-
PDF
- Split View
-
Views
-
Cite
Cite
Aikaterini Fotopoulou, Manos Tsakiris, Patrick Haggard, Angelique Vagopoulou, Anthony Rudd, Michael Kopelman, The role of motor intention in motor awareness: an experimental study on anosognosia for hemiplegia, Brain, Volume 131, Issue 12, December 2008, Pages 3432–3442, https://doi.org/10.1093/brain/awn225
Close - Share Icon Share
Abstract
Recent theories propose that anosognosia for hemiplegia (AHP) results from specific impairments in motor planning. However, no study has hitherto directly investigated the role of motor intention in the observed non-veridical awareness of action in AHP. We developed the following paradigm to investigate the role of motor planning in awareness in patients with AHP: Four hemiplegic patients with and four without anosognosia were provided with false visual feedback of movement in their left paralysed arm through a prosthetic rubber hand. We examined whether the ability to detect presence or absence of movement based on visual evidence varied according to whether the patient had planned to move their limb or not. Motor intention had a selective effect on patients with AHP; they were more likely than controls (U = 16, P< 0.001) to ignore the visual feedback of a motionless hand and claim that they moved it when they had the intention to do so (self-generated movement) than when they expected an experimenter to move their own hand (externally generated movement), or there was no expectation of movement. By contrast, patients without AHP were not influenced by these manipulations, and did not claim they moved their hand when the hand remained still. This is the first direct demonstration that altered awareness of action in AHP reflects a dominance of motor intention prior to action over sensory information about the actual effects of movement.
Introduction
Anosognosia is the apparent inability to acknowledge or recognize one's deficits. Babinski (1914) coined the term (from the Greek, α = without,  = disease,
= disease, 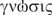 = knowledge) in order to describe the behaviour of right-brain-damaged patients with contralesional hemiplegia who denied their paralysis. Anosognosia for hemiplegia (AHP) occurs more frequently following right than left brain damage (Nathanson et al., 1952; Bisiach and Geminiani, 1991; Starkstein et al., 1992; Heilman et al., 1998).
= knowledge) in order to describe the behaviour of right-brain-damaged patients with contralesional hemiplegia who denied their paralysis. Anosognosia for hemiplegia (AHP) occurs more frequently following right than left brain damage (Nathanson et al., 1952; Bisiach and Geminiani, 1991; Starkstein et al., 1992; Heilman et al., 1998).
The manifestations of AHP can vary; some patients may admit their deficits but minimize their practical or emotional importance (anosodiaphoria) (Critchley, 1953). Others do not acknowledge any disability despite blatant evidence to the contrary (Feinberg and Roane, 2003). Moreover, some patients acknowledge their deficits in a general manner, while failing to detect specific motor inabilities and vice versa (Marcel et al., 2004). AHP can also be accompanied by delusional beliefs. For example, patients may reject the ownership of their limb completely (asomatognosia), attribute its ownership to someone else (somatoparahrenia) (Gerstmann, 1942), or, they may personify the paralysed limb (Juba, 1949).
Geschwind (1965) proposed that AHP is the result of inter-hemispheric disconnection (Adair et al., 1997). Others suggest that AHP is the result of a psychological defence against excessive depression or anxiety (Weinstein and Kahn, 1955; Bisiach and Geminiani, 1991; Heilman et al., 1998; Turnbull et al., 2005). AHP has also been seen as a secondary consequence of sensory feedback deficits, such as spatial or personal neglect (Cutting, 1978; Levine, 1990). Finally, AHP might result from the combination of sensory deficits and higher-order cognitive deficits like confusion, confabulation or memory impairments (Levine, 1991; Berti et al., 1996). However, double dissociations have been observed between AHP and each of the aforementioned deficits (Bisiach et al., 1986; Starkstein et al., 1992; Adair et al., 1995; Heilman et al., 1998; Berti et al., 2005; Coslett, 2005). Moreover, Marcel et al. (2004) recently found that neither sensory loss nor intellectual impairment, or their combination provides a complete explanation of AHP. Thus, although some of these impairments may be predisposing or aggravating factors, they do not appear to be sufficient causes of AHP.
More recent accounts, capitalizing on recent computational models of motor control (Frith et al., 2000), proposed that AHP results from specific impairments in motor planning. Under normal circumstances, the formation of an intention to move will be used by ‘forward models’ to generate accurate predictions about the impending sensory feedback. If an intended movement is not performed as planned, a comparator will detect a mismatch between the predicted sensory feedback and the absence of any actual sensory feedback. The error signal at the level of the comparator can be then used to inform motor awareness. Heilman and colleagues (1998) considered AHP a ‘motor intentional deficit’ arising from an inability to form motor intentions; if the formation of the intention to move is defective, the comparator (although properly functioning) does not receive any information about movement planning. Thus, the patient considers movement has been executed although no movement has been initiated (Gold et al., 1994).
Berti et al. (2007) investigated this hypothesis by studying the activity of proximal muscles in an EMG study. Specifically, they showed that left proximal muscle activation took place in a right-brain-damaged patient with left hemiplegia and AHP, even though left-side reaching movement could not be performed. They suggested that patients with AHP do have intact intention for voluntary action, and remain able to programme movements and form predictions. These findings go against the Heilman et al.'s hypothesis. Instead, Berti et al. (2007), following Frith et al. (2000) hypothesized that patients with AHP form appropriate representations of the desired and predicted positions of the limb, but they are not aware of the discrepancy between their prediction and the actual position. On this view, patients’ awareness is dominated by intention, and does not take account the failure of sensory evidence to confirm the execution of the intended action. AHP arises because action awareness is based on motor commands sent to the plegic limb and sensory evidence about lack of movement is not processed. Accordingly, AHP may involve damage to the brain areas that underpin the monitoring of the correspondence between motor outflow and sensory inflow are damaged (e.g. Brodmann premotor areas 6 and 44; Berti et al., 2005), or else contrary sensory information is neglected (Frith et al., 2000). Consequently, the mismatch between the predicted state (i.e. movement of the limb) and the actual state (i.e. no movement) is not registered.
Notwithstanding the theoretical interest of the above hypothesis, no study has hitherto directly investigated the role of motor intention in the generation of a positive symptom in AHP, namely, their non-veridical awareness of action. Berti and colleagues (2005, 2007) have provided convincing evidence that motor planning is intact in AHP (against the Heilman et al., hypothesis), but to our knowledge no study has been able to demonstrate that motor planning dominates awareness of action in AHP. Such a demonstration would require an experimental situation that sensory feedback relevant to the arm that the patient could normally detect was nevertheless not detected when patients formed a motor intention to move the arm. This was the aim of the current study.
Specifically, we developed an experimental paradigm to test the hypothesis that awareness of a movement is based primarily on motor planning, and that this information dominates over sensory feedback. Because AHP patients typically have somatsensory loss (Cutting, 1978), we focused on visual feedback from the limb rather than somatosenory feedback. Visual feedback of action plays an important role in action awareness, as shown in self-recognition studies with healthy participants (van den Bos and Jeannerod, 2002; Tsakiris et al., 2006).
We therefore used a realistic prosthetic hand to generate visual feedback of movements that the patient either commanded themselves (self-generated movement) or were controlled by an experimenter (externally generated movement). In some conditions, we gave visual feedback that was incompatible with the patient's predictions. In particular, in the critical condition, patients were instructed to move their left hand themselves, but the prosthetic hand remained still. These conditions essentially mirrored the classic anosognosic scenario within an experimentally controlled procedure (cf. Ramachandran, 1995). We hypothesized that if motor planning (motor intentions) dominates over sensory feedback in AHP, patients would fail to perceive the prosthetic hand's lack of movement. Thus, if asked to detect movements of their left arm, they would make more false alarms in this critical condition (self-generated movement) than in the externally generated movement conditions.
