-
PDF
- Split View
-
Views
-
Cite
Cite
Gwenaëlle Douaud, Stephen Smith, Mark Jenkinson, Timothy Behrens, Heidi Johansen-Berg, John Vickers, Susan James, Natalie Voets, Kate Watkins, Paul M. Matthews, Anthony James, Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia, Brain, Volume 130, Issue 9, September 2007, Pages 2375–2386, https://doi.org/10.1093/brain/awm184
Close - Share Icon Share
Abstract
Adolescent-onset schizophrenia provides an exceptional opportunity to explore the neuropathology of schizophrenia free from the potential confounds of prolonged periods of medication and disease interactions with age-related neurodegeneration. Our aim was to investigate structural grey and white matter abnormalities in adolescent-onset schizophrenia. Whole-brain voxel-wise investigation of both grey matter topography and white matter integrity (Fractional Anisotropy) were carried out on 25 adolescent-onset schizophrenic patients and 25 healthy adolescents. We employed a refined voxel-based morphometry-like approach for grey matter analysis and the recently introduced method of tract-based spatial statistics (TBSS) for white matter analysis. Both kinds of studies revealed widespread abnormalities characterized by a lower fractional anisotropy neuroanatomically associated with localized reduced grey matter in the schizophrenic group. The grey matter changes can either be interpreted as the result of a locally reduced cortical thickness or as a manifestation of different patterns of gyrification. There was a widespread reduction of anisotropy in the white matter, especially in the corpus callosum. We speculate that the anisotropy changes relate to the functional changes in brain connectivity that are thought to play a central role in the clinical expression of the disease. The distribution of grey matter changes was consistent with clinical features of the disease. For example, grey and white matter abnormalities found in the Heschl's gyrus, the parietal operculum, left Broca's area and the left arcuate fasciculus (similar to previous findings in adult-onset schizophrenia) are likely to relate to functional impairments of language and auditory perception. In addition, in contrast to earlier studies, we found striking abnormalities in the primary sensorimotor and premotor cortices and in white matter tracts susbserving motor control (mainly the pyramidal tract). This novel finding suggests a new potential marker of altered white matter maturation specific to adolescent-onset schizophrenia. Together, our observations suggest that the neuropathology of adolescent-onset schizophrenia involves larger and widespread changes than in the adult form, consistent with the greater clinical severity.
Introduction
Various models of the pathophysiological process in schizophrenia are still debated. Although a neurodevelopmental hypothesis for schizophrenia is now well established (Rapoport et al., 2005), some observations still suggest that a contribution from a degenerative process following the onset of psychosis is superimposed on the developmental impairments (Lieberman, 1999; Church et al., 2002; Perez-Neri et al., 2006). Other reports suggest that altered plasticity may also play a pathogenic role in the disease (Feinberg, 1982; Sporn et al., 2003; Vidal et al., 2006).
Early-onset schizophrenia offers a unique opportunity to explore the aetiology of this major mental disorder. In particular, an understanding of the interplay of the disease-associated pathology and normal brain development may offer crucial insights into the pathophysiological process of the disease. Age-related changes in grey matter throughout normal adolescence are dynamic, with substantial thinning of cortical grey matter starting initially in primary areas and occurring later in the secondary cortices of the frontal and parietal lobes and finally in the temporal lobes (Giedd et al., 1999; Sowell et al., 2001; Gogtay et al., 2004; Paus, 2005). In early-onset schizophrenia, the rate of grey matter loss appears greater, with larger changes found in parietal brain regions extending anteriorly into temporal lobes, involving also the sensorimotor and dorsolateral prefrontal cortices, as well as frontal eye fields (Thompson et al., 2001) and with a superior medial frontal grey matter loss later reaching the cingulate gyrus (Vidal et al., 2006).
Fractional anisotropy (FA), a proxy measure of white matter integrity, normally increases from the neonatal period to adulthood (Schneider et al., 2004; Barnea-Goraly et al., 2005; Ben Bashat et al., 2005; Ashtari et al., 2007). Two diffusion tensor imaging (DTI) studies of adolescent-onset schizophrenia have revealed reduced FA in the frontal and in the right occipital white matter, and in the left posterior hippocampus (Kumra et al., 2004; Kumra et al., 2005; White et al., 2007). Current theories of schizophrenia highlight the potential role of altered brain connectivity that may be manifest at a macro-anatomical level through structural changes of white matter tracts (Stephan et al., 2006).
However, as most cases are first diagnosed between the age of 20 and 25 years, the majority of structural brain imaging studies in schizophrenia thus far has been confined to adult subjects. We are aware of only a few studies that have explored whole-brain changes in early-onset schizophrenia on a voxel-by-voxel basis (Sowell et al., 2000; Thompson et al., 2001; Vidal et al., 2006). Moreover, despite a prolific literature, the structural cerebral changes revealed in adult-onset schizophrenia have previously shown great inconsistencies, partly due to the heterogeneity of the methods applied and to special methodological problems in working with this disease population, such as appropriately handling the enlargement of ventricles (Shenton et al., 2001; Honea et al., 2005; Kanaan et al., 2005; Kubicki et al., 2005; Walterfang et al., 2006; Kubicki et al., 2007). In performing the work described here, we took advantage of recent advances in voxel-based grey matter morphometry and white matter integrity analyses, as well as more appropriate statistical inferences.
The first aim of our study was to investigate differences in the topographic distribution of grey matter between adolescent-onset schizophrenic patients and healthy adolescent subjects, making no a priori assumptions about the location of possible abnormalities. Second, using diffusion-weighted images, we tested for alterations in the white matter integrity that could be related to grey matter changes, to work towards building a more comprehensive neuroanatomical characterisation of the disease.
Methods
The study was undertaken in accordance with the guidance of the Oxford Psychiatric Research Ethics Committee and written consent was obtained from all participants (and their parents if under the age of 16 years).
Subjects
Twenty-five adolescent-onset schizophrenic participants (18 men, 7 women, aged 13 to 18 years) were recruited from the Oxford regional adolescent unit and surrounding units. All were diagnosed as having DSM IV (APA, 1994) schizophrenia, using the Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al., 1997). In addition, the participants were administered the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1989). Age at onset of symptoms ranged from 11 to 17 years. All schizophrenic patients were receiving atypical antipsychotics (Table 1).
