-
PDF
- Split View
-
Views
-
Cite
Cite
Marcin Sliwa, Darko Markovic, Konrad Gabrusiewicz, Michael Synowitz, Rainer Glass, Malgorzata Zawadzka, Aleksandra Wesolowska, Helmut Kettenmann, Bozena Kaminska, The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A, Brain, Volume 130, Issue 2, February 2007, Pages 476–489, https://doi.org/10.1093/brain/awl263
Close - Share Icon Share
Abstract
The invasion of tumour cells into brain tissue is a pathologic hallmark of WHO grades II–IV gliomas and contributes significantly to the failure of current therapeutic treatments. Activated microglial cells are abundant in brain tumours and may support tumour invasiveness. We have previously demonstrated that cyclosporin A (CsA) can affect growth of glioma cells in vitro by inhibiting signalling pathways, which are essential for tumour proliferation and invasiveness. In this work, we demonstrate that migration of EGFP-transfected glioblastoma cells in organotypic brain slices was significantly inhibited by treatment with CsA. On average 77% of untreated cells migrated beyond 500 μm, while only 28–33% cells migrated as far in the brain slices treated with CsA (P < 0.001). This inhibitory effect on glioblastoma invasion was lost when glioblastoma cells were injected into microglia-depleted brain slices. Moreover, CsA significantly inhibits intracranial glioma growth in vivo. We demonstrate that microglia-derived factors increase glioma invasiveness in Matrigel assay in vitro and this is associated with activation of the PI-3K/Akt signalling pathway. The invasion promoting effect of microglia is abolished in the presence of CsA. Furthermore, glioma-derived soluble factors induce morphological transformation of microglia and activate MAPK signalling, although production of pro-inflammatory factors was not observed. Our findings that CsA interferes at clinically relevant concentrations with the tumour-promoting role of microglia and impairs invasive growth of glioma cells in vivo may provide a novel therapeutic strategy against gliomas.
Abbreviations
- CsA
cyclosporin A
- DAPI
4′,6-diamidino-2-phenylindole
- EGFP
enhanced green fluorescent protein
- ERK
extracellular signal-regulated kinase
- FCS
fetal calf serum
- G-CM
glioma-conditioned medium
- GBM
glioblastoma multiforme
- i.p.
intraperitoneally
- JNK
c-Jun-N-terminal kinase
- LPS
lipopolysaccharide
- LSC
laser scanning cytometry
- MAPK
mitogen-activated protein kinase
- MAPKAPK 2
MAP kinase-activated protein kinase 2
- MG-CM
microglia-conditioned medium
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- NCS
newborn calf serum
- PI-3K
phosphoinositide 3 kinase
- TBS-T
Tris-buffered saline/Tween-20
- TNFα
tumour necrosis factor α
Introduction
The invasion of tumour cells into brain tissue is a pathologic hallmark of WHO grade II–IV gliomas and contributes significantly to the failure of current therapeutic treatments. As a consequence, gross total resection or even hemispherectomy is rarely successful. Most patients diagnosed with glioblastomas survive less than a year despite intensive treatment (Ohgaki and Kleihues, 2005a, b). Proteases secreted during glioblastoma progression degrade extracellular matrix allowing tumour cells to spread and diffusely infiltrate the brain parenchyma (Rao, 2003; Demuth and Berens, 2004). Understanding the mechanisms responsible for the highly invasive potential of glioblastoma cells and their interactions with surrounding tissue may lead to identification of specific targets for a future treatment. Therapies that will effectively target invasive glioblastoma cells may significantly improve therapeutic outcome.
Microglia, resident brain macrophages, are highly abundant in gliomas and provide 10–34% of the brain tumour mass (Roggendorf et al., 1996; Badie and Schartner, 2000). Intratumoural microglia density is higher than in normal brain and abundance of microglia correlates with the grade of malignancy. Microglia in human gliomas appears mostly amoeboid and morphologically distinct from the resting microglia present in the intact brain (Wierzba-Bobrowicz et al., 1994; Graeber et al., 2002). However, it is postulated that defence functions of microglial cells against glioma are compromised by the tumour, e.g. through impaired surface expression of MHC class II (Flugel et al., 1999; Schartner et al., 2005), the immune-suppressed microglia may still display tumour-promoting features. Microglia can release many factors, including extracellular matrix proteases and cytokines, which directly or indirectly may influence tumour migration/invasiveness and proliferation (Platten et al., 2003; Rao, 2003; Watters et al., 2005). Glioma cell migration in Boyden chamber was stimulated by the presence of microglia or microglia-conditioned medium (Bettinger et al., 2002). It has been recently demonstrated in an organotypic brain culture that the invasive potential of glioblastoma was lower in microglia-depleted slices and addition of microglial cells to microglia-depleted slices restored the invasiveness (Markovic et al., 2005). These findings suggest that microglia in human gliomas may promote and support the invasive phenotype of these tumours.
We have previously demonstrated that cyclosporin A (CsA), an inhibitor of calcineurin and an immunosuppressive drug, affects growth/survival of rat (Mosieniak et al., 1997; Pyrzynska et al., 2000, 2002) and human glioblastoma cells (Zupanska et al., 2005). A strong inhibitory effect of CsA on PI-3K/Akt signalling in C6 glioma cells was demonstrated (Ciechomska et al., 2003). Akt kinase, major downstream PI-3K target, is frequently constitutively active in gliomas (Holland et al., 2000), and regulates proliferation, angiogenesis and invasion/metastasis (Kim et al., 2001; Testa and Bellacosa, 2001; Joy et al., 2003; Pu et al., 2004). Furthermore, we have recently demonstrated that FK506, an immunosuppressant and inhibitor of calcineurin, inhibits LPS-induced activation of primary microglia and affects pro-inflammatory cytokine gene expression (Zawadzka and Kaminska, 2005). Since CsA mimics many effects of FK506, we hypothesized that CsA—besides its direct effects on glioma cells—may also have indirect anti-tumourigenic effects by influencing microglial functions.
