-
PDF
- Split View
-
Views
-
Cite
Cite
Alessandro Stefani, Andres M. Lozano, Antonella Peppe, Paolo Stanzione, Salvatore Galati, Domenicantonio Tropepi, Mariangela Pierantozzi, Livia Brusa, Eugenio Scarnati, Paolo Mazzone, Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease, Brain, Volume 130, Issue 6, June 2007, Pages 1596–1607, https://doi.org/10.1093/brain/awl346
Close - Share Icon Share
Abstract
Gait disturbances and akinesia are extremely disabling in advanced Parkinson's disease. It has been suggested that modulation of the activity of the pedunculopontine nucleus (PPN) may be beneficial in the treatment of these symptoms. We report the clinical affects of deep brain stimulation (DBS) in the PPN and subthalamic nucleus (STN). Six patients with unsatisfactory pharmacological control of axial signs such as gait and postural stability underwent bilateral implantation of DBS electrodes in the STN and PPN. Clinical effects were evaluated 2–6 months after surgery in the OFF- and ON-medication state, with both STN and PPN stimulation ON or OFF, or with only one target being stimulated. Bilateral PPN-DBS at 25 Hz in OFF-medication produced an immediate 45% amelioration of the motor Unified Parkinson's Disease Rating Scale (UPDRS) subscale score, followed by a decline to give a final improvement of 32% in the score after 3–6 months. In contrast, bilateral STN-DBS at 130–185 Hz led to about 54% improvement. PPN-DBS was particularly effective on gait and postural items. In ON-medication state, the association of STN and PPN-DBS provided a significant further improvement when compared to the specific benefit mediated by the activation of either single target. Moreover, the combined DBS of both targets promoted a substantial amelioration in the performance of daily living activities. These findings indicate that, in patients with advanced Parkinson's disease, PPN-DBS associated with standard STN-DBS may be useful in improving gait and in optimizing the dopamine-mediated ON-state, particularly in those whose response to STN only DBS has deteriorated over time. This combination of targets may also prove useful in extra-pyramidal disorders, such as progressive supranuclear palsy, for which treatments are currently elusive.
Introduction
The pedunculopontine nucleus (PPN) plays an important role in the initiation and maintenance of locomotion in experimental animals (Skinner et al., 1990; Garcia-Rill et al., 1987). In MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-treated parkinsonian non-human primates, lesions of the PPN may produce akinesia (Nandi et al., 2002b); on the contrary, driving PPN activity by direct pharmacological activation or electrical stimulation increases motor activity (Jenkinson et al., 2005; Nandi et al., 2002a, b, 2004). Recently it has been suggested that PPN could be a therapeutic target to improve gait in certain parkinsonian patients (Mena-Segovia et al., 2004; Papahill and Lozano, 2000). Currently available therapies provide only variable degrees of control for axial signs of Parkinson's disease, such as deficits in gait and posture.
Two recent reports demonstrated that deep brain stimulation (DBS) in the PPN may be beneficial to patients with Parkinson's disease (Mazzone et al., 2005a; Plaha and Gill, 2005). The data to date suggest that PPN-DBS may be considered relatively safe and may improve motor function. While these preliminary reports are encouraging, longer term observations are required. In particular, the question arises as to whether PPN represents a fully alternative target area with respect to well-established implantation areas (e.g. the subthalamic nucleus, STN). This article reports our findings on six patients who underwent simultaneous bilateral PPN and STN-DBS implantation, followed up to 6 months postoperatively. Particular care was given to assessing both motor and gait subscores as well as the specific PPN versus STN mediated benefits.
Material and methods
The study refers to six Parkinson's disease patients double implanted in the PPN and STN. These subjects suffered from the advanced form of the disease as testified by severe Unified Parkinson's Disease Rating Scale (UPDRS; Fahn et al., 1987) section III impairment (>70) and rather disabling axial signs. Table 1 describes the patients’ main clinical and epidemiological characteristics. Table 2 provides their individual clinical evaluation scores in the presurgery phase. Our centre ensured each patient completely fulfilled the UK PDS Brain Bank diagnostic criteria for idiopathic Parkinson's disease. Table 2 demonstrates the unsatisfying impact of therapy on the activities of daily living (ADL, mean ON-state UPDRS section II is about 19) and axial symptoms. Freezing of gait in ON-state was eventually present, but quite inconsistent in at least three out of six subjects and, hence, not further investigated (but the analysis of PPN-DBS-mediated impact on freezing should represent a major research line as far as a larger series of patients will become available). None of these six patients exhibited tremor as a prominent clinical feature at time of surgery. Neither psychiatric nor cognitive deficits affected their quality of life. Implantation solely into the STN was unlikely to succeed, given the severe combination of peak dose dyskinesias plus major impairment of postural stability and walking. Therefore, we explored the possibility of a unique combination of targets, the STN plus the PPN. The patients were clearly informed by neurologists and neurosurgeons of the surgical risks due to the procedures. Written, informed consent was obtained from patients. The Local Ethics Committee approved the protocol and consent form describing the risks and potential benefits of the study.
