-
PDF
- Split View
-
Views
-
Cite
Cite
Penny A. MacDonald, Alex A. MacDonald, Ken N. Seergobin, Ruzbeh Tamjeedi, Hooman Ganjavi, Jean-Sebastien Provost, Oury Monchi, The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson’s disease: support from functional MRI, Brain, Volume 134, Issue 5, May 2011, Pages 1447–1463, https://doi.org/10.1093/brain/awr075
Close - Share Icon Share
Abstract
The central aim of our study was to elucidate functions mediated by the ventral and dorsal striatum, respectively, to better understand the cognitive effects of dopamine replacement in Parkinson’s disease. We proposed that the ventral striatum underlies general learning of stimulus associations, whereas the dorsal striatum promotes integration of various influences on selecting. In Parkinson’s disease, dopamine depletion is substantially less notable in the ventral relative to the dorsal striatum, and therefore greater improvements are expected for dorsal striatum-mediated functions with dopamine replacement. Using a simple selection task, we found that dopamine replacement impaired encoding and facilitation of consistent stimulus–stimulus relations across trials. This finding was in line with our contention that ventral striatum mediates learning stimulus associations, even when explicit feedback or reward is not provided. In contrast, dopamine replacement enhanced interference related to assimilating conflicting influences on selection across trials, consistent with our hypothesis that the dorsal striatum supports deciding in ambiguous contexts. We further confirmed these separable roles for the ventral and dorsal striatum in our selection task with healthy young volunteers using functional magnetic resonance imaging. In summary, we present a within-subject, double dissociation of the effects of dopamine replacement in patients with Parkinson’s disease for ventral striatum-mediated facilitation and dorsal striatum-mediated interference, confirmed in a separate functional magnetic resonance imaging experiment. Defining the distinct functions of the ventral and dorsal striatum will have direct clinical implications. Titration of therapy in Parkinson’s disease is generally geared towards optimizing dorsal striatum-mediated motor symptoms, possibly at the expense of ventral striatum operations, a consequence that is only beginning to be recognized. Enhanced awareness of these different processes will translate into medication strategies that take into account those symptoms that dopamine replacement might hinder, as well as improve. Here, we show impairments in learning new stimulus associations compared with improvements in integrating varied influences related to selection. Ultimately, this knowledge will lead clinicians to survey a broader range of symptoms in determining optimal therapy based on individual patient priorities.
Introduction
Learning associations among stimuli and concepts is central to our understanding of the world. Using these associations to guide behaviour, taking into account changing priorities, is an equally critical ability. Increasingly, the basal ganglia are implicated in these learning and executive functions (Monchi et al., 2001, 2004; Cools, 2006; Provost et al., 2010). Previous investigations suggest that individual segments of the basal ganglia mediate different elements of cognition. One approach for subdividing the striatum involves distinguishing the ventral from the dorsal striatum (Voorn et al., 2004; Wickens et al., 2007; Humphries and Prescott, 2010). In the present study, we define the ventral striatum as the nucleus accumbens and those portions of the caudate and putamen that are below the internal capsule. The dorsal striatum consists of the remaining portions of the caudate nuclei and putamen, constituting the bulk of these structures. In addition to subtle cyto-architectonic and neurochemical distinctions (Wickens et al., 2007), the ventral and dorsal striatum receive highly divergent glutamatergic and dopaminergic afferents. These anatomical differences likely implicate the ventral and dorsal striatum in different cognitive functions.
Previous studies sought to functionally differentiate the ventral and dorsal striatum. Atallah et al. (2007) demonstrated that stimulus–reward learning was impaired by γ-aminobutyric acid agonist or N-methyl-d-aspartic acid antagonist infusions to the ventral striatum. In a separate experiment, they showed that infusions of a γ-aminobutyric agonist to the dorsal striatum impaired a rat’s ability to consistently select a rewarded, relative to an unrewarded, arm in a Y-maze task on the basis of odour cues. However, it did not interfere with encoding the stimulus–reward relations as subsequent testing, when dorsal striatum infusions were stopped, revealed that associations were learned equally well for experimental and control animals. This study provided good evidence for distinct learning and performance functions of the ventral and dorsal striatum in non-human animals.
