-
PDF
- Split View
-
Views
-
Cite
Cite
L. Marquardt, W. Kuker, A. Chandratheva, O. Geraghty, P. M. Rothwell, Incidence and prognosis of ≥50% symptomatic vertebral or basilar artery stenosis: prospective population-based study, Brain, Volume 132, Issue 4, April 2009, Pages 982–988, https://doi.org/10.1093/brain/awp026
Close - Share Icon Share
Abstract
The higher risk of early recurrent stroke after posterior circulation transient ischaemic attack or minor stroke versus after carotid territory events could be due to a greater prevalence of large artery stenosis, but there have been few imaging studies, and the prognostic significance of such stenoses is uncertain. Reliable data are necessary to determine the feasibility of trials of angioplasty and stenting and to inform imaging strategies. In the first-ever population-based study, we determined the prevalence of ≥50% apparently symptomatic vertebral and basilar stenosis using contrast-enhanced MRA in consecutive patients, irrespective of age, presenting with posterior circulation transient ischaemic attack or minor ischaemic stroke in the Oxford Vascular Study and related this to the 90-day risk of recurrent transient ischaemic attack and stroke. For comparison, we also determined the prevalence of ≥50% apparently symptomatic carotid stenosis on ultrasound imaging in consecutive patients with carotid territory events. Of 538 consecutive patients, 141/151 (93%) had posterior circulation events and had vertebral and basilar imaging, of whom 37 (26.2%) had ≥50% vertebral and basilar stenosis, compared with 41 (11.5%) patients with ≥50% ipsilateral carotid stenosis in 357/387 (92%) patients with carotid events who had carotid imaging (OR = 2.74; 95% CI = 1.67–4.51; P = 0.002). Presence of ≥50% vertebral and basilar stenosis was unrelated to age, sex or vascular risk factors and, in contrast to ≥50% carotid stenosis was not associated with evidence of coronary/peripheral atherosclerosis. In patients with posterior circulation events, ≥50% vertebral and basilar stenosis was associated multiple transient ischaemic attacks at presentation (22% versus 3%; OR = 9.29; 95% CI = 2.31–37.27; P < 0.001) and with a significantly higher 90-day risk of recurrent events (OR = 3.2; 95% CI = 1.4–7.0; P = 0.006), reaching 22% for stroke and 46% for transient ischaemic attack and stroke. The prevalence of ≥50% vertebral and basilar stenosis in posterior circulation transient ischaemic attack or minor stroke is greater than the prevalence of ≥50% carotid stenosis in carotid territory events, and is associated with multiple transient ischaemic attacks at presentation and a high early risk of recurrent stroke. Trials of interventional treatment are therefore likely to be feasible, but more data are required on the long-term risk of stroke on best medical treatment.
Introduction
Posterior circulation transient ischaemic attack (TIA) and stroke account for ∼20% of all TIA and stroke (Cloud and Markus, 2003). Recent studies have shown that the early risk of recurrent stroke after TIA and minor stroke is as high as 8–10% in the first week (Rothwell et al., 2006), and is particularly high in patients with large artery atherosclerotic disease (Lovett et al., 2004), and after posterior circulation TIA and minor stroke (Flossmann and Rothwell, 2003; Flossmann et al., 2006). However, it is uncertain to what extent the high early risk of stroke in patients with posterior circulation events is due to atherosclerotic disease in the vertebral or basilar arteries.
There are few published data on the frequency of large artery disease in patients with posterior circulation ischaemic events (Bogousslavsky et al.1993; Caplan et al., 2004; Kim et al.2005). The two largest series were of selected patients who underwent conventional arterial angiography or time-of-flight MRA, often after major stroke (Bogousslavsky et al., 1993; Caplan et al., 2004). A more recent Korean study reported retrospectively collected data on contrast enhanced magnetic resonance angiography (MRA) in 72 patients with posterior circulation stroke (Kim et al., 2005). There have been no population-based studies of unselected patients with posterior circulation stroke, and there are no published data on patients with TIA.
There are also very limited data on the optimal management of large artery atherosclerosis in the posterior circulation stroke. Several small case series and a recent systematic review (Eberhardt et al., 2006) have described angioplasty and stenting of symptomatic vertebral and basilar stenosis, but only one small (16 patients) randomized trial of stenting for vertebral artery disease has been published (Coward et al., 2007). Large randomized trials of vertebral artery angioplasty/stenting versus best medical therapy alone are planned, but their feasibility will depend on the incidence of significant symptomatic vertebral stenosis in patients with recent posterior circulation TIA or stroke, and appropriate trial design and statistical power will depend on the risk of recurrent stroke on best medical therapy alone.