Materials and Methods
Subjects
Eight adult neurological patients were consecutively recruited from an acute stroke unit. Berti et al. (1996) have suggested that studying cases with denial of complete contralateral hemiplegia can generate more reliable findings than investigating patients with mild or moderate hemiparesis. Accordingly, inclusion criteria were: (i) complete left upper limb hemiplegia (completely paralysed left arm as reported by the responsible neurologist and confirmed by direct motor impairment examination; (ii) unilateral right hemisphere lesions (as detected by CT or MRI neuroimaging investigations); and (iii) <4 months from onset. Exclusion criteria were: (i) previous neurological or psychiatric history; (ii) <7 years of education or an estimated premorbid Full Scale Intelligence Quotient (FSIQ) based on the Wechsler Test of Adult Reading (WTAR, Wechsler, 2001) <70; (iii) medication with severe cog-nitive or mood side effects; and (iv) severe language impairment (i.e. insufficient communication).
Patients were classified as having AHP, based on an open interview that explored their awareness for motor deficits (Berti et al., 1996). This included (i) a first set of general questions: e.g. ‘Why are you in the hospital?’ (ii) specific questions about motor ability: e.g. ‘How is your left arm? Can you move it?’ and (iii) confrontation questions: e.g. ‘Please, touch my hand with your left hand. “Have you done it?” ’. The total score indicating awareness for upper limb motor impairment ranged from 0 to 2. A score of 0 was given if patients acknowledged their motor impairment; a score of 1 was given if patients did not acknowledge their motor impairment but recognized not having reached the examiner's hand; a score of 2 was given if patients denied motor impairment and the failure in reaching the examiner's hand. Patients were considered to be anosognosic when they did not acknowledge the contralesional hemiplegia after repeated and specific questioning by the examiner (scores 1 and 2). The scale developed by Feinberg et al. (2000) was used as a second measure of patients’ unawareness. This consists of 10 questions, including general open questions, e.g. ‘Do you have weakness anywhere?’ or ‘Is your arm causing you any problems?’ and confrontation questions, e.g. Left arm is lifted and dropped in right hemispace. ‘It seems there is some weakness. Do you agree?’ A score of 0 was given if the patient showed awareness of deficit; 0.5 for partial awareness; and 1.0 for complete unawareness or denial.
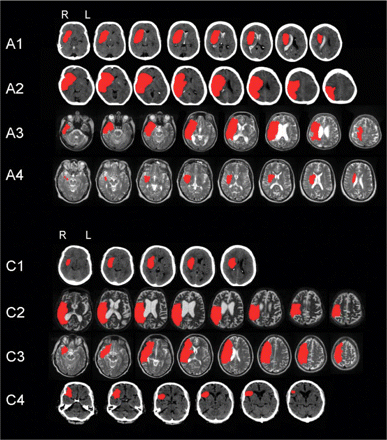
There were four right-hemisphere-damaged patients with complete left upper limb hemiplegia and AHP (AHP = experimental group) and four right-hemisphere-damaged patients with complete left upper limb hemiplegia but without AHP (HP = control group). All patients had lesions mainly in the territory of the right middle cerebral artery, involving frontal, temporal and parietal lobes and subcortical involvement of the basal ganglia, the insula and the internal capsule. Figure 1 illustrates the lesions of the patients as documented by clinical CT or MRI scans. The lesions showed variability typical of the literature (for an extensive meta-analysis see Pia et al., 2004). In the AHP group patients, A1 had a frontoparietal lesion extending to subcortical areas, Patients A2 and A3 showed large lesions affecting most of the right middle cerebral artery territory, and Patient A4 had focal subcortical damage mainly affecting the basal ganglia. The lesions of the control HP patients were equally varied. Due to the variation of the neuroradiological findings, precise volumetric analysis and detailed lesion mapping analysis and contrasts were not warranted (for recent volumetric studies of similar groups see Berti et al., 2005; Karnath et al., 2005). All patients underwent neurological and neuropsychological assessment. All participants gave informed consent and the study was approved by the local Trust's Ethical Committee.
Individual patients’ lesions in the two groups represented in red. Patients A1–A4 formed the AHP group and Patient C1–C4 formed the Control HP group. All patients had lesions affecting the territory of the right middle cerebral artery.
Neuropsychological assessment
In addition to the anosognosia tests mentioned above, the patients were assessed using the following standardized tests: pre-morbid intelligence was assessed using the WTAR (Wechsler, 2001); post-morbid intelligence was assessed using the following WAIS-III (Wechsler, 1998) subtests: vocabulary, similarities, digit span, arithmetic, matrix reasoning. Visual fields were tested with the customary ‘confrontation’ technique (Bisiach et al., 1986). Unilateral visuospatial neglect was assessed using the conventional sub-tests of the Behavioural Inattention Test (BIT), including line crossing, letter cancellation, star cancellation, figure and shape copying, line-bisection and representational drawing. The ‘One Item Test’ (Bisiach et al., 1986) and ‘Comb/Razor Test’ (McIntosh et al., 2000) were used for the assessment of personal neglect. Subtests of the RASP were used for the measurement of sensory functions: ‘Surface Touch’, ‘Tactile Extinction’ and ‘Proprioception’. Reasoning abilities were assessed using the Cognitive Estimates Test (Shallice and Evans, 1978) and the DK-EFS Proverbs Test (Delis et al., 2001) and inhibition of automatic responses was assessed with the Hayling Test (Burgess and Shallice, 1997). The Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith, 1983) was used to assess depression and anxiety.
Patients’ demographic characteristics and their performance on standardized neuropsychological tests are summarized in Table 1.