Such controlled dissociations are rarely achievable in human participants, given obvious ethical constraints. Goldenberg et al. (1999) reported a case of anterograde amnesia for verbal material following a left nucleus accumbens bleed despite unimpaired retrospective verbal memory, prospective and retrospective non-verbal memory, and cognitive flexibility. Conversely, lesions in the dorsal striatum impair decision making, particularly in ambiguous contexts [(Benke et al., 2003; Rieger et al., 2003; Troyer et al., 2004; Cools et al., 2006; Ell et al., 2006; Thoma et al., 2008; Yehene et al., 2008); but see (Aglioti et al., 1996; Troyer et al., 2004)]. In contrast, explicit (Vakil et al., 2000) and implicit learning [(Vakil et al., 2000; Exner et al., 2002; Schmidtke et al., 2002; Boyd and Winstein, 2004; Shin et al., 2005; Ell et al., 2006); but see (Keri et al., 2002; Vakil et al., 2004)] tend to be spared. Despite some inconsistencies, investigations of cognition in human patients with striatal lesions also suggest that the ventral striatum might be involved in encoding, whereas the dorsal striatum is critical for enacting decisions.
Functional MRI experiments show that the degree to which a motor sequence is implicitly learned correlates with activity in the ventral striatum (Reiss et al., 2005). This activity is greatest early in a novel task, dropping off as performance asymptotes (Reiss et al., 2005; Seger et al., 2010). Ventral striatum activity seems to parallel progression of learning. In contrast, the dorsal striatum is engaged in selection tasks, particularly when stimulus–response contingencies change (Rogers et al., 2000; Monchi et al., 2004, 2006; Grinband et al., 2006). Further, activity remains increased above baseline, well after sequences, categorization rules and stimulus- or response-reward relations have been acquired (Delgado et al., 2005; Reiss et al., 2005; Helie et al., 2010; Ohira et al., 2010; Seger et al., 2010). We interpret these results as suggesting that the dorsal striatum has a more primary role in decision execution than in learning.
From our survey of the literature, we hypothesized that the ventral striatum mediates learning associations between stimuli and that the dorsal striatum is implicated in performing selections. Most research aimed at understanding function of the ventral striatum has focused on its association with reward (Schultz, 2004; Delgado, 2007; Dagher and Robbins, 2009; Camara et al., 2010) and its role in processing affective information (Phan et al., 2004; Sabatinelli et al., 2007; Pessoa, 2009; Costa et al., 2010; Liang et al., 2010; Muhlberger et al., 2010). We hypothesized that the ventral striatum is an important encoding region, mediating learning associations in general, not simply the special case of reward learning. In contrast, the dorsal striatum is often linked to action selection and resolving stimulus–response conflict (Grahn et al., 2008, 2009; Yin et al., 2008; White, 2009; Balleine and O’Doherty, 2010). We proposed that it is implicated in selecting generally, even when stimulus–response couplings and motor responses are invariant.
To test our assumptions, we implemented a cognitive paradigm with conditions intended to differentiate the ventral and dorsal striatum. It consisted of a simple selection task, where associations between a repeated stimulus (i.e. a number) and a feature on which selections were based (i.e. magnitude relative to another number) were either congruent or incongruent for two consecutive events (i.e. the prime and the probe). Responding is typically faster when the association between the repeated stimulus and the relative magnitude is congruent across consecutive events relative to a control condition, where no stimuli repeat. Slower responses occur when the association between the repeated stimulus and the relative magnitude is incongruent for consecutive selections compared with the control condition (MacDonald and Joordens, 2000). This response facilitation in the congruent condition provides a straightforward measure of learning of the repeated relation across events (Park and Kanwisher, 1994; MacDonald and Joordens, 2000; Leboe et al., 2005), which we suggest is mediated by the ventral striatum. Response interference in the incongruent condition provides a measure of (i) detection; and (ii) integration of competing influences on selection, when stimulus-magnitude associations are discrepant across adjacent events (Park and Kanwisher, 1994; MacDonald and Joordens, 2000; Leboe et al., 2005). We propose that the dorsal striatum is implicated in surveying available information to enact more accurate selections and hence mediates interference. We planned to test our hypotheses about the differential reliance of performance in the congruent and incongruent conditions on the ventral and dorsal striatum, respectively, by (i) contrasting performance of patients with Parkinson’s disease ON and OFF dopamine-replacement medications compared with age-matched controls (Experiment 1); and (ii) using functional MRI in healthy young adults (Experiment 2).