Demographics data of adolescent-onset schizophrenic patients and controls
| . | AOS patients . | Controls . |
|---|---|---|
| Gender M/F | 18/7 | 17/8 |
| Age M (mean ± SD) | 16.5 ± 1.3 | 16.2 ± 1.7 |
| Age F (mean ± SD) | 15.9 ± 1.5 | 15.6 ± 1.3 |
| Handedness R/L | 20/5 | 21/4 |
| Full scale intelligence quotient (range, mean ± SD) | 66–123, 87 ± 14 | 64–127, 108 ± 15 |
| Socio-economic status (The national statistics socio-economic classification.http://www.statistics.gov.uk/methods____quality/ns____sec/) | 4 ± 1.5 | 3.5 ± 1.5 |
| Age at onset of symptoms (range, mean ± SD) | 11–16.8, 14.9 ± 1.6 | – |
| Disease duration (mean ± SD) | 1.4 ± 0.7 | – |
| PANSS: positive scores (mean ± SD) | 22 ± 5 | – |
| PANSS: negative scores (mean ± SD) | 16 ± 5 | – |
| Chlorpromazine equivalents (mean ± SD) | 340 ± 180 | – |
| Details of the treatment in mg. | O5; 4 × O10;O12.5; | – |
| O = olanzapine | 3 × O15; | |
| Q = quetiapine | 5 × O20;Q250; | |
| C = clozapine | C175; | |
| R = risperidone | 2 × C250;2 × C300; | |
| Rd = risperidone depot (injectable) | R1; R3; R4 + Rd37.5; O10 + Q375 + C25; | |
| Q350 + O15. |
| . | AOS patients . | Controls . |
|---|---|---|
| Gender M/F | 18/7 | 17/8 |
| Age M (mean ± SD) | 16.5 ± 1.3 | 16.2 ± 1.7 |
| Age F (mean ± SD) | 15.9 ± 1.5 | 15.6 ± 1.3 |
| Handedness R/L | 20/5 | 21/4 |
| Full scale intelligence quotient (range, mean ± SD) | 66–123, 87 ± 14 | 64–127, 108 ± 15 |
| Socio-economic status (The national statistics socio-economic classification.http://www.statistics.gov.uk/methods____quality/ns____sec/) | 4 ± 1.5 | 3.5 ± 1.5 |
| Age at onset of symptoms (range, mean ± SD) | 11–16.8, 14.9 ± 1.6 | – |
| Disease duration (mean ± SD) | 1.4 ± 0.7 | – |
| PANSS: positive scores (mean ± SD) | 22 ± 5 | – |
| PANSS: negative scores (mean ± SD) | 16 ± 5 | – |
| Chlorpromazine equivalents (mean ± SD) | 340 ± 180 | – |
| Details of the treatment in mg. | O5; 4 × O10;O12.5; | – |
| O = olanzapine | 3 × O15; | |
| Q = quetiapine | 5 × O20;Q250; | |
| C = clozapine | C175; | |
| R = risperidone | 2 × C250;2 × C300; | |
| Rd = risperidone depot (injectable) | R1; R3; R4 + Rd37.5; O10 + Q375 + C25; | |
| Q350 + O15. |
Demographics data of adolescent-onset schizophrenic patients and controls
| . | AOS patients . | Controls . |
|---|---|---|
| Gender M/F | 18/7 | 17/8 |
| Age M (mean ± SD) | 16.5 ± 1.3 | 16.2 ± 1.7 |
| Age F (mean ± SD) | 15.9 ± 1.5 | 15.6 ± 1.3 |
| Handedness R/L | 20/5 | 21/4 |
| Full scale intelligence quotient (range, mean ± SD) | 66–123, 87 ± 14 | 64–127, 108 ± 15 |
| Socio-economic status (The national statistics socio-economic classification.http://www.statistics.gov.uk/methods____quality/ns____sec/) | 4 ± 1.5 | 3.5 ± 1.5 |
| Age at onset of symptoms (range, mean ± SD) | 11–16.8, 14.9 ± 1.6 | – |
| Disease duration (mean ± SD) | 1.4 ± 0.7 | – |
| PANSS: positive scores (mean ± SD) | 22 ± 5 | – |
| PANSS: negative scores (mean ± SD) | 16 ± 5 | – |
| Chlorpromazine equivalents (mean ± SD) | 340 ± 180 | – |
| Details of the treatment in mg. | O5; 4 × O10;O12.5; | – |
| O = olanzapine | 3 × O15; | |
| Q = quetiapine | 5 × O20;Q250; | |
| C = clozapine | C175; | |
| R = risperidone | 2 × C250;2 × C300; | |
| Rd = risperidone depot (injectable) | R1; R3; R4 + Rd37.5; O10 + Q375 + C25; | |
| Q350 + O15. |
| . | AOS patients . | Controls . |
|---|---|---|
| Gender M/F | 18/7 | 17/8 |
| Age M (mean ± SD) | 16.5 ± 1.3 | 16.2 ± 1.7 |
| Age F (mean ± SD) | 15.9 ± 1.5 | 15.6 ± 1.3 |
| Handedness R/L | 20/5 | 21/4 |
| Full scale intelligence quotient (range, mean ± SD) | 66–123, 87 ± 14 | 64–127, 108 ± 15 |
| Socio-economic status (The national statistics socio-economic classification.http://www.statistics.gov.uk/methods____quality/ns____sec/) | 4 ± 1.5 | 3.5 ± 1.5 |
| Age at onset of symptoms (range, mean ± SD) | 11–16.8, 14.9 ± 1.6 | – |
| Disease duration (mean ± SD) | 1.4 ± 0.7 | – |
| PANSS: positive scores (mean ± SD) | 22 ± 5 | – |
| PANSS: negative scores (mean ± SD) | 16 ± 5 | – |
| Chlorpromazine equivalents (mean ± SD) | 340 ± 180 | – |
| Details of the treatment in mg. | O5; 4 × O10;O12.5; | – |
| O = olanzapine | 3 × O15; | |
| Q = quetiapine | 5 × O20;Q250; | |
| C = clozapine | C175; | |
| R = risperidone | 2 × C250;2 × C300; | |
| Rd = risperidone depot (injectable) | R1; R3; R4 + Rd37.5; O10 + Q375 + C25; | |
| Q350 + O15. |
Twenty-five healthy control participants, matched for age and sex to the patient group, were included in this study. These adolescent control participants were recruited from the community through their general practitioners and were screened for any history of emotional, behavioural or medical problems. Handedness was assessed with the Edinburgh Handedness Questionnaire (Oldfield, 1971). All participants attended normal schools. Exclusion criteria included moderate mental impairment (IQ <60), a history of substance abuse or pervasive developmental disorder, significant head injury, neurological disorder or major medical disorder (Table 1). Three schizophrenic patients and one control fulfilled criteria for mild learning disability according to DSM-IV (IQ ≤70) but showed no brain lesion on their respective scanning exams.