Groups demographic characteristics and neuropsychological profile
| . | AHP . | HP controls . | Mann–Whitney test . | |||
|---|---|---|---|---|---|---|
| . | Mean . | SD . | Mean . | SD . | Z . | P . |
| N | 4 | – | 4 | – | – | – |
| Age (years) | 63.75 | 5.95 | 61.00 | 22.79 | 0.00 | 1.00 |
| Education (years) | 12.75 | 2.87 | 12.00 | 2.83 | 0.73 | 0.30 |
| Days from onset | 20.0 | 13.23 | 19.75 | 3.30 | 0.00 | 0.40 |
| Premorbid IQ–WTAR | 112.67 | 13.05 | 104.75 | 7.72 | 0.00 | 0.40 |
| WAIS-III vocabulary | 9.75 | 4.65 | 8.75 | 2.99 | 0.30 | 0.38 |
| WAIS-III similarities | 9.50 | 3.11 | 10.25 | 2.50 | 0.29 | 0.38 |
| WAIS-III digit span | 10.25 | 3.50 | 9.25 | 1.71 | 0.44 | 0.36 |
| WAIS-III matrix reasoning | 6.33a | 2.52 | 8.33 | 2.52 | 0.89 | 0.27 |
| WAIS-III arithmetic | 7.67 | 3.21 | 8.67 | 2.31 | 0.70 | 0.31 |
| Visual fields R | 9.25 | 0.50 | 10.00 | 0.00 | 2.05 | 0.11 |
| Visual fields L | 3.25a | 3.40 | 6.25a | 2.06 | 1.48 | 0.20 |
| Visual fields both | 1.00a | 1.41 | 2.25a | 1.71 | 1.04 | 0.34 |
| RASP surface touch L—max 30 | 6.75a | 3.86 | 7.50a | 3.87 | 0.29 | 0.88 |
| RASP surface touch/spam max 10 | 5.00a | 2.44 | 4.75a | 1.70 | 1.00 | 1.00 |
| RASP extinction—max 12 | 3.66a | 6.35 | 6.50a | 2.12 | 0.59 | 0.80 |
| RASP proprioception—max 30 movement detection L | 6.75a | 3.30 | 6.50a | 4.04 | 0.15 | 0.89 |
| RASP proprioception—max 30 direction detection L | 4.45a | 2.87 | 3.50a | 3.69 | 0.44 | 0.69 |
| Comb/razor test R | 27.5 | 16.7 | 25.50 | 1.29 | 1.00 | 0.34 |
| Comb/razor test L | 13.75 | 17.35 | 20.00 | 5.42 | 1.16 | 0.34 |
| Comb/razor test ambiguous | 10.75 | 11.53 | 8.75 | 8.18 | 0.14 | 0.88 |
| Comb/razor test bias | −0.39a | 0.32 | −0.10 | 0.11 | 1.7 | 0.11 |
| Bisiach test | 1.00 | 0.50 | 0.00 | 0.00 | 1.53 | 0.13 |
| BIT total score | 84.75a | 29.54 | 96a | 35.31 | 0.43 | 0.66 |
| Line crossing R | 18 | 0 | 17.25 | 0.95 | 1.50 | 0.30 |
| Line crossing L | 6.75a | 6.16 | 15.75a | 3.3 | 2.00 | 0.06** |
| Letter cancellation R | 16 | 2.82 | 14.75 | 2.36 | 0.60 | 0.20 |
| Letter cancellation L | 6.25a | 3.3 | 12.25a | 5.5 | 1.40 | 0.70 |
| Star cancellation R | 21.5 | 7.59 | 23 | 4.08 | 0 | 1 |
| Star cancellation L | 8.5a | 10.63 | 20.75a | 4.11 | 1.60 | 0.10 |
| Copy | 1a | 1.41 | 3 | 1.15 | 1.80 | 0.08** |
| Representational drawing | 1.33a | 1.15 | 2.75 | 0.5 | 1.90 | 0.56 |
| Line bisection R | 3 | 0 | 2.5 | 1 | 1.00 | 0.30 |
| Line bisection Centre | 2 | 1.41 | 2.25 | 1.5 | 0.50 | 0.61 |
| Line bisection L | 0.75a | 0.95 | 1.5a | 1.73 | 0.60 | 0.50 |
| Berti awareness LUL | 1.5 | 0.6 | 0.00 | 0.00 | 2.50 | <0.05* |
| Berti awareness LLL | 1.75 | 0.50 | 0.00 | 0.00 | 2.50 | <0.05* |
| AHP questionnaire | 5.1 | 0.9 | 0.63 | 0.48 | 2.82 | <0.05* |
| Hayling test RTs | 2.00a | 1.15 | 3.00a | 2.31 | 0.62 | 0.33 |
| Hayling test errors | 4.00 | 1.83 | 3.50 | 2.89 | 0.30 | 0.40 |
| Proverbs | 8.00 | 3.61 | 11.50 | 5.74 | 1.08 | 0.22 |
| Cognitive estimates | 10.00a | 2.71 | 9.33a | 1.15 | 1.08 | 0.22 |
| HADS depression | 4.00 | 2.83 | 7.50 | 5.97 | 1.04 | 0.30 |
| HADS anxiety | 7.75 | 5.06 | 9.00 | 6.58 | 0.00 | 1.00 |
| . | AHP . | HP controls . | Mann–Whitney test . | |||
|---|---|---|---|---|---|---|
| . | Mean . | SD . | Mean . | SD . | Z . | P . |
| N | 4 | – | 4 | – | – | – |
| Age (years) | 63.75 | 5.95 | 61.00 | 22.79 | 0.00 | 1.00 |
| Education (years) | 12.75 | 2.87 | 12.00 | 2.83 | 0.73 | 0.30 |
| Days from onset | 20.0 | 13.23 | 19.75 | 3.30 | 0.00 | 0.40 |
| Premorbid IQ–WTAR | 112.67 | 13.05 | 104.75 | 7.72 | 0.00 | 0.40 |
| WAIS-III vocabulary | 9.75 | 4.65 | 8.75 | 2.99 | 0.30 | 0.38 |
| WAIS-III similarities | 9.50 | 3.11 | 10.25 | 2.50 | 0.29 | 0.38 |
| WAIS-III digit span | 10.25 | 3.50 | 9.25 | 1.71 | 0.44 | 0.36 |
| WAIS-III matrix reasoning | 6.33a | 2.52 | 8.33 | 2.52 | 0.89 | 0.27 |
| WAIS-III arithmetic | 7.67 | 3.21 | 8.67 | 2.31 | 0.70 | 0.31 |
| Visual fields R | 9.25 | 0.50 | 10.00 | 0.00 | 2.05 | 0.11 |
| Visual fields L | 3.25a | 3.40 | 6.25a | 2.06 | 1.48 | 0.20 |
| Visual fields both | 1.00a | 1.41 | 2.25a | 1.71 | 1.04 | 0.34 |
| RASP surface touch L—max 30 | 6.75a | 3.86 | 7.50a | 3.87 | 0.29 | 0.88 |
| RASP surface touch/spam max 10 | 5.00a | 2.44 | 4.75a | 1.70 | 1.00 | 1.00 |
| RASP extinction—max 12 | 3.66a | 6.35 | 6.50a | 2.12 | 0.59 | 0.80 |
| RASP proprioception—max 30 movement detection L | 6.75a | 3.30 | 6.50a | 4.04 | 0.15 | 0.89 |
| RASP proprioception—max 30 direction detection L | 4.45a | 2.87 | 3.50a | 3.69 | 0.44 | 0.69 |
| Comb/razor test R | 27.5 | 16.7 | 25.50 | 1.29 | 1.00 | 0.34 |
| Comb/razor test L | 13.75 | 17.35 | 20.00 | 5.42 | 1.16 | 0.34 |
| Comb/razor test ambiguous | 10.75 | 11.53 | 8.75 | 8.18 | 0.14 | 0.88 |
| Comb/razor test bias | −0.39a | 0.32 | −0.10 | 0.11 | 1.7 | 0.11 |
| Bisiach test | 1.00 | 0.50 | 0.00 | 0.00 | 1.53 | 0.13 |
| BIT total score | 84.75a | 29.54 | 96a | 35.31 | 0.43 | 0.66 |
| Line crossing R | 18 | 0 | 17.25 | 0.95 | 1.50 | 0.30 |
| Line crossing L | 6.75a | 6.16 | 15.75a | 3.3 | 2.00 | 0.06** |
| Letter cancellation R | 16 | 2.82 | 14.75 | 2.36 | 0.60 | 0.20 |
| Letter cancellation L | 6.25a | 3.3 | 12.25a | 5.5 | 1.40 | 0.70 |
| Star cancellation R | 21.5 | 7.59 | 23 | 4.08 | 0 | 1 |
| Star cancellation L | 8.5a | 10.63 | 20.75a | 4.11 | 1.60 | 0.10 |
| Copy | 1a | 1.41 | 3 | 1.15 | 1.80 | 0.08** |
| Representational drawing | 1.33a | 1.15 | 2.75 | 0.5 | 1.90 | 0.56 |
| Line bisection R | 3 | 0 | 2.5 | 1 | 1.00 | 0.30 |
| Line bisection Centre | 2 | 1.41 | 2.25 | 1.5 | 0.50 | 0.61 |
| Line bisection L | 0.75a | 0.95 | 1.5a | 1.73 | 0.60 | 0.50 |
| Berti awareness LUL | 1.5 | 0.6 | 0.00 | 0.00 | 2.50 | <0.05* |
| Berti awareness LLL | 1.75 | 0.50 | 0.00 | 0.00 | 2.50 | <0.05* |
| AHP questionnaire | 5.1 | 0.9 | 0.63 | 0.48 | 2.82 | <0.05* |
| Hayling test RTs | 2.00a | 1.15 | 3.00a | 2.31 | 0.62 | 0.33 |
| Hayling test errors | 4.00 | 1.83 | 3.50 | 2.89 | 0.30 | 0.40 |
| Proverbs | 8.00 | 3.61 | 11.50 | 5.74 | 1.08 | 0.22 |
| Cognitive estimates | 10.00a | 2.71 | 9.33a | 1.15 | 1.08 | 0.22 |
| HADS depression | 4.00 | 2.83 | 7.50 | 5.97 | 1.04 | 0.30 |
| HADS anxiety | 7.75 | 5.06 | 9.00 | 6.58 | 0.00 | 1.00 |
aScores below tests’ cut-off point, or >1 SD below the average mean.
