-
PDF
- Split View
-
Views
-
Cite
Cite
Nader Ghasemlou, Delphine Bouhy, Jingxuan Yang, Rubèn López-Vales, Michael Haber, Thusanth Thuraisingam, Guoan He, Danuta Radzioch, Aihao Ding, Samuel David, Beneficial effects of secretory leukocyte protease inhibitor after spinal cord injury, Brain, Volume 133, Issue 1, January 2010, Pages 126–138, https://doi.org/10.1093/brain/awp304
Close - Share Icon Share
Abstract
Secretory leukocyte protease inhibitor is a serine protease inhibitor produced by various cell types, including neutrophils and activated macrophages, and has anti-inflammatory properties. It has been shown to promote wound healing in the skin and other non-neural tissues, however, its role in central nervous system injury was not known. We now report a beneficial role for secretory leukocyte protease inhibitor after spinal cord injury. After spinal cord contusion injury in mice, secretory leukocyte protease inhibitor is expressed primarily by astrocytes and neutrophils but not macrophages. We show, using transgenic mice over-expressing secretory leukocyte protease inhibitor, that this molecule has an early protective effect after spinal cord contusion injury. Furthermore, wild-type mice treated for the first week after spinal cord contusion injury with recombinant secretory leukocyte protease inhibitor exhibit sustained improvement in locomotor control and reduced secondary tissue damage. Recombinant secretory leukocyte protease inhibitor injected intraperitoneally localizes to the nucleus of circulating leukocytes, is detected in the injured spinal cord, reduces activation of nuclear factor-κB and expression of tumour necrosis factor-α. Administration of recombinant secretory leukocyte protease inhibitor might therefore be useful for the treatment of acute spinal cord injury.
Introduction
The cellular immune response after injury in a variety of tissues is primarily mediated by neutrophils and macrophages, and is responsible for clearance of tissue debris and wound healing. Neutrophils and activated macrophages secrete proteases that degrade the tissue matrix, allowing for migration of cells, clearance of necrotic and apoptotic cells, and the effective remodelling and repair of the tissue after injury (Diegelmann and Evans, 2004). If unchecked, these immune cells can produce a variety of mediators that may cause bystander tissue damage. The pro-inflammatory response after spinal cord injury has been shown to contribute importantly to secondary tissue damage and worsening of functional deficits (Wells et al., 2003; Stirling et al., 2004; Brambilla et al., 2005; Oatway et al., 2005).
The immune cells that infiltrate the spinal cord after injury probably mediate their cytotoxic effects via the production of pro-inflammatory chemokines/cytokines, proteases and free radicals. As in other tissues, the inflammatory response also contributes to the process of wound healing after spinal cord injury. Secretory leukocyte protease inhibitor (SLPI; 12 kDa) is a serine protease inhibitor produced by neutrophils and macrophages that has anti-inflammatory and wound healing properties (Jin et al., 1997; Zhang et al., 1997; Taggart et al., 2002; Henriksen et al., 2004). Lipopolysaccharide-stimulated monocytes express SLPI, which acts to reduce inflammation by suppressing the production of pro-inflammatory mediators (Jin et al., 1997; Zhang et al., 1997). Wound healing in the skin is impaired by a lack of SLPI, while application of exogenous recombinant SLPI enhances repair (Ashcroft et al., 2000). Recombinant SLPI treatment also attenuates inflammation in a model of bacterial cell wall-induced arthritis (Song et al., 1999), and adenoviral expression of SLPI after cerebral ischaemia reduces lesion size (Wang et al., 2003). Expression of SLPI in the CNS has also recently been studied in experimental autoimmune encephalomyelitis in rats (Mueller et al., 2008). The role of SLPI in CNS trauma or disease, however, has not been examined.
In this study, we have assessed the expression of SLPI in the injured spinal cord and found it to be upregulated primarily in astrocytes surrounding the lesion epicentre and also expressed in some neutrophils in the injured spinal cord parenchyma. The effects of SLPI on spinal cord injury were assessed using SLPI over-expressing transgenic mice and wild-type mice treated with recombinant mouse SLPI. Our data show that SLPI plays a beneficial role in modulating the inflammatory response leading to reduced secondary damage and improved locomotor recovery after spinal cord injury.
Materials and methods
Animals and surgical procedures
Adult female mice (8–10 weeks old, 18–20 g) were used for all experiments. SLPI transgenic over-expressing and wild-type control mice were used. Transgenic mice were generated as follows: the cDNA encoding a full-length secreted mouse (m)SLPI was generated by PCR from a macrophage cDNA library and subcloned into an expression vector pIRES3-Neo (Clontech). After digestion with Mlu I, linearized plasmid cDNA, which contains complete expression elements (promoter, mSLPI encoding sequences, Poly A tail), was micro-injected into fertilized one-cell embryos derived from (C57BL/6J_CBA/CA) F1 males and females by the Transgenic Core Facility of Weill Medical College of Cornell. The genotyping of the resultant SLPI transgenic mice was confirmed by Southern blot (Supplementary Fig. 1A), and the heterozygous transgenic mice were backcrossed to C57BL/6J mice. A western blot of bone marrow cells from these mice shows up-regulation of SLPI in transgenic mice (Supplementary Fig. 1B).
In addition, female C57BL/6 mice (8–10 weeks of age) were treated with recombinant SLPI as described below, starting 1 h after contusion injury. Briefly, mice were anaesthetized with ketamine:xylazine:acepromazine (50:5:1 mg/kg) and a partial laminectomy made using Mouse Laminectomy Forceps [Fine Science Tools (FST), Vancouver] at the 11th thoracic vertebral level. A moderate contusion injury (50 ± 5 kDynes force, 400–600 µm displacement) was made on the exposed spinal cord using the Infinite Horizons impactor device (Precision Scientific Instrumentation, Lexington, KY), as described previously (Ghasemlou et al., 2005). All protocols were approved by the McGill University Animal Care Committee and followed the guidelines of the Canadian Council on Animal Care.