Image acquisition
The 50 participants underwent the same imaging protocol with a whole-brain T1-weighted and diffusion-weighted scanning using a 1.5 T Sonata MR imager (Siemens, Erlangen, Germany) with a standard quadrature head coil and maximum 40 mT.m−1 gradient capability.
The 3D T1-weighted FLASH sequence was performed with the following parameters: coronal orientation, matrix 256 × 256, 208 slices, 1 × 1 mm2 in-plane resolution, slice thickness 1 mm, TE/TR = 5.6/12 ms, flip angle α = 19°.
Diffusion-weighted images were obtained using echo-planar imaging (SE-EPI, TE/TR = 89/8500 ms, 60 axial slices, bandwidth = 1860 Hz/vx, voxel size 2.5 × 2.5 × 2.5 mm3) with 60 isotropically distributed orientations for the diffusion-sensitising gradients at a b-value of 1000 s.mm−2 and 5 b = 0 images. To increase signal-to-noise ratio, scanning was repeated three times and all scans were corrected for head motion and eddy currents using successive affine registrations before being averaged.
Image analysis
Grey matter preprocessing
As we wanted to investigate voxel-wise changes between schizophrenic patients and control participants across the whole brain, it was important that the use of non-linear deformations to register native scans into a common space was carried out with appropriate accuracy. The details of non-linear transformations may considerably influence the results, depending on the nature of the spatial registration itself or the dimensionality of the underlying model (Ashburner and Friston, 2001; Bookstein, 2001; Crum et al., 2003). Thus, two voxel-based analyses using different practical methodologies for the automated segmentation and registration of the brains were carried out for the investigation of the grey matter morphometry:
I. We conducted an ‘optimized’ VBM-style protocol (Good et al., 2001) using FSL tools (Smith et al., 2004, www.fmrib.ox.ac.uk/fsl) for brain extraction (Smith, 2002) and segmentation (Zhang et al., 2001) and the IRTK tool for non-rigid transformation using spline-based free-form deformation (Rueckert et al., 1999) to spatially register the native images.
II. We then verified that we were able to reproduce similar patterns of grey matter change with the optimized VBM protocol using the standard segmentation and registration tools available in the statistical parametric mapping software (SPM2, www.fil.ion.ucl.ac.uk/spm) (Ashburner et al., 2000; Ashburner and Friston, 2000).
The common optimized protocol carried out to assess differences in the topographic distribution of grey matter between adolescent-onset schizophrenic patients and controls was the following: first, a left–right symmetric study-specific grey matter template was built from the 50 grey matter-segmented native images and their respective mirror images that were all affine-registered to the ICBM-152 grey matter template. The 50 native grey matter volume images were then non-linearly normalized onto this template (Fig. 1). The optimized protocol also introduces a compensation (or ‘modulation’) for the contraction/enlargement due to the non-linear component of the transformation: each voxel of each registered grey matter image was divided by the Jacobian of the warp field. Finally, all 50 modulated normalised grey matter volume images were smoothed with an isotropic Gaussian kernel with a sigma of 3.5 mm (∼8 mm FWHM).
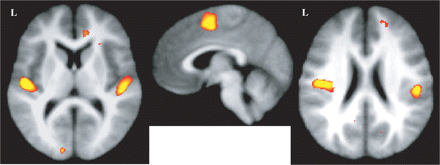
Reduced grey matter in patients in Heschl's gyri (left; z = 10), the SMA (middle; x = 4) and the parietal operculi (right; z = 22) obtained with the FSL-VBM approach overlaid on the average of the non-linearly registered T1-weighted images.
White matter preprocessing
FA, mean diffusivity (MD), λ//(first eigenvalue) and λ⊥ (average of the second and third eigenvalues) maps were generated using DTIFit within the FMRIB Diffusion Toolbox (part of FSL; Smith et al., 2004).
Voxel-wise differences in DTI indices were assessed using Tract-Based Spatial Statistics (TBSS, also part of FSL), a recent approach which increases the sensitivity and the interpretability of the results compared with voxel-based approaches based purely on non-linear registration (Smith et al., 2006). Ventricular enlargement caused by the pathophysiological process may for instance considerably mislead the interpretation of the voxel-based results. TBSS aims to solve the problematic issues of standard voxel-wise methods via the use of a carefully tuned non-linear registration (the same as was used for the grey matter analysis earlier), followed by the projection of the nearest maximum FA values onto a skeleton derived from a mean FA image. This projection step aims to remove the effect of cross-subject spatial variability that remains after the non-linear registration.
Statistical analyses
Finally, special care has also been given to the statistical method employed to investigate changes in the grey matter distribution and white matter integrity. To achieve accurate inference including full correction for multiple comparisons over space, we used permutation-based non-parametric inference within the framework of the general linear model (Nichols and Holmes, 2002) to investigate changes in the distribution of grey matter, FA and MD between both groups (5000 permutations). Results were all considered significant for P < 0.01 (after initial cluster-forming thresholding at P-uncorrected = 0.05), fully corrected for multiple comparisons. We also carried out exactly the same analyses with a subset of 15 right-handed schizophrenic males (mean age ± SD: 16.4 ± 1.4) and 15 age-matched right-handed control males (mean age ± SD: 16.3 ± 1.6) to account for any possible gender × disease, handedness × disease or gender × handedness × disease interaction in our grey and white matter results.
In addition, we performed a simple regression analysis with the antipsychotic dosage (chlorpromazine equivalent) within the patient group, to explore whether the therapy interacts with trait-related structural abnormalities.
Significant differences between patients and control participants in λ// and λ⊥ (within the clusters showing significant changes of anisotropy between both groups) were investigated by averaging the relevant eigenvalue data across ROIs identified by the FA analysis.