Using contrast enhanced magnetic resonance angiography, and CT angiography, it has now become possible to image the posterior circulation routinely at reasonable cost and negligible risk. A recently published meta-analysis of imaging studies showed that contrast enhanced MRA had good sensitivity and specificity for detection of 50–99% vertebral or basilar stenosis (Kahn et al., 2007). In the first ever population-based study, we used contrast enhanced MRA to determine the incidence of significant symptomatic vertebral and basilar stenosis in all patients with recent posterior circulation TIA or minor stroke, irrespective of age, in the Oxford Vascular Study population. We also determined the early risk of recurrent stroke on best medical therapy in patients with ≥50% vertebral or basilar stenosis. To further assess the likely feasibility of large randomized controlled trials, similar to those done for carotid endarterectomy and stenting, we also compared the incidence of with ≥50% vertebral or basilar stenosis with that of ≥50% recently symptomatic carotid stenosis in the Oxford Vascular Study population.
Methods
The study was nested within the Oxford Vascular Study, a population-based study of all acute vascular events. The study population comprised ∼91 000 individuals registered with 63 primary-care physicians in nine general practices in and around Oxford, UK. Oxford Vascular Study has been approved by the local research ethics committee.
Methods of Oxford Vascular Study have been reported previously (Rothwell et al., 2004, 2005). Briefly, multiple overlapping methods of ‘hot’ pursuit were used to achieve near complete ascertainment of all individuals with TIA or stroke. These include a daily, urgent open-access ‘TIA clinic’ to which participating general practitioners and the local accident and emergency department send all individuals with suspected TIA or stroke whom they would not normally admit to hospital; daily assessment of admissions to the medical, stroke, neurology and other relevant wards; and daily searches of the local accident and emergency attendance register. In order not to miss patients who presented late, patients who were referred to other services, or patients who were not referred to secondary care we also performed monthly computerized searches of family doctor diagnostic coding, hospital discharge codes, and all cranial and carotid imaging studies performed in local hospitals.
All patients were consented and seen by study physicians as soon as possible after their initial presentation. Event characteristics and risk factors were recorded and all cases were subsequently reviewed by the study senior neurologist (P.M.Rothwell) and classified as probable or definite TIA or stroke, or other condition using standard definitions (Rothwell et al., 2004, 2005). Vascular risk factors were defined as follows: Hypertension–blood pressure reported to be ≥140/90 mmHg at two readings before stroke or >5 days after stroke or on antihypertensive treatment. Diabetes Mellitus—elevated fasting blood glucose or on antidiabetic treatment. Hyperlipidaemia—total cholesterol >6.0 mmol/l or triglycerides 2.3 mmol/l or on lipid-lowering medication. Smoking—current or previous smoking.
All patients were followed up face-to-face at 30 days by a study nurse or physician. Patients were assessed for recurrent symptoms, medications and disability scores. All recurrent strokes that presented to medical attention would also be identified acutely by ongoing daily case-ascertainment within Oxford Vascular Study. All patients with recurrent events were reassessed by a study physician and reviewed by PMR. Vascular territory was assessed by the study neurologist who first assessed the patient and subsequently by PMR.
Consecutive patients with TIA or minor stroke in the carotid territory who were assessed between the April 1, 2002 and the March 31, 2007 in the Oxford Vascular Study outpatient clinic were investigated with carotid ultrasound performed by an experienced vascular technician using an ATL Ultramark HDI 5000 scanner. Symptomatic carotid stenosis was defined as ≥50% stenosis of the carotid artery on the appropriate side.
From December 1, 2005 until November 30, 2008 all patients who were seen at the Oxford Vascular Study TIA and stroke clinic with a suspected vertebral or basilar (VB) TIA or minor stroke had contrast enhanced MRA imaging of the posterior circulation. A Philips Aciva 1.5T scanner with a neurovascular coil was used (contrast enhanced MRA sequence: 20 ml ProHance® followed by 40 ml NaCl, flow rate 2 ml/s, TR 4.6 ms, TE 1.7 ms, Flip angle 40, slice thickness 1.2 mm, matrix 416/416, field of view 300/150/70 mm). In cases where contrast enhanced MRA was not possible (e.g. due to claustrophobia, pacemaker, frailty, etc.), we aimed to perform CT angiography instead.
