-
PDF
- Split View
-
Views
-
Cite
Cite
Claire L. Gibson, Laura J. Gray, Philip M. W. Bath, Sean P. Murphy, Progesterone for the treatment of experimental brain injury; a systematic review, Brain, Volume 131, Issue 2, February 2008, Pages 318–328, https://doi.org/10.1093/brain/awm183
Close - Share Icon Share
Abstract
Steroid sex hormones are potential neuroprotective candidates following CNS injury. All clinical trials to date have examined the effects of oestrogen alone or oestrogen-progestin combination therapy. Experimental studies have suggested that progesterone, in its own right, is a potential neuroprotective agent following acute cerebral injury. We performed a systematic review of controlled animal studies that administered progesterone before, or after, acute cerebral injury and measured lesion volume. Relevant studies were found from searching PubMed, Embase and Web of Science. From 119 identified publications, data from 18 studies using 480 experimental subjects met specific criteria and were analysed using the Cochrane Review Manager software. Following cerebral ischaemia, a significant benefit of progesterone was observed regardless of the assigned study quality score (P = 0.0002) whereas, following traumatic brain injury (TBI) a significant benefit of progesterone was only observed in studies that obtained the highest quality score of 5 (P = 0.02). Progesterone reduced lesion volume in a dose-dependent manner following either cerebral ischaemia (P< 0.001) or TBI (P = 0.03) with the most effective progesterone dose varying according to experimental injury model used. Progesterone treatment was only effective at reducing lesion volume when administered immediately following (i.e. 0–2 h) cerebral ischaemia (P = 0.0008). No studies using models of cerebral ischaemia or TBI assessed efficacy when progesterone was administered at later than 6 h following the onset of cerebral injury. Limited data were available for different groups of animals according to age/hormonal status and the full dose–response relationship was not available in all experimental groups. Although this systematic review provides some supporting evidence for a neuroprotective role of progesterone following either cerebral ischaemia or TBI importantly it highlights areas which need further pre-clinical investigation.
Introduction
Stroke is the leading cause of neurological disability and a major cause of death in the western world (Lo et al., 2003). In addition, traumatic brain injury (TBI) is the leading cause of disability in adults under the age of 40 (Djebaili et al., 2004). Despite advances in our understanding of the pathophysiological and pathochemical damage that occurs following either of these types of brain injury current treatments are limited both in their efficacy and utility. Both stroke and TBI result in massive neuronal loss and research has primarily focused on developing neuroprotective strategies in order to prevent/reduce this.
Evidence exists for a gender difference in vulnerability to either stroke or TBI in humans both in terms of risk and outcome. For example, pre-menopausal women have a lower risk of stroke (Kannel and Thom, 1994; Sacco et al., 1997) and a better outcome following stroke (Thorvaldsen et al., 1995) or TBI (Groswasser et al., 1998; Bounds et al., 1995) relative to men of the same age. Following the menopause, the incidence of stroke in women rapidly increases (Wenger et al., 1993) coincident with diminished circulating levels of the sex steroid hormones oestrogen and progesterone.
Whilst the majority of research has focused on oestrogen as the main source of neuroprotection seen in female animals (Gibson et al., 2006), there is increasing evidence that progesterone also plays a beneficial role in the injured brain. The neuroprotective potential of progesterone was initially revealed by the observation that female rats recover better from TBI than male rats in terms of functional impairment (Atella et al., 1987; Stein, 2001). Importantly, these studies were extended to demonstrate that females high in endogenous progesterone (i.e. pseudo-pregnant) at the time of injury recovered better in comparison to untreated males. The evidence for the neuroprotective effect of progesterone following TBI is substantial (Roof et al., 1993; Stein, 2001, 2005) and has led to the first randomized clinical trial for acute TBI (ProTECT). This trial has just been successfully completed and the investigators report that the use of progesterone after TBI was well tolerated in terms of safety (Wright et al., 2007). Although experimental evidence is beginning to reveal a neuroprotective effect of progesterone following cerebral ischaemia no clinical trials have examined the effects of acute administration of progesterone following cerebral stroke to date.
To evaluate the neuroprotective potential of progesterone we have conducted a systematic review of animal studies to investigate the neuroprotective properties of exogenously applied progesterone on lesion volume after experimental cerebral injury (i.e. cerebral ischaemia or TBI). Lesion volume was chosen as the most relevant outcome for the purpose of this review although some may argue it has limited value when considering whether a treatment is beneficial. The Stroke Academic Industry Roundtable (STAIR, 1999) suggested that drug efficacy in animals should be assessed against behavioural rather than volumetric end points. However, direct evidence is lacking to prove this approach results in better prediction of efficacy in clinical trial, and in fact in humans lesion volume determined by MRI diffusion-weighted imaging shows correlation both with impairment at 24 h (Tong et al., 1998) and with clinical outcome (Wardlaw et al., 2002; Engelter et al., 2003). The purpose of this review is to identify key factors, such as timing of treatment, therapeutic dose and effectiveness according to sex and age, which require further experimental investigation. Such experimental studies will help inform the design of future clinical trials aimed at assessing the neuroprotective potential of acute progesterone treatment following cerebral stroke or TBI.
