLS | 6 | 4 ± 1.9 | 1–6 |
| Last recurrent thunderclap headache | 63 | 7.4 ± 5.6 | 0–28 |
| Transient neurological deficit | 11 | 11.6 ± 4.9 | 0–23 |
| Diagnosis of cerebral infarction | 3 | 12 ± 3 | 9–15 |
Thunderclap headache and vasoconstriction also had different time courses. In the six subjects who had an initial normal MRA at a mean of 5.5 days (range 2–9) after headache onset, a repeat MRA showed visible narrowings at a mean of 13.6 days (range 9–20) while the last thunderclap headache had occurred at a mean of 7.5 days (range 0–12). Moreover, in 12 patients who underwent serial TCD the increase in arterial velocities was only moderate on the first TCD performed at a mean of 6.8 days after headache onset (range 0–14) but marked on the second TCD performed at a mean of 22.5 days (range 11–53), long after the last thunderclap headache which occurred at a mean of 8.7 days (range 3–18).
Blood and cerebrospinal fluid analysis
Blood analysis showed no significant abnormalities. Lumbar puncture was performed in 62 patients. CSF was abnormal in 36 patients: 16 had slightly elevated CSF WBC (mean: 12/mm3, range: 5–35) including 6 patients with more than 10 WBC/mm3; 20 patients had elevated CSF RBC (mean: 1560/mm3, range 60–17 500) and 24 had slightly elevated CSF protein levels (mean 59 mg/l, range 0.48–0.90).
Treatment
Vasoactive medications were stopped in all patients. No specific treatment was used in six patients including five who spontaneously improved and one who had low blood-pressure levels. Nimodipine was used in 61 patients with initial intravenous infusion (1–2 mg/h adapted to blood-pressure levels) followed by oral administration in 11 patients, and direct oral administration in the other 50 patients (30–60 mg every 4 h, adapted to blood-pressure levels). Duration of treatment ranged from 4 to 8 weeks. In 36 patients (59%), no new symptom occurred after treatment onset. The other 25 patients (41%) had recurrent headaches (22 patients) and/or new neurological deficits (5 patients). Twenty patients had headache only, thunderclap in 12, sudden but moderate in 8. Three patients had only TIAs. One had a thunderclap headache and a TIA. One (Table 5, case 5) had two new thunderclap headaches and multiple cerebral infarcts while on nimodipine for 8 days.
Follow-up and clinical outcome
By definition, follow-up investigations demonstrated the reversibility of arterial constriction in all 67 patients within 1–3 months after disease onset, with a normal MRA (n = 54) or conventional angiography (n = 13) (Fig. 1).
Vascular imaging in RCVS. (A) Catheter angiography at disease onset: multiple narrowing and dilatations, (B) Control catheter angiography at 3 months, (C) MRA at disease onset and (D) Control MRA.
Brain imaging in RCVS. (A) CT scan showing a small cSAH, (B) MRI (FLAIR sequence) showing a small cSAH, (C) CT scan showing an occipital intracerebral haemorrhage, (D) MRI showing sequelae of bilateral occipital infarcts and left frontal-parietal infarct, (E) MRI (FLAIR) showing hypersignals consistent with a RPLS and (F) Control MRI in the same patient after 28 days showing resolution of the RPLS.
Estimated event probability of haemorrhagic and ischaemic complications over time. TIA and cerebral infarction occurred significantly later than ICH and cSAH (P = 0.005). The mean delay between haemorrhagic events and ischaemic events was 8.2 days (95% CI: 4.2–12.2).
The mean duration of clinical follow-up was 16 ± 12.4 months (range: 2–38). Short-term outcome at the first follow-up visit 3–6 weeks after discharge was marked by a resolution of severe headaches and by the absence of new neurological deficits in all patients. The last thunderclap headache occurred 26 days and the last TIA 23 days after headache onset. However, 24 patients had mild persistent headaches and seven patients, including two with a previous history of mood disorders, developed a depressive syndrome. Four patients were discharged with persistent focal neurological deficits, respectively a thalamic sensory syndrome, hemianopia, left hemiparesis, and dysphasia with memory disturbance (Table 5, cases 2–5). Three of them were unable to resume work and two developed post-stroke epilepsy.