Recombinant SLPI treatment
To assess the role of exogenous SLPI treatment, recombinant mouse SLPI was generated as described previously (Yang et al., 2005a). The anti-elastase activity of recombinant mouse SLPI was assessed prior to its use. For this assay, human neutrophil elastase (15 mM, Sigma) was used. Recombinant mouse SLPI was pre-incubated with elastase for 20 min at 37°C before addition of the substrate [Suc-A-A-P-V-PNA (0.5 nM, Chromogenix)]. The enzymatic activity was measured by the initial rate of absorbance changes at 405 nm in 100 mM Tris–HCl, pH 6.5, 960 mM NaCl and 0.1% bovine serum albumin at 37°C. The activities of the two batches of recombinant mouse SLPI used for these experiments are similar, and illustrated in Supplementary Fig. 2. The endotoxin level of the recombinant mouse SLPI was also assessed using the QCL-1000 Chromogenic Limulus Amebocyte Lysate Endpoint Assay kit (Cambrex) using manufacturer's; instructions prior to its use in vivo. Recombinant mouse SLPI samples were found to have undetectable levels of endotoxin activity (<0.06 ng/µg protein). Protein concentration of recombinant mouse SLPI was determined using the bicinchoninic acid assay (Pierce) using bovine serum albumin as the standard. Recombinant mouse SLPI was diluted in sterile phosphate buffered saline (PBS) and mice injected intraperitoneally with 1µg recombinant mouse SLPI per gram initial body weight in 200 µl total solution. Control mice were injected intraperitoneally with an equal volume (200 µl) of sterile PBS. Mice were first injected 1 h after spinal cord injury and injections were repeated daily thereafter until Day 7.
Locomotor analysis
Mice were assessed for locomotor outcomes using the Basso Mouse Scale (BMS) (Basso et al., 2006), which is widely used to assess locomotor recovery after contusion injury in mice. Mice were scored in an open-field environment by two individuals who were blinded to experimental conditions and trained by the Basso group at Ohio State University to use the BMS system. Individual scores from each animal were pooled and averaged, for a maximum of 9 points for the BMS score and 11 points for the subscore, which assesses finer aspects of locomotion. The data were analysed using a two-way repeated-measures ANOVA and comparisons between groups were made using a post hoc Tukey test, where appropriate. All animals used for locomotor analysis were also used for Day 28 histological analysis.
Histology
Mice were deeply anaesthetized and sacrificed by transcardial perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer. A 10 mm length of spinal cord centred on the epicentre of injury was removed, post-fixed for 1 h and cryoprotected in 30% sucrose in 0.1 M phosphate buffer for 48 h. Serial cryostat cross-sections of the spinal cord (14 µm thickness) were obtained for histological analysis. C57BL/6 mice were sacrificed at 1, 3, 7 and 14 days after spinal cord injury for double immunofluorescence analysis of SLPI expression combined with cell type-specific labelling (n = 3–4 per time-point). The following primary antibodies were used: rabbit anti-mouse SLPI (Jin et al., 1997) (1:200); rat anti-CD11b (macrophage-1 antigen; recognizes macrophages/microglia) (1:200; Serotec, Raleigh, NC); rat anti-neutrophil 7/4 (1:200; Abcam, Cambridge, MA); rat anti-glial fibrillary acid protein (GFAP) (1:10 000; Dako, Mississauga, ON); and rabbit anti-5-hydroxytryptamine/serotonin (1:5000; Sigma–Aldrich, Oakville, ON). The specificity of the SLPI antibody was assessed by western blot of recombinant SLPI and adult mouse spinal cord homogenates (Supplementary Fig. 3A), and antibody depletion with recombinant mouse SLPI prior to immunostaining tissue sections (Supplementary Fig. 3B and C). Sections used for immunofluorescence were stained with the following secondary antibodies: donkey anti-rat immunoglobulin G conjugated to Alexa Fluor®594 (1:200; Molecular Probes, Eugene, OR) and goat anti-rabbit immunoglobulin G conjugated to AlexaFluor®488 (1:200; Molecular Probes). Histochemical staining with luxol fast blue and cresyl violet was used to assess myelin loss and ventral neuron loss, respectively.
Analysis of mRNA changes
Spinal cord tissue containing the lesion as well as regions rostral and caudal to epicentre of the injury (total length of 5 mm) was harvested from C57BL/6 female mice (8–10 weeks of age) at 1, 3, 7 and 14 days after spinal cord contusion injury; and from laminectomized controls (n = 3 mice per group) and RNA was extracted using the RNeasy Lipid Tissue kit (Qiagen, Mississauga, Ontario, Canada). A reverse transcription reaction was then carried out using Omniscript® RT kit (Qiagen, Mississauga, ON) according to the manufacturer's; protocol. Reverse transcription PCR was carried out using the GeneAmp RNA PCR kit (PerkinElmer Life Sciences, Wellesley, MA) as per manufacturer's; directions. Primers were designed using PrimerSelect (DNAStar Inc., Madison, WI) and peptidylprolyl isomerase A (PPIA) was used as a housekeeping gene. For quantitative real time-PCR, 1 µl of the reverse transcription product was added to 24 µl of Brilliant SYBR Green quantitative PCR Master Mix (Stratagene), and quantitative real-time PCR was used to analyse the expression of monocyte chemotactic protein-1, interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α (MX4000 apparatus, Stratagene). Glyceraldehyde 3-phosphate dehydrogenase was used as a housekeeping gene. Expression of chemokines and cytokines was assessed 12 h after spinal cord injury in mice treated 1 h after spinal cord injury with recombinant mouse SLPI or vehicle. This time point was chosen because it corresponds with the peak of cytokine expression after spinal cord injury (Pineau and Lacroix, 2007). The amount of cDNA was calculated based on the threshold cycle (CT) value, and was standardized by the amount of house-keeping gene using the 2-ΔΔCT method (Livak and Schmittgen, 2001).
Western blot
A 5 mm length of the spinal cord centred on the epicentre of injury was removed and snap-frozen in liquid nitrogen. The tissues were then homogenized in radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors. Protein concentration was determined and the samples (40 µg each) were separated on 4%–12% gradient or 10% sodium dodecyl sulfate-polyacrylamide gels (Invitrogen) as per manufacturer’s protocol. The samples were transferred onto polyvinylidene fluoride membranes (Millipore). The membranes were blocked for 4 h in 5% milk in PBS containing 0.05% Tween-20 (PBS-T), and then incubated with one of the following primary antibodies overnight at 4°C: rabbit anti-SLPI (1:400) and rabbit anti-Iκ-Bα (1:200). Membranes were washed in PBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit; Jackson ImmunoResearch) at 1:300 000. The membranes were washed again in PBS-T and the binding of horseradish peroxidase-conjugated antibodies detected by chemiluminescence (Western Lightning Chemiluminescence Reagent Plus, Perkin–Elmer Life Sciences). Membranes were re-probed with monoclonal mouse anti-β-actin (1:300; Sigma) to ensure equal loading of samples. The films were scanned and the changes in protein expression levels between samples quantified as a ratio of the β-actin levels using ImageQuant 5.0 software.