Clinical features of Parkinson's disease patients selected for surgery
| Patient no. . | Age (years) . | Disease duration (years) . | l-Dopa therapy (years) . | LTTS duration (years) . | l-Dopa equivalent before surgery (mg) . |
|---|---|---|---|---|---|
| 1 | 62 | 13 | 11 | 3 | 1150 |
| 2 | 61 | 10 | 8 | 3 | 1200 |
| 3 | 67 | 11 | 8 | 5 | 875 |
| 4 | 66 | 16 | 16 | 6 | 1325 |
| 5 | 62 | 8 | 6 | 3 | 750 |
| 6 | 69 | 15 | 12 | 7 | 1250 |
| Mean | 64.5 | 12.1 | 10.1 | 4.5 | 1091.6 |
| SD | 3.2 | 3.0 | 3.6 | 1.7 | 227.3 |
| Patient no. . | Age (years) . | Disease duration (years) . | l-Dopa therapy (years) . | LTTS duration (years) . | l-Dopa equivalent before surgery (mg) . |
|---|---|---|---|---|---|
| 1 | 62 | 13 | 11 | 3 | 1150 |
| 2 | 61 | 10 | 8 | 3 | 1200 |
| 3 | 67 | 11 | 8 | 5 | 875 |
| 4 | 66 | 16 | 16 | 6 | 1325 |
| 5 | 62 | 8 | 6 | 3 | 750 |
| 6 | 69 | 15 | 12 | 7 | 1250 |
| Mean | 64.5 | 12.1 | 10.1 | 4.5 | 1091.6 |
| SD | 3.2 | 3.0 | 3.6 | 1.7 | 227.3 |
Clinical features of Parkinson's disease patients selected for surgery
| Patient no. . | Age (years) . | Disease duration (years) . | l-Dopa therapy (years) . | LTTS duration (years) . | l-Dopa equivalent before surgery (mg) . |
|---|---|---|---|---|---|
| 1 | 62 | 13 | 11 | 3 | 1150 |
| 2 | 61 | 10 | 8 | 3 | 1200 |
| 3 | 67 | 11 | 8 | 5 | 875 |
| 4 | 66 | 16 | 16 | 6 | 1325 |
| 5 | 62 | 8 | 6 | 3 | 750 |
| 6 | 69 | 15 | 12 | 7 | 1250 |
| Mean | 64.5 | 12.1 | 10.1 | 4.5 | 1091.6 |
| SD | 3.2 | 3.0 | 3.6 | 1.7 | 227.3 |
| Patient no. . | Age (years) . | Disease duration (years) . | l-Dopa therapy (years) . | LTTS duration (years) . | l-Dopa equivalent before surgery (mg) . |
|---|---|---|---|---|---|
| 1 | 62 | 13 | 11 | 3 | 1150 |
| 2 | 61 | 10 | 8 | 3 | 1200 |
| 3 | 67 | 11 | 8 | 5 | 875 |
| 4 | 66 | 16 | 16 | 6 | 1325 |
| 5 | 62 | 8 | 6 | 3 | 750 |
| 6 | 69 | 15 | 12 | 7 | 1250 |
| Mean | 64.5 | 12.1 | 10.1 | 4.5 | 1091.6 |
| SD | 3.2 | 3.0 | 3.6 | 1.7 | 227.3 |
Clinical motor scores of Parkinson's disease patients selected for surgery
| Patient no. . | UPDRS II (off/on) . | UPDRS III (off/on) . | UPDRS III item 27 rising from chair (off/on) . | UPDRS III item 28 posture (off/on) . | UPDRS III item 29 gait (off/on) . | UPDRS III item 30 postural stability (off/on) . |
|---|---|---|---|---|---|---|
| 1 | 35/20 | 82/42 | 3/2 | 3/2 | 4/2 | 3/1 |
| 2 | 32/18 | 76/37 | 3/2 | 3/1 | 4/3 | 3/1 |
| 3 | 30/17 | 75/32 | 3/2 | 3/2 | 4/2 | 3/1 |
| 4 | 28/21 | 72/44 | 3/2 | 3/2 | 3/2 | 3/2 |
| 5 | 31/22 | 69/38 | 2/1 | 2/1 | 4/3 | 2/1 |
| 6 | 26/16 | 71/32 | 3/2 | 2/1 | 3/2 | 2/1 |
| Mean | 30.3/19.3 | 74.1/37.5 | 2.8/1.8 | 2.7/1.5 | 3.7/2.3 | 2.7/1.2 |
| SD | 2.9/2.2 | 4.6/4.9 | 0.4/0.4 | 0.5/0.5 | 0.5/0.5 | 0.5/0.4 |
| Patient no. . | UPDRS II (off/on) . | UPDRS III (off/on) . | UPDRS III item 27 rising from chair (off/on) . | UPDRS III item 28 posture (off/on) . | UPDRS III item 29 gait (off/on) . | UPDRS III item 30 postural stability (off/on) . |
|---|---|---|---|---|---|---|
| 1 | 35/20 | 82/42 | 3/2 | 3/2 | 4/2 | 3/1 |
| 2 | 32/18 | 76/37 | 3/2 | 3/1 | 4/3 | 3/1 |
| 3 | 30/17 | 75/32 | 3/2 | 3/2 | 4/2 | 3/1 |
| 4 | 28/21 | 72/44 | 3/2 | 3/2 | 3/2 | 3/2 |
| 5 | 31/22 | 69/38 | 2/1 | 2/1 | 4/3 | 2/1 |
| 6 | 26/16 | 71/32 | 3/2 | 2/1 | 3/2 | 2/1 |
| Mean | 30.3/19.3 | 74.1/37.5 | 2.8/1.8 | 2.7/1.5 | 3.7/2.3 | 2.7/1.2 |
| SD | 2.9/2.2 | 4.6/4.9 | 0.4/0.4 | 0.5/0.5 | 0.5/0.5 | 0.5/0.4 |
Clinical motor scores of Parkinson's disease patients selected for surgery
| Patient no. . | UPDRS II (off/on) . | UPDRS III (off/on) . | UPDRS III item 27 rising from chair (off/on) . | UPDRS III item 28 posture (off/on) . | UPDRS III item 29 gait (off/on) . | UPDRS III item 30 postural stability (off/on) . |
|---|---|---|---|---|---|---|
| 1 | 35/20 | 82/42 | 3/2 | 3/2 | 4/2 | 3/1 |
| 2 | 32/18 | 76/37 | 3/2 | 3/1 | 4/3 | 3/1 |
| 3 | 30/17 | 75/32 | 3/2 | 3/2 | 4/2 | 3/1 |
| 4 | 28/21 | 72/44 | 3/2 | 3/2 | 3/2 | 3/2 |
| 5 | 31/22 | 69/38 | 2/1 | 2/1 | 4/3 | 2/1 |
| 6 | 26/16 | 71/32 | 3/2 | 2/1 | 3/2 | 2/1 |
| Mean | 30.3/19.3 | 74.1/37.5 | 2.8/1.8 | 2.7/1.5 | 3.7/2.3 | 2.7/1.2 |
| SD | 2.9/2.2 | 4.6/4.9 | 0.4/0.4 | 0.5/0.5 | 0.5/0.5 | 0.5/0.4 |
| Patient no. . | UPDRS II (off/on) . | UPDRS III (off/on) . | UPDRS III item 27 rising from chair (off/on) . | UPDRS III item 28 posture (off/on) . | UPDRS III item 29 gait (off/on) . | UPDRS III item 30 postural stability (off/on) . |
|---|---|---|---|---|---|---|
| 1 | 35/20 | 82/42 | 3/2 | 3/2 | 4/2 | 3/1 |
| 2 | 32/18 | 76/37 | 3/2 | 3/1 | 4/3 | 3/1 |
| 3 | 30/17 | 75/32 | 3/2 | 3/2 | 4/2 | 3/1 |
| 4 | 28/21 | 72/44 | 3/2 | 3/2 | 3/2 | 3/2 |
| 5 | 31/22 | 69/38 | 2/1 | 2/1 | 4/3 | 2/1 |
| 6 | 26/16 | 71/32 | 3/2 | 2/1 | 3/2 | 2/1 |
| Mean | 30.3/19.3 | 74.1/37.5 | 2.8/1.8 | 2.7/1.5 | 3.7/2.3 | 2.7/1.2 |
| SD | 2.9/2.2 | 4.6/4.9 | 0.4/0.4 | 0.5/0.5 | 0.5/0.5 | 0.5/0.4 |
Neurosurgery