*Significant differences between the groups, P < 0.05.
**Trends towards significance, P < 0.10.
Wechsler (2001); Wechsler (1998); Visual-fields = the customary ‘confrontation’ technique (Bisiach et al., 1986); BIT Total score = sum of scores of the conventional sub-tests of the Behavioural Inattention Test; ‘One Item Test’ and ‘Comb/Razor Test’ = Tests of Personal Neglect. Bias on the latter is calculated according to McIntosh et al. (2000); RASP = The Rivermead Assessment of Somatosensory Performance Winward et al. (2000); Proverb Test = Delis Kaplan – Executive Functions System - Proverbs Subtest (Delis et al., 2001). Awareness Interview = Berti et al. (1996) Awareness Interview and Awareness Questionnaire = Feinberg et al. (2000) Awareness Questionnaire.
Groups demographic characteristics and neuropsychological profile
| . | AHP . | HP controls . | Mann–Whitney test . | |||
|---|---|---|---|---|---|---|
| . | Mean . | SD . | Mean . | SD . | Z . | P . |
| N | 4 | – | 4 | – | – | – |
| Age (years) | 63.75 | 5.95 | 61.00 | 22.79 | 0.00 | 1.00 |
| Education (years) | 12.75 | 2.87 | 12.00 | 2.83 | 0.73 | 0.30 |
| Days from onset | 20.0 | 13.23 | 19.75 | 3.30 | 0.00 | 0.40 |
| Premorbid IQ–WTAR | 112.67 | 13.05 | 104.75 | 7.72 | 0.00 | 0.40 |
| WAIS-III vocabulary | 9.75 | 4.65 | 8.75 | 2.99 | 0.30 | 0.38 |
| WAIS-III similarities | 9.50 | 3.11 | 10.25 | 2.50 | 0.29 | 0.38 |
| WAIS-III digit span | 10.25 | 3.50 | 9.25 | 1.71 | 0.44 | 0.36 |
| WAIS-III matrix reasoning | 6.33a | 2.52 | 8.33 | 2.52 | 0.89 | 0.27 |
| WAIS-III arithmetic | 7.67 | 3.21 | 8.67 | 2.31 | 0.70 | 0.31 |
| Visual fields R | 9.25 | 0.50 | 10.00 | 0.00 | 2.05 | 0.11 |
| Visual fields L | 3.25a | 3.40 | 6.25a | 2.06 | 1.48 | 0.20 |
| Visual fields both | 1.00a | 1.41 | 2.25a | 1.71 | 1.04 | 0.34 |
| RASP surface touch L—max 30 | 6.75a | 3.86 | 7.50a | 3.87 | 0.29 | 0.88 |
| RASP surface touch/spam max 10 | 5.00a | 2.44 | 4.75a | 1.70 | 1.00 | 1.00 |
| RASP extinction—max 12 | 3.66a | 6.35 | 6.50a | 2.12 | 0.59 | 0.80 |
| RASP proprioception—max 30 movement detection L | 6.75a | 3.30 | 6.50a | 4.04 | 0.15 | 0.89 |
| RASP proprioception—max 30 direction detection L | 4.45a | 2.87 | 3.50a | 3.69 | 0.44 | 0.69 |
| Comb/razor test R | 27.5 | 16.7 | 25.50 | 1.29 | 1.00 | 0.34 |
| Comb/razor test L | 13.75 | 17.35 | 20.00 | 5.42 | 1.16 | 0.34 |
| Comb/razor test ambiguous | 10.75 | 11.53 | 8.75 | 8.18 | 0.14 | 0.88 |
| Comb/razor test bias | −0.39a | 0.32 | −0.10 | 0.11 | 1.7 | 0.11 |
| Bisiach test | 1.00 | 0.50 | 0.00 | 0.00 | 1.53 | 0.13 |
| BIT total score | 84.75a | 29.54 | 96a | 35.31 | 0.43 | 0.66 |
| Line crossing R | 18 | 0 | 17.25 | 0.95 | 1.50 | 0.30 |
| Line crossing L | 6.75a | 6.16 | 15.75a | 3.3 | 2.00 | 0.06** |
| Letter cancellation R | 16 | 2.82 | 14.75 | 2.36 | 0.60 | 0.20 |
| Letter cancellation L | 6.25a | 3.3 | 12.25a | 5.5 | 1.40 | 0.70 |
| Star cancellation R | 21.5 | 7.59 | 23 | 4.08 | 0 | 1 |
| Star cancellation L | 8.5a | 10.63 | 20.75a | 4.11 | 1.60 | 0.10 |
| Copy | 1a | 1.41 | 3 | 1.15 | 1.80 | 0.08** |
| Representational drawing | 1.33a | 1.15 | 2.75 | 0.5 | 1.90 | 0.56 |
| Line bisection R | 3 | 0 | 2.5 | 1 | 1.00 | 0.30 |
| Line bisection Centre | 2 | 1.41 | 2.25 | 1.5 | 0.50 | 0.61 |
| Line bisection L | 0.75a | 0.95 | 1.5a | 1.73 | 0.60 | 0.50 |
| Berti awareness LUL | 1.5 | 0.6 | 0.00 | 0.00 | 2.50 | <0.05* |
| Berti awareness LLL | 1.75 | 0.50 | 0.00 | 0.00 | 2.50 | <0.05* |
| AHP questionnaire | 5.1 | 0.9 | 0.63 | 0.48 | 2.82 | <0.05* |
| Hayling test RTs | 2.00a | 1.15 | 3.00a | 2.31 | 0.62 | 0.33 |
| Hayling test errors | 4.00 | 1.83 | 3.50 | 2.89 | 0.30 | 0.40 |
| Proverbs | 8.00 | 3.61 | 11.50 | 5.74 | 1.08 | 0.22 |
| Cognitive estimates | 10.00a | 2.71 | 9.33a | 1.15 | 1.08 | 0.22 |
| HADS depression | 4.00 | 2.83 | 7.50 | 5.97 | 1.04 | 0.30 |
| HADS anxiety | 7.75 | 5.06 | 9.00 | 6.58 | 0.00 | 1.00 |
| . | AHP . | HP controls . | Mann–Whitney test . | |||
|---|---|---|---|---|---|---|
| . | Mean . | SD . | Mean . | SD . | Z . | P . |
| N | 4 | – | 4 | – | – | – |
| Age (years) | 63.75 | 5.95 | 61.00 | 22.79 | 0.00 | 1.00 |
| Education (years) | 12.75 | 2.87 | 12.00 | 2.83 | 0.73 | 0.30 |
| Days from onset | 20.0 | 13.23 | 19.75 | 3.30 | 0.00 | 0.40 |
| Premorbid IQ–WTAR | 112.67 | 13.05 | 104.75 | 7.72 | 0.00 | 0.40 |
| WAIS-III vocabulary | 9.75 | 4.65 | 8.75 | 2.99 | 0.30 | 0.38 |
| WAIS-III similarities | 9.50 | 3.11 | 10.25 | 2.50 | 0.29 | 0.38 |
| WAIS-III digit span | 10.25 | 3.50 | 9.25 | 1.71 | 0.44 | 0.36 |
| WAIS-III matrix reasoning | 6.33a | 2.52 | 8.33 | 2.52 | 0.89 | 0.27 |
| WAIS-III arithmetic | 7.67 | 3.21 | 8.67 | 2.31 | 0.70 | 0.31 |
| Visual fields R | 9.25 | 0.50 | 10.00 | 0.00 | 2.05 | 0.11 |
| Visual fields L | 3.25a | 3.40 | 6.25a | 2.06 | 1.48 | 0.20 |
| Visual fields both | 1.00a | 1.41 | 2.25a | 1.71 | 1.04 | 0.34 |
| RASP surface touch L—max 30 | 6.75a | 3.86 | 7.50a | 3.87 | 0.29 | 0.88 |
| RASP surface touch/spam max 10 | 5.00a | 2.44 | 4.75a | 1.70 | 1.00 | 1.00 |
| RASP extinction—max 12 | 3.66a | 6.35 | 6.50a | 2.12 | 0.59 | 0.80 |
| RASP proprioception—max 30 movement detection L | 6.75a | 3.30 | 6.50a | 4.04 | 0.15 | 0.89 |
| RASP proprioception—max 30 direction detection L | 4.45a | 2.87 | 3.50a | 3.69 | 0.44 | 0.69 |