Finally, we tested the potential for changes identified at the group level to distinguish cases from controls at an individual level. We therefore applied a simple multivariate discriminant analysis on the 50 smoothed and modulated grey matter images, using leave-one-out testing to form a discriminant vector from N-1 participants and testing this on the subject left out. This gives an unbiased estimation of discrimination ability between the groups of participants. We used the group-mean-difference t-statistic (from the two groups of participants within the N-1 subset) as the discriminant function.
Results
Grey matter results
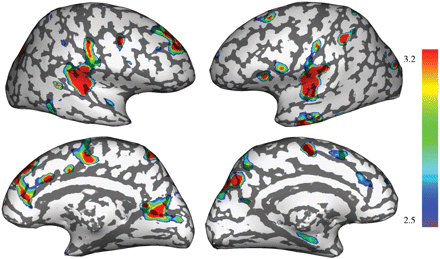
VBM-style comparison of control participants and adolescent-onset schizophrenic patients revealed a highly significant bilateral difference in grey matter volume distribution (patients < controls) in Heschl's gyrus, the parietal operculum and the supplementary motor area (SMA). It also showed significant bilateral differences (patients < controls) in the primary sensory and in the primary motor cortices. Significant reduced grey matter in the patients was also demonstrated in the left premotor cortex [including regions in Broca's area 44 and in the frontal eye field (FEF)] and in the right anterior cingulate gyrus, the right dorso-lateral prefrontal cortex (DLPFC), the precuneus and the temporal lobes (Figs 1, 2 and 5) (Table 2). When possible, these results were checked using a toolbox providing probabilistic cytoarchitectonic maps in the MNI standard space (Eickhoff et al., 2005).
3D representation of the significant grey matter loss found in patients overlaid on an inflated cortical surface (FSL-VBM).
Local peaks of the significant clusters (corrected P-value <0.01) showing reduced grey matter in the patient group (FSL-VBM) contrasted with the controls (secondary local maxima within a cluster are also presented when required)
| Cortical region (BA) . | Side . | MNI (mm) . | Local maximum t-value . | ||
|---|---|---|---|---|---|
| . | . | x . | y . | Z . | . |
| Auditory/Language areas | |||||
| Heschl gyrus | L | −48 | −18 | 10 | 5.29 |
| Parietal operculum (BA40) | L | −38 | −16 | 24 | 4.72 |
| Parietal operculum (BA40) | R | 50 | −24 | 20 | 4.52 |
| Heschl gyrus | R | 50 | −22 | 8 | 4.21 |
| Pars opercularis (BA44) | L | −46 | 18 | 12 | 3.23 |
| → Pars opercularis (BA44) | L | −50 | 8 | 20 | 3.07 |
| Sensori-motor/Premotor areas | |||||
| SMA | L/R | 1 | 0 | 52 | 4.19 |
| Post-central gyrus (BA2) | L | −40 | −36 | 46 | 3.79 |
| Pre-central gyrus (BA4) | R | 51 | −10 | 41 | 3.76 |
| Post-central gyrus (BA1) | R | 60 | −16 | 44 | 3.58 |
| Pre-central gyrus (BA4) | R | 38 | 10 | 38 | 3.56 |
| FEF | L | −22 | 18 | 48 | 3.13 |
| Prefrontal areas | |||||
| Anterior cingulate gyrus (BA24/32) | R | 14 | 8 | 34 | 3.85 |
| → Anterior cingulate gyrus (BA32) | R | 14 | 40 | 30 | 3.84 |
| → Anterior cingulate gyrus (BA24/32) | R | 10 | 22 | 25 | 3.61 |
| DLPFC (BA46) | R | 20 | 44 | 20 | 3.75 |
| → DLPFC (BA9) | R | 20 | 44 | 32 | 3.34 |
| Other areas | |||||
| Precuneus (BA7) | R | 24 | −70 | 32 | 4.03 |
| Parieto-occipital fissure | R | 20 | −60 | 14 | 4.14 |
| Precuneus (BA7) | L | −12 | −61 | 49 | 3.99 |
| Calcarine fissure (BA17) | L | −12 | −90 | 6 | 3.77 |
| Inferior temporal gyrus (BA20) | L | −50 | −16 | −22 | 3.90 |
| Middle temporal gyrus (BA20/21) | R | 58 | −11 | −16 | 3.61 |
| Cortical region (BA) . | Side . | MNI (mm) . | Local maximum t-value . | ||
|---|---|---|---|---|---|
| . | . | x . | y . | Z . | . |
| Auditory/Language areas | |||||
| Heschl gyrus | L | −48 | −18 | 10 | 5.29 |
| Parietal operculum (BA40) | L | −38 | −16 | 24 | 4.72 |
| Parietal operculum (BA40) | R | 50 | −24 | 20 | 4.52 |
| Heschl gyrus | R | 50 | −22 | 8 | 4.21 |
| Pars opercularis (BA44) | L | −46 | 18 | 12 | 3.23 |
| → Pars opercularis (BA44) | L | −50 | 8 | 20 | 3.07 |
| Sensori-motor/Premotor areas | |||||
| SMA | L/R | 1 | 0 | 52 | 4.19 |
| Post-central gyrus (BA2) | L | −40 | −36 | 46 | 3.79 |
| Pre-central gyrus (BA4) | R | 51 | −10 | 41 | 3.76 |
| Post-central gyrus (BA1) | R | 60 | −16 | 44 | 3.58 |
| Pre-central gyrus (BA4) | R | 38 | 10 | 38 | 3.56 |
| FEF | L | −22 | 18 | 48 | 3.13 |
| Prefrontal areas | |||||
| Anterior cingulate gyrus (BA24/32) | R | 14 | 8 | 34 | 3.85 |
| → Anterior cingulate gyrus (BA32) | R | 14 | 40 | 30 | 3.84 |
| → Anterior cingulate gyrus (BA24/32) | R | 10 | 22 | 25 | 3.61 |
| DLPFC (BA46) | R | 20 | 44 | 20 | 3.75 |
| → DLPFC (BA9) | R | 20 | 44 | 32 | 3.34 |
| Other areas | |||||
| Precuneus (BA7) | R | 24 | −70 | 32 | 4.03 |
| Parieto-occipital fissure | R | 20 | −60 | 14 | 4.14 |
| Precuneus (BA7) | L | −12 | −61 | 49 | 3.99 |
| Calcarine fissure (BA17) | L | −12 | −90 | 6 | 3.77 |
| Inferior temporal gyrus (BA20) | L | −50 | −16 | −22 | 3.90 |