Parkinson’s disease provides a model for dissociating functions of the ventral and dorsal striatum. The dopaminergic input to the ventral striatum derives from the ventral tegmental area, whereas dopaminergic innervation of the dorsal striatum arises from the substantia nigra (Haber, 2003). In Parkinson’s disease, the ventral tegmental area is relatively spared compared with the substantial cell loss that occurs in the substantia nigra at the time of clinical onset. Motor symptoms of tremor, bradykinesia and rigidity define Parkinson’s disease and are due to substantia nigra cell loss, producing dopamine deficiency and dysfunction in the dorsal striatum. This disparity is maintained as the disease progresses (Kish et al., 1988; Fearnley and Lees, 1991; McRitchie et al., 1997). Consequently, functions performed by the ventral and dorsal striatum are differentially impaired in Parkinson’s disease and by extension are dissimilarly affected by dopamine-replacement therapy. In fact, there is evidence that functions mediated by regions receiving dopaminergic input from the ventral tegmental area are impaired by dopaminergic therapy (Gotham et al., 1986, 1988; Swainson et al., 2000; Cools et al., 2001; Mehta et al., 2001, 2004; Cools, 2006). Titration of dopaminergic medications in Parkinson’s disease is aimed at normalizing dorsal striatum-mediated motor functions. It has been proposed that the less dopamine-depleted ventral striatum could be overdosed inadvertently, actually worsening ventral striatum operations that might be unimpaired at baseline (Gotham et al., 1986, 1988; Swainson et al., 2000; Cools, 2006).
For Experiment 1, we predicted that the ventral striatum-mediated facilitation in the congruent condition would be unchanged or reduced with dopamine replacement. In contrast, we expected that the dorsal striatum-mediated interference in the incongruent condition would be decreased for patients with Parkinson’s disease relative to healthy controls OFF medication, and that dopaminergic medication would enhance and therefore normalize interference.
Our next aim was to provide converging support for the findings obtained in Experiment 1. Using functional MRI, in Experiment 2, we could directly examine our contention that the ventral striatum mediates facilitation in the congruent condition and that the dorsal striatum underlies interference in the incongruent condition.
Experiment 1: contrasting facilitation and interference in patients with Parkinson’s disease ON and OFF dopamine-replacement therapy
Materials and methods
Participants
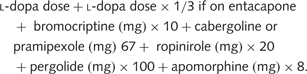
Experiment 1—demographics and clinical information, as well as screening cognitive and affective measures for patients with Parkinson’s disease and controls
| Group . | n . | Age . | Education . | Years of disease . | l-dopa (mg) . | DA (n) . | UPDRS ON . | UPDRS OFF . | ANART IQ . | BDI-II ON . | BDI-II OFF . | Apathy . | F-words . | Recall . | Clock . | Cube . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parkinson’s disease | 22 | 63.18 (2.00) | 13.82 (0.87) | 5.16 (1.27) | 480 (65.31) | 6 | 17.22 (1.60) | 22.36 (1.89) | 120.34 (1.81) | 7.55 (1.22) | 9.15 (1.63) | 10.68 (1.33) | 10.86 (1.83) | 6.27 (0.60) | 3 (0) | 1 (0) |
| Control | 22 | 62.27 (1.63) | 12.86 (0.65) | – | – | – | – | – | 121.69 (1.49) | 2.77 (0.77) | 3.16 (0.86) | 9.64 (1.06) | 14.31 (1.21) | 7.45 (0.68) | 3 (0) | 1 (0) |
| Group . | n . | Age . | Education . | Years of disease . | l-dopa (mg) . | DA (n) . | UPDRS ON . | UPDRS OFF . | ANART IQ . | BDI-II ON . | BDI-II OFF . | Apathy . | F-words . | Recall . | Clock . | Cube . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parkinson’s disease | 22 | 63.18 (2.00) | 13.82 (0.87) | 5.16 (1.27) | 480 (65.31) | 6 | 17.22 (1.60) | 22.36 (1.89) | 120.34 (1.81) | 7.55 (1.22) | 9.15 (1.63) | 10.68 (1.33) | 10.86 (1.83) | 6.27 (0.60) | 3 (0) | 1 (0) |
| Control | 22 | 62.27 (1.63) | 12.86 (0.65) | – | – | – | – | – | 121.69 (1.49) | 2.77 (0.77) | 3.16 (0.86) | 9.64 (1.06) | 14.31 (1.21) | 7.45 (0.68) | 3 (0) | 1 (0) |
Values are presented as group means (SEM). Screening cognitive and affective measures were completed by patients on medication unless indicated otherwise. Control participants did not receive dopaminergic therapy during any session of the experiment. Their data are presented here to correspond to the ON–OFF order of the patient with Parkinson’s disease to whom they were matched.
