-
PDF
- Split View
-
Views
-
Cite
Cite
Bon-Mi Gu, Ji-Young Park, Do-Hyung Kang, Seung Jae Lee, So Young Yoo, Hang Joon Jo, Chi-Hoon Choi, Jong-Min Lee, Jun Soo Kwon, Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder, Brain, Volume 131, Issue 1, January 2008, Pages 155–164, https://doi.org/10.1093/brain/awm277
Close - Share Icon Share
Abstract
A deficit in cognitive flexibility is acknowledged as a cognitive trait for obsessive-compulsive disorder (OCD). However, no investigations to date have used a cognitive activation paradigm to specify the neural correlates of this deficit in OCD. The objective of this study was to clarify how abnormal brain activities relate to cognitive inflexibility in OCD, using a task-switching paradigm. A task-switching paradigm which has two kinds of task-set was applied to 21 patients with OCD and 21 healthy subjects of matching age, IQ and sex, during an event-related functional magnetic resonance imaging experiment. Compared with the healthy subjects, patients with OCD exhibited a significantly higher error rate in task-switch trials (P < 0.05). Healthy controls showed significant activation in various areas, including dorsal frontal-striatal regions, during task-switching, whereas patients with OCD showed no activation in these areas. Significant differences were also observed in the dorsal frontal-striatal regions and ventromedial prefrontal and right orbitofrontal cortexes between patients with OCD and healthy controls. Correlation analysis indicated that the activations of orbitofrontal cortex were related with the performance in both groups and also with the activation of anterior cingulate cortex in the OCD group. These findings replicate previous studies of cognitive inflexibility in OCD and provide neural correlates related to a task-switching deficit in OCD. The results suggest that impaired task-switching ability in OCD patients might be associated with an imbalance in brain activation between dorsal and ventral frontal-striatal circuits.
Introduction
Recent studies of obsessive-compulsive disorder (OCD) have emphasized the essential role neurobiological abnormalities play in its aetiology and course and have provided biological models based on direct and indirect investigations of the possible brain circuits involved in the expression of obsessive-compulsive symptoms. Specifically, researchers have suggested that an imbalance between the direct and indirect pathways within frontal-striatal circuits results in a hyperactivated ventral and an inhibited dorsal frontal-striatal system, explaining both the clinical and neuropsychological symptoms of OCD (Saxena et al., 1998; Mataix-Cols and van den Heuvel, 2006). Supporting this hypothesis, previous functional neuroimaging studies of OCD have revealed hyperactivity in the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), thalamus and caudate nucleus, composing ventral cognitive circuits, using resting state or symptom provocation paradigms (Rauch et al., 1994; Breiter et al., 1996; Shin et al., 2006). In addition, recent functional neuroimaging studies using a cognitive activation paradigm have reported decreased responsiveness of dorsal frontal-striatal regions during executive performance among patients with OCD (van den Heuvel et al., 2005) and aberrant orbitofrontal-striatal and dorsal prefrontal activity among patients with OCD during a reversal learning task (Remijnse et al., 2006). Taken together, these results allow a conceptualization of OCD in terms of abnormalities within a distributed set of neural structures, including frontal-striatal circuitry.
Numerous studies have shown that impaired executive function is often evident in patients with OCD (Greisberg and McKay, 2003; Bannon et al., 2006; de Geus et al., 2007). ‘Executive function’ refers to a broad range of abilities such as mental set-shifting, information updating and monitoring, and inhibition (Miyake et al., 2000). Related with these abilities, cognitive inflexibility is frequently reported in the patients with OCD (Veale et al., 1996; Watkins et al., 2005; Bannon et al., 2006; Chamberlain et al., 2006). Among the various tasks to measure cognitive flexibility, task-switching (Monsell, 2003) is one promising paradigm and requires the ability to disengage attention from a previous task, resolve interference from previous stimuli or task sets, and update new task sets (Mayr and Keele, 2000). For executive functions such as switching a task set, a distributed neural network involving both subcortical and cortical regions should work in harmony (Alvarez and Emory, 2006), and considerable evidence indicates that the basal ganglia, in conjunction with the prefrontal cortex (PFC), plays a central role in task-switching (Cools et al., 2004; Monchi et al., 2006). Task-switching ability is an important factor in OCD because the rigid mental acts or behaviours of OCD patients imply problems with cognitive flexibility such as the ability to shift attention. A cognitive inflexibility might fix patients in a conceptual framework, preventing them from shifting from one thought to another and thus locking them within a specific thought and behavioural set (Graybiel and Rauch, 2000).
Recently, Bannon et al. reported that OCD patients exhibit persistent impaired set-shifting ability after symptom remission using computerized version of the Wisconsin Card Sorting Test (Bannon et al., 2006). In addition, Chamberlain et al. suggested that deficits in cognitive flexibility might represent a cognitive endophenotype for OCD, reporting impaired extradimensional set-shifting ability in OCD patients and in their unaffected first-degree relatives (Chamberlain et al., 2006, 2007). Together, these results could indicate that cognitive inflexibility is trait-like in nature among OCD patients and underlies complex clinical OCD symptomatology. Despite the importance of a deficit in cognitive flexibility to OCD pathophysiology, no studies have used a task-switching paradigm to directly investigate neural correlates of deficits in OCD patients.
The goals of this study were to clarify task-switching ability at the behavioural level and to identify the abnormal brain activities related to task-switching in OCD patients, using functional magnetic resonance imaging (fMRI). We focused primarily on differences in task-switching ability between OCD patients and healthy controls to probe cognitive inflexibility in OCD at the behavioural and brain-activation level. The study concurrently and cross-sectionally compared brain activation between drug-free patients and patients with a stable medication dosage to exclude any confounding effects of medication on task-switching ability in OCD patients.
Based on prior reports, we hypothesized that patients with OCD would exhibit a significantly higher error rate in task-switch trials and that impaired task-switching ability among OCD patients would be associated with an imbalance in brain activation between dorsal and ventral frontal-striatal circuits.