| Middle temporal gyrus (BA20/21) | R | 58 | −11 | −16 | 3.61 |
Local peaks of the significant clusters (corrected P-value <0.01) showing reduced grey matter in the patient group (FSL-VBM) contrasted with the controls (secondary local maxima within a cluster are also presented when required)
| Cortical region (BA) . | Side . | MNI (mm) . | Local maximum t-value . | ||
|---|---|---|---|---|---|
| . | . | x . | y . | Z . | . |
| Auditory/Language areas | |||||
| Heschl gyrus | L | −48 | −18 | 10 | 5.29 |
| Parietal operculum (BA40) | L | −38 | −16 | 24 | 4.72 |
| Parietal operculum (BA40) | R | 50 | −24 | 20 | 4.52 |
| Heschl gyrus | R | 50 | −22 | 8 | 4.21 |
| Pars opercularis (BA44) | L | −46 | 18 | 12 | 3.23 |
| → Pars opercularis (BA44) | L | −50 | 8 | 20 | 3.07 |
| Sensori-motor/Premotor areas | |||||
| SMA | L/R | 1 | 0 | 52 | 4.19 |
| Post-central gyrus (BA2) | L | −40 | −36 | 46 | 3.79 |
| Pre-central gyrus (BA4) | R | 51 | −10 | 41 | 3.76 |
| Post-central gyrus (BA1) | R | 60 | −16 | 44 | 3.58 |
| Pre-central gyrus (BA4) | R | 38 | 10 | 38 | 3.56 |
| FEF | L | −22 | 18 | 48 | 3.13 |
| Prefrontal areas | |||||
| Anterior cingulate gyrus (BA24/32) | R | 14 | 8 | 34 | 3.85 |
| → Anterior cingulate gyrus (BA32) | R | 14 | 40 | 30 | 3.84 |
| → Anterior cingulate gyrus (BA24/32) | R | 10 | 22 | 25 | 3.61 |
| DLPFC (BA46) | R | 20 | 44 | 20 | 3.75 |
| → DLPFC (BA9) | R | 20 | 44 | 32 | 3.34 |
| Other areas | |||||
| Precuneus (BA7) | R | 24 | −70 | 32 | 4.03 |
| Parieto-occipital fissure | R | 20 | −60 | 14 | 4.14 |
| Precuneus (BA7) | L | −12 | −61 | 49 | 3.99 |
| Calcarine fissure (BA17) | L | −12 | −90 | 6 | 3.77 |
| Inferior temporal gyrus (BA20) | L | −50 | −16 | −22 | 3.90 |
| Middle temporal gyrus (BA20/21) | R | 58 | −11 | −16 | 3.61 |
| Cortical region (BA) . | Side . | MNI (mm) . | Local maximum t-value . | ||
|---|---|---|---|---|---|
| . | . | x . | y . | Z . | . |
| Auditory/Language areas | |||||
| Heschl gyrus | L | −48 | −18 | 10 | 5.29 |
| Parietal operculum (BA40) | L | −38 | −16 | 24 | 4.72 |
| Parietal operculum (BA40) | R | 50 | −24 | 20 | 4.52 |
| Heschl gyrus | R | 50 | −22 | 8 | 4.21 |
| Pars opercularis (BA44) | L | −46 | 18 | 12 | 3.23 |
| → Pars opercularis (BA44) | L | −50 | 8 | 20 | 3.07 |
| Sensori-motor/Premotor areas | |||||
| SMA | L/R | 1 | 0 | 52 | 4.19 |
| Post-central gyrus (BA2) | L | −40 | −36 | 46 | 3.79 |
| Pre-central gyrus (BA4) | R | 51 | −10 | 41 | 3.76 |
| Post-central gyrus (BA1) | R | 60 | −16 | 44 | 3.58 |
| Pre-central gyrus (BA4) | R | 38 | 10 | 38 | 3.56 |
| FEF | L | −22 | 18 | 48 | 3.13 |
| Prefrontal areas | |||||
| Anterior cingulate gyrus (BA24/32) | R | 14 | 8 | 34 | 3.85 |
| → Anterior cingulate gyrus (BA32) | R | 14 | 40 | 30 | 3.84 |
| → Anterior cingulate gyrus (BA24/32) | R | 10 | 22 | 25 | 3.61 |
| DLPFC (BA46) | R | 20 | 44 | 20 | 3.75 |
| → DLPFC (BA9) | R | 20 | 44 | 32 | 3.34 |
| Other areas | |||||
| Precuneus (BA7) | R | 24 | −70 | 32 | 4.03 |
| Parieto-occipital fissure | R | 20 | −60 | 14 | 4.14 |
| Precuneus (BA7) | L | −12 | −61 | 49 | 3.99 |
| Calcarine fissure (BA17) | L | −12 | −90 | 6 | 3.77 |
| Inferior temporal gyrus (BA20) | L | −50 | −16 | −22 | 3.90 |
| Middle temporal gyrus (BA20/21) | R | 58 | −11 | −16 | 3.61 |
No significant differences were found when testing the opposite contrast (patients > controls).
Though it no longer survived the correction for multiple comparisons due to a reduction of the statistical power, the pattern of grey matter results found with the VBM analysis on the two subsets of right-handed males only was very similar to the one obtained with the gender and handedness mixed (but matched) populations.
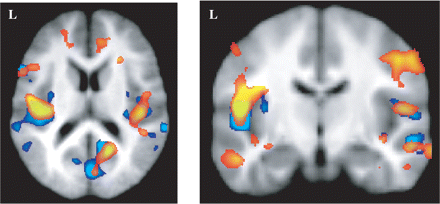
The pattern of grey matter results found with the SPM2-based VBM analysis was also similar to that obtained with the FSL software (Fig. 3). The difference was that we found slightly fewer significant clusters with SPM2-VBM than with FSL-VBM (see Supplementary Material, Table S1).
FSL-VBM results (red) and SPM-VBM results (blue) represented for the same range of t-values (>2.8). Results are very similar for instance in Brodmann area 44, the parietal operculi and the occipital lobe.
The spatial map resulting from the simple regression analysis of grey matter volume loss with dosage of antipsychotics in the patient group did not show any similarity to the patients–controls difference map (see Supplementary Material, Figure S1).
The discriminant analysis on the smoothed modulated grey matter images was able to detect adolescent-onset schizophrenic participants with 88% sensitivity and 80% specificity. Classification of the participants into one of the two groups demonstrated 84% accuracy (42/50 participants were correctly classified according to their diagnosis).