ANART IQ= National Adult Reading Test (Nelson and Willison, 1991) IQ estimation; Apathy= Apathy Evaluation Scale score; BDI-II OFF= Beck Depression Inventory II score measured for patients with Parkinson’s disease while they abstained from their usual dopamine-replacement therapy and for control participants during the session that corresponded to the OFF session of the patient with Parkinson’s disease to whom they were matched; BDI-II ON= Beck Depression Inventory II score measured for patients with Parkinson’s disease while they were treated with their usual dopamine-replacement therapy and for control participants during the session that corresponded to the ON session of the patient with Parkinson’s disease to whom they were matched; Clock= score on clock drawing component of Montreal Cognitive Assessment (MOCA); Cube= score on cube copying component of MOCA; DA= number of patients taking dopamine agonists; Education= years of education; F-words= number of words beginning with the letter F generated in 1 min; l-dopa= daily l-dopa equivalent dose in milligram; Recall= number of words recalled out of 15 after 30 min delay; UPDRS OFF= Unified Parkinson’s Disease Rating Scale motor score OFF medication; UPDRS ON= Unified Parkinson’s Disease Rating Scale motor score ON medication; Years of disease= years since diagnosis of Parkinson’s disease.
Experiment 1—demographics and clinical information, as well as screening cognitive and affective measures for patients with Parkinson’s disease and controls
| Group . | n . | Age . | Education . | Years of disease . | l-dopa (mg) . | DA (n) . | UPDRS ON . | UPDRS OFF . | ANART IQ . | BDI-II ON . | BDI-II OFF . | Apathy . | F-words . | Recall . | Clock . | Cube . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parkinson’s disease | 22 | 63.18 (2.00) | 13.82 (0.87) | 5.16 (1.27) | 480 (65.31) | 6 | 17.22 (1.60) | 22.36 (1.89) | 120.34 (1.81) | 7.55 (1.22) | 9.15 (1.63) | 10.68 (1.33) | 10.86 (1.83) | 6.27 (0.60) | 3 (0) | 1 (0) |
| Control | 22 | 62.27 (1.63) | 12.86 (0.65) | – | – | – | – | – | 121.69 (1.49) | 2.77 (0.77) | 3.16 (0.86) | 9.64 (1.06) | 14.31 (1.21) | 7.45 (0.68) | 3 (0) | 1 (0) |
| Group . | n . | Age . | Education . | Years of disease . | l-dopa (mg) . | DA (n) . | UPDRS ON . | UPDRS OFF . | ANART IQ . | BDI-II ON . | BDI-II OFF . | Apathy . | F-words . | Recall . | Clock . | Cube . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parkinson’s disease | 22 | 63.18 (2.00) | 13.82 (0.87) | 5.16 (1.27) | 480 (65.31) | 6 | 17.22 (1.60) | 22.36 (1.89) | 120.34 (1.81) | 7.55 (1.22) | 9.15 (1.63) | 10.68 (1.33) | 10.86 (1.83) | 6.27 (0.60) | 3 (0) | 1 (0) |
| Control | 22 | 62.27 (1.63) | 12.86 (0.65) | – | – | – | – | – | 121.69 (1.49) | 2.77 (0.77) | 3.16 (0.86) | 9.64 (1.06) | 14.31 (1.21) | 7.45 (0.68) | 3 (0) | 1 (0) |
Values are presented as group means (SEM). Screening cognitive and affective measures were completed by patients on medication unless indicated otherwise. Control participants did not receive dopaminergic therapy during any session of the experiment. Their data are presented here to correspond to the ON–OFF order of the patient with Parkinson’s disease to whom they were matched.