Methods
Participants
We recruited 21 patients from the OCD clinic at Seoul National University Hospital, all of whom fulfilled the DSM-IV criteria for OCD as diagnosed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) (First et al., 1996). We also recruited 21 comparison subjects matched for sex, age, IQ and handedness via internet advertisement. Exclusion criteria included a lifetime history of psychosis, bipolar disorder, substance abuse or dependence, Tourette's disorder or other tic-related conditions and history of significant head injury, seizure disorder, or mental retardation. Table 1 presents the demographic characteristics of the subjects. The Korean version of the Wechsler Adult Intelligence Scale (K-WAIS) (Yum et al., 1992) did not reveal any significant differences with regard to IQ. At the time of the study, eight patients were drug naive, and two patients were non-medicated. Eleven patients were medicated with a stable dosage: three with selective serotonin reuptake inhibitor (SSRI) monotherapy, two with an SSRI along with antianxiety medication, one with an SSRI with atypical antipsychotics. The remaining five were taking an SSRI in conjunction with antianxiety and antipsychotic medications. One patient had been diagnosed with co-morbid panic disorder; one, with an obsessive-compulsive personality disorder; and two, with schizotypal personality disorder. Eighteen patients had not been diagnosed with any co-morbid Axis I or II disorders (SCID II) (First et al., 1997). We tested all of the subjects using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (Goodman et al., 1989a, b) to assess the severity of the OCD symptoms and using the Beck Depression Inventory (BDI) (Beck et al., 1961) and Beck Anxiety Inventory (BAI) (Beck et al., 1988) to measure depression and anxiety levels, respectively. Eight patients scored over 16 in BDI self-reports, but no patient had been diagnosed with major depressive disorder by SCID I. We classified the patients according to the five clinical dimensions defined by Mataix-Cols and colleague (Mataix-Cols et al., 1999) and excluded patients with hoarding symptoms since they may be associated with different neural involvement from non-hoarding OCD patients (Saxena et al., 2004; Lochner et al., 2005). We found the following predominant obsession/compulsions among the patients: contamination/cleaning (N = 6), aggressive/checking (N = 11) and sexual/religious (N = 2). No classification could be assigned for two patients. All participants provided written informed consent, and the study was approved by the Institutional Review Board at Seoul National University Hospital.
Demographic characteristics of the subjects
| Variable . | OCD . | Control . | Statistics . |
|---|---|---|---|
| . | (N = 21) . | (N = 21) . | . |
| Sex(M/F) | 18/3 | 18/3 | χ2 = 0.00 |
| Handedness(R/L) | 20/1 | 20/1 | χ2 = 0.00 |
| Age(years) | 23.6 ± 4.5 | 24.8 ± 3.7 | t = 0.94 |
| IQa | 113.4 ± 9.2 | 114.7 ± 12.8 | t = 0.387 |
| BDI | 15.5 ± 11.3 | 4.5 ± 5.7 | t = −4.00b |
| BAI | 18.1 ± 10.8 | 5.1 ± 5.3 | t = −4.96b |
| Y-BOCS | 20.0 ± 5.8 (range 10–27 for medicated, 15–30 for unmedicated OCD) | ||
| Age of Onset (years) | 17.5 ± 5.0 | ||
| Duration of illness (years) | 6.1 ± 4.2 |
| Variable . | OCD . | Control . | Statistics . |
|---|---|---|---|
| . | (N = 21) . | (N = 21) . | . |
| Sex(M/F) | 18/3 | 18/3 | χ2 = 0.00 |
| Handedness(R/L) | 20/1 | 20/1 | χ2 = 0.00 |
| Age(years) | 23.6 ± 4.5 | 24.8 ± 3.7 | t = 0.94 |
| IQa | 113.4 ± 9.2 | 114.7 ± 12.8 | t = 0.387 |
| BDI | 15.5 ± 11.3 | 4.5 ± 5.7 | t = −4.00b |
| BAI | 18.1 ± 10.8 | 5.1 ± 5.3 | t = −4.96b |
| Y-BOCS | 20.0 ± 5.8 (range 10–27 for medicated, 15–30 for unmedicated OCD) | ||
| Age of Onset (years) | 17.5 ± 5.0 | ||
| Duration of illness (years) | 6.1 ± 4.2 |
Values are presented as mean ± SD. BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; Y-BOCS = Yale-Brown Obsessive Compulsive Scale.
aEstimated by Korean-Wechsler Adult Intelligence Scale-Revised (K-WAIS-R). bP<0.001.
Demographic characteristics of the subjects
| Variable . | OCD . | Control . | Statistics . |
|---|---|---|---|
| . | (N = 21) . | (N = 21) . | . |
| Sex(M/F) | 18/3 | 18/3 | χ2 = 0.00 |
| Handedness(R/L) | 20/1 | 20/1 | χ2 = 0.00 |
| Age(years) | 23.6 ± 4.5 | 24.8 ± 3.7 | t = 0.94 |
| IQa | 113.4 ± 9.2 | 114.7 ± 12.8 | t = 0.387 |
| BDI | 15.5 ± 11.3 | 4.5 ± 5.7 | t = −4.00b |
| BAI | 18.1 ± 10.8 | 5.1 ± 5.3 | t = −4.96b |
| Y-BOCS | 20.0 ± 5.8 (range 10–27 for medicated, 15–30 for unmedicated OCD) | ||
| Age of Onset (years) | 17.5 ± 5.0 | ||
| Duration of illness (years) | 6.1 ± 4.2 |
| Variable . | OCD . | Control . | Statistics . |
|---|---|---|---|
| . | (N = 21) . | (N = 21) . | . |
| Sex(M/F) | 18/3 | 18/3 | χ2 = 0.00 |
| Handedness(R/L) | 20/1 | 20/1 | χ2 = 0.00 |
| Age(years) | 23.6 ± 4.5 | 24.8 ± 3.7 | t = 0.94 |
| IQa | 113.4 ± 9.2 | 114.7 ± 12.8 | t = 0.387 |
| BDI | 15.5 ± 11.3 | 4.5 ± 5.7 | t = −4.00b |
| BAI | 18.1 ± 10.8 | 5.1 ± 5.3 | t = −4.96b |
| Y-BOCS | 20.0 ± 5.8 (range 10–27 for medicated, 15–30 for unmedicated OCD) | ||
| Age of Onset (years) | 17.5 ± 5.0 | ||
| Duration of illness (years) | 6.1 ± 4.2 |
Values are presented as mean ± SD. BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; Y-BOCS = Yale-Brown Obsessive Compulsive Scale.