The surgical procedure is described elsewhere (Mazzone et al., 2005a, b; Peppe et al., 2004; Stefani et al., 1997, 2002, 2006). Briefly, electrode implantation (Medtronic 3389) is performed in two target areas for each hemisphere through our ‘Maranello’ double arch system (Mazzone et al., 2004). To target the STN, the angle in the sagittal plane is 80–85° and 75–80° in the coronal plane, to obtain an extraventricular and extracapsular trajectory. The coordinates for this target are at the midpoint of the anterior commissure–posterior commissure (AC–PC) line, 11–12 mm lateral to the midline of the third ventricle, and 4 mm below AC–PC. To target the PPN, a simple indication of a fixed angle range in the sagittal plane is improper, given high interindividual variability. The key landmark to minimize surgical risks is the floor of the IVth ventricle (parallel to the brainstem axis). As a consequence, in each patient, the trajectory is performed strictly parallel to the floor of the IVth ventricle (and the angle is about 80–82° in the coronal plane). That said, the coordinates for PPN are –9/–13 mm lateral to the midline, 12.5/13 mm below PC; y = PC. The definitive choice of the more sensitive value (x coordinate) may also vary depending upon any patient's brainstem anatomy, the wideness of cisterna ambiens and the location of cerebral posterior artery with respect to these structures. Intra-operative microrecordings (MER) are performed routinely with FHC tungsten microelectrodes (1 MΩ) and are described in detail elsewhere (Galati et al., 2006; Stefani et al., 2006). By targeting the STN after PPN implantation (n = 4/6), we had the opportunity to study STN firing activity before and during PPN stimulation delivered at 10, 25 or 80 Hz (data not shown; Mazzone et al., 2006).
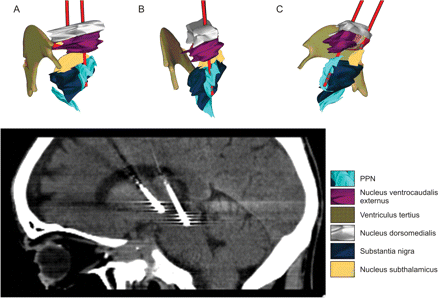
Postsurgery, the definitive electrode locations were verified by brain MRI or CT-scan. Figure 1 shows our presurgical planning, which includes a realistic reconstruction (based upon CT) of a patient's targets and their interrelations, and gives a representative sagittal postsurgical CT-scan, immediately following STN and PPN implantation. Figure 2 describes the PPN trajectories utilized in our cohort thus far. It is of interest that our targeting is never an ideal prolongation of distal STN regions but instead represents the region, well below the posterior ‘tail’ of the substantia nigra (SN), whose activation promises to modify both PPN-basal ganglia (BG) circuits and the descending pathway towards the spinal cord (Garcia-Rill, 1991). Although this targeting does not correspond to a certain clinical improvement, it unequivocally represents an alternative target, very distinct from a simple extension of the STN on mesencephalic subregions. Given these premises, the bottom of the 3389 lead is certainly below the anatomical boundary between the mesencephalon and pons, contact 0 or 1 is likely to involve the posterior SN (embedded inside the PPN), contact 1 (or 2) is commonly in the core of the PPN, and contacts 2 and 3 are near the lemniscus medialis and posterior to prelemniscal radiation (RAPRL); as a consequence, it is conceivable that our trajectory may modulate all different PPN functional subregions.
Top: 3D planning reconstructed by Maranello system in a representative patient (CT-based plans plus coordinate determination). Shown are axonometric projections in different spatial views focusing PPN and viciniori structures. The three images reflect a spatial representation from A (axonometric left oblique) through B (axonometric posterior–anterior) to C (right latero-lateral). Bottom: CT-scan (in the same patient), following monolateral implantation of STN and PPN (sagittal plane at 10.5 mm lateral to the midline).
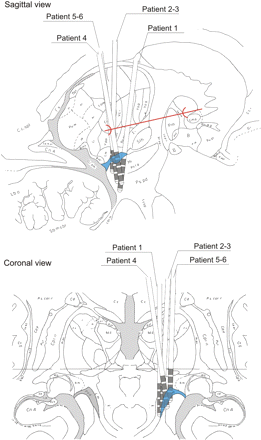
Schematic representation of PPN-implantation sites. Sagittal (12 mm lateral to the mid-sagittal plane) and coronal (around PC) diagrams, from the Schaltenbrand atlas, illustrating the PPN functional region with respect to surrounding major structures. Shown are the targeted implantation locations in all patients of the study (n = 6). Note the substantial supra-imposition of trajectories for patients 2/3 and 5/6. The PPN-targeted region was emphasized in cyan. The solid red line in sagittal view corresponds to the AC–PC line. Given the actual size of the Medtronic 3389, the leads traverse the whole region including portions of ZI and partially bounds the lemniscus medialis.
Patient evaluation
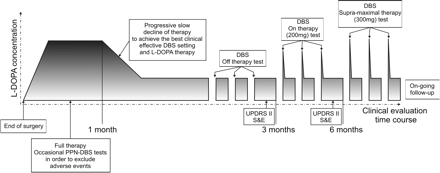
The careful clinical evaluation of this new combination of targets required, in each patient, a complex series of observations. In order to clarify our approach, Fig. 3 shows a flow-chart describing the postsurgery testing. Once Kinetra (Medtronic, Minneapolis, USA) is implanted, patients undergo a 3–4 week period dominated by (i) rapid restoration of presurgical therapy dosages (withdrawn before surgery) associated with (ii) random switching on of STN-DBS or PPN-DBS. The latter is of paramount importance in order to exclude adverse events for such a new target and to set reliable stimulation parameters. In this phase, we confirmed that there is no clinical benefit of PPN-DBS below 50 Hz (Mazzone et al., 2005a) and no major adverse events occurred (in particular, none affecting behaviour or cognition). The only commonly observed side effect was a disturbing paraesthesia following PPN activation (attributable to the lemniscus medialis; Fig. 2). Interestingly, this symptom always disappeared in <3 min, unless high voltage or high frequency stimulation was delivered. In two patients, for instance, paraesthesiae were extremely disturbing under 100 Hz even under 0.5–1 V, implying the recruitment of surrounding fibre pathways.