Leukocyte isolation and immunofluorescence labelling
Whole blood was collected from mice prior to spinal cord injury and at 2, 4 and 12 h after spinal cord injury and recombinant mouse SLPI or PBS injection (n = 3). Blood was removed from the tail vein using heparinized microhematocrit capillary tubes (Fisher) and centrifuged for 2 min. This separates the blood into three fractions: plasma, leukocytes and red blood cells. A diamond-tipped pen was used to cut away the red blood cell fraction and the plasma and leukocytes flushed out using a syringe into PBS. This was centrifuged at 1000× g for 1 min to remove platelets and plasma proteins. The leukocytes were then resuspended in PBS and distributed onto slides. After drying, the cells were fixed for 5 s in 100% methanol. After washing in PBS, cells were incubated with rabbit anti-mouse SLPI (1:100) for 30 min and washed in PBS. Antibody-binding was visualized using goat anti-rabbit immunoglobulin G conjugated to AlexaFluor®488 (1:100; Molecular Probes) for 30 min. Slides were then washed in PBS and incubated in 1 µg/ml propidium iodide for 10 min to visualize cell nuclei and then washed and mounted using a glycerol-based mounting media. Cells were photographed by static confocal imaging using an Ultraview spinning disk confocal system (Perkin–Elmer, Wellesley, MA) connected to an Eclipse TE2000 (Nikon, Tokyo, Japan). Low magnification images used to quantify the percentage of SLPI+ cells were obtained with a 20× lens (0.45 numerical aperture). High magnification images used to measure SLPI optical densities were obtained with a 60× oil immersion lens (1.25 numerical aperture with 1.5× optical zoom; Nikon).
Z-stacks were collected using MetaMorph imaging software (Molecular Devices, Palo Alto, CA) and deconvoluted in three dimensions using Autodeblur software (Auto-Quant, Media Cybernetics, Silver Spring, MD). Quantification of SLPI fluorescence signal intensity was carried out using MetaMorph imaging software by measuring SLPI fluorescence of propidium iodide-positive cells within an arbitrarily defined region of interest. Three-dimensional analysis of SLPI localization was carried out using deconvoluted high magnification confocal Z-stacks rendered in three dimensions using IMARIS 5.5.3 (Bitplane, Zurich, Switzerland). Nuclear SLPI was determined by masking SLPI fluorescence with a surface generated from nuclear propidium iodide fluorescence (IMARIS 5.5.3), leaving only the punctate labelling within the nucleus.
Quantification
All analyses of histological sections (other than for isolated leukocytes, above) were carried out using the BioQuant Image Analysis System (BioQuant, Nashville, TN) using a Zeiss AxioSkop2 microscope. The epicentre of injury in all spinal cords was identified using luxol fast blue, a stain for myelin, and GFAP immunoreactivity for astrocytes. To measure lesion size at 28 days, the total area of the spinal cord and the area of the GFAP-positive regions were outlined and measured at 200 µm intervals over a 2 mm distance, centred on the lesion epicentre. Myelin loss in the dorsal column was measured using luxol fast blue-stained cross-sections of the spinal cord. The threshold feature of BioQuant was used to measure the area of spared myelin within a preset area placed directly over and within the dorsal column. The ratio of spared myelin to the preset area was measured at 200 µm intervals over the 2 mm length of the cord. Ventral horn neurons were similarly counted, where values represent the sum of cresyl violet-stained neurons in the left and right horns. All the above quantification was done in vehicle and SLPI-treated C57BL/6 mice (n = 7–9 per group).
Serotonergic immunoreactivity was measured by using the threshold feature of the BioQuant Image analysis system to identify the pixel density representing all immunopositive axons in the ventral horn 1 mm caudal from the injury site in PBS and SLPI-treated mice (n = 7 per group). Proteins were analysed by quantifying scanned images of gels using ImageQuant 5.0 software.
Statistical analysis
Comparisons of two data sets were analysed by Student’s t-test, while data with more than two variables (i.e. over time or distance) were analysed by two-way repeated measures-ANOVA with post hoc Tukey analysis. All data are plotted as mean ± standard error unless otherwise specified.
Results
SLPI expression in the injured spinal cord
Increased SLPI expression at the mRNA and protein levels was seen in the first week after spinal cord contusion injury (Fig. 1A and B). The mRNA levels reached a peak 1 day after injury, returning to baseline by Day 14, with protein levels upregulated by 3 days as seen by western blot. Histological analysis revealed increased immunostaining at 7 and 14 days after injury, especially in reactive astrocytes at and near the lesion epicentre (Fig. 1C–F). This expression increased gradually over the first two weeks after injury. SLPI expression is also increased in neurons in the ventral horn (Fig. 1C and G, and Supplementary Fig. 4E, F). There is very little SLPI expression in the uninjured spinal cord with very low level staining in some neurons (Supplementary Fig. 4A–D). Double-immunofluorescence labelling also revealed that a proportion of neutrophils in the injured spinal cord parenchyma expressed SLPI (Fig. 1G–J). At 1 and 3 days after injury, about 50% of these neutrophils were SLPI+ while at 7 days ∼15% were SLPI+ (Fig. 1O). In contrast, a significantly higher percentage (75%–80%) of neutrophils located in the meninges covering the injured spinal cord were SLPI+ at 1 and 3 days and 60% at 7 days as compared to the numbers in the spinal cord parenchyma (P < 0.005) (Fig. 1O). The SLPI+ neutrophils in the meninges also appeared to show a greater intensity of SLPI immunoreactivity relative to those cells in the CNS parenchyma (data not shown). However, macrophages in the lesion core as well as microglia in the surrounding CNS tissue did not express SLPI (Fig. 1K–N).