Methods
Study identification
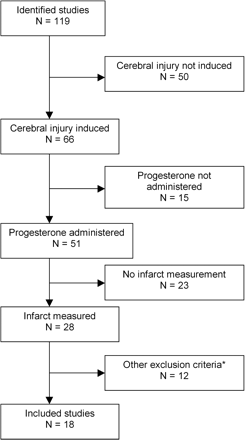
Experimental controlled studies of the effects of exogenously applied progesterone on lesion size in animal models of cerebral injury (i.e. stroke or TBI) were identified from PubMed, Embase, and Web of Science by searching for all articles published from 1980 to December 31, 2006. The earliest study included for analysis was from 1994 (Roof et al., 1994). Additional publications were identified from reference lists of all identified publications and non-systematic (i.e. literature-based reviews) review articles (Roof and Hall, 2000; Stein, 2001, 2005; Simpkins et al., 2005; Singh, 2005, 2006). The search strategy employed the following keywords: progesterone, traumatic or ischaemia, cerebral. However, studies were only included if they met all of the following criteria: experimental cerebral injury induced (i.e. cerebral ischaemia or TBI), progesterone administered and infarct volume measured (Fig. 1).
Search process showing reasons for exclusions of studies. A total of 18 studies were included. Studies were excluded if they did not report the following: induction of experimental brain injury, administration of progesterone, measurement of lesion volume or contain primary data*. N, number of studies.
Data extraction
Two authors (C.L.G. and L.J.G.) independently extracted data from relevant publications on animal species, number, gender, model of brain injury, intervention (dose of progesterone, timing relevant to induction of injury) and infarct volume (mm3,% of normal brain, mean,SD). A comparison (C) was defined as assessment of outcome (i.e. lesion volume) in treatment and control groups after treatment with an administered dose of progesterone starting at a stated time before or after the onset of cerebral injury. For each comparison, the data for mean outcome, SD and number of animals per group were extracted. If published studies (S) used multiple groups, for example to assess dose–response relationships, then the data from each group were individually extracted for analysis. Infarct volumes were classified as total and, if available, cortical and subcortical. Occasionally, numerical data were not reported in the text and these were extracted from enlarged, photocopied figures.
The methodological quality of each study was assessed using an eight-point rating as described previously (Macleod et al., 2005; Wilmot et al., 2005a, b; Gibson et al., 2006). One point was given for written evidence of each of the following criteria: presence of randomization, monitoring of physiological parameters, assessment of dose–response relationship, assessment of optimal time window, masked outcome measurement, assessment of outcome at Days 1–3, assessment of outcome at Days 1–30, combined measurement of lesion volume and functional outcome.
Data analysis
The data were analysed using Cochrane Review Manager (version 4.2) and Stata (version 7). The effect of progesterone compared with control on total lesion volume was assessed using the standardized mean difference (SMD); here the difference in effect between the progesterone and control group is divided by the total SD. This allows comparisons to be made if different methods of measurement or different animal species have been used. These estimates were pooled using the DerSimonian and Laird (1986) random effects model, which is a more conservative method than using a fixed-effects model and takes into account any statistical heterogeneity found between studies.All analyses were carried out by type of cerebral injury, ischaemia and TBI. To examine the effects of study characteristics and potential sources of heterogeneity on outcome, stratified meta-analyses were performed with experiments grouped according to: (i) study quality score; (ii) population grouping—all animals, adult males, ovariectomized females, intact females, aged females, aged males; (iii) progesterone dose and (iv) timing of progesterone administration in relation to onset of brain injury.
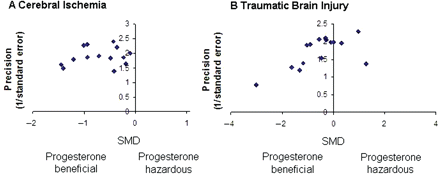
Publication bias was assessed by visually examining a funnel plot (Fig. 2) of precision (reciprocal of SE) against the SMD, with asymmetry being formally assessed using the Egger test (STATA function ‘metabias’; Egger et al., 1997). Significance was set at P < 0.05 and 95% confidence intervals (CI) are quoted throughout.
Funnel plot showing the existence of publication bias. Publication bias was assessed by visually examining a funnel plot of precision (reciprocal of SE) against the SMD and asymmetry was formally assessed using the Egger test. (A) Publication bias was absent for studies reporting the effect of progesterone administration on lesion volume in models of cerebral ischaemia (Egger's test statistic = −0.64, P = 0.53). (B) However, publication bias was present for studies using models of TBI (Egger's test statistic = −2.31, P = 0.038).
Results
Methodological design
The literature search identified 119 potential studies, although most had to be excluded for reasons given in Fig. 1. The characteristics of the remaining 18 studies are reported in Table 1. All of the included studies reported the effect of exogenously administered progesterone on lesion volume after acute cerebral injury. All included studies reported the effect of administering progesterone versus no progesterone (or vehicle) on infarct volume. The 18 studies represented the outcome from seven independent research groups. Five of the studies came from four different research groups, while the remaining 13 studies represent work from three research groups. Within the 18 studies, data from a total of 480 experimental subjects were included for analysis.