Schematic flow-chart of the postsurgery follow-up (x axis = time-course; y axis = l-dopa concentration). Note the following main phases: immediate postsurgery weeks (insertional period); DBS parameter optimal setting under stable drug-therapy; DBS testing in OFF-medication; DBS testing in ON-medication (distinguished in subdyskinetic and suprathreshold challenge tests). Note that, at least for these double-implanted PD patients, ADL scores and S&E scale were drawn from prolonged observations (1 week each) at around 3 and 6 months.
As this immediate postsurgery period ended, DBS was switched ON for 24 h/day either in the PPN or STN (or in both simultaneously) and anti-Parkinson's disease therapy was progressively reduced until the optimal stimulation setting was achieved. In order to ensure a reliable comparison between the pre- and postsurgical drug regimen (for the patient cohort investigated in this pioneering study), a small dose of non-ergot agonists (ropirinole and pramipexole) was re-introduced (mean 350 mg/day) along with an average of 425 mg l-dopa.
Comparative evaluations identified as optimal, standard stimulus parameters are the following: for PPN (bipolar contacts 0–1 and 4–5) = 60 μs pulse width, 25 Hz, 1.5–2 V; for STN (monopolar contact 1 or 2 and 5 or 6) = 90 μs pulse width, 185 Hz, 1.5–2.4 V. These stimulation parameters were consistently maintained throughout the clinical testing phase.
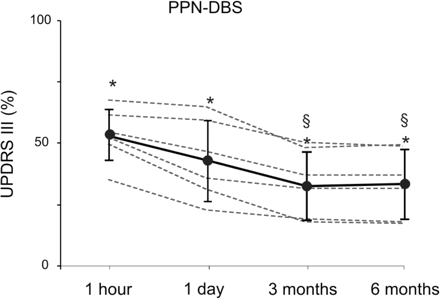
OFF-therapy evaluations were performed after an overnight therapy suspension (CAPIT) and started between 2 and 3 months postsurgery. The specific stimulation condition (STN or PPN or both) was activated randomly and blinded to the single neurologist in charge of the score. Each DBS (PPN or STN or combined activation) was maintained for about 24 h in order to avoid additive or artefactual responses (unless otherwise stated; Fig. 4). These early repetitive OFF-therapy testings, somehow unusual, were performed for three reasons: first, the need to acquire an average evaluation of the new targeting (only the anecdotal observations by ourselves and Plaha and Gill (2005) were previously available); second, we aimed to assess, without the possible interference of maximal/supramaximal DOPA concentrations, the chronic effectiveness of PPN and PPN plus STN-DBS on ADL; finally, this protocol allowed us to challenge the occurrence of a slight decline in PPN-mediated efficacy (as acknowledged in Fig. 4, see later).
Decline over time of PPN-DBS efficacy. The plot shows the percent (%) amelioration mediated by 1 h PPN-DBS in OFF-medication. The >40% clinical amelioration appreciable at 1 h decreases slightly at 1 day of postsurgery period and further in the subsequent follow-up. A steady-state response is reached only at 3 months. The UPDRS-III score at 3 and 6 months is, indeed, significantly worse (§) than the acute early assessments.
ON-medication assessments were considered reliable (and, hence, averaged) after 3 months following sufficient postsurgery stabilization and well-assessed DBS-mediated effects. Chronic therapy (mean 785 mg/day) was unchanged during this phase. Clinical challenge tests, performed in CAPIT, included the evaluation of maximal benefit after the administration of a standard 200 mg dose (Madopar dispersible) or following a supra-threshold dose of l-dopa methylester (50% higher = 300 mg).
Each clinical evaluation included the UPDRS-III. In order to evaluate gait and posture, we focused on the UPDRS-III dedicated items (27–30).
To assess the impact of PPN plus STN-DBS on ADL and patient self-sufficiency, the UPDRS II score (presurgery versus 3 and 6 months) and Schwab and England scale (S&E, presurgery versus 3 and 6 months) were performed. The UPDRS II and S&E scales were considered reliable only after prolonged observations; hence they were determined following 1 week free of CAPIT evaluations. Moreover, given that PPN-DBS alone had proven only slightly effective (consider, for instance, Fig. 5, upper plot), UPDRS II and S&E evaluations were limited to STN-DBS versus STN + PPN-DBS.
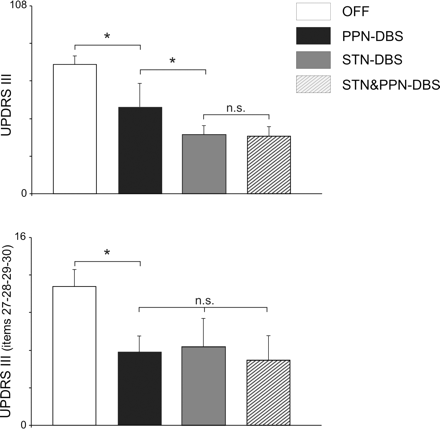
OFF-medication UPDRS-III score, under PPN-DBS versus STN-DBS versus PPN plus STN-DBS. Shown are the average UPDRS-III motor score (upper plot) or selected axial subscores (27–30 items, lower plot) in CAPIT with no stimulation (white columns) versus bilateral PPN-DBS alone, bilateral STN-DBS or STN-DBS plus PPN-DBS. Standard stimulus parameters were as follows: for PPN (bipolar contacts 0–1 and 4–5) = 60 μs pulse width, 25 Hz, 1.5–2 V; for STN (monopolar contact 1 or 2 and 5 or 6) = 90 μs pulse width, 185 Hz, 1.5–2.4 V. Note that PPN-DBS produced a significant 33% amelioration (clearly lower than STN alone = 54.1%). On the contrary, axial subscore evaluation (lower plot) demonstrates that PPN-DBS promoted a clinical change larger than the STN-DBS one (although not reaching significance).
Data analysis
The effects of different DBS modalities on the UPDRS score were studied by means of non-parametric one-way Friedman ANOVA in the OFF-medication state (Fig. 5, stimulation-OFF, STN-ON, PPN-ON and STN + PPN-ON). The ON-medication state was studied with a one-way Friedman ANOVA and compared to the basal condition in each modality (stimulation-OFF, STN-ON, PPN-ON and STN + PPN-ON).
When any statistically significant effect was present for the main factors, comparisons were made by means of the Wilcoxon matched pairs test. The accepted significance level was P < 0.05.