aEstimated by Korean-Wechsler Adult Intelligence Scale-Revised (K-WAIS-R). bP<0.001.
Task paradigm
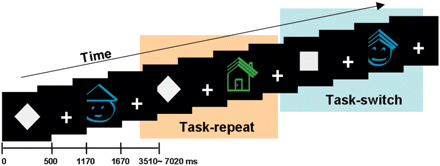
In this cued task-switching paradigm, stimuli consisted of four kinds of icons (green house, blue house, green face and blue face), and the subjects performed a task-switch requiring them to change a stimulus–response set (Fig. 1). During each trial, the subjects were cued explicitly (using a square or diamond cue) as to which task should be performed during the next stimulus. When the square cue was presented, the subjects were required to discriminate between face and house; when the diamond cue was represented, they were required to discriminate between blue and green. Prior to scanning, each subject learned and practiced stimulus–response mapping over a period of 10 min. Stimulus–response mapping was counterbalanced among the subjects. For some subjects, blue icons during the colour condition corresponded to the same finger as face icons during the object condition; for others, blue icons corresponded to the same finger as house icons. Half of the trials were task-switching conditions during which the subjects attended to a different dimension from the previous one, such as a square cue followed by a diamond cue, and half were task-repeat conditions during which the dimensions were identical to those in the preceding trial.
The task-switching paradigm. Each trial was categorized into task-switch or task-repeat depends on the previous task-set. The stimuli consist of four kinds of icons which were green house, blue house, green face and blue face. The square cue means that the subject should discriminate whether the upcoming stimulus is a house or a face and the diamond cue means a discrimination of green or blue colour.
At the beginning of each trial, a cue was presented for 500 ms, followed by a 670-ms fixation, and then a target appeared for 500 ms. In this event-related fMRI experiment, the intertrial interval (ITI) varied from 1840 to 5350 ms; the subjects could respond within this duration. We used the Optseq program (http://surfer.nmr.mgh.harvard.edu/optseq/) to find an efficient trial sequence for the event-related fMRI, and we presented a total of 264 trials.
Image acquisition
Whole-brain imaging was performed using a 1.5 Tesla whole body scanner (Siemens AVANTO, Germany). Functional images were acquired using an echo-planar imaging sequence (TR = 2.34 s, TE = 52 ms, FA = 90°, 3.28 × 3.28 × 4 mm3, 1 mm interslice gap, 25 axial slices). Functional images were acquired in four runs of 304 s each, and a total of 520 volumes were acquired for each subject. We presented task stimuli using the presentation program (http://www.neurobs.com) installed on a Windows-based PC and projected on a screen that was made visible to the subjects using a mirror mounted above the subject's head. The subjects responded to the target by pressing an MRI-compatible mouse button with index and middle finger of their right hand.
Functional imaging data analysis
Imaging data were analysed using SPM2 (Wellcome Department of Imaging Neuroscience, London, UK). The first three images in each run were discarded to eliminate non-equilibrium effects of magnetization. The functional images were corrected for differences in slice acquisition timing, followed by motion correction such as adjusting to the middle image of the second session. Spatial normalization into standard MNI space was performed using a statistical parametric mapping echoplanar imaging template. The images were re-sampled into 2 × 2 × 2 mm3 voxels and spatially smoothed using an 8 mm FWHM isotropic Gaussian kernel. Low-frequency signal drifts were removed using a 128 s high-pass filter, and temporal autocorrelation in the fMRI time series was corrected using a first-order autoregressive model.
Data were then analysed using a canonical hemodynamic response function in SPM2. Four event types (task-repeat, task-switch, error trials and post-error trials) were defined at the first level of analysis and the effect of error and post-error trials were excluded from the analysis because previous research has shown that even a small number of errors could alter activation maps (Murphy and Garavan, 2004) and because error-related abnormal activity in the ACC has been observed among OCD patients (Fitzgerald et al., 2005).
The contrasts for each subject from the first-level analysis were entered into a second-level random effects analysis. First, to determine the task-switching-related brain activation, we entered task-switch minus task-repeat contrast images of individuals into a one-sample t-test analysis in each subject group. The results of these analyses were reported at P < 0.0001 (uncorrected) and with a cluster size greater than 20 to prevent false positives due to multiple comparisons and to not lose statistically significant activities (Forman et al., 1995). Second, for the between-group analysis, we performed a one-way analysis of variance (ANOVA) using the contrast images of main effects as control switch, control repeat, OCD switch and OCD repeat. We used the following contrasts: (i) (control switch–control repeat)—(OCD switch–OCD repeat) for the effect of control minus OCD; and (ii) (OCD switch–OCD repeat)—(control switch–control repeat) for the OCD minus control. Significant areas in the between-group analysis were reported using the criteria of P < 0.001 (uncorrected) and cluster size greater than 20.