Expression of SLPI after spinal cord injury. (A) RT–PCR analysis of SLPI mRNA expression after spinal cord injury shows upregulation 1, 3 and 7 days after injury with levels returning to control [laminectomized (LAM)] levels by Day 14. Peptidylprolyl isomerase A (PPIA) is used as a control. (B) Western blot analysis also shows increased SLPI protein expression during the first week after spinal cord injury, reaching a peak at 3 days. β-actin is used as a loading control. (C) Double immunofluorescence labelling of a cross-section of the spinal cord for GFAP and SLPI. Note the increased expression of SLPI (green) in reactive astrocytes (red) bordering the lesion at 14 days post-injury (L = lesion). Note also the SLPI labelling of neuron-like cells (green) in the ventral horn (also see Supplementary Fig. 4E–G). The area outlined in the box is shown in higher magnification in panels D–F. (D–F) Higher magnification shows strong SLPI immunoreactivity (E) in reactive astrocytes (D) along the border of the lesion; the merged image is shown in panel F. (G) Double immunofluorescence labelling of spinal cord cross-section labelled for neutrophils (antibody 7/4) (red) and SLPI (green) at 1 day post-injury. Note also the SLPI labelling in the ventral horn, which appear to be neurons. (H–J) Higher magnification of the area outlined in the box in panel G is shown in H–J. Some neutrophils (H) (arrows) also express SLPI (I); the merged image is shown in panel J. The insert in J shows higher magnification of the area outlined in the dashed lines showing SLPI labelling in neutrophils. (K) Double immunofluorescence labelling of a cross-section of the spinal cord for CD11b (macrophage-1 antigen; Mac-1) and SLPI at 7 days post-injury. Note the lack of SLPI (green) expression in Mac-1+ (red) macrophages and microglia. The area outlined in the box in panel K is shown in higher magnification in panels L–N. (L–N) Mac-1+ macrophages (arrowheads) and microglia (arrows) do not express SLPI (M); the merged image is shown in panel N. The insert in N shows higher magnification of the area outlined in the dashed lines showing no SLPI labelling in macrophages. (O) Percentage of SLPI+ neutrophils in the meninges and spinal cord parenchyma at various times after spinal cord injury. Scale bars: C, G, K = 500 µm; F, J, N = 100 µm; inserts in J and N = 25 μm. A minimum of three mice were analysed for each time point for the immunofluorescence, RT–PCR and western blot analyses.
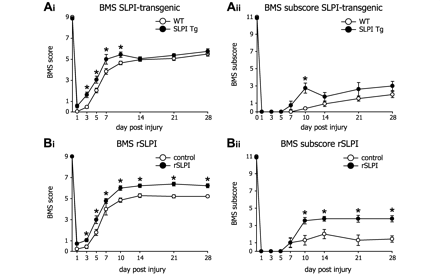
Over-expression of SLPI promotes recovery of locomotor function
SLPI over-expressing transgenic mice (Supplementary Fig. 1A and B) were first used to assess the role of SLPI in spinal cord injury. These transgenic mice showed significant improvement in locomotor recovery in the early phase after spinal cord contusion injury. Significant improvement in locomotor recovery as assessed by the BMS analysis was detected in SLPI transgenic mice between 3 and 10 days after spinal cord injury (Fig. 2A; *P < 0.02 for these time-points). Transient improvement was also seen in the BMS subscore, which measures finer aspects of locomotor control after the mice are able to bear weight on their hindlimbs (BMS Score > 4) (Fig. 2A). The BMS scores in SLPI transgenic mice reduced slightly on Day 14 and reached a plateau thereafter, with the scores being similar to wild-type mice between 14 and 28 days (Fig. 2A). No differences were found in various histological analyses on spinal cord tissue taken at 28 days after spinal cord injury to assess tissue sparing, myelin loss, neuron survival and serotonergic innervation in SLPI over-expressing transgenic mice as compared to wild-type controls (data not shown). These results suggest that the early beneficial effect of SLPI over-expression is not maintained after the first 7–10 days in SLPI transgenic mice. It is therefore possible that SLPI might have a beneficial effect during the first 7–10 days but its sustained expression after this time might have less desirable effects. To assess this more directly, we treated adult female C57BL/6 mice with recombinant mouse SLPI for the first 7 days after spinal cord injury.
Transgenic mice over-expressing SLPI and wild-type mice treated with recombinant SLPI, exhibit improved locomotor recovery after spinal cord injury. (A) Locomotor recovery was assessed after spinal cord contusion injury in wild-type and SLPI transgenic mice using the 9-point BMS. (Ai) SLPI transgenic mice (n = 8) show significant improvement in locomotor recovery, as compared to wild-type mice (n = 13), as early as 3 days after injury. However, this effect is lost by Day 14. (Aii) The BMS subscore, which measures finer aspects of locomotor control on an 11-point scale, also shows improved recovery in SLPI transgenic mice at Day 10 after injury, as compared to wild-type mice. (B) Locomotor recovery was also assessed after spinal cord injury in wild-type mice treated with recombinant mouse SLPI (rSLPI) or vehicle. (Bi) BMS analysis of mice treated with recombinant mouse SLPI shows significant improvement in locomotor recovery as early as 3 days after injury in wild-type mice treated daily with recombinant mouse SLPI (1 µg/g of body weight; n = 9) as compared to mice treated with vehicle (n = 7). This effect, unlike that observed in transgenic mice, is sustained over 28 days. (Bii) The BMS subscores were also improved, beginning at Day 10 after injury, in mice treated daily with recombinant SLPI as compared to vehicle-treated mice. This effect was also sustained until Day 28. *P < 0.05 for all graphs.
Treatment with recombinant SLPI promotes recovery of locomotor function
Mice were treated with 1 µg per gram body weight of recombinant mouse SLPI twice (at 1 and 7 days; n = 5 per group) or daily (for the first 7 days; n = 9 recombinant SLPI group and n = 7 vehicle group) after spinal cord contusion injury. The first injection was given 1 h after the injury and locomotor recovery followed using the BMS analysis for at least 14 days. Mice treated only twice with recombinant mouse SLPI did not show significant differences from vehicle-treated controls (data not shown), while mice treated daily with recombinant mouse SLPI improved locomotor recovery as early as 3 days after injury, and this recovery was maintained for the 28 day duration of the experiment (Fig. 2B). The BMS subscore was also increased significantly in recombinant mouse SLPI-treated mice from Day 10 onwards (Fig. 2B).