Results
As shown by images of the planned sites for implantation and the postsurgery CT-scan in Fig. 1, the PPN was targeted as a region well below and posterior to the STN macro-electrode. Figure 2 provides both sagittal and coronal illustrative diagrams [(from Schaltenbrand (1977) tables] showing the trajectories utilized in our patients (n = 6). Future larger series of PPN-implanted patients should clarify to what extent the slight differences in coordinates (mostly in the mediolateral axis) correlate with clinical efficacy. The most distal contacts (0 and 1) target the PPN with the obvious involvement (due to the actual lead size) of posterior SN segments. Coronal perspective (lower part) demonstrates that contact 1 (apparently misguided in sagittal view due to the peculiar PPN arch-like configuration) also targeted the appropriate dorsal PPN subregions.
As is routine, intra-operative MER were utilized in order to provide unequivocal identification of the PPN. In our previous paper (Mazzone et al., 2005a), we showed a peculiar feature of the PPN pars disseminata (PPNd) or PPN pars compacta (PPNc) subunits, as distinct from SN multi-units crossed at –7/–10 mm with respect to the AC–PC. At present, we are investigating the STN firing changes detectable after PPN implantation and activation. Low-frequency stimulation in the PPN (25 Hz, 2 V) promoted a clear-cut modulation in STN firing activity (data not shown; Mazzone et al., 2006).
Figure 4 details the steady-state (at 3 months postsurgery) UPDRS-III in OFF-medication. Single conditions (either PPN-DBS or STN-DBS or combined targets) were performed in different morning sections, randomly and blinded to the neurologists in charge (Fig. 3). Each stimulation condition produced a statistically significant reduction of the UPDRS-III in comparison with the OFF-condition (Friedman ANOVA: main effect Modality: P < 0.001). The Wilcoxon matched pairs test showed a statistically significant UPDRS-III reduction during PPN-DBS (P < 0.05), STN-DBS (P < 0.01) and STN + PPN-DBS (P < 0.01) in comparison with OFF-stimulation. The PPN-mediated effect promoted a slight reduction of UPDRS section III (black column, average 33%; Fig. 4), significantly lower than the impact of STN alone (about 54% mean amelioration). The combined activation of both targets showed a tendency to provide further improvement (56%), but no significant difference emerged between STN-DBS and STN + PPN-DBS (Fig. 5, upper plot). The Wilcoxon matched pairs test showed a statistically significant UPDRS mean reduction during STN-DBS (P < 0.01) and STN + PPN-DBS (P < 0.01), when compared to PPN-DBS alone (Fig. 4, upper plot).
If we take a closer look at axial signs (Fig. 5, lower plot), however, PPN-DBS seemed more effective. The UPDRS mean reduction (items 27–30) was statistically significant during PPN-DBS (P < 0.01), STN-DBS (P < 0.01) and STN + PPN-DBS (P < 0.01) as well. Table 3 provides an analytical description of each patient's score (items 27–30). No significant difference amongst the three DBS conditions was detected although the combined stimulation of both targets implemented a slightly better response (consider, for instance, ‘rising from a chair’ and gait, 27 and 29, respectively).
Effect of bilateral stimulation of PPN, STN, PPN + STN on OFF-medication UPDRS subscore
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 3 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 2 |
| 4 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| 5 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Mean | 1.0 | 1.0 | 0.8 | 1.5 | 1.5 | 1.3 | 1.0 | 1.1 | 0.6 | 1.3 | 1.3 | 1.1 |
| SD | 0.0 | 0.6 | 0.4 | 0.8 | 0.8 | 0.5 | 0.0 | 0.4 | 0.5 | 0.5 | 0.5 | 0.7 |
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 3 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 2 |
| 4 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| 5 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Mean | 1.0 | 1.0 | 0.8 | 1.5 | 1.5 | 1.3 | 1.0 | 1.1 | 0.6 | 1.3 | 1.3 | 1.1 |
| SD | 0.0 | 0.6 | 0.4 | 0.8 | 0.8 | 0.5 | 0.0 | 0.4 | 0.5 | 0.5 | 0.5 | 0.7 |
Effect of bilateral stimulation of PPN, STN, PPN + STN on OFF-medication UPDRS subscore
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 3 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 2 |
| 4 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| 5 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Mean | 1.0 | 1.0 | 0.8 | 1.5 | 1.5 | 1.3 | 1.0 | 1.1 | 0.6 | 1.3 | 1.3 | 1.1 |
| SD | 0.0 | 0.6 | 0.4 | 0.8 | 0.8 | 0.5 | 0.0 | 0.4 | 0.5 | 0.5 | 0.5 | 0.7 |
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 3 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 2 |
| 4 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| 5 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Mean | 1.0 | 1.0 | 0.8 | 1.5 | 1.5 | 1.3 | 1.0 | 1.1 | 0.6 | 1.3 | 1.3 | 1.1 |
| SD | 0.0 | 0.6 | 0.4 | 0.8 | 0.8 | 0.5 | 0.0 | 0.4 | 0.5 | 0.5 | 0.5 | 0.7 |
An intriguing aspect of PPN-DBS-mediated acute effects on motor score is highlighted by Fig. 4, which demonstrates a slight decline over time. Shown are the averaged section III score (after normalization) at 1 h following surgery; 1 day, 1 week, 3 months and 6 months. The very first clinical assessment was surprising, with a peculiar >40% mean benefit associated with the subject's enthusiasm (at least in five out of six patients). Yet, this ‘immediate’ dramatic PPN-driven impact cannot be consistently replicated with the following evaluations despite an ample interpatient variability. As any team familiar with postsurgery Parkinson's disease patients knows, well balanced and repeated clinical observations are necessary to avoid interference from placebo-like effects (Benedetti et al., 2003). There were no major differences in observations at 3 or 6 months and only then was the described benefit under the condition ON-PPN considered reliable (Fig. 5). A similar time-related effect with an early reduction of stimulation efficacy was not observed when STN-DBS was challenged at different postsurgery times (data not shown).
As therapy at a minimal effective dosage was stabilized (average 425 mg l-dopa/day, ranging from 375 to 650 mg and at least 1 month of a stable drug regimen plus a variable combination of non-ergot agonists for a final equivalent dosage of about 780 mg), a more comprehensive clinical assessment was performed, including UPDRS-II and S&E scale (chronic assessment) and acute ON-medication UPDRS-III (Fig. 6; Tables 4 and 5).