All scans were reviewed independently by an experienced study neuroradiologist and a study neurologist. Disagreements were resolved by consensus with a third observer (an experienced vascular neurologist). Apparently symptomatic VB stenosis (from here on referred to as ‘symptomatic’) was defined as ≥50% diameter reduction of the basilar or vertebral artery in a location that was considered likely to be responsible for the location of any acute infarct or the localization of the clinical syndrome. For example, a contralateral vertebral atery stenosis was not considered likely to be responsible for a posterior inferior cerebellar infarct, or a distal basilar stenosis for a medullary infarct. The estimate of the normal arterial diameter at the point of maximum stenosis was taken as the closest measurable section of non-diseased vertebral artery (or basilar artery), i.e. analagous to the ‘NASCET method’ of measurement of carotid stenosis (Rothwell et al., 1994). Inter- and intra-observer agreement in the identification of ≥50% diameter reduction of the basilar or vertebral artery was assessed on the full cohort.
After consensus was achieved on the presence of ≥50% symptomatic VB stenosis, two independent observers measured the exact degree of stenosis using a manual on-screen cursor tool. Inter-observer agreement was quantified using Bland and Altman analysis (Bland and Altman, 1986). One observer re-measured the same images 4 weeks later to determine intra-observer agreement.
All patients with the diagnosis of minor ischemic stroke or TIA were treated with aspirin, a statin and cases presenting in the acute phase were also given clopidogrel for the first month after they have been seen in the out-patient clinic. Hypertension was treated as clinically appropriate. Angioplasty and/or stenting were only considered if a patient had a recurrent ischaemic cerebrovascular event in the posterior circulation during follow-up.
Results
We included 538 consecutive patients in the study. A total of 151 were presented with TIA or minor stroke, 387 with TIA or minor stroke in the carotid territory. Demographic and clinical characteristics are shown in Table 1.
Demographic and clinical data of imaged patients presenting with posterior circulation versus carotid territory events, stratified according to the presence of ≥50% stenosis
| . | VB patients . | Carotid patients . | ||
|---|---|---|---|---|
| . | ≥50% VB stenosis, n = 37 (26.2%) . | No stenosis, n = 104 (73.8%) . | ≥50% carotid stenosis, n = 41 (11.5%) . | No stenosis, n = 316 (88.5%) . |
| Age (mean ± SD; years) | 69.9 (12.2) | 68.3 (12.4) | 75.4 (10.8) | 73.4 (11.5) |
| Age ≥ 80 years, n (%) | 11 (30) | 24 (23) | 16 (39) | 108 (34) |
| Female, n (%) | 13 (35) | 51 (49) | 18 (44) | 170 (54) |
| Hypertension, n (%) | 20 (54) | 60 (58) | 23 (56) | 168 (53) |
| Diabetes mellitus, n (%) | 6 (16) | 14 (13) | 4 (10) | 38 (12) |
| Hyperlipidaemia, n (%) | 11 (30) | 36 (35) | 13 (32) | 93 (29) |
| Current smoking, n (%) | 8 (22) | 11 (11) | 11 (27) | 44 (14) |
| Previous smoking, n (%) | 15 (41) | 49 (47) | 15 (37) | 130 (41) |
| Atrial fibrillation (prev. or curr.) (%) | 3 (8) | 7 (7) | 3 (7) | 45 (14) |
| NIHSS, median (quartiles) | 0 (0-1) | 0 (0-0) | 0 (0-1.5) | 0 (0-1) |
| Stroke as presenting event, n (%) | 19 (51) | 43 (41) | 21 (51) | 147 (47) |
| TIA as presenting event, n (%) | 13 (50) | 46 (58) | 20 (49) | 169 (53) |
| Previous PVD, n (%) | 2 (5) | 9 (9) | 7 (17) | 11 (3) |
| Previous MI, n (%) | 1 (3) | 8 (8) | 11 (27) | 31 (10) |
| Previous TIA, n (%) | 4 (11) | 12 (12) | 13 (32) | 58 (18) |
| Previous stroke, n (%) | 1 (3) | 5 (5) | 5 (12) | 51 (16) |