We also conducted a region of interest (ROI) analysis to examine the activation patterns in the dorsal or ventral frontal-striatal regions. We defined the functional ROIs from the result of between-group analysis and extracted the effect values using MarsBaR toolbox (http://marsbar.sourceforge.net/) in the normalized images. Among the significantly different areas between the OCD and the control group, the dorsolateral prefrontal cortex (DLPFC), the ventromedial prefrontal cortex (VMPFC), the ACC and the right OFC, which are directly connected to striatal regions (Haber et al., 2006), and the right caudate body were defined as ROIs; cluster criteria were P < 0.001 and k > 20 for the between-group analysis. We extracted beta values for each condition from individual ROIs and then calculated the task-switch minus task-repeat activation value.
Correlation analysis
To examine the relations between task performances and brain activations in each subject group, we ran a correlation analysis between the ROI values of previously defined five areas and the behavioural data of switch error rates, repeat error rates, switch reaction times (RT), repeat RT and switching costs of RT. Additionally, to determine the relationships between dorsal frontal areas and ventral frontal areas, correlation analysis was applied between the ROI values of DLPFC, ACC, OFC and VMPFC in each subject group. Finally, for the consideration of effects of symptom severity, depression or anxiety level on this task, we correlated the Y-BOCS, BDI and BAI values with the task performances and with brain activations in the OCD group.
Results
Behavioural performance
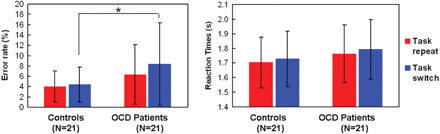
The rate of correct response was more than 80% for all subjects (OCD: 92.8 ± 6.3%; Control: 95.9 ± 2.4%), and we analysed error rates for the task-switch and task-repeat conditions. The error rates were significantly higher in the OCD group (8.44 ± 7.98%) than in the control group (4.44 ± 3.37%) for the task-switch conditions [t(40) = 2.12, P < 0.05], but no significant differences were observed between the two groups for the task-repeat conditions [OCD: 6.35 ± 5.76%, Control: 4.04 ± 3.00%, t(40) = −1.63, P = 0.11] (Fig. 2). The RT in the OCD patients was longer than that in the control group, but the difference was not significant in either in the task-repeat [OCD: 1.76 ± 0.20 s, Control: 1.70 ± 0.17 s, t(40) = −1.06, P = 0.30] or task-switch condition [OCD: 1.79 ± 0.20 s, Control: 1.73 ± 0.19 s, t(40) = −1.06, P = 0.29]. Switching cost (task-switch RT minus task-repeat RT) did not differ significantly [OCD: 0.030 ± 0.046 s, Control: 0.026 ± 0.039 s, t(40) = −0.31, P = 0.76].
Performances (mean ± SD) of the OCD and the control group during the task-switching paradigm. *t = 2.12, df = 40, P = 0.04.
fMRI results
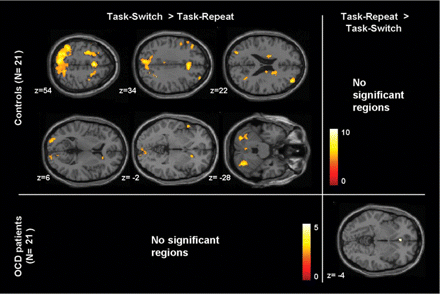
Although the control group exhibited increased BOLD signals in various regions, including the bilateral DLPFC, premotor area, cingulate cortex, parietal lobe, occipital cortex, temporal cortex and caudate nucleus during task-switching, the OCD patients exhibited no significant differences during the task-switch minus task-repeat contrast (Fig. 3 and Table 2). In the reversed contrast condition (such as task-repeat minus task-switch), the OCD group exhibited significant differences in VMPFC [coordinates = (0, 29, −5), Z-value = 4.3, cluster size = 22], whereas the control group exhibited no significant differences.
Increased activations associated with task-switch versus task-repeat contrast in the healthy controls and in the OCD patients. The activation is mapped on the MNI template (P<0.0001, uncorrected, k>20 voxels). Colour bars represent the T-value.
| Area of activation . | Left/right . | Brodmann's area . | Control (N = 21) . | Control (N = 21)>OCD (N = 21) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Coordinatesb . | Z-value . | Coordinatesb . | Z-value . | ||||