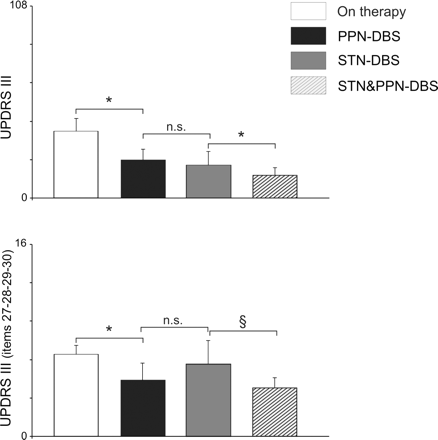
ON-medication (200 mg l-dopa) UPDRS score, under PPN-DBS, STN-DBS and PPN plus STN-DBS. Shown are the average UPDRS motor score (upper plot) or selected axial items (27–30, lower plot) in ON-medication (60 min following 200 mg l-dopa challenge, but see Table 5 for supra-threshold dose). Drug-mediated scores versus drug plus bilateral PPN-DBS alone, bilateral STN-DBS or the combined activation of both targets are compared. Standard parameters were: for PPN (bipolar contacts 0–1 and 4–5) = 60 μs pulse width, 25 Hz, 1.52 V; for STN (monopolar contact 1 or 2 and 5 or 6) = 90 μs pulse width, 185 Hz, 1.5–2.4 V. PPN-DBS ameliorated global section III by >44% and PPN-DBS plus STN-DBS produced an impressive 66.4%. More importantly, axial subscores (lower plot) demonstrated the significance of the combined targets stimulation with respect to either single target (P < 0.05).
Effect of bilateral stimulation of PPN, STN, PPN + STN on ON-medication (200 mg) UPDRS subscore
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 2 | 0 | 1 | 0 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Mean | 0.7 | 0.8 | 0.5 | 1.3 | 1.5 | 1.1 | 0.6 | 1.1 | 0.3 | 0.8 | 1.0 | 0.8 |
| SD | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 | 0.5 | 0.8 | 0.5 | 0.4 | 0.6 | 0.4 |
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 2 | 0 | 1 | 0 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Mean | 0.7 | 0.8 | 0.5 | 1.3 | 1.5 | 1.1 | 0.6 | 1.1 | 0.3 | 0.8 | 1.0 | 0.8 |
| SD | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 | 0.5 | 0.8 | 0.5 | 0.4 | 0.6 | 0.4 |
Effect of bilateral stimulation of PPN, STN, PPN + STN on ON-medication (200 mg) UPDRS subscore
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 2 | 0 | 1 | 0 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Mean | 0.7 | 0.8 | 0.5 | 1.3 | 1.5 | 1.1 | 0.6 | 1.1 | 0.3 | 0.8 | 1.0 | 0.8 |
| SD | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 | 0.5 | 0.8 | 0.5 | 0.4 | 0.6 | 0.4 |
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 2 | 0 | 1 | 0 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Mean | 0.7 | 0.8 | 0.5 | 1.3 | 1.5 | 1.1 | 0.6 | 1.1 | 0.3 | 0.8 | 1.0 | 0.8 |
| SD | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 | 0.5 | 0.8 | 0.5 | 0.4 | 0.6 | 0.4 |
Effect of bilateral stimulation of PPN, STN, PPN + STN on ON-medication (suprathreshold dose, 300 mg) UPDRS sub-score
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 2* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| 6 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Mean | 0.5 | 0.8 | 0.3 | 1.0 | 1.3 | 1.3 | 0.3 | 0.8 | 0.0 | 1.0 | 1.3 | 1.0 |
| SD | 0.6 | 0.5 | 0.5 | 0.0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.0 | 0.0 | 0.5 | 0.0 |
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 2* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| 6 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Mean | 0.5 | 0.8 | 0.3 | 1.0 | 1.3 | 1.3 | 0.3 | 0.8 | 0.0 | 1.0 | 1.3 | 1.0 |
| SD | 0.6 | 0.5 | 0.5 | 0.0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.0 | 0.0 | 0.5 | 0.0 |
*Patients manifesting disabling dyskinesias under 300 mg l-dopa.
Effect of bilateral stimulation of PPN, STN, PPN + STN on ON-medication (suprathreshold dose, 300 mg) UPDRS sub-score
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 2* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| 6 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Mean | 0.5 | 0.8 | 0.3 | 1.0 | 1.3 | 1.3 | 0.3 | 0.8 | 0.0 | 1.0 | 1.3 | 1.0 |
| SD | 0.6 | 0.5 | 0.5 | 0.0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.0 | 0.0 | 0.5 | 0.0 |
| Patient no. . | UPDRS III item 27 rising from chair . | UPDRS III item 28 posture . | UPDRS III item 29 gait . | UPDRS III item 30 postural stability . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . | PPN . | STN . | PPN + STN . |
| 1* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 2* | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 5 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| 6 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Mean | 0.5 | 0.8 | 0.3 | 1.0 | 1.3 | 1.3 | 0.3 | 0.8 | 0.0 | 1.0 | 1.3 | 1.0 |
| SD | 0.6 | 0.5 | 0.5 | 0.0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.0 | 0.0 | 0.5 | 0.0 |
*Patients manifesting disabling dyskinesias under 300 mg l-dopa.
In order to limit drug-induced involuntary movements, a l-dopa morning dose was a standard 200 mg; as a consequence, it produced a significant (P < 0.001) UPDRS mean decrease in the OFF-stimulus condition of 50.2% (not shown). PPN-DBS, during this l-dopa regimen, produced a significant (P < 0.01) additional UPDRS mean decrease of a surprising 44.3% (black column in Fig. 6, upper graph); STN-DBS promoted a similar (P < 0.01) additional UPDRS mean decrease of 51%. Noticeably, STN-DBS plus PPN-DBS induced a significant (P < 0.01) additional dramatic decrease around 66.4%.
Therefore, at variance from OFF-medication evaluations, illustrated in Fig. 4, the association of l-dopa and combined activation of both targets provided a significant (P < 0.05) performance gain compared to l-dopa associated with a single target activation. In other words, our patients experienced a better response (if compared to OFF-medication condition shown in Fig. 4 and Table 3) when both PPN and STN were activated. This applies to standard section III but, more effectively, to axial subscores (Fig. 6, lower plot and Table 4). In particular, the mean walking capability (item 29), above 2 (n = 6) in the best ON before surgery, and never <1 under STN-DBS, scored 0.3 (n = 6) following PPN plus STN-DBS (Table 4) with four out of six patients manifesting a normal gait.
The opportunity to determine unequivocally the selective role of PPN-DBS plus STN-DBS in ON-medication suggested the utilization of a suprathreshold dose that was a 50% higher (300 mg; Albanese et al., 2001). Table 5 shows the UPDRS III scores for rising from chair, posture, gait and posture stability. In two out of six patients, dyskinesias were extremely disabling, rendering clinical evaluation inconsistent. In one patient, involuntary movements had only a slight dystonic appearance in upper right arm, not impairing gait; in the other three, dyskinesias were negligible (n = 1) or limited to upper limbs (n = 2), allowing a reliable evaluation. As a consequence, it was confirmed that DBS-PPN + DBS-STN promoted a significant improvement when compared with DBS-STN alone.