Characteristics of included studies
| Study . | Year . | Species . | Sex/hormonal status . | Age . | Model of injury . | Dose range (mg/kg) . | Preparation . | First dose timing . | Route . | Measure of infarct . | Other outcome(s) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkayed et al. | 2000 | WR | RSF | A | FCI (T) | 10 | Progesterone | −7 d | s.c. | % | |
| Chen et al. | 1999 | WR | M | N | FCI (T) | 4–32 | Progesterone | +2 h | i.v. | % | Function |
| Choi et al. | 2004 | SDR | M | N | FCI (T) | 4 | Progesterone | −24 h | i.p. | mm3 | |
| Cutler et al. | 2006 | SDR | M | N | TBI | 16 | Progesterone | +1 h | i.p. | % | |
| Djebali et al. | 2004 | SDR | M | N | TBI | 16 | Progesterone | +1 h | i.p., s.c. | % | Function |
| Gibson and Murphy | 2004 | CM | M | N | FCI (T) | 8 | Progesterone | +1 h | i.p. | mm3 | Function |
| Gibson et al. | 2005 | CM | M | N | FCI (P) | 8 | Progesterone | +1 h | i.p. | mm3 | Function |
| Goss et al. | 2003 | SDR | M | N | TBI | 8–32 | Progesterone | +1 h | i.p. | % | |
| He et al. | 2004 | SDR | M | N | TBI | 4 | Metabolites | +1 h | i.p. | % | Function |
| Jiang et al. | 1996 | WR | M | N | FCI (T) | 4 | Progesterone | −0.5 h | i.p. | % | NS |
| Jones et al. | 2005 | CM | M | N | TBI | 8 | Progesterone | +0.03 h | i.p. | mm3 | Function |
| Kumon et al. | 2000 | SHRSP | M | N | FCI (T) | 4–8 | Progesterone | +2 h | i.p. | % | NS |
| Murphy et al. | 2000 | WR | OF | N | FCI (T) | 5–20 | Progesterone | −0.5 h | i.p. | % | |
| Murphy et al. | 2002 | WR | OF | N | FCI (T) | 30–60 | Progesterone | −7 d | i.p. | % | Function |
| Robertson et al. | 2006 | SDR | OF | N | TBI | NR | Progesterone | −7 d | s.c. | % | |
| Roof et al. | 1994 | SDR | M | N | TBI | 4 | Progesterone | +1 h | i.p. | % | |
| Sayeed et al. | 2006 | SDR | M | N | FCI (T) | 8 | Progesterone | +2 h | i.p., s.c. | % | |
| Toung et al. | 2004 | WR | RSF | A | FCI (T) | 5 | Progesterone | −0.5 h | i.p. | % |
| Study . | Year . | Species . | Sex/hormonal status . | Age . | Model of injury . | Dose range (mg/kg) . | Preparation . | First dose timing . | Route . | Measure of infarct . | Other outcome(s) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkayed et al. | 2000 | WR | RSF | A | FCI (T) | 10 | Progesterone | −7 d | s.c. | % | |
| Chen et al. | 1999 | WR | M | N | FCI (T) | 4–32 | Progesterone | +2 h | i.v. | % | Function |
| Choi et al. | 2004 | SDR | M | N | FCI (T) | 4 | Progesterone | −24 h | i.p. | mm3 | |
| Cutler et al. | 2006 | SDR | M | N | TBI | 16 | Progesterone | +1 h | i.p. | % | |
| Djebali et al. | 2004 | SDR | M | N | TBI | 16 | Progesterone | +1 h | i.p., s.c. | % | Function |
| Gibson and Murphy | 2004 | CM | M | N | FCI (T) | 8 | Progesterone | +1 h | i.p. | mm3 | Function |
| Gibson et al. | 2005 | CM | M | N | FCI (P) | 8 | Progesterone | +1 h | i.p. | mm3 | Function |
| Goss et al. | 2003 | SDR | M | N | TBI | 8–32 | Progesterone | +1 h | i.p. | % | |
| He et al. | 2004 | SDR | M | N | TBI | 4 | Metabolites | +1 h | i.p. | % | Function |
| Jiang et al. | 1996 | WR | M | N | FCI (T) | 4 | Progesterone | −0.5 h | i.p. | % | NS |
| Jones et al. | 2005 | CM | M | N | TBI | 8 | Progesterone | +0.03 h | i.p. | mm3 | Function |
| Kumon et al. | 2000 | SHRSP | M | N | FCI (T) | 4–8 | Progesterone | +2 h | i.p. | % | NS |
| Murphy et al. | 2000 | WR | OF | N | FCI (T) | 5–20 | Progesterone | −0.5 h | i.p. | % | |
| Murphy et al. | 2002 | WR | OF | N | FCI (T) | 30–60 | Progesterone | −7 d | i.p. | % | Function |
| Robertson et al. | 2006 | SDR | OF | N | TBI | NR | Progesterone | −7 d | s.c. | % | |
| Roof et al. | 1994 | SDR | M | N | TBI | 4 | Progesterone | +1 h | i.p. | % | |
| Sayeed et al. | 2006 | SDR | M | N | FCI (T) | 8 | Progesterone | +2 h | i.p., s.c. | % | |
| Toung et al. | 2004 | WR | RSF | A | FCI (T) | 5 | Progesterone | −0.5 h | i.p. | % |
WR = Wistar rats; SDR = Sprague-Dawley rats; CM = C57 mice; SHRSP = stroke-prone spontaneously hypertensive rats; RSF = reproductively senescent females; M = males; OF = ovariectomised females; A = aged; N = normal adult; FCI (T) = focal cerebral ischemia (transient); TBI = traumatic brain injury; FCI (P) = focal cerebral ischemia (permanent); NR = not reported; s.c. = subcutaneous; i.v. = intravenous; i.p. = intra-peritoneal; NS = neurological score.