| . | . | . | x . | y . | z . | . | x . | y . | z . | |
| Dorsolateral prefrontal cortex | Left | 9 | −50 | 7 | 31 | 4.1 | ||||
| Right | 10 | 38 | 52 | 21 | 5.6 | 36 | 51 | 18 | 3.9 | |
| Premotor area | Left | 6,4 | −30 | −2 | 46 | 4.9 | −36 | −12 | 37 | 4.1 |
| Right | 6 | 28 | 9 | 55 | 4.7 | 24 | 12 | 45 | 3.7 | |
| Ventrolateral prefrontal cortex | Left | 47 | −55 | 17 | −1 | 4.6 | −51 | 21 | −8 | 4.0 |
| Orbitofrontal cortex | Right | 11 | 32 | 34 | −15 | 4.3 | ||||
| Medial frontal cortex | Left | 6 | 0 | 12 | 47 | 5.5 | ||||
| Right | 6 | 4 | −20 | 69 | 4.1 | 18 | −15 | 58 | 4.1 | |
| Ventromedial prefrontal cortex | Left | 24 | 0 | 29 | −5 | 3.5 | ||||
| Anterior cingulate cortex | Left | 32 | −4 | 30 | 22 | 3.7 | ||||
| Right | 32 | 18 | 13 | 23 | 4.8 | |||||
| Posterior cingulate cortex | Left | 31 | −2 | −31 | 31 | 4.1 | −6 | −28 | 33 | 3.9 |
| Right | 29,31 | 20 | −41 | 26 | 4.5 | 18 | −35 | 31 | 3.6 | |
| Uncus | Right | 34 | 22 | 3 | −22 | 3.6 | ||||
| Insula | Right | 13 | 42 | −25 | −2 | 4.4 | ||||
| Parietal lobe | Left | 40,5,7 | −40 | −48 | 45 | 5.9 | −22 | −40 | 52 | 3.6 |
| Right | 40,5,7 | 42 | −45 | 35 | 4.2 | 24 | −38 | 53 | 3.8 | |
| Middle temporal gyrus | Right | 39 | 30 | −55 | 29 | 4.6 | 38 | −65 | 20 | 4.4 |
| Superior temporal gyrus | Right | 38,21 | 48 | 7 | −19 | 3.8 | ||||
| Occipital lobe | Left | 18 | −22 | −91 | 8 | 4.7 | 0 | −86 | 34 | 3.4 |
| Right | 17,18 | 14 | −93 | 6 | 4.5 | 18 | −79 | 9 | 3.5 | |
| Caudate head | Right | 18 | 21 | −4 | 4.6 | |||||
| Caudate body | Left | −19 | −1 | 20 | 4.3 | −21 | −3 | 20 | 3.9 | |
| Right | 18 | −9 | 21 | 4.4 | 20 | 5 | 18 | 4.1 | ||
| Caudate tail | Left | −30 | −34 | 11 | 3.8 | |||||
| Area of activation . | Left/right . | Brodmann's area . | Control (N = 21) . | Control (N = 21)>OCD (N = 21) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Coordinatesb . | Z-value . | Coordinatesb . | Z-value . | ||||
| . | . | . | x . | y . | z . | . | x . | y . | z . | |
| Dorsolateral prefrontal cortex | Left | 9 | −50 | 7 | 31 | 4.1 | ||||
| Right | 10 | 38 | 52 | 21 | 5.6 | 36 | 51 | 18 | 3.9 | |
| Premotor area | Left | 6,4 | −30 | −2 | 46 | 4.9 | −36 | −12 | 37 | 4.1 |
| Right | 6 | 28 | 9 | 55 | 4.7 | 24 | 12 | 45 | 3.7 | |
| Ventrolateral prefrontal cortex | Left | 47 | −55 | 17 | −1 | 4.6 | −51 | 21 | −8 | 4.0 |
| Orbitofrontal cortex | Right | 11 | 32 | 34 | −15 | 4.3 | ||||
| Medial frontal cortex | Left | 6 | 0 | 12 | 47 | 5.5 | ||||
| Right | 6 | 4 | −20 | 69 | 4.1 | 18 | −15 | 58 | 4.1 | |
| Ventromedial prefrontal cortex | Left | 24 | 0 | 29 | −5 | 3.5 | ||||
| Anterior cingulate cortex | Left | 32 | −4 | 30 | 22 | 3.7 | ||||
| Right | 32 | 18 | 13 | 23 | 4.8 | |||||
| Posterior cingulate cortex | Left | 31 | −2 | −31 | 31 | 4.1 | −6 | −28 | 33 | 3.9 |
| Right | 29,31 | 20 | −41 | 26 | 4.5 | 18 | −35 | 31 | 3.6 | |
| Uncus | Right | 34 | 22 | 3 | −22 | 3.6 | ||||
| Insula | Right | 13 | 42 | −25 | −2 | 4.4 | ||||
| Parietal lobe | Left | 40,5,7 | −40 | −48 | 45 | 5.9 | −22 | −40 | 52 | 3.6 |
| Right | 40,5,7 | 42 | −45 | 35 | 4.2 | 24 | −38 | 53 | 3.8 | |
| Middle temporal gyrus | Right | 39 | 30 | −55 | 29 | 4.6 | 38 | −65 | 20 | 4.4 |
| Superior temporal gyrus | Right | 38,21 | 48 | 7 | −19 | 3.8 | ||||
| Occipital lobe | Left | 18 | −22 | −91 | 8 | 4.7 | 0 | −86 | 34 | 3.4 |
| Right | 17,18 | 14 | −93 | 6 | 4.5 | 18 | −79 | 9 | 3.5 | |
| Caudate head | Right | 18 | 21 | −4 | 4.6 | |||||
| Caudate body | Left | −19 | −1 | 20 | 4.3 | −21 | −3 | 20 | 3.9 | |
| Right | 18 | −9 | 21 | 4.4 | 20 | 5 | 18 | 4.1 | ||
| Caudate tail | Left | −30 | −34 | 11 | 3.8 | |||||
aOCD patients did not show any significant difference in this contrast. bx, y, z refer to coordinates in the Talairach and Tournoux stereotactic space. *P<0.001, uncorrected, k>20 voxels.