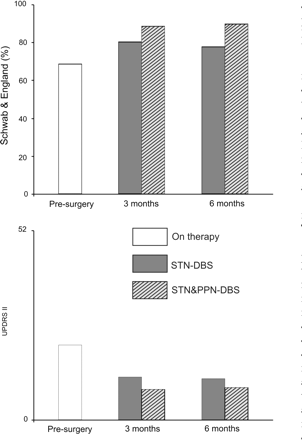
Finally, S&E scale for ADL provided a further reassessment on the chronic beneficial effect achieved by adding PPN-DBS to standard STN-DBS (Fig. 7, upper plot). Consistently, UPDRS II confirmed the effectiveness of PPN-DBS when added to STN alone (Fig. 7, lower plot).
S&E scale (upper plot) and UPDRS II score (lower plot) following a week of observation under chronic drug therapy. The following conditions are illustrated: presurgery ON therapy, postsurgery STN-DBS and postsurgery STN-DBS plus PPN-DBS (at 3 and 6 months). The combined target activation provided a better performance on ADL and UPDRS-II scores.
Discussion
This article confirms that the PPN, implantable through a safe trajectory (Mazzone et al., 2005a), may represent a new target area in association with the standard STN when aiming at optimal control of axial motor impairment.
In all of the 12 tegmentum pontis studied, neither impairment of ocular saccades nor changes in vigilance were observed. In contrast with previous experience in primates (Nandi et al., 2002a, b, 2004), we have not detected any ‘stun’ effect related to the mechanical insertion. In fact, no temporary reduction of motor activity was detected during the peri-operative or immediate post-operative phases. On the contrary, intra-operative, low-frequency stimulation was followed by a sort of pleasant arousal and beneficial effect on akinesia (Mazzone et al., 2005a). Neuropsychological evaluations, although preliminary due to the limited observation time, so far have excluded any cognitive impact of PPN implantation and activation. The only relevant event suffered by our patients was a transient paraesthesia (involving inferior limbs) as the PPN implant was switched on, mostly in patients with a more medial trajectory (for example patient 1). More importantly, clinical evaluations at 3 and 6 months highlighted the beneficial effect of PPN + STN double implantation, mostly on gait items (see later).
OFF-medication evaluation
Clinical assessments in the OFF-medication state have shown that PPN low-frequency stimulation is beneficial, although its impact on hypokinetic signs was clearly less impressive than STN-DBS alone. The combined activation of both targets did not promote a substantial additional benefit to STN stimulation, at least when UPDRS-III was examined (Fig. 4). This finding implies that the PPN alone is not an alternative target for Parkinson's disease patients. As a consequence, following the very first OFF-medication evaluation (first 3 months) the condition ‘PPN-DBS alone’ was commonly avoided unless otherwise stated (i.e. 24-h testing in ON-medication state). In brief, we do not regard PPN as a substitute for STN as a common DBS target in idiopathic Parkinson's disease, due to its high cost and complex procedure. Nevertheless, the benefit in ADL, promoted by standard STN-DBS in OFF-medication state, tends to decline with the natural course of the disease (see, for instance, Krack et al., 2003). At present, longer evaluation (1–5 years) is required to determine whether the combination of PPN- and STN-DBS will help to render patients’ performance more stable. The additive effect of PPN-DBS (when combined with STN-DBS) on the UPDRS-II and S&E scales are, in this context, promising.
PPN activation also proved significantly effective on items whose sensitivity to common pharmacological agents or standard STN-DBS is variable and frequently unsatisfactory (Lang and Lozano, 1998), at least after several years of disease. The small but significant influence on postural stability and gait (as detailed in Fig. 4, lower plot and Fig. 6), otherwise only slightly improved by dopaminergic medication, represents a key advance for severe Parkinson's disease patients and possibly also for extrapyramidal syndromes such as progressive supranuclear palsy (PSP).
Earlier postsurgical tests (1 h, 1 day) showed more impressive PPN-mediated benefits on rigidity and akinesia. These promising ‘immediate’ responses to PPN activation (Fig. 5) were not maintained as the clinical assessment proceeded. In this regard, our observations are in conflict with Plaha and Gill (2005). This may reflect the shorter follow-up period (16 or 42 days), or the more dorsal implantation site in that study. Since they suggested a robust clinical response to the monopolar stimulation of contact 3, we studied the best combination (either bipolar or monopolar) in our six subjects. In our hands, lower contacts (0–1 bipolar), whose embedding in PPN is indisputable, provided the best clinical response. We are aware that any contact stimulation (even bipolar) is likely to affect functional regions well beyond the mere ‘core’ of any targeted area (Plaha et al., 2006). Thus, our final implantation ‘inside’ PPN (Fig. 2) implies the involvement of other bordering areas. The modulation of PPN fibres outwardly impinging on internal pallidus (GPi)-STN as well as SN portions or other brainstem subregions is basically unavoidable.
At present, in Parkinson's disease patients, the concurrent or competitive role of PPN-related ascending versus descending pathways is far from being fully understood. Our preliminary intra-operative data suggest that PPN-stimulation modulates STN firing activity, facilitating a train-like pattern (Mazzone et al., 2006). In principle, this finding is not surprising, since it is well known that the ascending projections of PPN are abundant and widespread, the PPN-thalamic pathway (mostly to parafascicular nucleus, Capozzo et al., 2003) and PPN-GPi being two of the most prominent ones. In particular, an extensive projection from PPN to STN is well documented in monkeys (Lavoie and Parent, 1994) and is presumed to be bilateral and composed of PPN efferent collaterals to the pallidal complex.
Ongoing neurophysiological investigations (in our patients) are also clarifying to what extent the excitability threshold of the soleus Hoffmann reflex (HR) is modified by PPN-DBS. Of particular interest in this group of patients with bilateral STN and PPN implantation is the specific role of each region in directly inducing significant changes on spinal cord circuitries connected to lower limb muscles, whose activity is clearly involved in posture and locomotion (Spann and Grofova, 1989).