White matter results
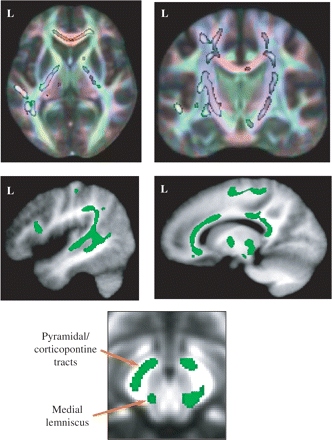
TBSS mapping of anisotropy differences between the adolescent-onset schizophrenic patients and the healthy controls demonstrated a highly significant bilateral decrease of FA in the pyramidal and the corticopontine tracts, the superior thalamic radiations and the medial lemniscus in patients (Figs 4 and 5) and reduced anisotropy in the corpus callosum (from the splenium to the genu), the left arcuate fasciculus and the left optic radiations (Figs 4 and 5). The pyramidal and corticopontine tracts could be differentiated from the medial lemniscus in the brainstem (Fig. 4).
Significant reduction of FA in the corticospinal/corticopontine tracts, the superior thalamic radiations, the left optic radiations, the corpus callosum, the left arcuate fasciculus and in the brainstem (distinction of the corticospinal/corticopontine tracts and the medial lemniscus) of the adolescent-onset schizophrenic patients overlaid on the mean FA map. The skeletonized results have been thickened for better visibility. A red–green–blue rendering of the orientation of the white matter tracts (red = x-axis; green = y-axis; blue = z-axis) has been overlaid to help identifying the corticospinal tract (blue).
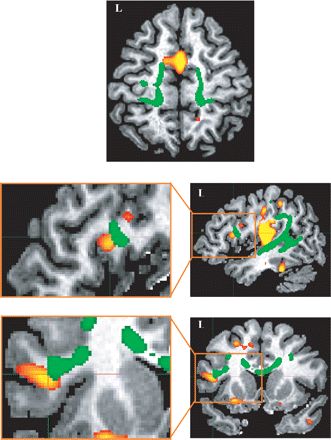
Examples demonstrating the correspondence between grey and white matter results: decrease of FA in the corticospinal/corticopontine tracts (green) and grey matter loss in the SMA (red) in the top row on an axial view; decrease of FA in the left arcuate fasciculus (green) and grey matter loss in Brodmann area 44 (red) in the middle row on a sagittal view and in the bottom row on a coronal view. Results were overlaid on a single control subject for a better identification of the regions involved.
There were no relative increases in FA or MD in the patients.
The pattern of reduced anisotropy found with the TBSS analysis on the two subsets of right-handed males only was similar to the one obtained with the gender and handedness mixed populations, but did not survive the correction for multiple comparisons.
The spatial map resulting from the simple regression analysis of reduced FA with dosage of antipsychotics in the patient group did not show any significant result.
Both the mean λ// and mean λ⊥, averaged across the clusters of significantly decreased FA in the patients compared with controls, were significantly different between the two groups: λ//(×10−3 mm2.s−1) was relatively reduced in the patient group (controls: 1.218 ± 0.008; patients: 1.186 ± 0.008, P < 0.03), while λ⊥ (×10−3 mm2.s−1) was increased (controls: 0.570 ± 0.006; patients: 0.587 ± 0.006, P < 0.04).
Correspondence between grey and white matter results
There was good concordance between the reduced grey matter and the decrease of anisotropy found in the left Brodmann area 44 and the left arcuate fasciculus of the patients (Fig. 5). The primary sensori-motor, premotor and supplementary motor cortex changes are related anatomically with reduced FA in the pyramidal and the corticopontine tracts, the posterior superior thalamic radiations and the medial lemniscus (see example in Fig. 5). Both a region in the left primary visual cortex and in the left optic radiations showed significant abnormalities in the patients.
Discussion
Patients with early-onset schizophrenia present with more severe symptoms and signs than adult-onset patients (Rapoport et al., 2005; White et al., 2006). Our study allows us to relate the clinical pattern of deficits to trait-associated differences in grey matter distribution and in white matter microstructure. A comparison between this study and previous imaging investigations of adult-onset schizophrenia suggests qualitatively similar patterns of change relative to age-matched healthy controls, consistent with the hypothesis that there is a continuum of disease with a common neuropathological substrate. However, an unexpected observation in our study of the adolescent population was the remarkably large grey matter changes consistent with altered white matter integrity in motor control regions. These have not traditionally been considered as major sites of pathological change. They might possibly represent a surrogate marker of disturbed dynamics of white matter maturation particular to adolescent-onset schizophrenia.
Grey matter morphological changes in adolescent-onset schizophrenia
The topography of grey matter changes in the adolescent-onset schizophrenic subjects suggests a structural substrate for the relatively severe functional impairments in language and working memory for early-onset schizophrenic patients (White et al., 2006). The VBM-style analysis revealed differences in the grey matter topographic distribution bilaterally in Heschl's gyri, the parietal operculum and in the left Brodmann area 44 in Broca's area of adolescent-onset patients. The different patterns of grey matter distribution found in the Heschl's gyri are consistent with previous ROI approaches showing a bilateral decrease of volume in male patients with paranoid schizophrenia (Rojas et al., 1997) and in both female and male first-episode schizophrenic patients (Hirayasu et al., 2000). A voxel-wise approach using deformation-based morphometry also suggested that there may be a correlation of local volume decrease in left Heschl's gyrus and severity of auditory hallucinations (Gaser et al., 2004). Remarkably, concomitant with the increased activation in Heschl's gyri of patients having verbal auditory hallucinations, significant correlations between BOLD signal and temporal hallucination pattern were also found in the left parietal operculum and Broca's area (Dierks et al., 1999). The left parietal operculum, sometimes referred as the anterior supramarginal gyrus, was also found involved in greater rate of clinical improvement for subjects with auditory/verbal hallucinations when targeted by transcranial magnetic stimulation (Hoffman et al., 2007).
We found loss of grey matter in the right anterior cingulate gyrus. This finding is in line with two previous reports using ROI analysis that demonstrated a reduced volume in the right anterior cingulate cortex in schizophrenia (Zhou et al., 2005) and in first-episode schizophrenia (Lopez-Garcia et al., 2006). Interestingly, using dynamic causal modelling in schizophrenic patients relative to healthy subjects, Mechelli and coworkers showed a bilateral reduction in the functional intrinsic connectivity between Heschl's gyrus and the anterior cingulate cortex (Mechelli et al., 2007). This suggests functional interactions between these regions, which we speculate may be related to the core symptoms of auditory hallucinations.