**P<0.0001, uncorrected, k>20 voxels.
| Area of activation . | Left/right . | Brodmann's area . | Control (N = 21) . | Control (N = 21)>OCD (N = 21) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Coordinatesb . | Z-value . | Coordinatesb . | Z-value . | ||||
| . | . | . | x . | y . | z . | . | x . | y . | z . | |
| Dorsolateral prefrontal cortex | Left | 9 | −50 | 7 | 31 | 4.1 | ||||
| Right | 10 | 38 | 52 | 21 | 5.6 | 36 | 51 | 18 | 3.9 | |
| Premotor area | Left | 6,4 | −30 | −2 | 46 | 4.9 | −36 | −12 | 37 | 4.1 |
| Right | 6 | 28 | 9 | 55 | 4.7 | 24 | 12 | 45 | 3.7 | |
| Ventrolateral prefrontal cortex | Left | 47 | −55 | 17 | −1 | 4.6 | −51 | 21 | −8 | 4.0 |
| Orbitofrontal cortex | Right | 11 | 32 | 34 | −15 | 4.3 | ||||
| Medial frontal cortex | Left | 6 | 0 | 12 | 47 | 5.5 | ||||
| Right | 6 | 4 | −20 | 69 | 4.1 | 18 | −15 | 58 | 4.1 | |
| Ventromedial prefrontal cortex | Left | 24 | 0 | 29 | −5 | 3.5 | ||||
| Anterior cingulate cortex | Left | 32 | −4 | 30 | 22 | 3.7 | ||||
| Right | 32 | 18 | 13 | 23 | 4.8 | |||||
| Posterior cingulate cortex | Left | 31 | −2 | −31 | 31 | 4.1 | −6 | −28 | 33 | 3.9 |
| Right | 29,31 | 20 | −41 | 26 | 4.5 | 18 | −35 | 31 | 3.6 | |
| Uncus | Right | 34 | 22 | 3 | −22 | 3.6 | ||||
| Insula | Right | 13 | 42 | −25 | −2 | 4.4 | ||||
| Parietal lobe | Left | 40,5,7 | −40 | −48 | 45 | 5.9 | −22 | −40 | 52 | 3.6 |
| Right | 40,5,7 | 42 | −45 | 35 | 4.2 | 24 | −38 | 53 | 3.8 | |
| Middle temporal gyrus | Right | 39 | 30 | −55 | 29 | 4.6 | 38 | −65 | 20 | 4.4 |
| Superior temporal gyrus | Right | 38,21 | 48 | 7 | −19 | 3.8 | ||||
| Occipital lobe | Left | 18 | −22 | −91 | 8 | 4.7 | 0 | −86 | 34 | 3.4 |
| Right | 17,18 | 14 | −93 | 6 | 4.5 | 18 | −79 | 9 | 3.5 | |
| Caudate head | Right | 18 | 21 | −4 | 4.6 | |||||
| Caudate body | Left | −19 | −1 | 20 | 4.3 | −21 | −3 | 20 | 3.9 | |
| Right | 18 | −9 | 21 | 4.4 | 20 | 5 | 18 | 4.1 | ||
| Caudate tail | Left | −30 | −34 | 11 | 3.8 | |||||
| Area of activation . | Left/right . | Brodmann's area . | Control (N = 21) . | Control (N = 21)>OCD (N = 21) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Coordinatesb . | Z-value . | Coordinatesb . | Z-value . | ||||
| . | . | . | x . | y . | z . | . | x . | y . | z . | |
| Dorsolateral prefrontal cortex | Left | 9 | −50 | 7 | 31 | 4.1 | ||||
| Right | 10 | 38 | 52 | 21 | 5.6 | 36 | 51 | 18 | 3.9 | |
| Premotor area | Left | 6,4 | −30 | −2 | 46 | 4.9 | −36 | −12 | 37 | 4.1 |
| Right | 6 | 28 | 9 | 55 | 4.7 | 24 | 12 | 45 | 3.7 | |
| Ventrolateral prefrontal cortex | Left | 47 | −55 | 17 | −1 | 4.6 | −51 | 21 | −8 | 4.0 |
| Orbitofrontal cortex | Right | 11 | 32 | 34 | −15 | 4.3 | ||||
| Medial frontal cortex | Left | 6 | 0 | 12 | 47 | 5.5 | ||||
| Right | 6 | 4 | −20 | 69 | 4.1 | 18 | −15 | 58 | 4.1 | |
| Ventromedial prefrontal cortex | Left | 24 | 0 | 29 | −5 | 3.5 | ||||
| Anterior cingulate cortex | Left | 32 | −4 | 30 | 22 | 3.7 | ||||
| Right | 32 | 18 | 13 | 23 | 4.8 | |||||
| Posterior cingulate cortex | Left | 31 | −2 | −31 | 31 | 4.1 | −6 | −28 | 33 | 3.9 |
| Right | 29,31 | 20 | −41 | 26 | 4.5 | 18 | −35 | 31 | 3.6 | |
| Uncus | Right | 34 | 22 | 3 | −22 | 3.6 | ||||
| Insula | Right | 13 | 42 | −25 | −2 | 4.4 | ||||
| Parietal lobe | Left | 40,5,7 | −40 | −48 | 45 | 5.9 | −22 | −40 | 52 | 3.6 |
| Right | 40,5,7 | 42 | −45 | 35 | 4.2 | 24 | −38 | 53 | 3.8 | |
| Middle temporal gyrus | Right | 39 | 30 | −55 | 29 | 4.6 | 38 | −65 | 20 | 4.4 |
| Superior temporal gyrus | Right | 38,21 | 48 | 7 | −19 | 3.8 | ||||
| Occipital lobe | Left | 18 | −22 | −91 | 8 | 4.7 | 0 | −86 | 34 | 3.4 |
| Right | 17,18 | 14 | −93 | 6 | 4.5 | 18 | −79 | 9 | 3.5 | |
| Caudate head | Right | 18 | 21 | −4 | 4.6 | |||||
| Caudate body | Left | −19 | −1 | 20 | 4.3 | −21 | −3 | 20 | 3.9 | |
| Right | 18 | −9 | 21 | 4.4 | 20 | 5 | 18 | 4.1 | ||
| Caudate tail | Left | −30 | −34 | 11 | 3.8 | |||||
aOCD patients did not show any significant difference in this contrast. bx, y, z refer to coordinates in the Talairach and Tournoux stereotactic space. *P<0.001, uncorrected, k>20 voxels.
**P<0.0001, uncorrected, k>20 voxels.
Between-group analysis using switch minus repeat contrast revealed significantly lower levels of activation in the OCD group compared with the control group for distributed areas including DLPFC, ACC, caudate nucleus, VMPFC and right OFC (Fig. 4A and Table 2). OCD patients did not exhibit significantly higher activation than healthy controls in any area.
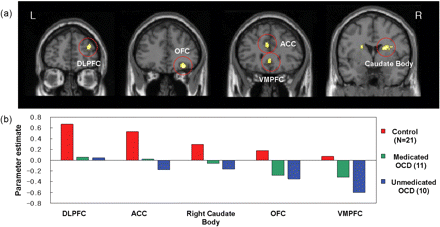
(A) The significantly different activation regions between the OCD patients and the healthy controls are mapped on the MNI template. P<0.001, uncorrected, k>20 voxels. DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; VMPFC, ventromedial prefrontal cortex. (B) Individual activation values of task-switch minus task-repeat in the ROI were extracted.