ANART IQ= National Adult Reading Test (Nelson and Willison, 1991) IQ estimation; Apathy= Apathy Evaluation Scale score; BDI-II OFF= Beck Depression Inventory II score measured for patients with Parkinson’s disease while they abstained from their usual dopamine-replacement therapy and for control participants during the session that corresponded to the OFF session of the patient with Parkinson’s disease to whom they were matched; BDI-II ON= Beck Depression Inventory II score measured for patients with Parkinson’s disease while they were treated with their usual dopamine-replacement therapy and for control participants during the session that corresponded to the ON session of the patient with Parkinson’s disease to whom they were matched; Clock= score on clock drawing component of Montreal Cognitive Assessment (MOCA); Cube= score on cube copying component of MOCA; DA= number of patients taking dopamine agonists; Education= years of education; F-words= number of words beginning with the letter F generated in 1 min; l-dopa= daily l-dopa equivalent dose in milligram; Recall= number of words recalled out of 15 after 30 min delay; UPDRS OFF= Unified Parkinson’s Disease Rating Scale motor score OFF medication; UPDRS ON= Unified Parkinson’s Disease Rating Scale motor score ON medication; Years of disease= years since diagnosis of Parkinson’s disease.
There were no statistically significant demographic differences between patients with Parkinson’s disease and controls and all participants’ performance on screening cognitive measures confirmed that they were not clinically cognitively impaired. Patients scored significantly higher on the Beck Depression Inventory II than controls. However, there were no differences ON or OFF medication for patients with Parkinson’s disease in terms of the depressive symptoms endorsed.
Apparatus
The experiment was conducted on a 12.1 widescreen laptop (Lenovo X201) running at a resolution of 1280 × 800 on the Windows 7 operating system. A secondary millisecond keyboard (Razor Arctosa) was employed for all responses. The screen was angled for optimal viewing at a distance of ∼50 cm.
Experimental design and procedure
All patients performed a simple selection task, during which they repeatedly chose either the smaller or larger number in a pair depending on a simultaneously presented cue. All patients performed the task both ON and OFF dopamine-replacement therapy on two consecutive days. The ON–OFF order was counterbalanced across patients with half performing the task first ON medication and the other half performing the first session OFF treatment. During ON testing sessions, patients with Parkinson’s disease took their dopamine-replacement medication as prescribed by their treating neurologist. During OFF testing sessions, patients with Parkinson’s disease abstained from dopamine-replacement therapy for a minimum of 12 and a maximum of 18 h prior to testing. Control participants also performed the selection task on two consecutive days. Although control patients did not take dopamine-replacement medication during either testing session, their data were analysed to parallel the ON–OFF order of the patient with Parkinson’s disease to whom they were matched. For example, for participants acting as controls for patients with Parkinson’s disease who were tested first ON, then OFF dopamine-replacement medication, their data obtained in the first testing session were treated as ON results, whereas the data from the second testing session were regarded as OFF findings. Therefore, we controlled for order, fatigue and possible practice effects.
During both ON and OFF testing sessions, participants performed 576 number selections, which were organized into 288 number-selection couples as explained below. Number selections were separated by rest periods into four equal blocks. Participants received 10 practice trials before each experimental session to ensure that they understood the task. All number selections proceeded as follows: (i) four crosses appeared in the centre of a computer screen for 500 ms; (ii) a blank screen was presented for 500 ms; (iii) two number words were presented in the centre of the computer screen, one above the other, surrounded by a large or small box; (iv) the participant spoke his/her response into a microphone, stopping the timer; (v) the number stimuli disappeared from the screen; and (vi) a blank screen appeared for 500 ms while the experimenter coded the accuracy of the response. On half of the number selections, the box was small, with thin lines, instructing the participant to read aloud the smaller number in the pair. In the other half of the selections, the box was large, with thick lines, cueing the participant to read aloud the larger number in the pair. Participants were asked to respond as quickly, yet as accurately as possible. Response times were calculated as the time of the spoken response minus the onset of the number pair in milliseconds. An experimenter coded the accuracy of responses online. All sessions were recorded and reviewed to ensure coding accuracy.