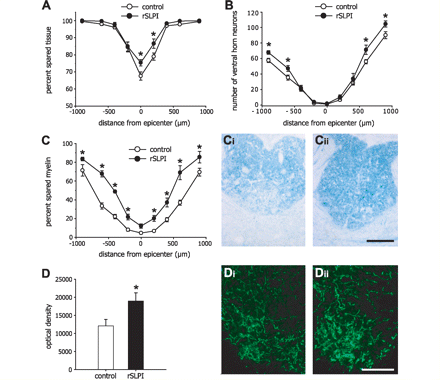
Recombinant SLPI treatment reduces secondary tissue damage and improves serotonergic innervation
Tissue sections from the spinal cords of mice treated daily for the first week with recombinant mouse SLPI or vehicle were analysed 28 days after spinal cord injury for several markers of tissue damage (n = 7 per group for all histological analyses). Quantification of GFAP immunoreactivity, which was used to demarcate the area of surviving CNS tissue, revealed significant reduction in the lesion size at the epicentre of the injury and 200 µm caudally in recombinant mouse SLPI-treated mice as compared to vehicle-treated controls (Fig. 3A), indicating improved tissue sparing at the core of the injury site in the recombinant mouse SLPI-treated group. Myelin loss, another marker for secondary tissue damage, was assessed by luxol fast blue histochemistry and also showed reduced damage in recombinant mouse SLPI-treated mice at all distances rostral and caudal to the lesion epicentre, relative to the vehicle-treated group (Fig. 3C). These results indicate that treatment with recombinant mouse SLPI improves tissue sparing and reduces secondary tissue damage in the spinal cord after contusion injury in mice.
As ventral horn neurons play an important role in locomotor function, we quantified their survival 28 days after spinal cord injury. The recombinant mouse SLPI-treated mice showed significantly greater numbers of neurons in the ventral horn at 600 and 900 µm rostral and caudal to the lesion epicentre (Fig. 3B). The extent of serotonergic innervation of the ventral horn 1 mm caudal to the injury epicentre was also assessed. The serotonergic fibres extend from raphe neurons in the brainstem and are an important mediator of locomotor control (Ribotta et al., 2000). The density of serotonergic immunoreactivity, which provides a measure of serotonergic innervation in the ventral horn, was 60% greater in the recombinant mouse SLPI-treated mice relative to vehicle-treated controls (Fig. 3D).
Reduced secondary tissue damage and increased innervation of serotonergic fibres in recombinant SLPI-treated mice after spinal cord injury. (A) The lesion size, measured as a ratio of the area bounded by GFAP-immunoreactive astrocytes to the total area of the spinal cord in cross-section, shows significantly reduced tissue damage in recombinant SLPI-treated mice at and near the epicentre of injury. (B) There is also increased survival of neurons in the ventral horn of the spinal cord at distances rostral and caudal to the site of injury. (C) The percentage of the area of spared myelin in the dorsal columns detected by luxol fast blue staining. Graph shows that myelin sparing at different distances from the lesion epicentre is significantly greater in recombinant SLPI-treated mice as compared to vehicle-treated controls. Micrographs show luxol fast blue staining of the dorsal columns of vehicle-treated (Ci) and recombinant SLPI-treated (Cii) mice. (D) Quantification of serotonergic immunoreactivity 1 mm caudal to the injury reveals significantly increased serotonergic fibre innervation caudal to the lesion in recombinant SLPI-treated mice. Micrographs of serotonergic innervation of the ventral horn of vehicle-treated (Di) and recombinant SLPI-treated (Dii) mice. *P < 0.05 for all graphs; n = 7 per group. Scale bars in Cii and Dii = 200 µm.
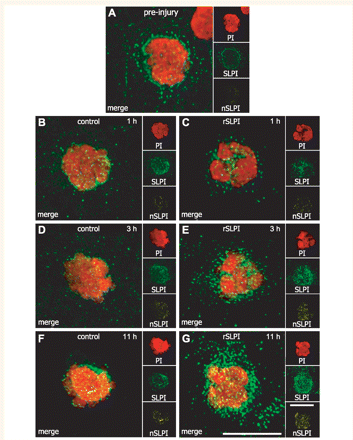
SLPI localizes to the nucleus of leukocytes
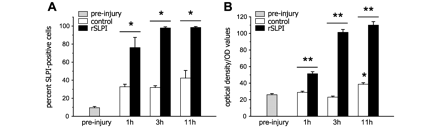
Taggart and colleagues reported that SLPI could enter leukocytes in vitro, localize to the nucleus, and bind to NF-κB binding sites on pro-inflammatory immune response genes (Taggart et al., 2005). This results in diminished p65 binding and a subsequent reduction in the inflammatory response. We therefore examined whether in vivo, recombinant mouse SLPI injected intraperitoneally is internalized by leukocytes in the peripheral circulation and subsequently becomes localized to the nucleus. Prior to contusion injury, 9.5% of leukocytes are SLPI+ (Figs 4A and 5A). In vehicle-treated spinal cord injury mice, the percentage of SLPI+ leukocytes increases 2.5- to 3-fold rapidly, relative to leukocytes purified from naïve mice, and remains unchanged over the 12 h period studied (Figs 4B, D, F and 5A). The percentage of SLPI+ leukocytes in recombinant mouse SLPI-treated mice, however, increases 8-fold 1 h after injection (P = 0.004) and 10-fold (>98% of cells) at 3 and 11 h after SLPI injection (Figs 4C, E, G and 5A). Interestingly, the average intensity of the intracellular SLPI-labelling was elevated by 1.48-fold in vehicle-treated mice 12 h after spinal cord injury as compared to uninjured controls after vehicle injection (Figs 4F and 5B) suggesting a slight increase in de novo expression of SLPI or uptake or both. In contrast, the increase in fluorescence intensity per cell was almost 5-fold in recombinant mouse SLPI-treated mice at 3 and 11 h after injection (Figs 4E, G and 5B).
SLPI localizes to the nucleus of circulating leukocytes isolated after recombinant mouse SLPI treatment. In all figures, ‘PI’ (red) shows the 3D reconstruction of the nucleus (labelled with propidium iodide), ‘SLPI’ (green) shows the total intracellular SLPI immunoreactivity, and ‘nSLPI’ (yellow) shows the SLPI immunoreactivity within the nucleus only. (A) A very small amount of SLPI is localized to the nucleus of leukocytes obtained from mice prior to contusion injury. (B, D and F) Vehicle-treated mice at 1, 3 and 11 h after injection show little change in nuclear SLPI. (C, E and G) In contrast, there is a marked increase in total intracellular and nuclear SLPI in leukocytes obtained from recombinant mouse SLPI-treated mice as early as 3 h and up to 11 h after injection. Vehicle and recombinant mouse SLPI were injected 1 h after spinal cord injury. All scale bars = 10 µm.