| Previous TIA/stroke, n (%) | 5 (14) | 17 (16) | 16 (39) | 101 (32) |
| . | VB patients . | Carotid patients . | ||
|---|---|---|---|---|
| . | ≥50% VB stenosis, n = 37 (26.2%) . | No stenosis, n = 104 (73.8%) . | ≥50% carotid stenosis, n = 41 (11.5%) . | No stenosis, n = 316 (88.5%) . |
| Age (mean ± SD; years) | 69.9 (12.2) | 68.3 (12.4) | 75.4 (10.8) | 73.4 (11.5) |
| Age ≥ 80 years, n (%) | 11 (30) | 24 (23) | 16 (39) | 108 (34) |
| Female, n (%) | 13 (35) | 51 (49) | 18 (44) | 170 (54) |
| Hypertension, n (%) | 20 (54) | 60 (58) | 23 (56) | 168 (53) |
| Diabetes mellitus, n (%) | 6 (16) | 14 (13) | 4 (10) | 38 (12) |
| Hyperlipidaemia, n (%) | 11 (30) | 36 (35) | 13 (32) | 93 (29) |
| Current smoking, n (%) | 8 (22) | 11 (11) | 11 (27) | 44 (14) |
| Previous smoking, n (%) | 15 (41) | 49 (47) | 15 (37) | 130 (41) |
| Atrial fibrillation (prev. or curr.) (%) | 3 (8) | 7 (7) | 3 (7) | 45 (14) |
| NIHSS, median (quartiles) | 0 (0-1) | 0 (0-0) | 0 (0-1.5) | 0 (0-1) |
| Stroke as presenting event, n (%) | 19 (51) | 43 (41) | 21 (51) | 147 (47) |
| TIA as presenting event, n (%) | 13 (50) | 46 (58) | 20 (49) | 169 (53) |
| Previous PVD, n (%) | 2 (5) | 9 (9) | 7 (17) | 11 (3) |
| Previous MI, n (%) | 1 (3) | 8 (8) | 11 (27) | 31 (10) |
| Previous TIA, n (%) | 4 (11) | 12 (12) | 13 (32) | 58 (18) |
| Previous stroke, n (%) | 1 (3) | 5 (5) | 5 (12) | 51 (16) |
| Previous TIA/stroke, n (%) | 5 (14) | 17 (16) | 16 (39) | 101 (32) |
PVD = peripheral vascular event; MI = myocardial infarction.
Demographic and clinical data of imaged patients presenting with posterior circulation versus carotid territory events, stratified according to the presence of ≥50% stenosis
| . | VB patients . | Carotid patients . | ||
|---|---|---|---|---|
| . | ≥50% VB stenosis, n = 37 (26.2%) . | No stenosis, n = 104 (73.8%) . | ≥50% carotid stenosis, n = 41 (11.5%) . | No stenosis, n = 316 (88.5%) . |
| Age (mean ± SD; years) | 69.9 (12.2) | 68.3 (12.4) | 75.4 (10.8) | 73.4 (11.5) |
| Age ≥ 80 years, n (%) | 11 (30) | 24 (23) | 16 (39) | 108 (34) |
| Female, n (%) | 13 (35) | 51 (49) | 18 (44) | 170 (54) |
| Hypertension, n (%) | 20 (54) | 60 (58) | 23 (56) | 168 (53) |
| Diabetes mellitus, n (%) | 6 (16) | 14 (13) | 4 (10) | 38 (12) |
| Hyperlipidaemia, n (%) | 11 (30) | 36 (35) | 13 (32) | 93 (29) |
| Current smoking, n (%) | 8 (22) | 11 (11) | 11 (27) | 44 (14) |
| Previous smoking, n (%) | 15 (41) | 49 (47) | 15 (37) | 130 (41) |
| Atrial fibrillation (prev. or curr.) (%) | 3 (8) | 7 (7) | 3 (7) | 45 (14) |
| NIHSS, median (quartiles) | 0 (0-1) | 0 (0-0) | 0 (0-1.5) | 0 (0-1) |
| Stroke as presenting event, n (%) | 19 (51) | 43 (41) | 21 (51) | 147 (47) |
| TIA as presenting event, n (%) | 13 (50) | 46 (58) | 20 (49) | 169 (53) |
| Previous PVD, n (%) | 2 (5) | 9 (9) | 7 (17) | 11 (3) |
| Previous MI, n (%) | 1 (3) | 8 (8) | 11 (27) | 31 (10) |
| Previous TIA, n (%) | 4 (11) | 12 (12) | 13 (32) | 58 (18) |
| Previous stroke, n (%) | 1 (3) | 5 (5) | 5 (12) | 51 (16) |
| Previous TIA/stroke, n (%) | 5 (14) | 17 (16) | 16 (39) | 101 (32) |
| . | VB patients . | Carotid patients . | ||
|---|---|---|---|---|
| . | ≥50% VB stenosis, n = 37 (26.2%) . | No stenosis, n = 104 (73.8%) . | ≥50% carotid stenosis, n = 41 (11.5%) . | No stenosis, n = 316 (88.5%) . |
| Age (mean ± SD; years) | 69.9 (12.2) | 68.3 (12.4) | 75.4 (10.8) | 73.4 (11.5) |
| Age ≥ 80 years, n (%) | 11 (30) | 24 (23) | 16 (39) | 108 (34) |
| Female, n (%) | 13 (35) | 51 (49) | 18 (44) | 170 (54) |
| Hypertension, n (%) | 20 (54) | 60 (58) | 23 (56) | 168 (53) |
| Diabetes mellitus, n (%) | 6 (16) | 14 (13) | 4 (10) | 38 (12) |