In this work, we demonstrate a significant inhibitory effect of CsA on glioma invasion in organotypic brain slice cultures and in vivo. This effect was lost when brain slices were depleted of microglia. Using microglia–glioma co-cultures, we describe a phenomenon named ‘glioma-associated microglia stimulation’ characterized by amoeboid transformation of microglia, selective activation of mitogen-activated protein kinase signalling pathways, promoting effect on glioma invasiveness concomitant with a lack of pro-inflammatory responses. CsA inhibits both glioma-associated microglia stimulation and microglia-promoting effects on glioma invasiveness in vitro. Our results demonstrate that counteracting the pro-tumourigenic activity of microglial cells by CsA may significantly affect glioma invasion.
Material and methods
Cell culture and treatment
C6 glioma cell line (American Type Culture Collection, Manassas, VA, USA) was cultured in Dulbecco modified Eagle's medium (DMEM) supplemented with 10% newborn calf serum (Gibco, MD, USA) and antibiotics (50 U/ml penicillin, 50 μg/ml streptomycin) at a density of 5–8 × 104 cells/cm2 in a humidified atmosphere of CO2/air (5%/95%) at 37°C (Heraeus, Hanau, Germany). GL261 glioma cell line (National Cancer Institute, Frederick, MD, USA) was stably transfected with the p-EGFP-N1 vector. GL261 glioblastoma cells were cultured in DMEM with 10% heat inactivated FCS, 0.2 mM glutamine, and antibiotics. Genetecin–G418 (Gibco, Gaithersburg, MD, USA) 1.2 mg/ml was used to keep stably transfected clones.
Rat microglia cultures were obtained from cerebral cortices of 1-day-old Wistar rats as described (Zawadzka and Kaminska, 2005). Briefly, cerebral cortices were mechanically dissociated, treated with 0.025% trypsin at 37°C for 20 min and collected by centrifugation at 110 r.p.m. Cells were plated at the density of 3 × 105 cells/cm2 in DMEM, high-glucose formula (4.5 mg/l), supplemented with 10% FCS, 2 mM glutamine and antibiotics on poly-l-lysine coated culture flasks. Nine days after plating, microglial cells were collected by a mild shaking (1 h at 125 r.p.m.) and centrifugation. Cell viability was determined by trypan blue exclusion and cells were plated at a final density of 2 × 105 cells/cm2 onto 24-well plates or 60 mm dishes. Non-adherent cells were removed after 30 min by changing the medium. More than 96% of the adherent cells were positive for isolectin B4, a specific microglial marker. Primary cultures of murine microglia were prepared from cerebral cortex of newborn C57/BL6 mice using similar procedure (Markovic et al., 2005).
CsA was obtained from Novartis (a clinical formulation, Sandimmum 50 mg/ml, Basel, Switzerland). The indicated concentrations of CsA were added to the culture medium for indicated time periods or every 48 h to the medium of organotypic brain slice cultures.
Organotypical brain slice model
The organotypical brain slice cultures were obtained as described (Glass et al., 2005; Markovic et al., 2005) from 16-day-old male C57/BL6 mice (animal breeding facility, Schönwalde, Germany). The brain slices cut into 250 μm sections were transferred to a Transwell insert in a 6-well plate (Becton Dickinson, Lincoln Park, NJ, USA). The brain slices were incubated in 1 ml of culture medium containing DMEM (Gibco, Gaithersburg, MD, USA) supplemented with 10% FCS (Atlanta Biological, USA), 0.2 mM glutamine and antibiotics. After overnight equilibration, medium was exchanged for the cultivation medium containing 25% heat inactivated horse serum, 50 mM sodium bicarbonate, 2% glutamine, 25% Hanks balanced salt solution, 1 μg/ml insulin (all from Gibco, Gaithersburg, MD, USA), 2.46 mg/ml glucose (Braun Melsungen AG, Germany), 0.8 μg/ml vitamin C (Sigma-Aldrich, Germany), 5 mM Tris and antibiotics.
Tumour cell injection into brain slices
About 5000 GL261 glioblastoma cells (in 0.5 μl) were inoculated into the slice using a syringe mounted to a micromanipulator. An injection canal was formed, reaching 150 μm deep into the 250 μm thick slice. Then, the needle was retracted by 50 μm and cell suspension was slowly injected over 30 s. To ensure identical experimental conditions, the cells were always inoculated into the same area.
Preparation of microglia-depleted slices
Liposomes were obtained from GOT Therapeutics (Berlin, Germany) and from the Department of Molecular Cell Biology of the Free University of Amsterdam. For the preparation of clodronate-liposomes, 86 mg of phosphatidylcholine (Lipoid EPC; Lipoid, Ludwigshafen, Germany) and 8 mg of cholesterol (Sigma Chemical Co., St Louis, MO) were combined with 10 ml of a clodronate (0.7 M; a gift of Roche Diagnostics, Mannheim, Germany) solution and sonicated gently. The resulting liposomes were washed to eliminate free drug. All liposomes were passed through a 12 μm filter immediately prior to use in order to eliminate large lipid aggregates as described (Markovic et al., 2005).
Confocal microscopy
2-Photon microscopy (Till Photonics GmbH, Muenchen, Germany) was used to study 3-dimensional distribution of glioblastoma cells. The tumour cells were consistently inserted into the central part of the 250 μm thick slices and did not migrate on the surface of the cultures. Migratory distances were then investigated using a confocal microscope (Sarastro 2000, Molecular Dynamics, Sunnyvale, USA).
Multi-photon microscopy (Leica, Germany) was used to study morphological changes of microglial cells after G-CM treatment. Phase-contrast and fluorescent images were obtain using a microscope (OLYMPUS IX-71) equipped with a digital camera (DP50) driven by Camedia software.