| Comb/razor test R | 27.5 | 16.7 | 25.50 | 1.29 | 1.00 | 0.34 |
| Comb/razor test L | 13.75 | 17.35 | 20.00 | 5.42 | 1.16 | 0.34 |
| Comb/razor test ambiguous | 10.75 | 11.53 | 8.75 | 8.18 | 0.14 | 0.88 |
| Comb/razor test bias | −0.39a | 0.32 | −0.10 | 0.11 | 1.7 | 0.11 |
| Bisiach test | 1.00 | 0.50 | 0.00 | 0.00 | 1.53 | 0.13 |
| BIT total score | 84.75a | 29.54 | 96a | 35.31 | 0.43 | 0.66 |
| Line crossing R | 18 | 0 | 17.25 | 0.95 | 1.50 | 0.30 |
| Line crossing L | 6.75a | 6.16 | 15.75a | 3.3 | 2.00 | 0.06** |
| Letter cancellation R | 16 | 2.82 | 14.75 | 2.36 | 0.60 | 0.20 |
| Letter cancellation L | 6.25a | 3.3 | 12.25a | 5.5 | 1.40 | 0.70 |
| Star cancellation R | 21.5 | 7.59 | 23 | 4.08 | 0 | 1 |
| Star cancellation L | 8.5a | 10.63 | 20.75a | 4.11 | 1.60 | 0.10 |
| Copy | 1a | 1.41 | 3 | 1.15 | 1.80 | 0.08** |
| Representational drawing | 1.33a | 1.15 | 2.75 | 0.5 | 1.90 | 0.56 |
| Line bisection R | 3 | 0 | 2.5 | 1 | 1.00 | 0.30 |
| Line bisection Centre | 2 | 1.41 | 2.25 | 1.5 | 0.50 | 0.61 |
| Line bisection L | 0.75a | 0.95 | 1.5a | 1.73 | 0.60 | 0.50 |
| Berti awareness LUL | 1.5 | 0.6 | 0.00 | 0.00 | 2.50 | <0.05* |
| Berti awareness LLL | 1.75 | 0.50 | 0.00 | 0.00 | 2.50 | <0.05* |
| AHP questionnaire | 5.1 | 0.9 | 0.63 | 0.48 | 2.82 | <0.05* |
| Hayling test RTs | 2.00a | 1.15 | 3.00a | 2.31 | 0.62 | 0.33 |
| Hayling test errors | 4.00 | 1.83 | 3.50 | 2.89 | 0.30 | 0.40 |
| Proverbs | 8.00 | 3.61 | 11.50 | 5.74 | 1.08 | 0.22 |
| Cognitive estimates | 10.00a | 2.71 | 9.33a | 1.15 | 1.08 | 0.22 |
| HADS depression | 4.00 | 2.83 | 7.50 | 5.97 | 1.04 | 0.30 |
| HADS anxiety | 7.75 | 5.06 | 9.00 | 6.58 | 0.00 | 1.00 |
aScores below tests’ cut-off point, or >1 SD below the average mean.
*Significant differences between the groups, P < 0.05.
**Trends towards significance, P < 0.10.
Wechsler (2001); Wechsler (1998); Visual-fields = the customary ‘confrontation’ technique (Bisiach et al., 1986); BIT Total score = sum of scores of the conventional sub-tests of the Behavioural Inattention Test; ‘One Item Test’ and ‘Comb/Razor Test’ = Tests of Personal Neglect. Bias on the latter is calculated according to McIntosh et al. (2000); RASP = The Rivermead Assessment of Somatosensory Performance Winward et al. (2000); Proverb Test = Delis Kaplan – Executive Functions System - Proverbs Subtest (Delis et al., 2001). Awareness Interview = Berti et al. (1996) Awareness Interview and Awareness Questionnaire = Feinberg et al. (2000) Awareness Questionnaire.
As shown in Table 1, the groups did not differ in age (range in years 38–83), education or post-onset assessment time. As expected the two groups differed significantly in the two awareness interviews used; the Awareness Interview (Berti et al., 1996) and in AHP Questionnaire (Feinberg et al., 2000). Interestingly, three of the four AHP patients claimed they did move their paralysed left limbs during the awareness tests, while the HP patients immediately acknowledged that the task was impossible for them. Their awareness scores were consistent with patients’ spontaneous behaviour; the HP patients acknowledged their paralysis spontaneously and never attempted to move without assistance. By contrast, the AHP patients tended to deny or minimize their motor deficits in spontaneous behaviour. All four patients explicitly stated that they could move their left arm, had attempted to stand on their own at least once and repeatedly expressed hostility towards staff and relatives for forbidding them from getting up and walking on their own or, performing bimanual tasks. In addition, all four patients falsely claimed (confabulated) that they had been moving their left side while in hospital (e.g. ‘Of course, I can use my left arm. How do you think I scratch my right arm when it is itchy during the night?’). One patient showed somatoparaphrenia in spontaneous behaviour in that she insisted her left arm was her husband's arm (Patient A2 in Fig. 1).
The two groups did not differ in their general pre-morbid or post-morbid intelligence scores and there were no significant differences in their reasoning abilities as measured by two frontal tasks. However, it should be noted that both groups performed worse than expected by their premorbid IQ on the WAIS-III Performance subtest Matrix Reasoning and on the Cognitive Estimates Test (normal range is 2–6). Both groups showed some degree of visuospatial, sensory and personal neglect but this appeared more severe in the AHP. It is of interest that the AHP scored below cut-off levels in the Comb/Razor Test, while the HP group did not. The differences between the groups on two visuospatial Neglect Tests (left-side line cancellation and copy) approximated significant levels (P < 0.10) (Table 1). It thus seemed that AHP had overall more severe neglect than HP patients, but variability was noted among patients of both groups and given the small samples assessed, differences were non-significant. Finally, AHP patients showed lower scores for depression and anxiety on the HADS comparing to HP patients. However, the scores of the two groups did not significantly differ and they were considered to be within normal limits for a patient population in a general hospital.