We also provide evidence for reduced grey matter volume in the right dorso-lateral prefrontal cortex. This result extends observations from two earlier studies investigating insight in first-episode antipsychotic-naive schizophrenic patients, in which a volume reduction in the right DLPFC was reported for patients presenting with poor insight compared with those who had good insight and there was a negative correlation of this volume with awareness of symptoms (Shad et al., 2004, 2006).
However, functional deficits in schizophrenia are not confined to cognitive domains of language and auditory perception. White and coworkers have highlighted greater relative motor performance deficits in adolescent patients compared with adult-onset patients (White et al., 2006). More severe impairments of motor control have been related to an earlier age of diagnosis (Manschreck et al., 2004). Consistent with these clinical findings, we found striking differences in the distribution of grey matter in sensorimotor and premotor areas (S1, M1, SMA, Brodmann area 44 and FEF). These findings are consistent with the results of an earlier deformation-based approach which showed accelerated loss of grey matter in early-onset schizophrenic patients in the sensorimotor, supplementary motor and frontal eye fields relative to matched healthy controls (Thompson et al., 2001). In our study, we found also a lower fractional anisotropy in the corticospinal tract (and in a white matter region that we presume to be in the corticopontine tract) and in the superior thalamic radiations.
Although sensorimotor and premotor areas appear not to have been a major focus of attention for a long time in structural analyses of schizophrenia, some studies have recently reported a bilateral reduction of grey matter volume in line with our findings in the SMA (Suzuki et al., 2005; Exner et al., 2006; Lopez-Garcia et al., 2006) and in the pre-central and the post-central gyri (Zhou et al., 2005, 2007). Loss of grey matter in the primary motor area and the SMA might be related to the impaired psychomotor performance, extrapyramidal symptoms and the presence of neurological soft signs (Dazzan and Murray, 2002; Bachmann et al., 2005) that have been detected in schizophrenic patients (Jahn et al., 2006; Putzhammer and Klein, 2006). Interestingly, these disturbances have been also found in never-medicated patients and in adolescents who later developed schizophreniform disorders (Gupta et al., 1995; Chatterjee et al., 1995; Flyckt et al., 1999; Cannon et al., 2006).
It is notable that most of the premotor and motor regions revealed by the grey matter analysis can be related to speech production. In addition to our finding in Brodmann area 44, we have provided evidence for a significantly reduced grey matter in the SMA, a region that plays a role in word production (Ziegler et al., 1997; Blank et al., 2002; Alario et al., 2006; Tremblay and Gracco, 2006). Finally, the bilateral local peaks of grey matter volume reduction in the primary motor cortex are localized in the middle of the functional representation of the mouth based on a meta-analysis of PET studies (Fox et al., 2001). Interestingly, parietal operculum at its junction with the temporal lobe is considered to act as an interface between posterior temporal cortex (speech perception) and motor cortex (speech production) (Wise et al., 2001).
Interpretation of grey matter changes defined by voxel-wise analysis
The similar pattern of the grey matter abnormalities found with two different practical methodologies reinforce our confidence in these results. We found a few more significant clusters with FSL-VBM than with SPM2-VBM, analysing the data with the same statistical model. Because there could be a continuum of results, dependent on the degrees of freedom of the non-rigid registration (Crum et al., 2003), this small divergence may be due to the slightly more accurate non-linear registration used within the FSL-VBM preprocessing (free-form deformation with 20 mm initial control point spacing in this analysis, Rueckert et al., 1999) than in the SPM2-VBM (discrete cosine transform basis functions decomposition with a 25 mm cutoff in our study, Ashburner et al., 2000). Interpretation of such voxel-wise analyses in the grey matter has inherent limitations, however. Indeed, although consistent with some previous volumetric findings, it is not possible to clearly determine if the results we found in early-onset schizophrenia are the consequence of developmentally reduced thickness or atrophy or rather an indirect reflection of a different gyrification pattern associated with this disease. It might be possible that a misalignment of the gyri/sulci or even different folding patterns may lead to the difference of grey matter distribution that we found between healthy and patient groups. White and colleagues have found significant changes of the sulco-gyral morphology in adolescent-onset schizophrenia (White et al., 2003). Many other studies have found either a localised increase of the gyrification index (GI) (Vogeley et al., 2000, 2001; Harris et al., 2004; Narr et al., 2004; Falkai et al., 2006) or a decrease of the gyrification complexity (Kulynych et al., 1997; Sallet et al., 2003; Jou et al., 2005; Wheeler and Harper, 2007; Bonnici et al., 2007). Among these different analyses, one study has explored whole-brain cortical folding in the largest population of schizophrenic patients (N = 40), confirming the increase of GI in the prefrontal cortex identified by Vogeley and colleagues and also showing a decrease of GI in the rest of the cortex (Sallet et al., 2003). Study of cortical thickness (and cortical area labelling) in these participants should allow the confound of potential sulci misalignment to be overcome to identify among our results those representing an effective loss of grey matter from those characterising difference in sulco-gyral patterns (Voets et al., in preparation).
Changes in white matter integrity are related anatomically to the grey matter pathology
We believe that our approach to defining the white matter pathology represents an advance over previously applied strategies (Smith et al., 2006). This may contribute to the greater extent and clinico-pathologically more consistent changes that we have observed relative to earlier DTI studies in early-onset schizophrenia (Kumra et al., 2004, 2005; White et al., 2007). In addition, the combined application of diffusion- and T1-weighted imaging has allowed us to directly relate grey and white matter pathology (Fig. 5).
Associated with reduced grey matter found in the caudal end of superior temporal and inferior parietal parts of the Sylvian fissure (Heschl's gyri and parietal operculum) and in the left pars opercularis of Broca's area, we found that a part of the superior longitudinal fasciculus, presumably the arcuate fasciculus, showed a left-lateralized reduced degree of anisotropy. This white matter change is consistent with two reports in adult-onset schizophrenia (Burns et al., 2003; Pugliese et al., 2007). The arcuate fasciculus has been investigated recently in detail in vivo by DTI tractography studies in human brains (Catani et al., 2005; Makris et al., 2005; Parker et al., 2005; Powell et al., 2006; Schmahmann et al., 2007) and confirmed to connect Wernicke's area (rostrally bordered by Heschl's gyrus) and Broca's area through the parietal operculum (or supramarginal gyrus) more extensively on the left than the right hemisphere (Parker et al., 2005; Powell et al., 2006).