In vivo studies
DBA/2J mice from the breeding house of the Medical Research Center were housed and maintained in the animal facility of the Nencki Institute. EGFP-GL261 glioma cells were implanted into the brains of 16-week-old males. Briefly, mice were anaesthetized with 80 mg/kg xylazine and 4 mg/kg ketamine mixture, mounted into a stereotactic head holder (Stoelting Co., USA) and 2 × 104 GL261 cells in 1 μl of DMEM were inoculated to the striatum (1.5 mm posterior, 1.5 mm lateral to the bregma) using a 1 μl Hamilton syringe (Hamilton) advanced to a depth of 3 mm from the cortical surface. Starting from the second day after cell implantation, 2 or 10 mg/kg CsA was administrated intraperitoneally (i.p.) every 2 days.
After 14 days, before the sacrifice, the animals were anaesthetized and perfused through the heart with a phosphate-buffer saline (PBS) then 4% paraformaldehyde. The brains were removed, immersed in the same fixative for 24 h, and cryoprotected in 30% sucrose solution in PBS. Next, brains were frozen with dry CO2 and 40 μm thick sections were cut using a cryostat. Images were captured and tumour volumes were calculated. The experimental protocol was approved by the Local Animal Care and Use Committee and conforms to the national guidelines for the care and use of animals in research.
Protein isolation and western blotting
Whole-cell protein extracts were prepared, resolved by electrophoresis and transferred to a nitrocellulose membrane (Amersham Biosciences, Germany) as described (Ciechomska et al., 2003). After blocking with 5% non-fat milk in TBS-T (Tris-buffered solution pH 7.6/0.005% Tween-20) the membranes were incubated overnight with primary antibodies diluted in a blocking buffer. The primary antibody reaction was followed by 1 h incubation with relevant secondary antibodies. Antibodies recognizing phosphorylated kinases: Akt (Thr308/Ser473), p38 MAP kinase (Thr180/Tyr182), ERK1/2 (Thr202/Tyr204), JNK (Thr183/Tyr185), as well as corresponding total kinases (all diluted 1:1000), and horseradish peroxidase-conjugated anti-rabbit IgG (diluted 1:2000) were from Cell Signaling (MA, USA). Horseradish peroxidase-conjugated anti-mouse IgM antibodies and antibody against β-actin (diluted 1:2000) were from Oncogene Research Products (MA, USA). Immunocomplexes were detected using an enhanced chemiluminescence detection system (ECL) and membrane exposure to X-ray films. The molecular weight of proteins was estimated with prestained protein markers (Sigma, St Louis, MO, USA). Band intensities were measured by a densitometric analysis of immunoblots with BioRad Molecular Imager FX and Quantity One software (CA, USA).
Co-culture system and conditioned medium treatment
Microglial cells were shaken off from glial cultures for 1 h at 125 r.p.m. and seeded on 24-wells plates. C6 glioma cells were seeded on Falcon cell culture inserts with 0.4 μm pore size at the density of 5 × 104 cells/insert. After 48 h of equilibration, inserts containing glioma cells were added to the plates with microglia in the lower compartment. In another series of experiments, microglia (5 × 104 cells/cm2) were plated onto 24-well plates, incubated for 48 h to silence and subsequently treated with the glioma-conditioned medium (G-CM) for various times. A fresh medium or microglia-conditioned medium (24 h) was added as a control.
The microglia-conditioned medium (MG-CM) was collected after 24 h of culture and applied to glioma cells in a volume of 3 ml (60 mm dish) or 100 μl (96-wells plate). In these experiments, we also used two different controls: a fresh medium and glioma-conditioned medium. No significant differences between controls were observed.
Living cell number was determined using MTT metabolism test. MTT stock solution was added to glioma cell cultures exposed to various conditions at concentration 0.5 mg/ml. After 2 h of incubation at 37°C, water-insoluble dark blue formazan crystals were dissolved in a lysis buffer containing 20% SDS and 50% DMF. Optical densities were measured at 570 nm using a scanning multi-well spectrophotometer.
Immunohistochemistry, immunofluorescence and laser scanning cytometry
Microglial cells co-cultured with glioma cells were fixed with 4% paraformaldehyde and stained overnight at 4°C with the FITC-labelled isolectin B4 (10 μg/ml, Sigma, Germany) as described (Zawadzka and Kaminska, 2005). Subsequently, cells were stained for 10 min with 10 μg/ml DAPI (a 4′,6-diamidino-2-phenylindole, Sigma-Aldrich, Germany) to visualize cell nuclei. Cell body area was measured using a laser scanning cytometry (LSC, CompuCyte, Cambridge, MA, USA).
In some experiments, microglia co-cultured with glioma cells or G-CM-treated were fixed using 4% paraformaldehyde and microglial cells were incubated for 4 h at room temperature with the peroxidase-conjugated isolectin B4 (10 μg/ml, Sigma, Germany) from Bandeiraea simplicifolia. The peroxidase activity was visualized with 0.1 mol/l phosphate-buffer containing 0.025% 3,3′-diaminobenzidine (DAB), 0.02% hydrogen peroxide and 0.6% ammonium nickel II sulphate hexahydrate. Non-specific peroxidase activity was blocked by pre-incubating cells in 3% hydrogen peroxide in 10% methanol for 30 min.
Matrigel Matrix invasion assay
Invasion assay was a modification of the previously published protocol (Albini et al., 1987). Inserts containing 8 μm pore size (Transwell, Corning, NY, USA) were coated with 1 mg/ml Growth Factor Reduced Matrigel Matrix (BD Biosciences, San Diego, CA, USA). Matrigel Matrix was added in volume of 50 μl per insert, dried in sterile conditions (37°C) for 2–3 h and reconstituted for 15 min with 50 μl of medium containing 2% FCS. Afterwards, C6 glioma cells were seeded at a density of 5 × 104 per insert. At the time of experiment, 50 μl of medium containing 2% FCS with or without CsA was added to the upper compartment and 500 μl to the lower compartment. In some experiments, microglia were seeded in the lower compartment 48 h before inserts with glioma cells were added. After 48 h of incubation Matrigel Matrix was precisely removed, glioma cells on membranes were fixed with 95% methanol/5% PBS and DAPI staining was performed (Sigma, MO, USA). Then, the membranes from Transwell inserts were cut out and cells were counted under fluorescent microscopy. Histograms of migrating glioma cells in control and CsA-treated cultures in the presence or absence of microglia were obtained. Data are expressed as means from three independent experiments performed in duplicate.