Experimental investigations
Design
The experiment assessed whether the intention to act (i.e. move one's left hemiplegic hand) influenced the perception of movement of the same hand in hemiplegic patients with or without AHP. Motor intention was manipulated by instruction at three different levels: self-generated movement (patients were instructed to raise their left arm), externally generated movement [patients were told that an experimenter will lift their (the patient's) left arm] and no-movement instruction. Visual feedback of movement was manipulated at two levels: Movement, no movement. This allowed for a 2 × 2 × 3 design (Table 2). The experimental factors were: (i) group (patients with AHP, patients with HP); (ii) the visual feedback of hand movement (movement/no movement); and (iii) the motor intention (self, external, none).
Table summarizing the experimental design
| Instruction . | Rubber-hand . | |
|---|---|---|
| . | Movement . | No movement . |
| Self-generated movement | Six trials | Six trials |
| Externally- generated movement | Six trials | Six trials |
| No movement instruction | Six trials | Six trials |
| Instruction . | Rubber-hand . | |
|---|---|---|
| . | Movement . | No movement . |
| Self-generated movement | Six trials | Six trials |
| Externally- generated movement | Six trials | Six trials |
| No movement instruction | Six trials | Six trials |
Table summarizing the experimental design
| Instruction . | Rubber-hand . | |
|---|---|---|
| . | Movement . | No movement . |
| Self-generated movement | Six trials | Six trials |
| Externally- generated movement | Six trials | Six trials |
| No movement instruction | Six trials | Six trials |
| Instruction . | Rubber-hand . | |
|---|---|---|
| . | Movement . | No movement . |
| Self-generated movement | Six trials | Six trials |
| Externally- generated movement | Six trials | Six trials |
| No movement instruction | Six trials | Six trials |
Dependent variables
Dependent variables included (i) movement detection scores: ‘Did your left hand move?’ (YES/NO response); (ii) confidence rating on measure a (Likert-type scale 1–7); (iii) agency scores over the observed movement: ‘Did you or someone else move your hand?’ (‘Me/Someone else’ response).
Materials and procedure
The aim of the experiment was to assess whether patients’ intention to act influenced their awareness of movement of the same hand. As hemiplegic patients cannot move their left arm, a life-sized rubber model of a left hand and arm was used to create false visual feedback of left-hand movement. Prosthetic hands were used because they can be easily manipulated by the experimenters, they are quite realistic and moving them does not elicit somatosensory signals. A suitable rubber hand was selected for each patient in order to resemble each patient's own (real) hand in terms of size, shape, skin tone and freckles. The rubber hand was placed on a hospital table in front of the patients (by an assisting experimenter seated at the left of the patient and holding the proximal end of the rubber hand), while their vision was temporarily blocked. The rubber hand was aligned to the patient's midline, adopting the canonical position that their own left hand would occupy. The rubber hand's proximal end, held by the assisting experimenter, and the patient's left hand were hidden from the patient's view under a pillow. The ‘ownership’ of the rubber hand was tested as described below, the experimental procedure was explained to patients and they were then given two examples of each trial of the experiment (see below).
In 12 trials patients were instructed to slightly raise their left hand (self-generated movement condition) following a tap at a pre-determined spot on the table in front of them (about 20 cm to the right of the rubber hand). In another 12 trials they were told to stay motionless and to anticipate that, following the tap, the assisting experimenter (seated at the left of the patient and holding the proximal end of the rubber hand under a pillow—see above) would passively lift the patient's left hand upwards for them (externally generated movement condition). In a final set of 12 trials they were told to stay motionless following the tap and that the assisting experimenter would not attempt to move their left hand either (no-movement instruction condition). Self-generated movement, externally generated movement and no-movement instruction trials were presented in mixed, random order. For each of these levels of the variable Intention, the assisting experimenter was manipulating the rubber hand in two levels: movement (six trials for each intention condition), or no movement (six trials for each intention condition) (Table 2). Movement and no-movement trials were presented in random order. Patients had to answer in each trial the movement detection, confidence and movement agency questions described above.
Our hypothesis was that AHP patients would make more errors in their perception of rubber hand movement or non-movement in the self-generated movement condition, than in the externally generated movement or no-movement instruction conditions.
Control and ownership baseline questions
Control questions (‘Please move your right hand upwards; did your right hand move?’) were asked before the experiment and every 10 trials to ensure patients were paying attention to and understanding properly the examiner's instructions. In addition, questions that verified that the patients believed their rubber hand was their own were asked before, during (approximately every eight trials), and after the experiment. These included the following questions: (i) please point to your left hand using your right hand (while their left hand was hidden and the left rubber hand was placed in front of the patient). (ii) Is this your hand? (pointing to the rubber hand). Patients performed flawlessly on the right-hand control task and the ownership control questions (i.e. they did not doubt the rubber hand was theirs), and therefore these results are not presented below.
It should also be noted that at the end of the experiment and while the participants own left hand was out of sight, we tried placing the rubber hand in positions incongruent to the canonical position of patients’ own left hands. These included positions further to the left, to the right and rotated rubber hand (180° upside down and back to front) positions. All patients (including the patient with a somatoparaphrenic delusion) denied ownership of the rubber hand in all incongruent positions, replicating previous findings in healthy participants (Tsakiris and Haggard, 2005; Costantini and Haggard, 2007). As soon as the rubber hand was placed closer to the canonical position of the left hand and in the correct orientation (irrespective of where their own left hand was lying) patients stated that they thought the rubber hand was their own. In one of the control HP patients, we assessed whether this acquisition of ownership of the rubber hand would apply even after we showed him the rubber hand and explained the use we made of it during the experiment. After this briefing, the patient spontaneously commented that we had ‘tricked’ him in believing that the rubber hand was his own. However, when minutes later we presented the rubber hand in the canonical position of his left arm as in the experiment and asked him if it was the rubber hand or his own, he once again stated the rubber hand was his own arm.
Results
Movement detection
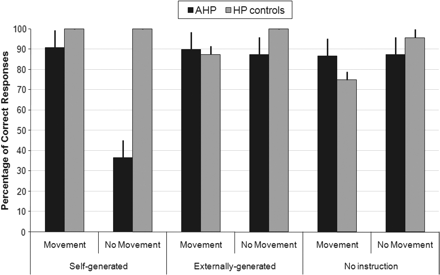
The HP (control) group showed overall high levels of correct responses, i.e. they responded ‘yes’ when the prosthetic hand moved and ‘no’ when it did not. The AHP group showed also a relative high level of correct responses with one exception: a dramatic decrease in their correct responses in the case where they were told to move their left hand (self-generated movement) and there was no movement of the rubber hand. These results are depicted in Fig. 2.
Percentage of correct responses across groups (means and SEs) for ‘Self-generated’ (self-generated movement), ‘Externally generated’ (externally generated movement) and ‘No instruction’ (no movement instruction) Intention conditions and for Rubber hand movement and No movement conditions. Patients with AHP show a selective dramatic decrease of correct responses in the ‘Self-generated/No Movement’ condition.