No recurrent RCVS or thunderclap headache occurred during the rest of follow-up. All patients were strongly advised to abstain from all vasoconstrictive drugs but one woman with SSRI-induced RCVS attempted suicide 6 weeks after discharge, by ingesting 800 mg of paroxetine and a bottle of whisky. She was found comatous the next morning. Her vascular and neuroimaging were normal and she completely recovered in a few days.
Discussion
Reversible cerebral vasoconstriction is a rare and still poorly understood syndrome (Call et al., 1988; Bousser et al., 2001; Singhal and Bernstein, 2005; Calabrese et al., 2007). The present series of 67 consecutive patients is the largest so far reported. In all patients, MRA and/or conventional angiography documented both the initial vasoconstriction and its disappearance in less than 3 months. The stringent diagnostic criteria that we used allowed us to rule out other disorders responsible for headache and cerebral arterial constrictions such as primary or secondary cerebral angiitis or vasospam complicating an aneurismal subarachnoid haemorrhage. Our 67 patients were collected over 3 years in a single institution suggesting that RCVS is probably underdiagnosed. This important recruitment is explained by the presence in Lariboisière hospital of both an emergency headache centre in which about 8000 patients are seen every year and a neurology department with both a longstanding interest in headache and a stroke unit. Moreover, all patients presenting to our institution with a chief complaint of thunderclap or acute severe headaches undergo a standardized diagnosis procedure including CT scan, LP, MRI with MRA, cervical and transcranial ultrasound investigations and eventually conventional angiography (Bousser et al., 2001; Ducros, 2005; Schwedt et al., 2006; Valade, 2006).
About two-thirds of our patients had a secondary RCVS. This is much more than previously reported: from 0%, excluding postpartum (Chen et al., 2006a), to 25% (Hajj-Ali et al., 2002). The precipitating factors encountered in our series were the classical ones (Table 2) (Bousser et al., 2001; Singhal, 2004a; Calabrese et al., 2007; Williams et al., 2007), i.e. postpartum and exposure to various vasoactive substances. However, the most frequent substance was cannabis, which, though the most commonly used illicit drug (Moussouttas, 2004), has rarely been incriminated so far (Alvaro et al., 2002; Mateo et al., 2005). In addition, our data suggest two new precipitating factors: binge-drinking, at least in combination with vasoactive substances, already suspected to be a risk-factor for stroke but not reported in RCVS (Bruno, 2003), and interferon alpha, previously incriminated in a patient with RPLS (Hinchey et al., 1996), and in another one with multiple cerebral infarcts (Drapier et al., 2000), but both without proven cerebral vasoconstriction. A case-control study is now needed to assess the strength of the association between these various risk factors and RCVS.
Our data further confirm that recurrent thunderclap headaches over a few days to 2 weeks is the clinical hallmark of RCVS (Day and Raskin, 1986; Slivka and Philbrook, 1995; Dodick et al., 1999; Sturm and Macdonell, 2000; Garg, 2001; Singhal et al., 2002; Dodick et al., 2003; Liao et al., 2003; Loewen et al., 2004; Valenca et al., 2004; Mak et al., 2005; Kirton et al., 2006; Chen et al., 2006a; Calabrese et al., 2007). Consistent with previous case-reports (Slivka and Philbrook, 1995), these hyper-acute (peaking in <1 min) and excruciating headaches were immediately recognized as totally different from their usual headache attacks by the patients who had a history of migraine or cluster headache. In addition to headache, 21% of the patients had focal neurological deficits of variable severity, whereas seizures were not frequent (<5%). The frequency of these other neurological symptoms seems to vary according to patterns of recruitment. In a retrospective study of 16 severe cases initially thought to have primary angiitis of the central nervous system, 88% had severe headache, 63% focal neurological deficits and 21% seizures (Hajj-Ali et al., 2002). In contrast, in a prospective study of 56 patients presenting to a headache clinic with recurrent thunderclap headaches of unknown aetiology, only 9% had focal neurological deficits and none had seizure (Chen et al., 2006a).