Nitric oxide release assay and cytokine quantification
Microglial nitric oxide (NO) production was assessed as the nitrite accumulation in the supernatant using the Griess reagent. Murine microglia was plated in 96-well plates with a density of 105 cells/well cultured in 100 μl of DMEM/FCS medium. Cells were stimulated with LPS (100 ng/ml; Sigma, Deisenhofen, Germany), GL261 conditioned medium (G-CM) or in the combination of G-CM and CsA (1 μM). Control cells were treated with DMEM/FCS medium only. Hundred microlitres of the supernatant were mixed with 100 μl of Griess reagent and incubated for 10 min at room temperature. Absorption was measured at 540 nm in a microplate reader (Perkin Wallac GmbH, Freiburg, Germany). The level of interleukin-6 (IL-6) production in the cell culture supernatants was assayed using mouse-specific antibody pairs and protein standards for ELISA (R&D Systems, Wiesbaden, Germany). The colorimetric reaction was analysed at 450 nm in a microplate reader.
Statistical analysis
The results are expressed as means ± standard deviation (SD). Statistical analyses were performed using Statistica (ver. 7.1 StatSoft. Inc, OK, USA) software. Statistical significance was determined by Newman–Keuls analysis.
Results
CsA affects migration/invasion of glioblastoma cells in brain slices
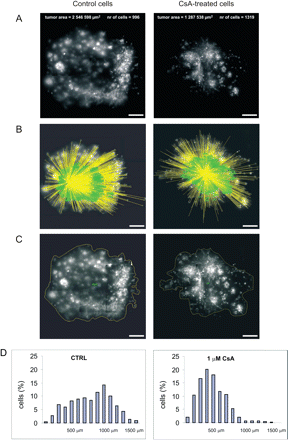
Approximately 5000 EGFP-transfected GL261 glioblastoma cells were inoculated into cultured brain slices. The distance between the individual fluorescent cell and the centre of the injection canal was measured (Fig. 1A and B) and total tumour area was calculated from confocal images (Fig. 1C). After injection, the tumour cells were spread ∼250 μm around the injection site. In the first 2 days, tumours grew to a radius of ∼500 μm and only few cells had migrated beyond this border. After 4 or 5 days, many cells had migrated more than 500 μm, some up to 1.5 mm. Accumulated data from up to 1000 cells per injection site allowed us to assemble distance histograms showing a distribution of cells migrating from the injection site (Fig. 1D).
Migration of glioblastoma cells in organotypic brain slice cultures. (A) Representative images of murine EGFP-GL261 glioblastoma cells in brain slices 5 days after inoculation, untreated or treated with 1 μM CsA. Scale bar = 250 μm. The number of visualized cells and the area of glioma cell migration in organotypic brain slice from a representative experiment are indicated. (B) From the confocal images the distance of individual glioblastoma cells from the inoculation site was determined. The measured distances are superimposed to the image shown in (A). From the distances measured, a distance histogram of individual experiment was constructed (D). (C) From the image (A), a tumour size consisting of all glioblastoma cells detected in the 3-dimensional space was calculated. (D) A distance histogram of individual experiments indicating data obtained from fluorescent images is presented. The percentages of cells migrating for particular distances are indicated; indicative interval is 100 μm.
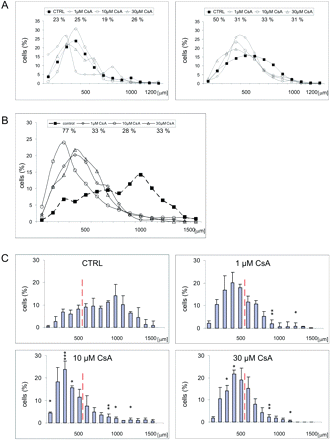
We have compared tumour cell migration in the slices cultured in the absence or presence of different doses of CsA 2, 4 and 5 days after EGFP-GL261 inoculation. There was no significant difference in migratory distances in untreated and CsA-treated slices at Day 2 after tumour cell inoculation. However, 4 days post-inoculation, the difference in migratory distances in control slices or CsA-treated slices became apparent (Fig. 2A). Five days after inoculation, statistically significant differences in migratory distances between control or CsA-treated slices were observed (Fig. 2B). In brain slices treated with CsA, the bulk population of glioblastoma cells remained around the inoculation site within a radius of 500 μM, with only few cells dispersed out of this site.
CsA inhibits invasiveness of glioblastoma cells in brain slice cultures. (A) The invasiveness of glioblastoma cells within brain slices in the absence or presence of CsA was determined and distance histograms were constructed. The percentage of cells, which migrated >500 μm after 2 (left) or 4 (right) days of culture was calculated. Data are expressed as means from three independent experiments. (B) Distance histograms from control slices and CsA-treated slices (1 μM, 10 μM or 30 μM) 5 days after glioblastoma inoculation. The percentage of cells migrating >500 μm was calculated (means from three experiments). (C) Distance histograms from control slices and CsA-treated slices (1 μM, 10 μM or 30 μM) 5 days after glioblastoma inoculation; indicative interval is 100 μm. The data show that CsA inhibits invasiveness of glioblastoma cells in cultured brain slice cultures. The panel shows means ± SD from three independent experiments. A statistical significance of differences between control or CsA-treated slices was determined using Newman–Keuls analysis, *P < 0.05, **P < 0.01.
To identify the effect of CsA on glioma invasion we defined two cell populations: a first population that stayed within the radius of 500 μm regarded as resident cells; a second population, which migrated beyond that radius consisted of invasive cells. The percentage of cells migrating beyond 500 μm is depicted on diagram in the Fig. 2B. On average 77% of untreated cells migrated beyond 500 μm, while only 28–33% cells migrated as far in the brain slices treated with CsA (Fig. 2B). The observed differences were statistically significant. There was no significant difference in the inhibitory effects of CsA applied at various concentrations (Fig. 2C). The cumulative data demonstrate that the migration of glioblastoma cells was strongly inhibited in CsA-treated brain slices.