| Hyperlipidaemia, n (%) | 11 (30) | 36 (35) | 13 (32) | 93 (29) |
| Current smoking, n (%) | 8 (22) | 11 (11) | 11 (27) | 44 (14) |
| Previous smoking, n (%) | 15 (41) | 49 (47) | 15 (37) | 130 (41) |
| Atrial fibrillation (prev. or curr.) (%) | 3 (8) | 7 (7) | 3 (7) | 45 (14) |
| NIHSS, median (quartiles) | 0 (0-1) | 0 (0-0) | 0 (0-1.5) | 0 (0-1) |
| Stroke as presenting event, n (%) | 19 (51) | 43 (41) | 21 (51) | 147 (47) |
| TIA as presenting event, n (%) | 13 (50) | 46 (58) | 20 (49) | 169 (53) |
| Previous PVD, n (%) | 2 (5) | 9 (9) | 7 (17) | 11 (3) |
| Previous MI, n (%) | 1 (3) | 8 (8) | 11 (27) | 31 (10) |
| Previous TIA, n (%) | 4 (11) | 12 (12) | 13 (32) | 58 (18) |
| Previous stroke, n (%) | 1 (3) | 5 (5) | 5 (12) | 51 (16) |
| Previous TIA/stroke, n (%) | 5 (14) | 17 (16) | 16 (39) | 101 (32) |
PVD = peripheral vascular event; MI = myocardial infarction.
Of the 151 patients events in the VB territory, 141 (93%) underwent imaging of the posterior circulation (contrast enhanced MRA in 135 cases and CT angiography in six cases). Reasons for non-imaging were that seven patients were too frail to attend hospital and were assessed only at home or in a nursing home, one patient refused investigation and one agreed but did not attend. Of 387 patients with carotid territory events, 357 (92%) had carotid imaging (339 with ultrasound, 15 with contrast enhanced MRA, three with CT angiography).
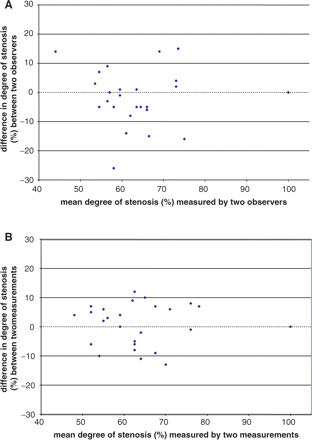
Inter-observer agreement on the presence of ≥50% symptomatic VB stenosis was good, with 94% agreement between the study neuroradiologist and the study neurologist. In cases where there was a consensus about the presence of ≥50% stenosis, two independent observers then measured the exact degree of stenosis as detailed in the methods. Figure 1A shows a Bland and Altman plot for the inter-observer agreement. Figure 1B shows a Bland and Altman plot for the intra-observer agreement for one of the observers. The mean absolute difference in measured percent stenosis between the two independent observers was 6%, with no overall bias between the observers. The corresponding for intra-observer difference was 5%.
Observer agreement for measurement of the degree of vertebral and/or basilar stenosis—Bland and Altman plots for: (A) inter-observer agreement; (B) intra-observer agreement.
The prevalence of ≥50% symptomatic VB stenosis in posterior circulation TIA or minor stroke was greater than the prevalence of ≥50% ipsilateral carotid bifurcation stenosis in carotid territory events. Of the 141 patients with VB events who were imaged, 37 (26.2%) had ≥50% symptomatic vertebral or basilar stenosis compared with 41 (11.5%) out of 357 imaged patients with carotid events (OR = 2.74; 95% CI = 1.67–4.51; P = 0.002).
There were no significant differences in age, sex and traditional treatable vascular risk factors of patients with versus without ≥50% symptomatic VB stenosis (Table 1). There were also no differences in age, sex and traditional treatable vascular risk factors between those patients with carotid territory events with ≥50% symptomatic carotid stenosis versus those without. However, patients with ≥50% symptomatic carotid stenosis were significantly more likely to have had a previous myocardial infarction (17% versus 3%, P = 0.002), peripheral vascular event (27% versus 10%, P = 0.012) and previous TIA (32% versus 18%, P = 0.041) than patients without carotid stenosis. However, we could not show similar differences regarding history of myocardial infarction, peripheral vascular event and TIA for VB patients with and without ≥50% stenosis.