Figure 4B presents the beta values of task-switch minus task-repeat in each group for the ROI analysis. In areas of the dorsal frontal-striatal circuit, including the DLPFC, ACC and caudate body, these results indicate that the OCD group exhibited little difference between task-switch and task-repeat conditions, whereas the control group exhibited significantly increased activation during task-switch trials. However, OCD patients exhibited decreased activation in ventral frontal-striatal regions of the VMPFC and right OFC during task-switching, whereas the control group exhibited little difference between two conditions. We subdivided the OCD group into medicated and non-medicated groups and calculated the ROI values for each group; both the medicated and non-medicated OCD groups exhibited decreased task-switch minus task-repeat values in these areas compared with the control group.
Correlation analysis
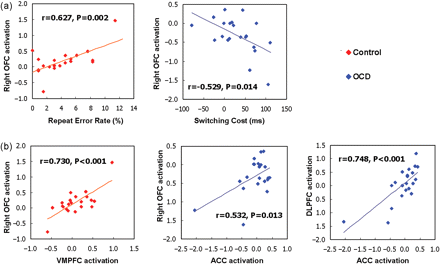
Correlation analysis between performance and brain activation revealed that right OFC activation was significantly correlated with error rate of repeat trials in controls (r = 0.627, P = 0.002) and with switching cost of RT in OCD patients (r = −0.529, P = 0.014) (Fig. 5A). Other areas of DLPFC, ACC, VMPFC and right caudate body did not show any significant correlation with behavioural data.
(A) The significant correlations between performances and right OFC activations are represented in each subject group. (B) The significant correlations between the brain activation values (task-switch minus task-repeat) of DLPFC, ACC, OFC and VMPFC are shown in each subject group.
Between the previously defined ROIs, we found a significant correlation between the right OFC and the VMPFC activations in control group (r = 0.730, P < 0.001) and the activations of ACC was correlated with right OFC (r = 0.532, P = 0.013) and DLPFC (r = 0.748, P < 0.001) in OCD group (Fig. 5B). Scores of OCD symptom severity (Y-BOCS), depression level (BDI) and anxiety level (BAI) did not show any significant correlation with the brain activations of the previously defined ROIs and with the performances.
Discussion
To the best of our knowledge, this is the first study to investigate the neural correlates of cued task-switching in patients with OCD. Of the various cognitive flexibility tasks available, we applied a cued task-switching paradigm because the design simplicity of this paradigm and external cues can help to exclude confounding factors, such as the effects of negative feedback when providing instructions for the next task. Moreover, the participants concentrated on categorizing the trials as colour or shape discrimination tasks, rather than as switch or repeat tasks, thereby reducing the possibility that they would use explicit strategies to switch a task set. In addition, owing to our concern about error-related abnormal activation in OCD patients, we excluded the error and post-error trials during imaging analysis. In this task-switching paradigm, OCD patients had significantly greater error rates than the control group during task-switch trials; this result is consistent with the previous reports of cognitive inflexibility in OCD patients (Veale et al., 1996; Watkins et al., 2005; Bannon et al., 2006; Chamberlain et al., 2006).
In terms of functional data, the brain activations in the two groups clearly differed on the switch minus repeat contrast. In the healthy volunteers, the task-switch trials activated widespread networks in both cortical and subcortical brain regions. In contrast, the activation in these brain areas, including the frontal-striatal circuit, was reduced significantly in OCD patients during the same tasks. During task-switching, healthy controls recruited regions of activation that included the PFC, ACC, parietal lobe, temporal cortex and caudate nucleus. This result agrees with the previous imaging studies reporting that task-switching is related to the PFC (Brass and von Cramon, 2002; Braver et al., 2003; Cools et al., 2004), basal ganglia (Cools et al., 2004; Monchi et al., 2006) and parietal lobe (Bunge et al., 2002). Wallis et al. (2001) showed that the DLPFC is involved in task representation and abstract rule information. In particular, the right DLPFC (Braver et al., 2003) is involved in sustained cognitive control during task-switching. Monchi et al. (2006) showed that the caudate nucleus is involved in planning and set-shifting and may be involved in cognitive manipulation. Neuroimaging studies have reported that activation of the parietal cortex is related to representing response contingencies (Bunge et al., 2002) and that ACC activation plays a role in monitoring conflict processing (Kerns et al., 2004) and in changing an action choice with a decrease in reward (Bush et al., 2002). Our findings of decreased activation in neural networks, including the dorsal prefrontal-striatal circuits, are consistent with a functional neuroimaging study which reported that OCD patients exhibited decreased dorsal frontal-striatal responsiveness during planning (van den Heuvel et al., 2005). Combined, these findings support the hypothesis that impaired task-switching ability in OCD patients is also associated with decreased dorsal prefrontal-striatal responsiveness.