Inhibitory effect of 1 μM CsA on migration/invasion of glioblastoma cells in organotypic brain slices is mediated by microglia
The effects of 1 μM CsA could reflect a direct drug effect on invasiveness since CsA affects proliferation and viability of glioma cells when applied in vitro at concentrations 10–30 μM (Pyrzynska et al., 2002; Zupanska et al., 2005). We sought to determine whether an inhibitory effect of 1 μM CsA on invasion of glioblastoma cells is mediated by modulation of tumour-promoting activity of microglia.
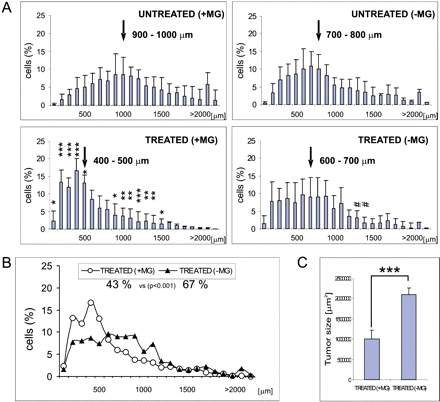
Clodronate-filled liposomes have been used to prepare microglia-depleted slices, as previously described (Markovic et al., 2005). While 1 μM CsA strongly and significantly affected glioblastoma invasion in control slices, the effect of CsA was weak and not statistically significant in microglia-depleted slices. Approximately 86% of inoculated GL261 cells migrated beyond 500 μm in control slices and 72% in microglia-depleted slices at Day 5 after cell inoculation (Fig. 3A, top). In the presence of CsA, the percentage of cells, which migrated >500 μm was 43% in control slices (n = 10) and 67% in microglia-depleted slices (n = 13). The Fig. 3B shows histogram summarizing the data from control and microglia-depleted slices in the presence of 1 μM CsA. The tumour size in CsA-treated brain slices containing microglia was considerably reduced and no reduction was observed in microglia-depleted slices (Fig. 3C). These results demonstrate that an inhibitory effect of CsA on glioblastoma migration in the brain organotypic slices was lost when slices were depleted of microglia by pre-treatment with clodronate-filled liposomes. It strongly suggests that the inhibitory effect of CsA on tumour invasion in the organotypic brain slice cultures is mediated by microglia.
Inhibitory effect of CsA on glioblastoma invasiveness is mediated by microglia. (A) The effect of 1 μM CsA on the invasiveness of glioblastoma cells within control or microglia-depleted brain slices. On the left, the histograms indicate data from brain slices containing microglia: untreated (n = 12) or 1 μM CsA-treated (n = 14) 5 days after glioblastoma inoculation. On the right, the histograms indicate the invasiveness of glioblastoma cells within microglia-depleted slices: untreated (n = 10) or 1 μM CsA-treated slices (n = 13). A statistical significance of differences between untreated or CsA-treated slices was determined using Newman–Keuls analysis at three levels: *P < 0.05, **P < 0.01 and ***P < 0.001 for slices containing microglia and #P < 0.05 for microglia-depleted slices. An arrow indicates distance range where 50% of cells migrated; indicative interval is 100 μm. (B) Cumulative index of data demonstrating that glioblastoma cells migrate for shorter distance in CsA-treated normal slices (43%) than in CsA-treated, microglia-depleted slices (67%). (C) The histogram depicts tumour size calculated in CsA-treated normal slices (n = 14) and microglia-depleted slices (n = 13); ***P < 0.001.
CsA impairs glioma growth in vivo
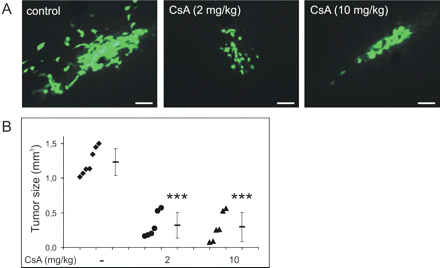
Since tumour regression in animal experimental tumour models represents an important endpoint of clinical relevance, we evaluated the ability of CsA to reduce tumour growth invivo. To address this point, gliomas were induced in mice by an inoculation of murine EGFP-GL261 glioma cells into the brain of the animals. CsA was delivered i.p. every 2 days at doses 2 or 10 mg/kg of body weight starting from the second day after inoculation. After 14 days tumour-bearing mice were perfused and brain slices were analysed using fluorescent microscopy. We found that 14 days after implantation, CsA-treated mice had significantly smaller tumours than control mice (Fig. 4). The tumour reduction was of ∼75% (0.31 ± 0.18 mm3 and 0.29 ± 0.20 mm3 in 2 or 10 mg/kg CsA-treated mice, respectively, n = 6, versus 1.23 ± 0.19 mm3, n = 7, in control mice). There was no difference between groups of tumour-bearing mice treated with 2 or 10 mg/kg CsA.
CsA impairs growth of intracranial gliomas. (A) Murine EGFP-GL261 glioma cells were inoculated into the striatum of the brain of 16-week-old male DBA/2J mice. Saline or CsA was injected i.p. every 2 days, starting from the second day after EGFP-GL261 inoculation. Examples of gliomas from the brain of saline or CsA-treated mice 14 days of treatment are presented. Scale bar = 20 μm. (B) Quantification of tumour volumes in saline or CsA-treated mice was performed and results for individual animals are presented. Results represent the mean of 6–7 mice in each group and are expressed as mean volume ± SD. ***P < 0.001, versus tumour volumes in control mice.