Quantification of SLPI expression in leukocytes. (A) The percentage of SLPI-immunoreactive leukocytes isolated from the tail vein prior to injury (pre-Injury) and at 1, 3 and 11 h after intraperitoneal injection of recombinant mouse SLPI or vehicle (n = 3). There is an increase in SLPI+ leukocytes after injury in recombinant SLPI- and vehicle-treated mice (all times significantly increased relative to pre-Injury; P < 0.05). However, the percentage of SLPI+ leukocytes is significantly higher in recombinant SLPI-treated mice, relative to vehicle-treated controls at all time-points. (B) Quantification of the intensity of SLPI immunofluorescence measured by confocal microscopy in over 150 cells per group per time-point shows that SLPI intensity is significantly higher in recombinant SLPI-treated mice as compared to vehicle-treated controls at all time-points examined (P < 0.05). There is a 96% increase in SLPI intensity as early as 1 h after recombinant SLPI-treatment. This intensity increases to about 3.2-fold of control at 11 h after treatment. *P < 0.05 versus pre-injury and **P < 0.05 at specific time-points; >150 cells analysed in each of three mice per group (n = 3 per group).
Based on 3D analysis of confocal images, a substantial portion of SLPI immunoreactivity was localized in the nucleus of leukocytes, beginning as early as 3 h after recombinant mouse SLPI injection (Fig. 4E). The increase in SLPI localized to the nucleus was maintained at the 11 h time-point in mice treated with recombinant mouse SLPI (Fig. 4G). Vehicle-treated mice, on the other hand, showed SLPI localization primarily in the cytoplasm (Fig. 4B, D and F).
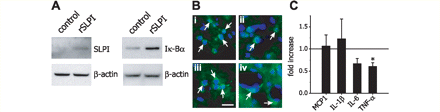
Recombinant mouse SLPI treatment results in increased SLPI and Iκ-Bα and reduced TNF-α expression in the injured spinal cord
Recombinant SLPI also appeared to enter the spinal cord tissue after spinal cord injury as western blot analysis of spinal cord tissue taken 12 h after spinal cord injury from the same mice used for the leukocyte analysis described above showed increased SLPI in two out of the three recombinant mouse SLPI-treated mice as compared to vehicle-treated controls. In these two mice there was a 2-fold increase in SLPI (Fig. 6A; n = 3 per group). This increase in SLPI may be due in part to the presence in the spinal cord of leukocytes which have internalized recombinant mouse SLPI, as SLPI+ small, round cell that appear to be leukocytes could be detected within the lesioned dorsal part of the spinal cord 12 h after recombinant mouse SLPI injection and spinal cord injury (Fig. 6B). In addition, entry of recombinant mouse SLPI into the injured spinal cord due to disruption of the blood–brain barrier cannot be ruled out as some diffuse SLPI staining could also be detected in the injured tissue at this time. These results suggest that recombinant mouse SLPI is taken up by leukocytes and localizes to the nucleus, and that higher levels of SLPI can be detected in the lesioned spinal cord after recombinant mouse SLPI treatment. We therefore examined whether recombinant mouse SLPI treatment reduces NF-κB activation and pro-inflammatory cytokine expression that can modulate the inflammatory response after spinal cord injury. We examined NF-κB activity by assessing Iκ-Bα levels in the injured spinal cord tissue from mice 11 h after injection of recombinant mouse SLPI or vehicle (n = 3 per group). Iκ-Bα exerts its effects by inhibiting nuclear localization signals of NF-κB, keeping the transcription factor masked and sequestered in the cytoplasm (Natoli and Chiocca, 2008). Western blot analysis revealed a 65% increase in Iκ-Bα in spinal cord homogenates in recombinant mouse SLPI-treated mice, as compared to vehicle-injected controls (n = 3; *P < 0.02) (Fig. 6A).
Altered SLPI, Iκ-Bα, and cytokine levels in the spinal cord of recombinant mouse SLPI-treated mice after spinal cord injury. (A) Western blots for SLPI and Iκ-Bα show increased levels of both proteins in the spinal cord of mice 12 and 24 h, respectively, after spinal cord injury in mice injected with recombinant mouse SLPI, as compared to vehicle-treated controls. β-actin is used as a loading control (n = 3 per group). (B) Micrographs of small rounded SLPI+ cells ∼10–12 μm in diameter (arrows in panels i–iii) that appear to be leukocytes located in the dorsal injured part of the spinal cord from mice 12 h after recombinant mouse SLPI injection and spinal cord injury. In panel (iv) SLPI+ cells (arrows) are seen on the outer side of blood vessels that may be cells that have migrated out of the circulation. Scale bar = 15 µm. (C) Quantitative real-time PCR analysis of mRNA expression of pro-inflammatory chemokine and cytokine expression 12 h after spinal cord injury shows a significant reduction in TNF-α mRNA levels (*P < 0.05; n = 3 per group) in mice treated with recombinant mouse SLPI. No changes are seen in monocyte chemotactic protein-1, IL-6 and IL-1β mRNA levels (n = 3 per group). Data represent fold increase over vehicle-treated controls (horizontal line) also taken 12 h after spinal cord injury.
We next examined the expression of four pro-inflammatory chemokines and cytokines known to be regulated by SLPI and NF-κB, namely monocyte chemotactic protein-1, IL-1β, IL-6 and TNF-α (Libermann and Baltimore, 1990; Ohlsson et al., 1996; Jin et al., 1997; Ueda et al., 1997; Greene et al., 2004) in the spinal cord 12 h after spinal cord injury in mice treated with recombinant mouse SLPI or vehicle. TNF-α mRNA levels were significantly reduced by 40% (*P < 0.05) as compared to vehicle-treated control mice as detected by quantitative real-time PCR (Fig. 6C). The expression of monocyte chemotactic protein-1, IL-6 and IL-1β were unchanged. Our results indicate that treatment with exogenous SLPI reduces expression of a key pro-inflammatory cytokine shown to have detrimental effects after spinal cord injury (Lee et al., 2000; Pearse et al., 2004; Ferguson et al., 2008; Genovese et al., 2008).
Discussion
Previous studies have demonstrated an important role for SLPI in reducing the pro-inflammatory response in non-CNS tissues in vitro and in vivo (Jin et al., 1997; Sallenave et al., 1997; Zhang et al., 1997; Song et al., 1999; Ashcroft et al., 2000; Taggart et al., 2005). These anti-inflammatory effects of SLPI are primarily mediated via its direct inhibition of NF-κB activity (Taggart et al., 2005), which leads to reduced cytokine/chemokine production. We now demonstrate an important role for SLPI in mediating tissue sparing after spinal cord injury. Using both SLPI over-expressing mice and wild-type mice treated with recombinant mouse SLPI, we show that SLPI has a beneficial effect in the early phase after spinal cord injury (1 week). We show that systemically delivered recombinant SLPI translocates to the nucleus of circulating leukocytes, where it can be detected for at least 11 h after injection. Moreover, in mice treated with recombinant mouse SLPI, increased SLPI is detected in the injured spinal cord, and is associated with an increase in Iκ-Bα levels and reduced TNF-α expression in the injured tissue. These changes are accompanied with a significant improvement in locomotor recovery and reduction in secondary tissue damage. The latter include increased sparing of spinal cord tissue, of ventral horn neurons, and myelin; and increased serotonergic innervation of the ventral horn caudal to the lesion at 28 days after spinal cord injury.