Signal detection theory was applied to investigate differences between groups in the ability to detect movements of the rubber hand in each condition (non-parametric analyses of total correct responses were also performed and confirmed the results of the analysis based on signal detection theory presented below). For this purpose, a hit was defined as the patient reporting a movement of their left hand when the prosthetic hand moved. A false alarm involved reporting movement when the prosthetic hand did not move. D′ values were calculated for each subject and each condition. Hit rates of 1.0 and false alarm rates of 0.0 were replaced by 0.99 and 0.01, respectively to allow d′ prime to be calculated despite floor and ceiling performance. D′ values in each condition were compared between the AHP and control groups, using Mann–Whitney tests. An alpha level of 0.017 was applied to correct for three comparisons. In the own-intention condition, d′ values were significantly lower for the AHP than the control group (median d′ 1.24 versus 4.65, U = 16, P < 0.001). The groups did not differ in sensitivity in the externally applied and no-intention conditions (externally applied, median d′ 3.03 versus 4.65, U = 10.5, P = 0.23; no-intention, median d′ 2.96 versus 2.54, P = 0.39). We conclude that the AHP group was impaired in detecting the movement of the rubber hand only in the condition where they had intended to move. Interestingly, the AHP group did not show a ‘general’ impairment in perceptual sensitivity, since their d′ values were in fact higher than the control group in the no-intention condition.
Confidence scores
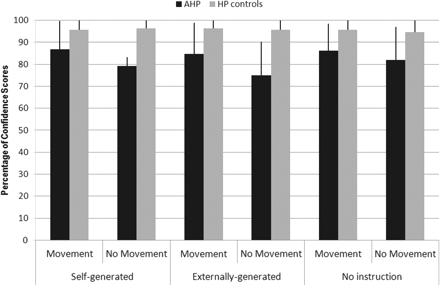
All patients showed high levels of confidence in their answers (Fig. 3). A non-parametric Mann–Whitney test revealed an overall effect of group (Z = 2.29, P < 0.05) with AHP patients feeling less confident for their answers than the HP patients. A Wilcoxon Signed Rank Test showed that more confidence was shown overall in response to rubber hand movement than to non-movement conditions (Z = 2.46, P < 0.05). The interaction of group × movement was analysed by calculating the difference between confidence scores of movement and no movement and a non-parametric Mann–Whitney test showed that the group had a significant effect on this difference (Z = 0.24, P < 0.05), with AHP patients showing less confidence than HP patients in their answers to no-movement than to movement conditions. There were no other observed differences.
Percentage of Confidence Scores across groups (means and SEs) for ‘Self-generated’ (self-generated movement), ‘Externally generated’ (externally generated movement) and ‘No instruction’ (no movement instruction) ‘Intention Conditions’ and for ‘Rubber Hand Movement’ and ‘No-movement Conditions’.
Agency questions
We assessed the extent to which patients experienced agency over the movement of the hand by asking them ‘Did you or someone else move this hand?’ in each trial. The anosognosic patient who also showed somatoparaphrenia in spontaneous behaviour (see A2 in Fig. 1) found this question very challenging and either remained quiet when asked or, stated she did not know. We discontinued the question after six to seven trials as she appeared to get considerably upset by it. Two of the three remaining AHP patients stated that they had performed the movement in all of the ‘self-generated movement’ trials that they had ‘perceived’ a movement, even if the rubber hand had in fact remained motionless. The fourth patient experienced agency in all of the ‘self-generated movement’ trials that he had ‘perceived’ a movement, except in one of the four trials in which he had ‘perceived’ a movement but the rubber hand had in fact remained motionless. In the ‘externally generated movement’ and ‘no movement’ conditions, these three anosognosic patients denied agency of the movement.
All control patients denied agency of movement in all the ‘externally generated movement’ and ‘no movement instruction’ trials that they had perceived a movement. However, to our surprise three out of four of the HP control subjects stated that they had performed the movement in all of the ‘self-generated movement’ trials that the rubber hand moved. Given that these patients were not anosognosic in spontaneous behaviour and in formal assessment (Table 1), we asked them the following questions after the completion of the experiment: what made them believe they had moved their left hand, whether they had moved it at all during their hospitalization and, if not, and what allowed them to move it now. All three patients answered that they have not moved their left hand at all since the stroke, they could not explain why they were able to do so during the experiment, and they based their answers mostly on what they saw but also to an extent on an ‘internal feeling of having moved it’ during some of the trials. Characteristically, one subject also commented: ‘Sometimes I had the impression that even if I had closed my eyes I would feel it move. I mean I felt the movement in my hand. I didn't just see it’. None of the patients had recovered any of their left-arm motor power at the time of the experiment.
Discussion
In order to investigate the role of motor intention in AHP, we provided patients with and without AHP substituted visual feedback of movement in their left paralysed upper limb. We assessed whether their ability to visually detect this substituted movement varied according to whether patients had planned to move their limb or not. We found that all patients, including patients who were aware of their hemiplegia, believed that they generated movements when they were presented with visual feedback of a moving prosthetic hand that they thought was their own. Crucially, motor intention had a selective effect on patients with AHP; they systematically disregarded visual information of a motionless rubber hand on trials where they had the intention to move, compared with trails where they expected the experimenter to move the rubber hand, or when there was no expectation of movement. In other terms, patients with AHP were unable to detect absence of movement correctly in the self-generated condition, but were able to detect it in the externally generated condition. By contrast, patients without AHP were not influenced by these manipulations and they did not claim that they had moved their hand when the rubber hand had remained still.
These results confirm that AHP is influenced by motor planning, and in particular that motor ‘awareness’ in AHP derives from the processing of motor intentions. This finding is consistent with the proposals made by Frith et al. (2000; see also Berti et al., 2007) that the illusory ‘awareness’ of movement in anosognosic patients is created on the basis of a comparison between the intended and predicted positions of the limbs, and not on the basis of a mismatch between the predicted and actual sensory feedback. According to this hypothesis, patients with AHP are able to form appropriate representations of the desired and predicted positions of the limb. However, conflicting information derived from sensory feedback that would indicate a failure of movement is not normally available, because of brain damage to regions that would register the actual state of the limbs, or else because this contrary information is neglected. A recent lesion mapping study suggested that premotor areas BA6 and 44, which are implicated in action monitoring, are the most frequently damaged areas in patients with AHP (Berti et al., 2005). This finding may explain why these patients fail to register their inability to move, but it does not address the functional mechanism that underpins their illusory awareness of action per se. Our study provides direct evidence for the hypothesis that awareness of action is based on the stream of motor commands and not on sensory inflow.
Patients with AHP have diverse somatosensory deficits. Therefore assessing the exact role of sensory feedback on AHP can be challenging. To resolve this problem, we substituted visual feedback from a prosthetic hand, which patients believed was their own, for somatosensory feedback from the patient's own hand. Accordingly, we predicted that the detection of the patient's movement based on this substitute feedback depended on their motor intention. Thus, patients’ errors were unrelated to proprioceptive sensory feedback. Crucially, visual information showing a lack of movement was selectively ignored when the patients were instructed to generate themselves the feed-forward motor signals to move. To the best of our knowledge our study is the first to test directly the hypothesis of dominant motor intention in AHP patients. Interestingly, accumulating evidence suggests that even neurologically healthy people demonstrate a remarkably limited awareness of actual movements and their sensory feedback (Fourneret and Jeannerod, 1998). In fact, it is only when the discrepancy between predicted and actual consequences exceeds a certain threshold that we become aware of an error signal from the comparator. On that view, AHP may represent an exaggerated form of the normal function of the internal models of the motor system.
Could our result be explained by alternative accounts of AHP? The selective deficit that AHP patients showed in our experiment cannot be accounted for by the proposal that AHP results from a lack of motor intention (Heilman et al., 1998). That hypothesis does explain the clinical observation that patients cannot ‘discover’ their impairment, but it is not sufficient to explain why patients may claim that they have moved when actually they have not (see also Frith et al., 2000 and Berti et al., 2007 for discussion).