Microglia-promoting effect on glioma invasiveness is blocked by CsA
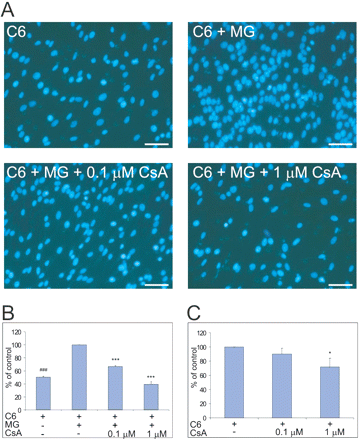
To understand molecular mechanisms underlying the inhibitory effects of CsA on tumour invasion, we investigated mutual interactions between glioma and microglia, and their influence on glioma invasiveness. Invasion of glioma cells through Matrigel, a matrix extract of non-crosslinked ECM macromolecules, has been evaluated. Cells migrating through the Matrigel were stained and counted (Fig. 5A). Under control conditions glioma cells migrated through the Matrigel within 48 h, but in the presence of microglia the number of invading cells was considerably higher (Fig. 5B). Addition of 0.1 and 1 μM CsA abolished the promoting effect of microglia on glioma cell invasion (Fig. 5B). In the absence of microglia 0.1 μM CsA had no effect and 1 μM CsA only partially inhibited invasion of glioma cells through Matrigel (Fig. 5C).
Cyclosporin A abolishes microglia-promoting effects on glioma invasiveness. (A) C6 glioma cells were seeded at a density of 5 × 104/insert in 24-well Transwell coated with the Growth Factor Reduced Matrigel Matrix. In the lower compartment microglia were seeded in DMEM/2% FCS; under control conditions only medium was present. After 48 h, the Matrigel invading cells were fixed in methanol, stained with DAPI and counted using a fluorescent microscope. Fluorescent images from a representative experiment are shown. Scale bar = 50 μm. (B) The percentage of invading C6 glioma cells without or with CsA in the presence or absence of microglia. The number of cells migrating in the presence of microglia was taken as 100%. Results are expressed as means ± SD from three independent experiments (each in duplicate). Statistical significance was determined using Newman–Keuls analysis; ***P < 0.001 for glioma cells co-cultured with microglia and treated with CsA; ###P < 0.001 for glioma cells cultured alone as compared with cells co-cultured with microglia. (C) Invading C6 cells in the absence or presence of CsA were calculated and number of control cells was taken as 100%. Results are expressed as means ± SD from three independent experiments (each in duplicate). Statistical significance was determined using Newman–Keuls analysis; *P < 0.05.
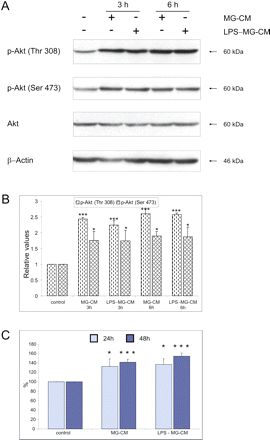
To address mechanisms responsible for invasion promoting effects of microglia, we determined the influence of the microglia-conditioned medium on PI-3K/Akt signalling pathway. The exposure of glioma cells to MG-CM activated PI-3K/Akt signalling pathway in glioma cells. The levels of active, phosphorylated Akt were increased in glioma cells after the exposure to MG-CM (Fig. 6A). Pre-treatment of microglia with 100 ng/ml lipopolysaccharide (LPS), an immunostimulator of microglia, did not influence Akt signalling pathway in glioma cells. A densitometric analysis of immunoblots from three independent experiments confirmed that activation of PI-3K/Akt signalling in glioma cells exposed to MG-CM was statistically significant (Fig. 6B).
Microglia-derived soluble factors stimulate Akt signalling pathway and increase proliferation of glioma cells. (A) Increase of the levels of phosphorylated Akt in C6 glioma cells treated with the microglia-conditioned medium (MG-CM). Additionally, glioma cells were treated with a conditioned medium collected from microglial cultures pre-treated with 100 ng/ml lipopolysaccharide (LPS-MG-CM). Equal loading of proteins was ensured by β-actin immunodetection on the same blots. Similar results were obtained in three independent experiments. (B) Densitometric analysis of phospho-Akt levels in glioma cells. Phosphorylated Akt kinase level was related to β-actin level; the data are shown as means ± SD from three independent experiments; *P < 0.05, ***P < 0.001. (C) Microglia-conditioned medium increases glioma proliferation. MG-CM or conditioned medium from LPS-treated microglial cultures (LPS-MG-CM) was added to glioma cells; control cells were treated with a fresh medium. The number of living cells was determined using MTT metabolism test 24 and 48 h after changing medium. The data are shown as means ± SD from three independent experiments, each in triplicate; *P < 0.05; ***P < 0.001.
Furthermore, an addition of MG-CM to C6 glioma cell cultures significantly increased the number of glioma cells at 24 h and even more at 48 h post-treatment (Fig. 6C). Pre-treatment of microglia with 100 ng/ml LPS did not have an additive effect on glioma proliferation.
Glioma-associated microglia stimulation is blocked by CsA
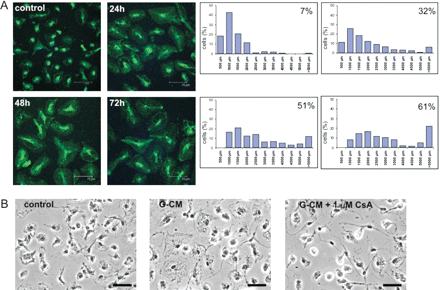
Co-culture of microglial cells with glioma cells or exposure to the glioma-conditioned medium (G-CM) induced morphological changes such as enlargement of the cell body and acquiring an amoeboid cell shape (Fig. 7A). Microglial cells were stained with the fluorescein-labelled isolectin B4 to visualize morphological changes. Counterstaining with DAPI facilitated the quantification of microglial cells undergoing morphological transformation using LSC. A progressive increase in the number of microglial cells with enlarged bodies during co-culture with glioma cells was observed (Fig. 7A). Similar changes were observed when the G-CM was added to microglia cultures (not shown). Morphological transformation of microglial cells into amoeboid microglia after exposure to the G-CM was blocked by 1 μM CsA (Fig. 7B).