In patients with posterior circulation events, VB stenosis was strongly associated with multiple TIAs shortly prior to first seeking medical attention (22% versus 3%; OR = 9.29; 95% CI = 2.31–37.27; P < 0.001).
Of 37 patients with VB stenosis, 23 stenoses (62%) were located in the extracranial vertebral artery, 11 (30%) in the intracranial vertebral artery and three (8%) in the basilar artery. Of the 23 (62%) extracranial stenoses, nine (24%) were at the vertebral origin, seven (19%) were near the origin and seven (19%) were in the V2 or V3 segment. There were no significant differences in age, traditional treatable vascular risk factors of patients with intracranial versus extracranial VB stenosis. However, patients with intracranial VB stenosis were significantly less likely to be female (21% versus 43%, P = 0.043), and more likely to have a past history of TIA (21% versus 4%, P = 0.039). However, there were no differences in past history of myocardial infarction, peripheral vascular event and stroke between patients with intracranial and extracranial VB stenosis.
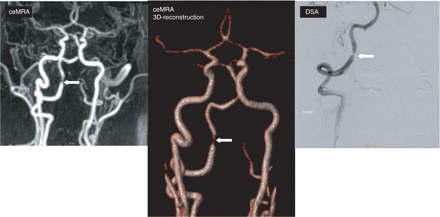
Seventeen patients had only one vertebral affected (≥50% stenosis), five both vertebral arteries (Fig. 2), four one vertebral artery plus one carotid artery, seven one vertebral artery and both carotid arteries, two both vertebral arteries and one carotid artery and two patients had only the basilar artery affected. In summery, nine patients (24%) had triple vessel disease of the main brain supplying arteries. Of 23 patients with extracranial VB stenosis, 10 (43%) had evidence of carotid disease on at least one side, whereas of 14 patients with intracranial VB stenosis only four (29%) had carotid disease. However, this difference is not statistically significant.
Contrast enhanced MRA and digital subtraction angiography showing tight stenosis of the right distal vertebral artery (arrow) and occlusion of left vertebral artery.
In imaged patients with a posterior circulation event, the presence of carotid stenosis (assessed as the mean of both sides) was associated with the presence of vertebral stenosis. For example, an average degree of carotid stenosis of ≥50% was present in 6/37 (16%) patients with VB stenosis compared to 2/104 (2%) without VB stenosis (OR = 9.5; 95% CI = 1.8–49.4). The same association was seen for average carotid stenosis of ≥30% (12/37 versus 6/104; OR = 7.5; 95% CI = 2.6–22.0), and for average carotid stenosis of ≥10% (22/37 versus 38/104; OR = 2.4; 95% CI = 1.1–5.2). However, the majority of patients with ≥50% VB stenosis had very little carotid disease and so the sensitivity of carotid imaging in prediction of VB disease was low.
Eighteen patients had an acute ischaemic infarct at an appropriate location on MRI brain imaging. Seven were in the occipital lobe (three left, two right, two bilateral), three in the cerebellum (one left, one right, one bilateral), three in the thalamus (one left, two right) and five were in the brainstem. Of 19 patients without evidence for acute ischaemia on imaging, clinical presentation was suggestive of an event in the occipital lobe in seven, in the cerebellum in two, in the thalamus in one and the brainstem in nine.
Overall, in patients with TIA and minor ischaemic stroke in our population, the annual incidence of symptomatic ≥50% VB stenosis (13.5 patients per 100 000) was higher than the incidence of symptomatic carotid stenosis (9.0 per 100 000).
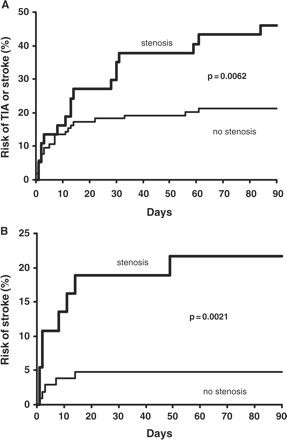
Seventeen (46%) of the 37 patients who had symptomatic ≥50% VB stenosis had a recurrent TIA or ischaemic stroke in the posterior circulation in the first 90 days after the initial event compared with 22 (21%) of the 104 patients with a posterior circulation event without VB stenosis (OR = 3.2; 95% CI = 1.4–7.0; P = 0.006; Fig. 3A). The difference remained when the analysis was confined to risk of recurrent stroke alone: Eight (22%) versus five (5%), P = 0.002, Fig. 3B. However, of these eight early recurrent strokes in patients who had symptomatic ≥50% VB stenosis, four happened before patients sought medical attention. No patient with ≥50% VB stenosis received any interventional treatment during the 90-day risk period.