In our series the most frequent complication of RCVS was cSAH (22%), which consisted of small localized bleedings at the surface of the brain that could not explain the diffuse vasoconstriction, which involved mostly arteries without direct contact with the subarachnoid blood. It thus differed from aneurismal vasospam, which usually correlates with the location and amount of bleeding, and is not multifocal. Such cSAH have already been reported in several isolated RCVS cases (Ursell et al., 1998; Buxton and McConachie, 2000; Cantu et al., 2003; Singhal, 2004b) and in up to 13% in a retrospective series (Hajj-Ali et al., 2002), but they have not been emphasized as a main feature of RCVS in recent reviews (Calabrese et al., 2007; Singhal, 2004a). RCVS may be an underestimated cause of cSAH, as suggested by a recent series of 12 cases of which 4 were due to RCVS (Spitzer et al., 2005).
Stroke occurred in 9% of our patients, consistent with the 7% found in the only previously published prospective series (Chen et al., 2006a). Higher percentages of stroke, up to 54%, have been reported in literature reviews and retrospective series, with ICH in 14–25% and infarctions in 14–31% (Hajj-Ali et al., 2002; Calabrese et al., 2007; Williams et al., 2007). These figures are possibly an overestimation because of a potential publication bias in favour of severe cases and because cases with headache only might have been overlooked.
A RPLS was found in 9% of our patients and has already been reported in RCVS (Jackson et al., 1993; Slivka and Philbrook, 1995; Meschia et al., 1998; Sturm and Macdonell, 2000; Dodick et al., 2003; Liao et al., 2003; Nowak et al., 2003; Singhal, 2004b; Chen et al., 2006b). These reversible posterior-predominant white matter lesions visible on MRI are thought to be due to a transient vasogenic cerebral oedema related to small-vessel dysfunction and breakdown of the blood–brain barrier, usually in the setting of severe hypertension (Hinchey et al., 1996; Singhal, 2004b; Chen et al., 2006b). In half of our cases, blood-pressure levels were under 160/90 mmHg, suggesting that RPLS may occur in the absence of hypertension.
A new finding in our study is the association of RCVS and vertebral artery dissections. Only one case has so far been reported of RCVS with an associated dissection involving the internal carotid artery, but it was considered as incidental (Singhal, 2004b). Both RCVS and dissections can present with thunderclap headache, but dissections are not responsible for a flurry of recurrent thunderclap headaches (Arnold et al., 2006). The percentage of patients with both vascular disorders in our series (6%) favours a non-incidental association. One hypothesis is that dissection of an extra-cranial artery could precipitate RCVS as reported after carotid endarterectomy (Rosenbloom and Singhal, 2007).
In our study, treatment with nimodipine did not seem as efficient as previously suggested (Lu et al., 2004; Zuber et al., 2006): more than 30% of patients had headache recurrences, 7% had TIAs and one had multiple brain infarctions. In the only published prospective series, of 52 patients (of which only 22 had proven vasoconstriction): 17% had recurrent thunderclap headaches and 7% a brain infarction (Chen et al., 2006a). Given the spontaneous reversibility of RCVS, the real benefit of nimodipine cannot be assessed in such open studies.
Consistent with the literature (Hajj-Ali et al., 2002; Singhal, 2004a; Chen et al., 2006a; Calabrese et al., 2007), clinical outcome in our patients was globally good, except for 4% who had one or multiple strokes responsible for persistent neurological deficits and were unable to resume their previous work. In contrast to a few reported cases, we observed no death (Buckle et al., 1964; Geraghty et al., 1991; Williams et al., 2007) and no relapse (Ursell et al., 1998; Hajj-Ali et al., 2002).