Soluble factors released by glioma cells induce morphological transformation of microglial cells. (A) Left: rat microglial cells were cultured alone or co-cultured with rat C6 glioma cells for various times in 24-well culture inserts. Afterwards, microglial cells were stained with FITC-labelled isolectin B4 followed by DAPI and analysed under a confocal multi-photon microscope. Images from a representative experiment (out of 5) are presented. Scale bar = 75 μm. Right: stained microglial cells were analysed by a laser scanning cytometry (LSC). Cell body area of microglial cells was measured and the percentage of cells with body area >2000 μm2 was calculated. Histograms representing the area covered by microglial cells (x axis, μm2) were prepared. Data are expressed as means from 40 fields in a representative experiment. (B) CsA blocks morphological alterations of microglial cells induced by glioma-conditioned medium. Microglia cultures were exposed to the microglia- or glioma-conditioned medium in the absence or presence 1 μM CsA. After 48 h, microglial cells were counterstained with the peroxidase-conjugated isolectin B4 and analysed under a light microscope. Representative images are presented; scale bar = 50 μm. Similar results were observed in three independent experiments.
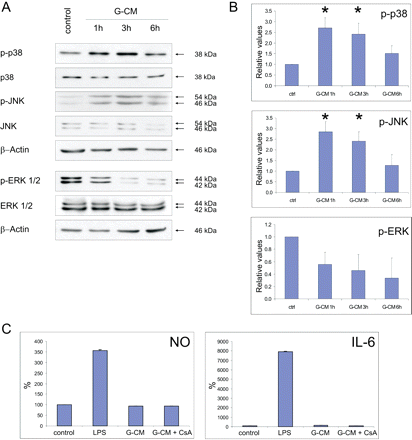
Morphological transformation of microglia is often associated with activation of MAPKs, which mediate inflammatory responses, such as production of NO, tumour necrosis factor-alpha (TNFα), IL-6, monocyte chemotactic protein-1 (MCP-1) in vitro and in vivo (Ciallella et al., 2005; Lund et al., 2005; Waetzig et al., 2005). Thus, we evaluated the levels of active MAP kinases: ERK, JNK and p38 in microglia exposed for various times to the G-CM. While the levels of phosphorylated JNK and p38 MAP kinases were increased, the level of phospho-ERK was reduced following the G-CM addition (Fig. 8A). A densitometric analysis of immunoblots from three independent experiments (Fig. 8B) demonstrates a reproducibility of changes in the MAPK levels in microglia exposed to glioma-derived factors.
Glioma-conditioned medium induces MAPK signalling but not inflammatory responses in microglia. (A) Immunoblots show the levels of active, phosphorylated MAP kinases in microglial cells treated with G-CM for various times. Microglial cells were seeded on 60 mm plates, silenced for 48 h and cells were exposed to the microglia- or glioma-conditioned medium. Blots probed with an antibody against total and phosphorylated kinases and re-probed with an antibody against β-actin. (B) A densitometric analysis of phosphorylated MAP kinases in microglial cells exposed to G-CM. The data are shown as means ± SD from three independent experiments; *P < 0.05 using Newman–Keuls analysis as compared to control. (C) NO release and IL-6 production were measured in microglial cell cultures under various conditions. Murine microglia was plated in 96-well plates with a density of 105 cells/well cultured in 100 μl of DMEM/FCS medium. Microglial nitric oxide (NO) and IL-6 production were assessed with a NO release assay and cytokine ELISA, respectively. NO release and IL-6 production were measured relative to control (cultured in fresh medium), after addition of either 0.1 μg/ml LPS to the medium or G-CM (GL261 glioma conditioned medium) without or with 1 μM CsA. The data are shown as means ± SD from three independent experiments (n = 8 in each experiment).
We determined NO release and production of IL-6 by microglial cells exposed to G-CM or LPS (used as reference), as well as possible effect of 1 μM CsA. NO production was elevated in microglial cells incubated with 100 ng/ml LPS for 24 h. Exposure of microglial cultures to G-CM did not result in nitrite accumulation (Fig. 8C). Similarly, the level of IL-6 was highly elevated in LPS-treated microglia but not in G-CM-treated microglia. While LPS treatment produced 8000% increase of IL-6 production in microglial cells, G-CM treatment resulted in the 36% increase of IL-6 production. 1 μM CsA did not modulate NO and IL-6 production in G-CM-treated microglia. Our findings demonstrate that glioma cells secrete factors, which induce morphological transformation of microglia, activate JNK and p38 MAPK signalling pathways, but do not induce the classical microglial inflammatory responses.
DISCUSSION
CsA affects invasion of glioblastoma cells and tumour growth in brain slice cultures and in vivo
Current treatments for glioblastomas are disappointing for their lack of effectiveness. Malignant glioblastomas are characterized by a diffuse infiltration into the brain and migrating glioblastoma cells are often resistant to apoptosis (Ohgaki and Kleihues, 2005a, b). We have previously reported that 30–60 μM CsA, induces growth arrest or/and programmed cell death of cultured rat and human glioma cells (Pyrzynska et al., 2002; Zupanska et al., 2005). Our findings prompted us to evaluate a potential of CsA against experimental gliomas in the brain slice model that is suitable for studies of tumour growth and invasion (Jung et al., 2002), and in animal model of gliomas. In this study, we demonstrate that 1, 10 and 30 μM CsA significantly decreased migration/invasion of glioma cells in organotypic brain slice cultures (Figs 1 and 2). In the consequence, the tumours were significantly smaller in CsA-treated brain slice cultures than under control conditions. While the effects of 10 and 30 μM CsA on tumour invasiveness may involve to some extent anti-proliferative or cytotoxic action of the drug (Zupanska et al., 2005), an inhibitory effect of 1 μM CsA manifests drug effect on migration/invasiveness of glioma cells. CsA at concentrations lower that 10 μM has no cytostatic or cytotoxic effect on glioma cells.