In addition to the dorsal frontal-striatal circuit areas, we found that activity (task-switch minus task-repeat) in the VMPFC and right OFC differed significantly between the two groups. Abnormalities in the VMPFC (Irle et al., 1998; Cavedini et al., 2002) and right OFC (Rauch et al., 1994; Breiter et al., 1996; Choi et al., 2004) have frequently been reported in OCD patients, and previous research has suggested that the OFC and VMPFC are associated with reward-related aspects of behavioural control (Rogers et al., 1999, 2000; Zalla et al., 2000; Breiter et al., 2001; Fellows and Farah, 2003) and that lesions in the OFC lead to difficulties in extinguishing or switching a response from a previously rewarded stimulus (Rolls et al., 1994; Dias et al., 1996). In addition, recent OCD neuroimaging research using reversal learning in OCD patients has reported aberrant activation of the OFC (Remijnse et al., 2006). Importantly, using a probabilistic reversal task, O’Doherty et al. (2003) reported that the right OFC and VMPFC activations were related to response selection in response maintenance conditions, as compared to merely looking at the rewarded stimulus chosen by a computer. Therefore, these activations were distinguished from those in response to rewarding and punishing feedback itself. While the OFC and VMPFC were engaged under response maintenance conditions, the dorsal ACC was activated in response-switching conditions when a behavioural choice was required. Although this occurred in a reversal learning task, in terms of the behavioural choice between switching and repeating, the result leads to postulate that when a behavioural choice is required, the OFC and VMPFC activation is related to maintaining the response or reward contingency, and the dorsal ACC activation is related to switching them. In this view, we suggest that increased activation of OFC and VMPFC in OCD during repeat compared to switch could be related to the tendency to maintain the previous task-set. Moreover, previous research reported that the hypoactivation of the VMPFC during attention shifting was associated with an increased shifting cost, which reflected poor shifting performance (Wager et al., 2005), and that the activation of ACC was related to a decrease in reward, which was a precursor to a change in action choice (Bush et al., 2002). In agreement with this view, our correlation analysis revealed that the ACC activations for switch, compared to repeat, were negatively correlated with the OFC activations for repeat, compared to switch, in OCD patients; this suggests that the subjective weights of the switch and repeat conditions differ among subjects. In addition, the analysis of the correlation between the behavioural data and OFC accords with this view, implying that, with the greater activation of the OFC during repeat trials, the OCD patients showed a greater switching cost, which suggested that they had a bias toward keeping the previous response set rather than switching, and had difficulty in switching from the previous set. Also, the relationship of the repeat error rate with the OFC activity in controls showed that those who have reduced OFC activation in repeat trials, as compared to switch trials, tend to show a higher error rate during repeat previous task-set, which could reflect an internal weight between maintaining the previous task-set and switching to a new set.
The activation values determined in ROI analysis revealed the activation patterns in each area for each group. Most of the areas with activation differences between the two groups, especially the dorsal regions of the ACC and DLPFC, were activated more in healthy controls than in OCD patients. However, while the OFC and VMPFC were activated only slightly in the control group at the switch minus repeat contrast, these areas in OCD patients were activated during the repeat condition or hypoactivated (Fig. 4). These results indicate increased responsiveness of the ventral frontal-striatal regions to repeating a previous task and decreased responsiveness of the dorsal circuit to switching a set in OCD patients. Consequentially, during the task, healthy controls would place more value on switch trials, whereas OCD patients might have an opposite approach, or might have difficulty in discarding the previous set, making it difficult to update the new set efficiently. Therefore, the task-switching ability is related not only to the dorsal but also to the ventral frontal-striatal areas. The imbalance between the dorsal and ventral frontal-striatal regions may be a neural correlate of the cognitive inflexibility seen in patients with OCD. However, further investigation is required because the direct relationship between the OFC and the subjective weights between repeat and switch has not been examined using a task-switching paradigm in a control group. However, the increased response of the ventral region with an inhibited dorsal frontal-striatal circuit during task-switching in OCD patients accords with the current neurobiological models of OCD (Saxena et al., 1998; Mataix-Cols and van den Heuvel, 2006) and, using event-related fMRI, offers a neurobiological view of the impaired cognitive flexibility that might be one of the core features of OCD.
This study has several limitations. First, depression and anxiety levels, which were significantly higher in the OCD patients, could have confounding effects. Thus, we attempted to address the effects of depression or anxiety levels based on a correlation analysis with behaviour or brain activation but found that no significant relationship appeared in the OCD group. However, we still cannot rule out the possibility that these confounding factors, especially depression, could affect the behaviour flexibility even though it was not detectable on correlation analysis. Also, since we did not include clinical control group, it is hard to know whether these results are specific to OCD or not. It could be interesting to examine the task-switching-related activation in other patients groups like depression or schizophrenia, since they share some common features like dorsal frontal-striatal dysfunction with OCD. Second, we included patients who were taking medication. However, the ROI analysis indicated that both the medicated and non-medicated OCD groups showed less activation in the regions of interest than the control group. Moreover, non-medicated OCD patients showed less activation than medicated OCD patients and this could give important implications for the effects of treatments or SSRI medications related with previous study of cognitive inflexibility after prefrontal serotonin depletion in primate. Clarke et al. (2004) have shown that selective serotonin depletion of the marmoset PFC produced perseveration during reversal learning and implicated importance of prefrontal serotonin in behavioural flexibility. Future research should examine more precisely how the effects of treatment affect these levels. Third, the severity of the obsessive-compulsive symptoms measured using the Y-BOCS did not show any significant relationships with task performance or brain activation. However, in accord with our result, cognitive inflexibility has been reported in OCD patients after symptom remission (Bannon et al., 2006), and in the unaffected first-degree relatives of OCD patients (Chamberlain et al., 2007). Therefore, we speculate that the cognitive inflexibility could be related to traits rather than symptoms. Fourth, our patient group is heterogeneous in terms of symptom clusters and thus the result could be influenced by the various symptoms among the patients. Finally, since the majority of patients in this study were male and it has been suggested that gender may contribute to the clinical and biological heterogeneity of OCD (Lochner et al., 2004), it is hard to generalize this result to female patients with OCD.
In conclusion, this study has shown the neural correlates of the impaired task-switching ability of the OCD patients directly, using fMRI. Our results suggest that an imbalance between the dorsal and ventral frontal-striatal circuits is responsible for the cognitive inflexibility of OCD patients. Further research to clarify the dynamic functional connectivity between these networks should increase our understanding of the pathophysiology of OCD.
Acknowledgements
This research was supported by a grant (M103KV010012-06K2201-01210) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea. The authors thank to Yong-Sik Jung for his help in MR scanning.
References
Abbreviations:
- OCD
obsessive-compulsive disorder
- OFC
orbitofrontal cortex
- ACC
anterior cingulate cortex
- PFC
prefrontal cortex
- fMRI
functional magnetic resonance imaging
- BDI
Beck depression inventory
- BAI
Beck anxiety inventory