Characteristics of included studies
| Study . | Year . | Species . | Sex/hormonal status . | Age . | Model of injury . | Dose range (mg/kg) . | Preparation . | First dose timing . | Route . | Measure of infarct . | Other outcome(s) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkayed et al. | 2000 | WR | RSF | A | FCI (T) | 10 | Progesterone | −7 d | s.c. | % | |
| Chen et al. | 1999 | WR | M | N | FCI (T) | 4–32 | Progesterone | +2 h | i.v. | % | Function |
| Choi et al. | 2004 | SDR | M | N | FCI (T) | 4 | Progesterone | −24 h | i.p. | mm3 | |
| Cutler et al. | 2006 | SDR | M | N | TBI | 16 | Progesterone | +1 h | i.p. | % | |
| Djebali et al. | 2004 | SDR | M | N | TBI | 16 | Progesterone | +1 h | i.p., s.c. | % | Function |
| Gibson and Murphy | 2004 | CM | M | N | FCI (T) | 8 | Progesterone | +1 h | i.p. | mm3 | Function |
| Gibson et al. | 2005 | CM | M | N | FCI (P) | 8 | Progesterone | +1 h | i.p. | mm3 | Function |
| Goss et al. | 2003 | SDR | M | N | TBI | 8–32 | Progesterone | +1 h | i.p. | % | |
| He et al. | 2004 | SDR | M | N | TBI | 4 | Metabolites | +1 h | i.p. | % | Function |
| Jiang et al. | 1996 | WR | M | N | FCI (T) | 4 | Progesterone | −0.5 h | i.p. | % | NS |
| Jones et al. | 2005 | CM | M | N | TBI | 8 | Progesterone | +0.03 h | i.p. | mm3 | Function |
| Kumon et al. | 2000 | SHRSP | M | N | FCI (T) | 4–8 | Progesterone | +2 h | i.p. | % | NS |
| Murphy et al. | 2000 | WR | OF | N | FCI (T) | 5–20 | Progesterone | −0.5 h | i.p. | % | |
| Murphy et al. | 2002 | WR | OF | N | FCI (T) | 30–60 | Progesterone | −7 d | i.p. | % | Function |
| Robertson et al. | 2006 | SDR | OF | N | TBI | NR | Progesterone | −7 d | s.c. | % | |
| Roof et al. | 1994 | SDR | M | N | TBI | 4 | Progesterone | +1 h | i.p. | % | |
| Sayeed et al. | 2006 | SDR | M | N | FCI (T) | 8 | Progesterone | +2 h | i.p., s.c. | % | |
| Toung et al. | 2004 | WR | RSF | A | FCI (T) | 5 | Progesterone | −0.5 h | i.p. | % |
| Study . | Year . | Species . | Sex/hormonal status . | Age . | Model of injury . | Dose range (mg/kg) . | Preparation . | First dose timing . | Route . | Measure of infarct . | Other outcome(s) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkayed et al. | 2000 | WR | RSF | A | FCI (T) | 10 | Progesterone | −7 d | s.c. | % | |
| Chen et al. | 1999 | WR | M | N | FCI (T) | 4–32 | Progesterone | +2 h | i.v. | % | Function |
| Choi et al. | 2004 | SDR | M | N | FCI (T) | 4 | Progesterone | −24 h | i.p. | mm3 | |
| Cutler et al. | 2006 | SDR | M | N | TBI | 16 | Progesterone | +1 h | i.p. | % | |
| Djebali et al. | 2004 | SDR | M | N | TBI | 16 | Progesterone | +1 h | i.p., s.c. | % | Function |
| Gibson and Murphy | 2004 | CM | M | N | FCI (T) | 8 | Progesterone | +1 h | i.p. | mm3 | Function |
| Gibson et al. | 2005 | CM | M | N | FCI (P) | 8 | Progesterone | +1 h | i.p. | mm3 | Function |
| Goss et al. | 2003 | SDR | M | N | TBI | 8–32 | Progesterone | +1 h | i.p. | % | |
| He et al. | 2004 | SDR | M | N | TBI | 4 | Metabolites | +1 h | i.p. | % | Function |
| Jiang et al. | 1996 | WR | M | N | FCI (T) | 4 | Progesterone | −0.5 h | i.p. | % | NS |
| Jones et al. | 2005 | CM | M | N | TBI | 8 | Progesterone | +0.03 h | i.p. | mm3 | Function |
| Kumon et al. | 2000 | SHRSP | M | N | FCI (T) | 4–8 | Progesterone | +2 h | i.p. | % | NS |
| Murphy et al. | 2000 | WR | OF | N | FCI (T) | 5–20 | Progesterone | −0.5 h | i.p. | % | |
| Murphy et al. | 2002 | WR | OF | N | FCI (T) | 30–60 | Progesterone | −7 d | i.p. | % | Function |
| Robertson et al. | 2006 | SDR | OF | N | TBI | NR | Progesterone | −7 d | s.c. | % | |
| Roof et al. | 1994 | SDR | M | N | TBI | 4 | Progesterone | +1 h | i.p. | % | |
| Sayeed et al. | 2006 | SDR | M | N | FCI (T) | 8 | Progesterone | +2 h | i.p., s.c. | % | |
| Toung et al. | 2004 | WR | RSF | A | FCI (T) | 5 | Progesterone | −0.5 h | i.p. | % |
WR = Wistar rats; SDR = Sprague-Dawley rats; CM = C57 mice; SHRSP = stroke-prone spontaneously hypertensive rats; RSF = reproductively senescent females; M = males; OF = ovariectomised females; A = aged; N = normal adult; FCI (T) = focal cerebral ischemia (transient); TBI = traumatic brain injury; FCI (P) = focal cerebral ischemia (permanent); NR = not reported; s.c. = subcutaneous; i.v. = intravenous; i.p. = intra-peritoneal; NS = neurological score.
The majority of studies employed a model of transient focal ischaemia (10 studies), with one study using a model of permanent focal ischaemia (Gibson et al., 2005) and seven studies using a model of TBI. Various rat strains (Wistar, Sprague-Dawley, Spontaneously Hypertensive and Reproductively Senescent) were used in 15 out of the 18 studies; three studies used mice. Methodological design was variable as far as drug administration was concerned. Several routes of administration (subcutaneous, intraperitoneal, intravenous) were used with first-dose timings in relation to onset of cerebral injury ranging from 7 days before, to 6 h after. In terms of outcome measure, lesion volume was visualized by histological staining and reported as; lesion volume (mm3), percentage of total cross-sectional area, or percentage of ipsilateral non-ischaemic total/region.