After inflammation in peripheral tissues, macrophages and neutrophils are the major immune cells expressing SLPI (Jin et al., 1997; Sallenave et al., 1997; Zhang et al., 1997; Taggart et al., 2002). In the spinal cord, however, we show that reactive astrocytes and neutrophils express SLPI after injury. Macrophages, which had previously been shown in other non-CNS injury and disease models to express high levels of SLPI (Jin et al., 1998; Song et al., 1999; Thuraisingam et al., 2006) did not express SLPI in the injured spinal cord. The same was true for activated microglia at and near the site of spinal cord injury. However, neurons in the injured spinal cord grey matter expressed increased levels of SLPI. A somewhat similar expression pattern was also seen after cerebral ischaemia, namely that macrophages in the necrotic core did not express SLPI but astrocytes and neurons expressed SLPI (Wang et al., 2003). In contrast, in experimental autoimmune encephalomyelitis, the mouse model of multiple sclerosis, SLPI was expressed by astrocytes, as well as by activated microglia/macrophages and neurons at the first peak of the clinical attack (Mueller et al., 2008). The mechanisms controlling the differential regulation of SLPI expression in microglia/macrophages under different inflammatory conditions are not known at present. However, the lack of expression of SLPI by microglia/macrophages in the injured spinal cord may make them more pro-inflammatory and increase their cytotoxic potential, which may contribute to the secondary tissue damage after spinal cord injury. In contrast, macrophages that enter the injured cord in mice treated with recombinant mouse SLPI may be less pro-inflammatory, as SLPI was shown to be internalized by circulating leukocytes and SLPI+ leukocytes could be found in the injured cord. The entry of recombinant mouse SLPI into the spinal cord after injury due to breakdown of the blood–brain barrier may also have an effect on astrocytes and neurons, which are known to express various pro-inflammatory cytokines after spinal cord injury (Brambilla et al., 2005; Pineau and Lacroix, 2007; de Rivero Vaccari et al., 2008).
SLPI was expressed by neutrophils located within the injured spinal cord and in the overlying meninges, with the percentage of SLPI+ neutrophils being lower in the spinal cord parenchyma. Neutrophils have been shown to secrete a variety of pro-inflammatory mediators in the injured CNS, including chemokines/cytokines (Tonai et al., 2001; Gorio et al., 2007) and proteases such as matrix metalloproteinases and elastases (Taoka et al., 1997; Tonai et al., 2001; Noble et al., 2002). These proteases released by neutrophils help them to migrate into the tissue; however, such proteases may also have unwanted by-stander cytotoxic effects in the context of spinal cord injury. The neutrophil oxidative burst, an important anti-microbial mechanism, results in the rapid production of free radicals—an important mediator of secondary tissue damage after CNS injury (Bao et al., 2004; Xiong et al., 2007; Rathore et al., 2008). The inhibition of protease activity by SLPI may thus help to reduce the harmful effects of neutrophils in the injured spinal cord. A majority of reports indicate that a partial reduction of neutrophil influx into the injured spinal cord leads to increased tissue sparing and improved locomotor recovery after injury (Taoka et al., 1997; Tonai et al., 2001; Gorio et al., 2007). A more recent study, however, indicates that extensive depletion of neutrophils can be detrimental to locomotor recovery and tissue sparing after spinal cord injury (Stirling et al., 2009). These data suggest that neutrophils may have both detrimental and beneficial roles in the injured spinal cord. Therefore, the reduced SLPI expression by neutrophils that enter the spinal cord parenchyma, as compared to those in the meninges, may confer a cytotoxic phenotype to the cells in the spinal cord tissue. In the injured spinal cord, reactive astrocytes that line the lesion express high levels of SLPI. This may be of particular significance because SLPI has been shown to reduce NF-κB activation (Lentsch et al., 1999a, b; Taggart et al., 2002; Henriksen et al., 2004). Interestingly, a transgenic mouse with inactivation of NF-κB in astrocytes showed significantly improved locomotor recovery, reduced secondary damage, and increased axonal sparing after spinal cord injury (Brambilla et al., 2005, 2009). Several groups have reported, based on in vitro and in vivo studies, that SLPI can alter cytokine levels by suppressing NF-κB activation (Jin et al., 1997; Lentsch et al., 1999a; Song et al., 1999). More recently, Taggart and colleagues reported that SLPI is rapidly internalized in less than 1 h by monocytes in vitro, and translocated into the nucleus. In the nucleus, SLPI binds directly to the p65 subunit of NF-κB, which leads to reduced expression of pro-inflammatory cytokines, such as TNF-α (Taggart et al., 2005). We have now extended these in vitro findings to an in vivo model, and show that recombinant mouse SLPI delivered systemically is taken up by leukocytes in the peripheral circulation, and then translocates to the nucleus of these cells. We also show that the systemically administered recombinant mouse SLPI enters the injured spinal cord and increases Iκ-Bα levels which is reciprocally related to reduced NF-κB activation (Beg et al., 1992; Henkel et al., 1993). The increased SLPI in the injured cord after intraperitoneal injection might be due in part to the presence of SLPI+ leukocytes in the injured cord and to the direct entry of injected recombinant mouse SLPI into the CNS tissue due to breakdown of the blood–brain barrier. This would suggest that cells that take up the recombinant mouse SLPI within the injured spinal cord may be less pro-inflammatory and cytotoxic, which may underlie the reduced secondary tissue damage and improved locomotor recovery. Our finding that recombinant mouse SLPI treatment reduces TNF-α expression in the injured spinal cord 12 h after injury lends some measure of support for this possibility. There are several studies that point to a detrimental role for TNF-α after spinal cord injury and CNS damage. TNF-α has been shown to mediate neuronal cell death in vitro and in vivo via N-methyl-d-aspartic acid- and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-mediated mechanisms (Hermann et al., 2001; Ferguson et al., 2008; Lebrun-Julien et al., 2009). Although some studies have shown beneficial effects for TNF-α in spinal cord injury (Farooque et al., 2001; Kim et al., 2001), there is evidence from studies in which blocking or reducing TNF-α signalling after spinal cord injury led to reduced cell death (Lee et al., 2000; Yune et al., 2003; Pearse et al., 2004; Genovese et al., 2008) and improved locomotor recovery (Genovese et al., 2008). Although there are some differences in spinal cord injury pathology between humans and mouse, particularly in terms of the formation of cavitation, it is worthy to note the time course of the cellular immune response (microglia macrophage and neutrophil) and the cytokine response after spinal cord injury appears to be similar in humans and rodent models (Sroga et al., 2003; Yang et al., 2004, 2005b; Fleming et al., 2006; Donnelly and Popovich, 2008; Stirling and Yong, 2008).