Moreover, 14 days after implantation of glioma cells into the brain, CsA-treated mice had significantly smaller tumours than did control mice (Fig. 4). The tumours were reduced by 75% in mice after a systemic injection of CsA (2 or 10 mg/kg). The indicated concentrations of CsA correspond to blood concentration of 0.2 or 1 μM, respectively. We have previously reported that systemic injections of 100 mg/kg CsA result in a blood concentration of 10 μM that the animals tolerated well (Ciechomska et al., 2003). Although, CsA shows a limited permeability through the intact blood–brain barrier, a breakdown of this barrier has been observed in brain tumours. High-grade astrocytomas secrete vascular endothelial growth factor, which stimulates angiogenesis, opening of tight junctions in high-grade astrocytoma microvessels and increases endothelial cell permeability (Davies, 2002). The leaky blood–brain barrier in brain tumours may result in similar CsA concentrations in the brain as in blood. Therapeutic efficacy of CsA for experimental gliomas was apparent at clinically relevant concentrations. According to medical data bases, a daily CsA dose of 3–7 mg/kg was used in most transplantation patients (Frei et al., 1998; Levy et al., 2002) or patients with severe psoriasis (Koo, 1998) for many months without significant side effects.
It is noteworthy that other immunosuppressants, rapamycin, as well as related drugs temsirolimus (CCI-779) and everolimus have been shown to inhibit glioblastoma cell proliferation in culture and intracerebral xenografts (Heimberger et al., 2005). Temsirolimus demonstrated modest activity in recurrent gliomas in recent phase I/II studies (Galanis et al., 2005). Our results suggest that similarly to CsA, rapamycin and related drugs besides a direct inhibition of glioblastoma cell proliferation in culture may affect intratumoural microglia and modulate their influence on tumour invasiveness.
Tumour-promoting role of microglia and effects of CsA
Intratumoural density of amoeboid microglia is higher than in normal brain and correlates with the grade of malignancy (Wierzba-Bobrowicz et al., 1994; Graeber et al., 2002). The presence of microglia in and around tumours may reflect the immune response or microglia recruitment by tumours to promote growth and invasion (Watters et al., 2005). Due to impaired expression of MHC class II, brain tumour-infiltrating microglia are not efficient in inducing an effective anti-tumour T-cell response (Flugel et al., 1999; Schartner et al., 2005). Microglia produces many factors that influence glioma proliferation and migration (Watters et al., 2005). TGFβ and TNFα produced by microglia and glioma itself may promote tumour proliferation and migration/invasion, via induction of matrix degrading enzymes such as cathepsins, metalloproteinases: MMP-2, MMP-3 and MMP-9 allowing glioma cells to infiltrate the brain (Rao, 2003; Lakka et al., 2004; Pu et al., 2004). Studies by Markovic et al. (2005) demonstrated that microglia release MMP-2 and convert the pre-MMP-2 released by glioblastoma cells to the active form.
We demonstrated that microglial cells release factors that stimulate PI-3K/Akt signalling pathway in glioma cells. Enhancing of Akt signalling in glioma cells by microglia-derived factors may be important in promoting effects on glioma invasiveness and proliferation. It has been shown that PI-3K/Akt signalling pathway regulates glioma cell migration, invasiveness and MMP expression (Kim et al., 2001; Kubiatowski et al., 2001).
We found that CsA, applied at low doses 0.1 or 1 μM, abolished the promoting effect of microglia on glioma invasiveness. Since 1 μM CsA has only moderate effect on glioma invasion in the Matrigel assay in the absence of microglia, we suggest that the inhibitory effect of CsA is mediated by inhibition of tumour-supporting functions of microglia. This interpretation is strengthened by our observation that the inhibitory effect of 1 μM CsA on glioma invasiveness was lost in microglia-depleted brain slices. At higher concentrations CsA may directly affect glioma proliferation and invasiveness.
Glioma-released factors stimulate microglia without inducing inflammatory responses
Studying glioma–microglia co-cultures, we found mutual interactions between glioma and microglial cells. We describe a novel phenomenon named ‘glioma-associated microglial stimulation’ characterized by amoeboid transformation of microglia, selective activation of MAPK signalling pathways, release of factors promoting glioma invasiveness with co-current lack of pro-inflammatory responses such as NO and IL-6 production.
It is still unknown how tumour cells interact with microglia to stimulate them to produce factors that facilitate tumour cell invasion. Our results demonstrate an activation of p38 MAPK and JNK signalling pathways in microglial cells. MAPK are a family of serine/threonine protein kinases that mediate many cellular and inflammatory responses (Kyriakis and Avruch, 2001; Pearson et al., 2001; Kaminska, 2005). p38 MAPK plays a prominent role in the upregulation of metalloproteinases in many cell types, including Raw 264.7 murine macrophages (Woo et al., 2004).
There is increasing clinical and experimental evidence that inflammation and cancer are causally linked, and tumour-educated macrophages may promote tumour progression and metastasis (Mantovani et al., 2004; Pollard, 2004). For example, it has been shown recently that ovarian cancer cells switch co-cultured macrophages to a ‘tumour-associated phenotype’ characterized by changes in macrophage cytokine, chemokine and MMP expression (Hagemann et al., 2006). We demonstrate a similar phenomenon in glioma–microglia co-cultures; however, a detailed molecular characterization awaits further studies.
Our findings demonstrate that glioma-secreted factors are responsible for stimulation of microglial cells, which in turn support tumour growth and invasiveness. CsA blocks both glioma-associated microglia stimulation, as well as microglia-promoting effects on glioma invasiveness in vitro, in brain slices and in vivo. We hypothesize that blocking unfavourable microglia stimulation with CsA (or similarly acting drugs) will inhibit glioma invasion and in consequence, tumour growth.
This work was supported by PBZ-MIN-001/P05/2002/10 from the Ministry of Science and Information Technology, Foundation for Polish Science (BK) and by BMBF (H.K.). Funding to pay the Open Access publication charges for this article was provided by Grant PBZ-MIN-107-/P04/2004 from the Ministry of Science and Education.