Publication bias was absent for studies reporting the effect of lesion volume in models of stroke (Egger's test statistic = −0.64, P = 0.53, Fig. 2A) but present for studies using models of TBI (Egger's test statistic = −2.31, P = 0.038, Fig. 2B).
Reported study quality
The median quality rating for included articles that used a model of cerebral ischaemia was 4 out of 8 (range 2–7) whilst the median quality rating for included articles using a model of TBI was 5 (range 2–5). Animals were allocated treatment by randomization in six out of the 11 included ischaemia articles (Jiang et al., 1996; Kumon et al., 2000; Gibson and Murphy 2004; Toung et al., 2004; Gibson et al., 2005; Sayeed et al., 2006) and all but one (Robertson et al., 2006) of the seven included TBI articles did so. Whereas only one TBI article that was included (Goss et al., 2003) reported the monitoring of physiological parameters, all of the ischaemia articles did so (although the majority of these only monitored body temperature). Only four ischaemia articles (Chen et al., 1999; Kumon et al., 2000; Murphy et al., 2000, 2002) and three TBI articles (Goss et al., 2003; Djebali et al., 2004; Robertson et al., 2006) assessed dose–response relationships. Three ischaemia articles (Jiang et al., 1996; Murphy et al., 2000, 2002) investigated the optimal time window of administering progesterone whereas no included articles using a TBI model reported doing so. However, one published study has demonstrated that progesterone is still effective at reducing oedema volume when administered at 24 h following TBI (Roof et al., 1996). All 11 included articles that used a model of ischaemia assessed outcome at Days 0–3; three of those also assessed outcome at Days 7–30 (Chen et al., 1999; Kumon et al., 2000; Gibson and Murphy 2004) and four included articles reported the combined measurement of lesion volume and functional outcome (Jiang et al., 1996; Chen et al., 1999; Kumon et al., 2000; Gibson and Murphy 2004). All of the seven included articles that used a model of TBI assessed outcome at Days 7–30 whereas only three (Djebali et al., 2004; Jones et al., 2005; Cutler et al., 2006) assessed outcome at Days 1–3 and all but one (Robertson et al., 2006) of those included combined lesion volume measurements with measures of functional outcome. Only four of the ischaemia articles (Gibson et al., 2005; Kumon et al., 2000) and three of the TBI articles (He et al., 2004; Jones et al., 2005; Cutler et al., 2006) reported outcome measures being blinded to treatment.
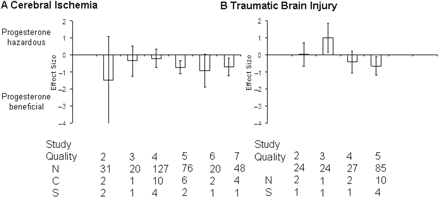
Following cerebral ischaemia, a beneficial effect of progesterone treatment was observed when the effects of quality score are discounted (effect size = −0.51, 95% CI = −0.82 to −0.19, P = 0.0002). The beneficial effect of administering progesterone increased with increasing reported quality score (Fig. 3A); indeed, there was no apparent beneficial effect of administering progesterone in studies that obtained a quality score of 4 or less. Following TBI progesterone did not have a beneficial effect on lesion volume when the effects of quality score are discounted (−0.37, −0.79 to 0.06, P = 0.09); however, this result was dependent on a low quality study (score 3) reporting a detrimental effect of progesterone (P = 0.02). Higher quality studies (median quality score 5) reported a beneficial effect of progesterone administration (P = 0.02, Fig. 3B).
Standardized mean difference and 95% CI by reported quality score after cerebral ischaemia and TBI. (A) Effect size was independent of quality score after cerebral ischaemia. (B) However, a beneficial effect of progesterone was only present after TBI in studies with a reported quality score of 4 or 5. N, number of animals; C, number of comparisons; S, number of published studies.
Infarct volume according to hormonal status/age
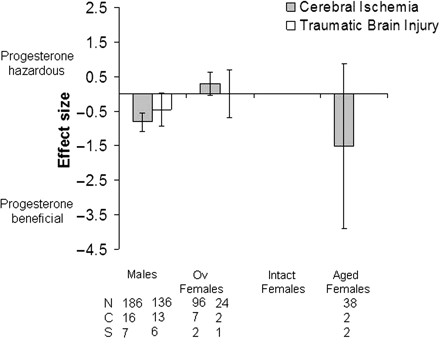
The effect of progesterone on lesion volume following cerebral ischaemia or TBI was analysed according to population groupings based on hormonal status i.e. males, ovariectomized females, intact females, aged females and aged males (Fig. 4).
Standardized mean difference and 95% CI for lesion volume following either cerebral ischaemia or TBI. Data shown are grouped according to hormonal status/age of animals; males, ovariectomized females, intact females and aged females. When grouped according to hormonal status/age progesterone only had a significant beneficial effect in male animals that had undergone cerebral ischaemia. However, studies were limited to similar groups of animals being used in terms of hormonal status/age. Ov, ovariectomized; N, number of animals; C, number of comparisons; S, number of published studies.