ON-medication evaluation
The key finding of our study is that PPN low-frequency activation potently ameliorates the ON-drug/ON-STN condition (Fig. 6). This result is striking, in light of the assumption that the best drug-mediated ON-state should be comparably as effective as drug plus DBS (and not significantly less effective). On the other hand, evidence-based treatment recommendations, based on all available pharmacological and surgical approaches, support the usefulness of STN-DBS for improving motor function and reducing off-time, albeit with a finite period of postsurgical utility (Pahwa et al., 2006). We are aware that a clinical improvement of almost 50% under STN-DBS or STN- plus PPN-DBS (in ON-state and with respect to the l-dopa response, Fig. 6) may sound unusual. Yet, 200 mg l-dopa might not represent the maximal dose, implying a possible underestimation of drug-mediated peak effect, at least in some of our patients. As only four out of six patients tolerated supramaximal concentrations (Table 5), the potential comparison was therefore not possible. Previous reports have described conflicting results on this issue. Anderson et al. (2003) found that DOPA alone and DOPA plus STN-DBS provided a similar degree of benefit (at least on motor UPDRS). In contrast, Rodriguez-Oroz et al. (2005) estimated an additional effect of STN-DBS to the best drug-induced ON-state (about 30% at 1 year observation). Intriguingly, STN-DBS provided an improvement to some axial signs implying a synergistic effect between l-dopa and STN stimulation (not found for limb signs; Bejjani et al., 2000).
The effect of PPN-DBS plus STN-DBS in ON-state (versus OFF-state) may appear paradoxical, since axial symptoms, if developing late in the natural course of the disease, are generally ascribed to ‘non-dopaminergic’ brainstem degeneration. In fact, the PPN-mediated benefits (exceeding those of l-dopa) support the possibility that PPN-mediated ascending projections—either direct to the SN or indirect through the parafascicular nucleus and/or STN—modulate endogenous dopamine signalling pathways.
Considering our protocol (bipolar stimulation of more distal contacts), stimulus parameters (60 µs, 2 V, 25 Hz) and the size of the electrocatheter, it is likely that the electrical dipole does not act exclusively on small or specific subpopulations of neurons. Although some degree of clustering (mostly for cholinergic neurons) was described in rodent PPN, our clinical observations are likely to derive from a combined stimulation of both glutamate and acetylcholine cell bodies in the densely cellular (PPNc) and diffuse (PPNd) areas (Bevan et al., 1995; Lavoie and Parent, 1994; Mesulam et al., 1989; Takakusaki et al., 1996). Future utilization of smaller devices, active on specific pathways, should be tested on MPTP-intoxicated non-human primates (and eventually in patients), in order to assess performance under the activation of more selective neuronal or fibre pathways. Since, however, double-labelled neurons (for glutamate and acetylcholine) are abundant in PPN (Lavoie and Parent, 1994b), a microdialysis study (Fedele et al., 2001; Stefani et al., 2005) seems well suited to addressing the extracellular release of PPN projections on target areas.
Conclusions
Parkinsonian axial deficits represent a challenging task when disease progresses, although some degree of l-dopa-sensitivity may persist even in severe patients with motor fluctuations (Hughes et al., 1994). Traditional STN-DBS may provide some benefits and a synergistic effect of DOPA and STN-DBS was documented, as outlined by walking and postural stability score (Bejjani et al., 2000). Yet, another series of STN-implanted patients showed substantial equivalence amongst l-dopa and STN-mediated benefit on posture subscores (Maurer et al., 2003). In a large cohort of severe Parkinson's disease patients, the STN-DBS-mediated impact on limb signs seems more striking than gait (Rodriguez-Oroz et al., 2005); and the latter tends to decline in prolonged follow-up (5–10 years). Our patients manifested already an unsatisfactory control of axial signs even when the best combination of dopamine-mimetic agents was utilized (compare, for instance, 50% amelioration of global UPDRS section III versus 35% for item 27 and 37% for item 29).
The postsurgery study demonstrates that PPN may represent a strong candidate to provide additional amelioration of items otherwise difficult to treat. More importantly, the two-target stimulation proved more effective than the stimulation of each target alone. In brief, the combination of targets adds value to the control of axial signs. This evidence might support the selection of previously STN-implanted patients (with poor therapeutic response) for additional PPN targeting. That said, the unimpressive effect of PPN-DBS, when stimulated alone, on UPDRS-III in OFF-therapy suggests that PPN is not a fully alternative target. In this respect, preliminary data (Plaha and Gill, 2005) should be viewed with extreme caution. Nevertheless, extended follow-up [(as in the 5-year post-STN implantation prospective study by Krack et al. (2003)] documented some degree of deterioration in the STN-mediated impact on axial signs and akinesia. At present, it is unclear whether STN + PPN combination will show an analogous pattern, with the same decline in efficacy.
PPN-DBS may increase the efficacy of exogenously administered therapy. Combined DBS (PPN + STN) was significantly more efficacious when motor performance was assessed in ON-medication (under both subdyskinetic and suprathreshold concentrations). Moreover, despite the short follow-up, PPN-DBS plus STN-DBS significantly improved the ADL and patient's self-sufficiency. It is hard to determine the possible mechanisms underlying this PPN-mediated benefit. Speculations involve an active role of PPN-DBS on the endogenous release of dopamine or on the intrastriatal dopamine and acetylcholine balance.
Undesired involuntary movements occurred in two out of six patients with l-dopa supramaximal dose (together with PPN-DBS and STN-DBS). This is not unexpected if PPN-DBS exceeds the l-dopa response by recruiting endogenous amines. On one hand, this clinical observation points out that PPN should never be considered a good candidate for treating dyskinesias; on the other, it reinforces the need for prolonged follow-up observations, comparing different drug regimens.
The surprising, although slight, decline of PPN-mediated efficacy on motor items, when comparing early versus late OFF response in CAPIT raises a major question, concerning the possible occurrence of complex adaptive changes. Nevertheless, analogous response deterioration did not feature PPN-mediated effects on gait and posture. Given the relatively small number of patients (and the difference in the time dependency of the response's decline) it is difficult to identify a conclusive explanation. It is possible that unconventional (i.e. cyclic) protocol of stimulation would be preferable for maintaining larger effectiveness of PPN activation.
At present, we are testing the efficacy of PPN cyclic stimulation, together with continuous delivery of STN-DBS, in idiopathic Parkinson's disease patients. In addition, we are also investigating to what extent PPN-DBS could be an effective option in otherwise untreatable advanced forms of extrapyramidal disorders such as PSP.
Acknowledgements
We express our gratitude to Dr Atticus H. Hainsworth (St George's Hospital, London) for critical reading and editing of the manuscript. We are profoundly thankful to Stefano Sposato MD and Giuseppe Gattoni for radiological and technical assistance. This manuscript has been supported by grants from Ministero Della Salute, Regione Piemonte and Friuli Venezia Giulia to A.S. and P.S.
References
Abbreviations:
- PPN
pedunculopontine nucleus
- STN
subthalamic nucleus
- DBS
deep brain stimulation
- SN
substantia nigra