The female preponderance was less marked in our series (1.8 : 1) than in the previous reports, 4.3 : 1 (Hajj-Ali et al., 2002) to 10.2 : 1 (Chen et al., 2006a), but we had only five postpartum cases. We show for the first time significant sex differences. In women, RCVS occurred at an older age close to 50 years, was more frequently secondary to single drug exposure, mostly SSRI or nasal decongestants, and had a higher frequency of cSAH and strokes. In men, RCVS was characterized by a younger age of onset, in the early 30s, by the preponderance of multiple-substance exposure mostly with cannabis, and by the low incidence of cSAH and strokes. Only one major ICH occurred among the 24 males in our series, and notably, this patient took three different vasoactive substances: cannabis, SSRI and interferon alpha. It seems that the spontaneous susceptibility to RCVS is higher in women than in men, in whom exposure to multiple vasoactive substances, sometimes with binge-drinking, is often required for a RCVS to develop.
Our series allows to delineate the remarkable temporal pattern of RCVS, which may give insight into the pathophysiology. Thunderclap or acute severe headaches, which were the presenting symptoms usually recurred over a mean period of 1 week. ICH, cSAH, seizures and RPLS were early features occurring mainly during the first week, while ischaemic events, including TIAs and cerebral infarction, occurred significantly later, mainly during the second week. This temporal pattern suggests that the underlying disturbance in the control of cerebral arterial tone first involves small distal arteries responsible for haemorrhages and RPLS, and then progresses towards medium and large-sized arteries responsible for ischaemic events. This centripetal progression could also account for the different time course of headaches and vasoconstriction, since firstly thunderclap headaches sometimes occurred before any detectable vasoconstriction and secondly vasoconstriction outlasted thunderclap headaches by a mean of 14 days. This suggests that thunderclap headaches may primarily be due to the involvement of small distal arteries, with sudden changes in calibre (constriction or dilatation) that could stimulate perivascular pain-sensitive fibres.
In conclusion, our results suggest that RCVS is more frequent than previously thought and confirm that the main pattern of presentation is that of recurrent thunderclap headaches over 1–3 weeks. They show that the condition is not benign, with symptomatic ischaemic or haemorrhagic stroke in 7% and cSAH in 22%. They illustrate the difficulty in diagnosis with 21% of the patients having a normal initial MRA and 9% having both normal MRA and TCD. The different time courses of thunderclap headaches, vasoconstriction, cSAH and strokes suggest that the responsible vasospastic disorder starts in small distal arteries and progresses towards medium sized and large arteries. Nimodipine seemed less effective than reported so far but needs to be evaluated in a placebo-controlled study. No relapse was observed in the present series but a long-term follow-up is ongoing. Although the exact pathophysiology remains speculative, it seems appropriate to firmly advise the patients to avoid all vasoactive substances.
References
Abbreviations:
- cSAH
cortical subarachnoid haemorrhage
- ICH
intracerebral haemorrhage
- MRA
magnetic resonance angiography
- MRI
magnetic resonance imaging
- RCVS
reversible cerebral vasoconstriction syndromes
- RPLS
reversible posterior leukoencephalopathy syndrome
- TCD
transcranial Doppler
- TIA
transient ischaemic attack
- neuroimaging
- ischemia
- transient ischemic attack
- vascular constriction
- seizures
- serotonin
- cerebrovascular accident
- cerebral infarction
- cerebral hemorrhage
- subarachnoid hemorrhage
- nimodipine
- headache
- cerebral artery
- follow-up
- infarction
- nasal decongestants
- neurologic manifestations
- postpartum period
- sex characteristics
- cannabis
- call-fleming syndrome
- reversible posterior leukoencephalopathy syndrome
- symptom onset