Nor can the observed results simply be a confounding effect of contralesional neglect. Even though our anosognosic group did show higher neglect scores than non-anosognosic patients with similar lesions and motor deficits, patients did perceive the lack of movement in the externally generated movement and no-movement instruction conditions, and they showed only a selective difference for self-generated movement. This suggests that neglect cannot be the primary cause of the anosognosic errors performed by our AHP patients (see also Bisiach et al., 1986; Marcel et al., 2004 for dissociations between AHP and neglect).
It is also unlikely that the observed results reflect a general deficit in detecting abnormalities and contradictions (Ramachandran, 1995), or a greater suggestibility, because AHP patients were able to detect the similar discrepancies between instruction and no rubber hand movement in the externally generated movement conditions. In addition, our patient groups were well matched in terms of cognitive deficits, such as confusion or intellectual impairment ruling out a general cognitive deficit explanation (Levine, 1991).
Interestingly, a number of investigators have suggested that emotional factors may influence AHP and explain some of its delusional elements (Marcel et al., 2004; Vuilleumier, 2004; Turnbull, 2005). These emotional factors could be directly linked to brain dysfunction, rather than being secondary (psychogenic) consequences as previously suggested (Weinstein and Kahn, 1955). For example, AHP may result from abnormal affective regulation (Turnbull et al., 2005; Nadrone et al., 2008). Alternatively, right brain damage may alter emotional and attitudinal processes implicated in self-attribution of perceptual experiences (Marcel et al., 2004), and spatial deficits may undermine the representation of self-other separateness (Solms, 1999; Feinberg et al., 2005). Although our experiment did not test these hypotheses directly, it is possible that the emotional relevance of self-generated movement condition (and the relevant noted feelings of agency of movement) was higher than that of the externally generated movement trials.
However, it should be noted that an abnormality in motor planning cannot explain all the features of AHP. For example, it is unclear why some patients deny their motor deficits even when not attempting to move (see also Frith et al., 2000 for discussion), or why other patients may selectively forget their failures to perform everyday tasks and even instances of self-harm (Ramachandran, 1995). These observations indicate that at least some features of AHP cannot be explained by disruption of sensorimotor mechanisms (Ramachandran, 1995; Feinberg and Roane, 2003; Frith et al., 2000; Marcel et al., 2004; Vuileumier, 2004; Turnbull et al., 2005).
Additional findings
Two further findings merit discussion. First, hemiplegic and hemianaesthetic patients, with and without AHP, accepted a rubber hand as their own if it was placed at a canonical position. The attribution of ownership of the rubber hand points to the fragility of our ownership judgements and is relevant to studies of the ‘rubber-hand illusion’ in healthy volunteers (Botvinick and Cohen, 1998). These experiments have demonstrated that the sense of body ownership can be disrupted in normal volunteers by showing them a rubber arm that is seen to be stroked exactly in time with tactile stroking of their real arm. Furthermore, movements of the real arm may become inaccurate because the volunteers assume the starting position of their arm is that of the rubber arm. Given our patients’ severely impaired proprioception and tactile sensation, we anticipated that visual feedback of a realistic rubber hand in the canonical position could make them unusually ready to attribute the rubber hand to themselves. Indeed, we showed that patients were willing to accept a rubber hand as theirs when placed in the canonical position and they did not doubt the ownership of the rubber hand throughout the experiment. Furthermore, one of our patients was somatoparaphrenic in spontaneous behaviour (Patient A2 claimed that her own left hand was her husband's) but she did accept the rubber hand as hers when it was placed in the canonical position of her own left hand and her own left hand was out of sight.
Frith (2005) has speculated that patients with asomatognosia or somatoparaphrenia think the hand they see is not their own hand because it is in a different place from where they know and expect their own hand to be. Indeed, we observed that patients only self-attributed the rubber hand when it appeared in the position they expected their own left hand to be. Previous studies in healthy volunteers have shown that passive visual exposure to artificial hands in a congruent posture induces a visual recalibration of proprioception of the participant's real hand position toward the position of the artificial hand (Holmes et al., 2006). However, simply viewing an artificial hand does not create a strong illusion of ownership in healthy participants. For that, synchronized visual and tactile experience is required, as in the rubber hand illusion. Our patients’ tactile and proprioceptive deficit may have made them unusually willing to accept merely visual evidence of ownership.
A second interesting observation concerns our control group. Patients with hemiplegia but no AHP believed they had moved their plegic arm in conditions of self-generated movement/rubber hand movement. In other words, when they were asked to move their left arm and an experimenter was moving the rubber hand congruently with the instructions but unbeknownst to them, the majority of our control patients claimed they had moved their arm themselves (unlike the AHP patients who believed they had moved even when the rubber hand was motionless). Remarkably, these patients were fully and simultaneously aware of their paralysis. This misattribution of the agency of rubber hand movement highlights both how patients’ beliefs may be altered by experimental manipulations, and the roles of both vision and motor intention in these belief formations. The effects of illusory visual feedback on action recognition have been similarly explored using mirrors (Ramachandran, 1995) and video projections (Daprati et al., 1997; Sirigu et al., 1999). Sirigu and colleagues (1999) showed that incongruent visual feedback can lead apraxic patients with left parietal lesions to falsely believe they have performed normal instead of inaccurate movements. Interestingly, in the same study, when healthy control participants received visual feedback of the examiner's hand performing the same simple action they were instructed to perform (simultaneous and congruent visual feedback), their performance in distinguishing between the visual image of their hand and that of the examiner's was not significantly better than that of the parietal patients. This difference became significant only when complex actions were tested. These observations suggest that the match between intention and visual feedback is important in self-attribution of action.
Finally, the possibility of an effect of suggestibility should be taken into account in both of these observations, but it should also be noted that both were selective and thus could not fully be accounted for by suggestibility. Patients did not accept the rubber hand as theirs when it was placed in non-canonical positions and control patients did not report being able to move their paralysed arms when they received contrary visual feedback.
Conclusion
We have tested the hypothesis that AHP involves a dominance of forward motor planning over sensory feedback in the awareness of action and have produced experimental evidence in support of this hypothesis. Specifically, we found that patients with AHP are more likely to falsely detect movement of their plegic arm when they have the intention to move it, than when movements are externally generated or when no movement is anticipated. We have interpreted these findings according to a model of motor control that emphasises the role of motor anticipation and prediction in motor awareness (Frith et al., 2000); the non-veridical awareness of action in AHP is formed on the basis of intact representations of the intended and predicted position of the limbs. In AHP these are not appropriately constrained by sensory information about the actual position of the limbs. While previous studies have suggested that conflicting sensory information may not capable of altering anosognosic beliefs (Berti et al., 2005), they did not demonstrate that sensory feedback about the affected limb was ignored even when it was demonstrably available. Accordingly, this study demonstrated for the first time why anosognosic beliefs are formed in the first place: the altered awareness of action in AHP depends predominantly on sequences of motor outflow, rather than sensory inflow. Actual sensory feedback has a remarkably limited role in the experience of action in neurologically healthy individuals (Sarrazin et al., 2007). To this extent, AHP may be a pathological exaggeration of the dominance of proactive and predictive information in motor awareness.
Funding
ESRC/MRC fellowship (to A.K); Neuropsychoanalysis Foundation fellowship (to A.K.); Volkswagen Foundation ‘European Platform for Life Sciences, Mind Sciences and Humanities’ grant for the ‘Body-Project’ (to A.K. and M.T.).
Acknowledgements
We would like to thank the patients and their families for their participation. We are also grateful to Clare Jacobson, Sarah Gregory and the staff at St Thomas's Hospital, Mark Ward, for their assistance with this study.
References
Abbreviations:
- AHP
anosognosia for hemiplegia
- BIT
Behavioural Inattention Test
- HP
hemiplegia
- HADS
Hospital Anxiety and Depression Scale
- WTAR
Wechsler Test of Adult Reading