Following cerebral ischaemia progesterone treatment had the largest beneficial effect when administered to males (P ≤ 0.00001), a trend to benefit was also seen in aged females (P = 0.21). In contrast, progesterone appeared to have a detrimental effect when administered to ovariectomized females although this was not significant (P = 0.07). Overall, significantly different estimates were found within hormonal status groups (males, ovariectomized females, intact females) for lesion volume after cerebral ischaemia (χ2 = 57.44, df = 24, P = 0.0001). Following TBI progesterone treatment had a trend to benefit when administered to males (P = 0.07).
Dose of progesterone
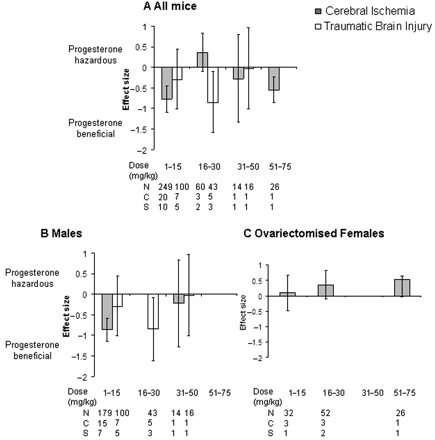
The effects of progesterone dose on lesion volume were also analysed irrespective of hormonal status (Fig. 5). For articles that utilized a model of cerebral ischaemia a beneficial effect of progesterone on lesion volume was only observed in the lowest dose treatment group, i.e. 1–15 mg/kg (−0.77, −1.09 to −0.45, P < 0.00001, Fig. 5A). Following TBI, a significant effect of progesterone was seen in a higher dose range i.e. 16–30 mg/kg than that seen for cerebral ischaemia (−0.85, −1.59 to −0.10, P = 0.03, Fig.5A). Significantly different estimates were found within progesterone dose groups for cerebral ischaemia (χ2 = 57.39, df = 24, P = 0.0001) and TBI (χ2 = 27.55, df = 12, P = 0.006).
Standardized mean difference and 95% CI for lesion volume following cerebral ischaemia or TBI in all animals (A), males (B) and ovariectomized females (C). Data are grouped according to the dose of progesterone administered (mg/kg, where reported). Following cerebral ischaemia progesterone had a significant effect on reducing lesion volume after administration of the lowest dose range of progesterone reported i.e. 1–15 mg/kg whereas a beneficial effect of progesterone following TBI was reported following progesterone administration within the dose range of 16–30 mg/kg. N, number of animals; C, number of comparisons; S, number of published studies.
In order to identify any gender differences in response to different progesterone doses, the data were analysed for males (Fig. 5B) and ovariectomized females (Fig. 5C). For males, the only dose range to have any beneficial effect on lesion volume was 1–15 mg/kg for cerebral ischaemia (P < 0.00001) and 16–30 mg/kg for TBI (P = 0.03). Following cerebral ischaemia progesterone appeared to have an increasing detrimental effect with increasing dose when administered to ovariectomized females (Fig. 5C) however this effect was not significant and not all dose ranges have been explored in all groups.
Timing of progesterone administration in relation to onset of ischaemia
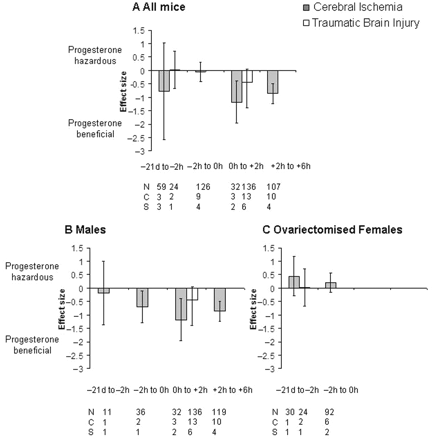
Only post-injury administration of progesterone was effective at reducing lesion volume when comparing all animals (Fig. 6). Progesterone was effective at reducing lesion volume when administered 0–2 h (−1.18, −1.97 to −0.38, P = 0.004, Fig. 6A) or 2–6 h (−0.86, −1.23 to −0.49, P = 0.0008, Fig. 6A) following cerebral ischaemia. In males, progesterone treatment also had a significant effect when administered immediately prior to the onset (i.e. −2 to 0 h) of cerebral ischaemia (−0.70, −1.29 to −0.11, P = 0.004, Fig. 6B). In contrast, progesterone appeared to have a detrimental effect (non-significant) on lesion volume when administered prior to ischaemia in ovariectomized females (Fig. 6C). No studies reported administering progesterone to ovariectomized females following the onset of cerebral ischaemia. No studies reported the effects of administering progesterone on lesion volume beyond 6 h following the onset of ischaemia.
Standardized mean difference and 95% CI for lesion volume following cerebral ischaemia or TBI in all animals (A), males (B) and ovariectomized females (C). Data are grouped according to the time of the first administration of progesterone in relation to the onset of either cerebral ischaemia or TBI. Progesterone was only effective when administered following the onset of cerebral ischaemia. N, number of animals; C, number of comparisons; S, number of published studies.
Although progesterone appeared to be effective when administered between 0 and 2 h post-TBI this effect was not significant (P = 0.07, Fig. 6A). Additionally, no studies reported the effects of administering progesterone on lesion volume at later than 2 h following the onset of TBI or immediately prior (i.e. −2 to 0 h) to the onset of TBI. The majority of TBI studies used male animals (Fig. 6B) with ideal timing of administration not being fully investigated for other groups. Significantly different estimates were found within the timing of progesterone groups for cerebral ischaemia (χ2 = 57.44, df = 24, P = 0.0001) and TBI (χ2 = 29.19, df = 14, P = 0.01).