Risk for recurrent events in the first 90 days after initial event for patients with and without ≥50% VB stenosis: (A) stroke and TIA; (B) stroke.
Discussion
Until recently patients with VB territory TIAs and minor strokes were generally thought to have a better prognosis than patients with carotid territory events (Marshall, 1964; Baker et al., 1968; Acheson, 1971; Ziegler and Stephenson Hassanein, 1973; Heyman et al., 1984). However, a systematic review of all available data on prognosis after TIA and minor stroke showed that there was actually little overall difference in risk of recurrent stroke and that the risk was higher in patients with VB territory events in the acute phase (Flossmann and Rothwell, 2003). This latter observation of a particularly high early risk of stroke was subsequently confirmed in the Oxford Vascular Study study (Flossmann et al., 2006). However, it has been uncertain whether this high risk is due to a greater prevalence of large artery stenosis in patients with posterior circulation events than carotid territory events or some other factor. This question is important in that if the risk of stroke was particularly high in the subset of patients with vertebral or basilar stenosis then randomized trials of angioplasty and/or stenting would be appropriate. However, reliable data on the natural history of apparently symptomatic VB stenosis is a pre-requisite for any such trial.
In the first ever population-based study of the frequency of apparently symptomatic stenoses in unselected patients who had a posterior circulation TIA or minor stroke, we have shown that the prevalence of ≥50% VB stenosis was significantly greater than the frequency of ≥50% carotid stenosis in patients who had an anterior circulation event. Overall, the annual incidence of TIA and minor stroke associated with symptomatic ≥50% VB stenosis was higher than the incidence of TIA and minor stroke associated with symptomatic carotid bifurcation stenosis. We also showed that in patients with posterior circulation events, ≥50% VB stenosis was associated with multiple TIAs at presentation and with a high early risk of recurrent stroke. These findings suggest that randomized trials of interventional treatment in patients with symptomatic VB stenosis are both appropriate and feasible.
In terms of trying to triage patients with an increased likelihood of having ≥50% VB stenosis and thereby focussing investigation, we did not find any associations that were sufficiently sensitive and specific to be clinically useful. However, the presence of multiple posterior circulation TIAs should certainly be regarded as a pointer towards possible large artery aetiology. Interestingly, there was absolutely no association between VB stenosis and either known coronary artery disease or peripheral vascular disease—in contrast to carotid stenosis. This observation raises questions about possible differences in susceptibility to carotid and VB stenosis. Although, there was an association between the presence or carotid stenosis and the presence of VB stenosis, about half of patients with VB stenosis had little or no carotid plaque.
Several previous imaging studies have also reported data on the prevalence of VB stenosis in selected non-consecutive cohorts. Kim et al. (2005) imaged a variety of patients with TIA or stroke as well as some asymptomatic patients. In a subgroup analysis only looking at patients with posterior circulation (n = 72) infarcts they found a prevalence of ≥50% stenosis of the proximal vertebral artery of 44.4%, distal vertebral artery/basilar artery of 36.1%. In an older study, Bogousslavsky et al. (1993) reported 70 patients with posterior circulation stroke imaged with time-of-flight MRA, of whom 27 (39%) had ≥50% basilar stenosis and 19 (27%) had ≥50% vertebral artery stenosis. In the New England Medical Center Posterior Circulation Registry, Caplan et al. (2004) reported 407 patients with posterior circulation ischaemic events of whom ∼80% underwent contrast catheter angiography. One hundred forty-eight patients had ≥50% stenosis in more than one large artery of the posterior circulation. However, all of these studies were done in selected cohorts and none report data on prognosis.