The differences observed in locomotor recovery in SLPI-over-expressing transgenic mice and mice treated with recombinant mouse SLPI point to a potential dual role for SLPI after spinal cord injury. Our data suggest that SLPI over-expression has a protective effect during the first 7–10 days after injury that leads to improved locomotor control, while the continued over-expression beyond this period might be detrimental. This was supported by the finding that treatment with recombinant mouse SLPI for only the first week after spinal cord injury results in improvement of locomotor recovery that is sustained for the 28 day duration of the study. We found that daily delivery of recombinant mouse SLPI for 1 week was required to produce improvement after spinal cord injury whereas two injections on Days 1 and 7 were not effective. This differs from other in vivo murine models, including arthritis and skin wound healing, in which one or two injections of recombinant mouse SLPI were effective (Song et al., 1999; Ashcroft et al., 2000; Wang et al., 2003). This may reflect the time course of neutrophil and macrophage influx, the extent of microglia/macrophage and astrocyte activation in the first week after spinal cord injury, or differences in the activity of the recombinant proteins used.
Our data suggest that SLPI can modulate the inflammatory response after spinal cord injury. We show that an increased level of SLPI in the first week after spinal cord injury has beneficial effects in terms of histological outcomes and locomotor function. The anti-inflammatory effects observed in the spinal cord may be mediated through the effects of SLPI in reducing NF-κB activation and TNF-α expression in the first few days after spinal cord injury. Our results suggest that treatment with recombinant mouse SLPI could be a useful therapy in the acute phase after spinal cord injury, for which there is currently no effective treatment.
Funding
Canadian Institutes of Health Research (CIHR) (MOP 14828) to SD, and the National Institutes of Health [GM061710 to AD. NG was the recipient of CIHR and Fonds de la recherche en santé du Québec Doctoral Studentships, and RLV a recipient of a CIHR Post-Doctoral Fellowship.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank Ourania Tsatas for technical support and Margaret Attiwell for help with the illustrations.
Abbreviations
- BMS
Basso Mouse Scale
- GFAP
glial fibrillary acidic protein
- IL
interleukin
- NF-κB
nuclear factor-κB
- PBS
phosphate buffered saline
- SLPI
secretory leukocyte protease inhibitor
- TNF
tumour necrosis factor
References
Author notes
*These authors contributed equally to this work.

![Expression of SLPI after spinal cord injury. (A) RT–PCR analysis of SLPI mRNA expression after spinal cord injury shows upregulation 1, 3 and 7 days after injury with levels returning to control [laminectomized (LAM)] levels by Day 14. Peptidylprolyl isomerase A (PPIA) is used as a control. (B) Western blot analysis also shows increased SLPI protein expression during the first week after spinal cord injury, reaching a peak at 3 days. β-actin is used as a loading control. (C) Double immunofluorescence labelling of a cross-section of the spinal cord for GFAP and SLPI. Note the increased expression of SLPI (green) in reactive astrocytes (red) bordering the lesion at 14 days post-injury (L = lesion). Note also the SLPI labelling of neuron-like cells (green) in the ventral horn (also see Supplementary Fig. 4E–G). The area outlined in the box is shown in higher magnification in panels D–F. (D–F) Higher magnification shows strong SLPI immunoreactivity (E) in reactive astrocytes (D) along the border of the lesion; the merged image is shown in panel F. (G) Double immunofluorescence labelling of spinal cord cross-section labelled for neutrophils (antibody 7/4) (red) and SLPI (green) at 1 day post-injury. Note also the SLPI labelling in the ventral horn, which appear to be neurons. (H–J) Higher magnification of the area outlined in the box in panel G is shown in H–J. Some neutrophils (H) (arrows) also express SLPI (I); the merged image is shown in panel J. The insert in J shows higher magnification of the area outlined in the dashed lines showing SLPI labelling in neutrophils. (K) Double immunofluorescence labelling of a cross-section of the spinal cord for CD11b (macrophage-1 antigen; Mac-1) and SLPI at 7 days post-injury. Note the lack of SLPI (green) expression in Mac-1+ (red) macrophages and microglia. The area outlined in the box in panel K is shown in higher magnification in panels L–N. (L–N) Mac-1+ macrophages (arrowheads) and microglia (arrows) do not express SLPI (M); the merged image is shown in panel N. The insert in N shows higher magnification of the area outlined in the dashed lines showing no SLPI labelling in macrophages. (O) Percentage of SLPI+ neutrophils in the meninges and spinal cord parenchyma at various times after spinal cord injury. Scale bars: C, G, K = 500 µm; F, J, N = 100 µm; inserts in J and N = 25 μm. A minimum of three mice were analysed for each time point for the immunofluorescence, RT–PCR and western blot analyses.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/133/1/10.1093_brain_awp304/1/m_awp304f1.gif?Expires=1716413006&Signature=g1uR7mstliVw3d3j2Mh7rEr8ytw6ard0fkWuPE10iDDn-e9txvGgnEwbTt2pmXo41U7EzXBSAH2VJpSHsTbqhre~aO5bX-GFhtZeH3vO-c53uOr7e6PtwkzGEpaxk1~mPTA8b3mN-2-e3TUTpVtahZxf7mmrFt6ovP7mZ9mIY7vhM8hzkkv2KsSiUiztLfmsSyHsOASPico8eswoyw7tewh156UUhbSJoNVZPAm~WIV8r7dCYP2~a0AEArpeksoHb5hbziTKSSPb7FcSu13nmUlGGfiFUSStF-a-Tnl2s9DsV35XznvFZ0suzm5wnI8-R6WjtjRhd3ze8QrDXtZczQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)