Discussion
This systematic review indicates that progesterone is neuroprotective, in terms of reducing lesion volume, following either cerebral ischaemia or TBI. However, progesterone was only effective at reducing lesion volume following TBI in those studies that achieved the highest quality score whereas progesterone was effective following cerebral ischaemia regardless of quality score. To identify any limitations of the effectiveness of progesterone, data were grouped according to hormonal status and age, dose and timing of progesterone treatment in relation to the onset of cerebral injury. When data were grouped according to hormonal status and age, progesterone treatment following cerebral ischaemia was only effective when administered to males; an effect not seen following TBI. However, there were insufficient studies of progesterone in other various groups such as ovariectomized females, intact females, and aged animals. Although this review did identify potentially efficacious dose ranges following cerebral ischaemia and TBI, adequate dose–response relationships were not fully examined in all experimental groups according to hormonal status. In addition, no included studies administered progesterone treatment at a time point later than 6 h following the onset of cerebral ischaemia or TBI.
Systematic reviews and meta-analyses are fundamental tools in the interpretation of the effectiveness of a particular treatment across a large number of studies. Historically they have been used exclusively for reviewing clinical trials and only a few have applied systematic review techniques to data obtained from animal experiments. However, the need to conduct systematic reviews on animal experiments aimed at modelling clinically relevant problems, such as stroke and TBI, has been highlighted recently (Sandercock and Roberts, 2002; Dirnagl, 2006). Systematic reviews allow for a more objective appraisal of the research evidence than is allowed by the traditional narrative reviews more commonly associated with animal research. It would appear, from the papers analysed in this review, that the methodological quality of a large number of animal studies is poor; in particular few studies randomize animals to treatment groups. Systematic reviews allow an insight into the cause of any bias present in animal experiments. The long-term aim of animal studies examining candidate neuroprotective factors is to inform the design of clinical trials. Some would argue the need for a much more systematic review of animal data before proceeding to clinical trials (Pound et al., 2004; Dirnagl, 2006) to reveal not only that a drug can have neuroprotective activity under certain conditions but also to give an insight into potential limitations of the drug (e.g. time to treatment), which are likely to affect its clinical usefulness.
However, systematic reviews do have various limitations. Firstly, analyses can only include available data, usually only available in published studies. Negative or neutral studies are less likely to be published so results may overstate effect size. Interestingly, positive studies of recently patented compounds may also not be published. In this respect, Egger's asymmetry test revealed that publication bias was absent in studies of cerebral ischaemia but present in studies of TBI. Consequently, the benefits of progesterone on lesion volume following TBI may have been overestimated which may be attributable to the low number of TBI studies included for analysis. Additionally, non-publication will limit available information on the effect of treatment within certain protocol aspects such as dose or time of administration. The technique of extracting multiple pieces of information from single publications has the potential to introduce bias into the review because the results have been generated from the same laboratories and investigators.
This review focuses only on the effect of progesterone on lesion volume following either cerebral stroke or TBI, largely due to insufficient data regarding other outcomes such as behaviour. When comparing TBI with focal ischaemia, there may appear to be a large amount of literature supporting the neuroprotective effect of progesterone following injury. However, only those studies that measured lesion volume were included in this review whereas a large number of papers support the neuroprotective properties of progesterone following TBI when looking at various outcomes including oedema volume or various molecular and functional tests. To date, only one paper (Gibson et al., 2006) has measured oedema volume following cerebral ischaemia and very few have measured functional ability. Functional outcome, in combination with effects on histopathology, may be as important in terms of assessing benefit (STAIR, 1999). In terms of cerebral ischaemia, infarct size has been reported to correlate (Rogers et al., 1997) or not correlate (Hattori et al., 2000; Reglodi et al., 2003) with neurological impairment.
The aim of this review is to demonstrate whether progesterone is effective at reducing lesion volume following cerebral injury, i.e. ischaemia or TBI. Importantly, this review also aims to give an insight into the limitations of the effectiveness of progesterone. We assessed quality score in accordance with previously published protocols (Macleod et al., 2005; Wilmot et al., 2005a, b; Gibson et al., 2006) which are all based upon recommendations by the STAIR (1999). The publication of these standards for pre-clinical neuroprotective drug development were organized by an expert panel of stroke researchers in order to address why so many clinical trials of neuroprotective drugs for acute ischaemic stroke have failed. Although this panel of experts recognized the importance of pre-clinical testing of neuroprotective drugs guidelines concerning how to perform pre-clinical development of potential neuroprotective treatments were lacking. Without rigorous, robust and detailed pre-clinical evaluation, it is unlikely that novel neuroprotective drugs will prove to be effective when tested in large, time-consuming and expensive clinical trials. The current study is the first to apply such an assessment to experimental studies using models of TBI. Reassuringly, the majority of TBI papers did rate relatively high in terms of quality score and all eight measures of quality were deemed relevant to TBI studies. However, we could only assess the studies as reported, so a low quality score may indicate either that the authors did not undertake a procedure, for example randomization, or that they did not report it. Lack of randomization can introduce bias and may result in overestimation of treatment efficacy. Randomization is necessary in order to exclude systematic errors of sampling. As with randomization, blinding was only reported in a fraction of included studies. In order to remove experimenter bias all outcome measures (e.g. lesion volume) should be assessed blinded to treatment of the experimental groups. Factors such as randomization and blinded assessment seem to be important in terms of affecting outcome in experimental stroke studies. For example, a recent systematic review of hypothermia in experimental stroke studies found that non-randomized studies overstated the decrease in lesion volume and studies which were not blinded to outcome overstated efficacy when compared to randomized and blinded outcome studies respectively (van der Worp et al., 2007). Although sources of variation between experimental animals might be small the literature does support the notion that randomization can influence outcome in experimental animal stroke studies (Macleod et al., 2004, 2005; Sena et al., 2007). In addition, it is important that the experimenter has no knowledge of the assigned experimental group when inducing ischaemia as knowledge of experimental group may affect management, albeit subtly, of any complications associated with the ischaemia (e.g. ability to induce sufficient ischaemia, perioperative hypotension) and therefore influence outcome. Additionally, while most studies reported monitoring physiological parameters, the majority only measured body temperature. Although body temperature is a useful parameter, additional physiological parameters also give invaluable information about the physiological effects of a particular treatment, which could be crucial when considering the design of clinical trials.