Although, we believe that the results of our study are reliable, there are a number of methodological issues that need further discussion. Firstly, it can be very difficult sometimes to reliably distinguish clinically between carotid territory and posterior circulation TIA and minor stroke (Caplan, 2000), intra-observer agreement between neurologists is only moderate (Kraaijeveld et al., 1984), and even a clinical consensus between neurologists does not correlate particularly well with the relative gold standard of an acute ischaemic lesion on diffusion-weighted MRI (Flossman et al., 2008). However, all our patients were initially seen and examined by an experienced neurologist specializing in stroke medicine and have been reviewed by an additional independent stroke specialist. Moreover, any inaccuracy of diagnosis of vascular territory would be expected to have diluted and differences between the groups, suggesting that the differences that we report are likely, if anything, to be underestimates. Second, we only included patients with TIA or minor stroke, and it is possible that findings might differ in patients with disabling stroke. However, previous studies of VB stenosis in selected patients with more severe stroke report similar prevalences of apparently symptomatic stenosis (Bogousslavsky et al., 1993; Caplan et al., 2004). Third, the number of patients with VB events (n = 151) and particularly the number with ≥50% VB stenosis (n = 37) was not sufficiently large to provide narrow confidence intervals around the estimate of risk of early recurrent stroke. However, our study is nevertheless the largest such study published to date, it was population-based with as a high a rate of imaging as is possible in practice, and perhaps most importantly none of patients received any interventional treatment during the 90-day risk period. Thus, albeit with wide confidence intervals, the risk estimates are therefore likely to be unbiased estimates of the natural history of recently symptomatic VB stenosis on best medical treatment alone. Fourth, although we found that the overall incidence of TIA and minor stroke associated with ≥50% symptomatic VB stenosis was higher than that of TIA and minor stroke associated with ≥50% symptomatic carotid bifurcation stenosis, we only imaged the carotid arteries by bifurcation ultrasound in patients with carotid territory events. It is likely, therefore, that some patients with proximal common carotid stenosis or distal internal carotid stenosis were missed. Fifth, it is possible that some of the presenting TIAs or strokes were due to a different mechanism than large artery disease. For example, three (8%) out of 37 patients with VB stenosis had previous or current atrial fibrillation. However, we had no hard evidence of cardioembolic aetiology, such as evidence of infarction in multiple territories. In the first case, there was no evidence of stroke on the cerebral MRI, in the other two cases the MRI revealed occipital cortical infarcts only that did not suggest an embolic aetiology. Sixth, the carotid and VB imaging protocol were only partly contemporaneous (i.e. 2002–07 versus 2005–08, respectively). However, in both periods all patients presenting to medical attention with TIA or minor stroke were recruited and it is unlikely that there were such rapid temporal trends in the frequency of atherosclerotic disease during this period that our comparison was substantially biased. Moreover, any small bias due to increased pre-morbid use of statins with time would have tended to impact more on the slightly more recent VB imaging cohort.
Finally, contrast enhanced MRA is less accurate in delineating the degree of stenosis than digital subtraction angiography, particularly for stenoses at the origin of the vertebral artery. However, recent comparative studies have shown good overall agreement between digital subtraction angiography and contrast enhanced MRA (Leclerc et al., 1998; Cosottini et al., 2003; Randoux et al., 2003; Yang et al., 2005). For example, Yang et al. (2005) reported that contrast enhanced MRA had a sensitivity of 88% and a specificity of 98% for 50–99% VB stenosis on digital subtraction angiography. Another digital subtraction angiography—contrast enhanced MRA comparison study of 48 patients with stroke and TIA of all territories published by Cosottini et al. (2003) reported observer agreement of 96% (κ = 0.84) for detection of vertebral artery stenosis, which was similar to the agreement found for detection of carotid artery stenosis. Similar findings have also been published by Leclerc et al. (1998), who concluded that contrast enhanced MRA is a useful tool for assessing atherosclerotic lesions in supra-aortic vessels. Finally, in a recent meta-analysis, Kahn et al. (2007) showed that contrast enhanced MRA has a higher sensitivity and diagnostic odds ratio than CT angiography, ultrasound or time of flight MRA for detection of 50–99% VB stenosis.
In conclusion, we have shown that the frequency of ≥50% VB stenosis in posterior circulation TIA or minor stroke is greater than the frequency of ≥50% carotid stenosis in carotid territory events, such that the incidence rate of symptomatic ≥50% VB stenosis was higher than that of symptomatic carotid stenosis. Symptomatic ≥50% VB stenosis was associated with multiple TIAs at presentation and a high early risk of recurrent stroke. Our results, therefore, suggest that trials of interventional treatment in patients with symptomatic large artery disease in the posterior circulation are appropriate and feasible. However, more data are required on the long-term risk of stroke on best medical treatment, both overall and in subgroups with widespread occlusive disease, such as the 24% of patients in our cohort who had ‘triple-vessel-disease’.
Funding
UK Medical Research Council (to OXVASC); the Dunhill Medical Trust; the Stroke Association; the BUPA Foundation; the National Institute for Health Research; the Thames Valley Primary Care Research Partnership; Oxford Partnership Comprehensive Biomedical Research Centre.
References
- atherosclerosis
- stents
- transient ischemic attack
- ultrasonography
- magnetic resonance angiography
- carotid stenosis
- cerebrovascular accident
- basilar artery stenosis
- ischemic stroke
- angioplasty
- constriction, pathologic
- diagnostic imaging
- vertebrae
- stroke risk
- oxford vascular study
- medical management
- carotid artery imaging