In correspondence with the grey matter abnormalities found bilaterally in the primary motor, the premotor and the supplementary motor cortices, TBSS analysis of FA shows a reduced anisotropy in the pyramidal tract of adolescent-onset schizophrenic patients. A third of the pyramidal tract neurons originate from M1, the rest of them arising from premotor area and SMA (Nolte, 1999). The corticospinal tract changes may be specifically related to the early age at symptom onset (Manschreck et al., 2004). The changes may be especially prominent as they occur in brains that are still developing during adolescence, especially in the (sensori)motor-related areas (Sowell et al., 2001; Paus, 2005; Toga et al., 2006). Particularly, the posterior limb of the internal capsule is thought to be one major area of white matter development during childhood and adolescence (Paus et al., 1999; Schmithorst et al., 2002; Barnea-Goraly et al., 2005; Ashtari et al., 2007). Hence, it is likely that this exceptional finding in the corticospinal tract may be the marker of a delay in white matter maturation specific to adolescent-onset schizophrenia, as none of the DTI studies investigating adult-onset schizophrenia have found a change of anisotropy in this tract or, even more generally, in any tract present in the posterior limb of the internal capsule (Kanaan et al., 2005; Kubicki et al., 2005, 2007).
We indeed also found a bilateral lower degree of anisotropy in the corticopontine tract, the superior thalamic radiations and the medial lemniscus, together composing the cortico-cerebellar-thalamo-cortical loop, the functional disconnection of which is generally considered as a fundamental abnormality in schizophrenia (Andreasen et al., 1996; Honey et al., 2005). The posterior part of the superior thalamic radiations together with the medial lemniscus (the ‘posterior column-medial lemniscus system’ in Mettler, 1948) and the posterior part of the corticopontine tract are the two major ascending and descending pathways of the primary somatosensory cortex. The reduced integrity found in these tracts may thus be closely related to the bilateral grey matter loss demonstrated in the primary somatosensory cortex. This latter result, in line with the first recent report of a grey matter atrophy in the post-central gyrus (Zhou et al., 2007), gives a probable structural substrate for the subtle somatosensory disturbances observed in schizophrenic patients (Ritzler et al., 1977; Javitt et al., 1999; Tanno et al., 1999).
Functional consequences of loss of white matter integrity
Inter- and intra-hemispheric connectivity disturbances have been suggested to play a major role in schizophrenia (McGlashan and Hoffman, 2000; Stephan et al., 2006). Our findings of a widespread reduction of anisotropy in the white matter seem to further support the hypoconnectivity hypothesis, which suggests that neuronal interactions could be altered by subtle microstructural abnormalities in the spatial distribution of synapses, length or calibre of axons or the geometry of axonal branches (Beaumont and Dimond, 1973; Friston and Frith, 1995; Innocenti et al., 2003). The decrease of anisotropy revealed in the white matter can be interpreted either as a loss of organization of the fibres (which is expected to be associated with a reduction of the longitudinal diffusivity λ//) or as an alteration of the myelin (which should be associated with an increase of the transverse diffusivity λ⊥) (Beaulieu, 2002; Song et al., 2002; Concha et al., 2006). Both types of change were found in the adolescent-onset schizophrenia group. Previous work has demonstrated impairments of myelination in chronic schizophrenia and a lack of a significant relationship between myelin water fraction and age, suggesting the absence or the delay in ongoing brain maturation (Davis et al., 2003; Flynn et al., 2003). Particularly, in line with the observation of a substantial reduction of myelin water fraction in the corpus callosum in adult-onset schizophrenia, we also found altered white matter integrity distributed from the splenium to the genu in our schizophrenic adolescents (Foong et al., 2000; Agartz et al., 2001; Ardekani et al., 2003; Kanaan et al., 2006). The corpus callosum undergoes major microstructural changes during healthy adolescence (Barnea-Goraly et al., 2005; Ben Bashat et al., 2005; Snook et al., 2005; Ashtari et al., 2007) and disruption of its development is expected to have consequences for brain connectivity and plasticity (Innocenti et al., 2003).
Summary
In summary, with study of adolescent-onset schizophrenia, we have been able to characterize widespread neuropathological changes that can plausibly be related to symptoms and signs of the disease. Care was taken to optimise the methodology to allow investigation of both grey matter distribution and white matter integrity and to relate them at the level of whole brain structure. The changes suggest both pathology affecting grey matter morphology and inter/intra-hemispheric white matter connections and would be consistent with molecular pathogenesis involving myelin. A post hoc analysis suggested that 42/50 participants could be correctly classified as schizophrenic patients or healthy controls on the basis of the nature and extent of changes seen in the grey matter.
At present, we cannot distinguish whether the greater changes found in our study compared with the previous literature arise from the good sensitivity of the methods employed here (Sowell et al., 2000) or from the greater severity of disease with the early age of symptom onset (Manschreck et al., 2004). We therefore aim in future work to explore adult-onset schizophrenia with the same methodological approaches. Another issue will be to determine with a longitudinal study whether the brain abnormalities demonstrated in these schizophrenic adolescents will show dynamic evolution (Thompson et al., 2001; Vidal et al., 2006) towards the less marked changes previously observed in adult-onset schizophrenia, particularly in the sensorimotor-related grey and white matter.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We would like to thank the participants and their families, referring psychiatrists and the Donnington Health Centre, Oxford. We would also like to thank Dr Clare MacKay at the University of Oxford Centre for Clinical Magnetic Resonance Research for providing helpful comments on this manuscript. This study is supported by the MRC, OHSRC, UK EPSRC, BBSRC and Wellcome Trust.
References
Abbreviations:
- DLPFC
dorso-lateral prefrontal cortex
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FEF
frontal eye field
- FSL
FMRIB (functional magnetic resonance imaging of the brain centre) software library
- IRTK
image registration toolkit
- MD
mean diffusivity
- ROI
region of interest
- SMA
supplementary motor area
- SPM
statistical parametric mapping
- TBSS
tract-based spatial statistics
- VBM
voxel-based morphometry
- BA
Brodmann area.