In order to assess the limitations of the effectiveness of progesterone we evaluated the effects of hormonal status and age. The majority of studies used young adult males. This is in contrast to the majority of experimental studies evaluating the neuroprotective effect of oestrogens which tend to use ovariectomized females (Gibson et al., 2006). It is important that the effectiveness of steroid hormones, such as progesterone, are assessed in males as they represent the population group most at risk of TBI and, in conjunction with post-menopausal women, represent the group at highest risk of a cerebral stroke. However, clinical trials aimed at testing the effectiveness of hormone therapy on the occurrence and outcome following vascular events have reported conflicting results. In fact, a systematic review of completed clinical trials found that hormone therapy was associated with an elevated risk of stroke, which was ischaemic in type and of increased severity (Bath and Gray, 2005). However, all clinical trials have assessed the effects of either combined (oestrogen–progestin) hormone therapy or oestrogen-only hormone therapy following stroke. Thus, the effects of progestin-only hormone therapy on incidence and outcome following stroke are unknown.
This review highlights that very few studies have assessed the neuroprotective effectiveness of progesterone in either female animals (ovaroectomized or intact) or aged animals. Although cerebral stroke is considered a disease of aging experimental studies using aged animals are rarely undertaken because of financial and logistical costs. However, it is important that the effectiveness of progesterone treatment is assessed in aged animals, especially following stroke, prior to the initiation of any clinical trials. This review also highlights the lack of studies, which fully explore the effectiveness of progesterone with regards to timing of first administration. As the majority of patients at greatest risk from TBI or cerebral stroke (i.e. men and post-menopausal women) will not be receiving hormone therapy it is clinically relevant to extend the first time of application. Although one study has shown that progesterone treatment delayed up to 24 h following TBI was still effective at reducing brain oedema (Roof et al., 1996) no studies looking at lesion volume have initiated progesterone treatment at later than up to 6 h following the onset of cerebral ischaemia or TBI. All included studies applied progesterone within the dose range of 4–60 mg/kg but the majority of studies did not measure circulating plasma progesterone levels. The few that have indicate that intraperitoneal absorption of 4 mg/kg resulted in plasma progesterone levels between 41.9 and 70.7 ng/ml 4 following injection (Jiang et al., 1996) whereas intraperitoneal administration of 30 mg/kg resulted in an average plasma progesterone level of 133 ng/ml (Murphy et al., 2000). In the rat, plasma progesterone levels range from basal levels of 2–18 ng/ml to 120–130 ng/ml in pregnancy, with intermediate values of 40–90 ng/ml during late proestrus (Wiest, 1970; Nequin et al., 1979). In the current study, those studies which used a lower dose of progesterone would have achieved physiological levels in intact female animals but supraphysiological levels in male animals. Thus, there is a need for adequate dose-response relationships to be examined more fully in all experimental groups.The effects of progesterone following various types of CNS injury are described as being pleiotropic in that it has been shown to reduce necrotic damage and cell loss, reduce oedema formation, and restore cognitive performance (Gibson et al., 2005; Stein, 2005). In terms of the mechanisms of progesterone-induced neuroprotection in vitro studies or studies using models of TBI or spinal cord injury have revealed that progesterone; has antioxidant properties (Roof et al., 1997); regulates the expression of trophic factors such as brain-derived neurotrophic factor (Gonzalez et al., 2004); activates intracellular signalling pathways involved in the promotion of cell survival (Singh, 2001); increases the expression of anti-apoptotic molecules such as Bcl-2 and Bcl-XL (Yao et al., 2005); and, decreases the expression of pro-apoptotic molecules such as Bax, Bad and caspase-3 (Djebali et al., 2004). Some of these effects of progesterone may be mediated by the activation of classical progestin receptors, which are widely expressed in the brain (Guerra-Araiza et al., 2003). In addition, rapid membrane effects of progesterone may be mediated by membrane progestin receptors, or by the membrane-associated progesterone-binding protein 25-Dx (Meffre et al., 2005). Despite all these beneficial effects of progesterone in the CNS its specific mechanisms following acute cerebral injury are not completely understood.
Overall this review has emphasized the need for bi-directional translational research between experimental and clinical studies. Progesterone is a potential neuroprotective treatment following either cerebral stroke or TBI. In fact, the first clinical trial aimed at assessing the neuroprotective effect of progesterone following TBI has just been completed (Wright et al., 2007). In that clinical trial, the investigators report that progesterone resulted in improved outcome and was well tolerated in terms of safety after TBI. Larger scale trials to assess outcome following progesterone treatment for TBI are planned although clinical trials following cerebral stroke have yet to be initiated. The design of such clinical trials relies fundamentally upon animal studies to inform them with regards to both potential hazards and the optimal progesterone treatment in terms of dosing and timing among different populations. This review has identified fundamental areas where experimental evidence is still required.
Acknowledgements
PMWB is Stroke Association Professor of Stroke Medicine. Supported by NIH grant NS29226 (to S.P.M.).