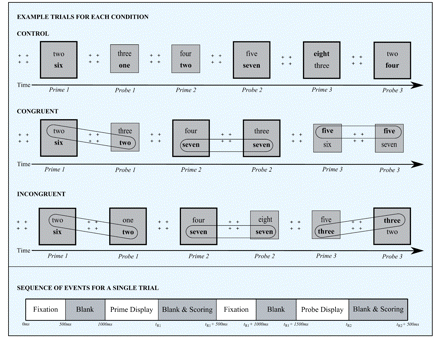
The numbers ‘one’ through to ‘eight’ were presented repeatedly, in pairs throughout the experiment. Although from the participants’ perspective the task comprised recurring, independent, randomly-ordered number pairings and comparisons, trials were actually organized into prime–probe couples to create the conditions that we expected would differentially engage the ventral and dorsal striatum. In the control condition, no numbers were repeated between a prime event and a subsequent probe event (e.g. ‘two’ and ‘six’, choose larger, respond ‘six’, followed by ‘one’ and ‘three’, choose smaller, respond ‘one’). This provided a baseline measure of speed and accuracy for judgements of relative magnitude of numbers. The top row in Fig. 1 presents three consecutive control trials, each comprising a prime and a probe event. The target number that matches the selection criterion, which is designated by the size of the surrounding box (i.e. large or small), is presented in bold font in all figures. In the congruent condition, one number was repeated and was consistently either the smaller or larger number across the prime–probe couple (e.g. ‘two’ and ‘six’, choose larger, respond ‘six’, followed by ‘three’ and ‘two’, choose smaller, respond ‘two’). In the example, the repeated item (i.e. ‘two’) is consistently the smaller number on both the prime and probe events. The second row in Fig. 1 presents three consecutive congruent trials, each comprising a prime and a probe event. The repeated number is circled to highlight its consistent relative magnitude (i.e. smaller or larger) across prime and probe events. In the incongruent condition, one number was repeated and was inconsistent in terms of its magnitude from the prime to the probe event (e.g. ‘two’ and ‘six’, choose larger, respond ‘six’, followed by ‘one’ and ‘two’, choose larger, respond ‘two’). In the example, the repeated item (i.e. ‘two’) is first the smaller and then the larger number in a pair. The third row in Fig. 1 presents three consecutive incongruent trials, each comprising a prime and a probe. The repeated number is circled to draw attention to its inconsistent relative magnitude across these consecutive events. Figure 1 also presents the sequence of events that constitute a single, complete trial in Experiment 1.
Three consecutive control (top), congruent (middle) and incongruent (bottom) trials, each consisting of a prime and probe event, are presented. Four plus signs were presented to orient the participant’s attention to the centre of the computer screen, prior to each event. A large box with thick lines signalled that the larger number in the pair was the target. A small box with thin lines indicated that the smaller number in the pair was the target. Participants were instructed to read aloud the target number, for each event, as quickly yet as accurately as possible. For illustrative purposes in the figure, the target is presented in bold, whereas the distracter appears in regular font. In the control condition, no numbers repeated from the prime to the probe event. In the congruent condition, a number repeated from the prime to the probe event and was consistent in terms of its relative magnitude (i.e. larger or smaller). In the incongruent condition, a number repeated from the prime to the probe event and was inconsistent in terms of its relative magnitude. To highlight the relation between the prime and the probe events in the congruent and incongruent conditions, we have circled the repeated item in the figure. Below the example trials for each of the conditions, we present a timeline showing the sequence and durations of events for a single trial. Each trial began with a fixation stimulus (i.e. four plus signs) for 500 ms, followed by a blank screen for 500 ms. A pair of numbers was presented one above the other, within a large or a small box, constituting the prime event. The stimuli remained on the screen until the participant gave a response into a microphone, ending the timer. A blank screen was presented for 500 ms during which the experimenter scored the participant’s response. A fixation stimulus and a blank screen were presented again, each for 500 ms, prior to the probe event, which consisted of two numbers one above the other within a large or a small box. The probe display ended when the participant gave a response into a microphone. A blank screen occurred during which the experimenter scored performance on the probe event.
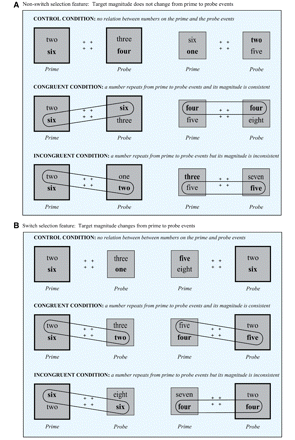
In both ON and OFF testing sessions, there were 96 control, 96 congruent and 96 incongruent trials. As previously mentioned, each session was divided into four blocks of trials separated by rest breaks, with 24 control, 24 congruent and 24 incongruent trials per block. For both the congruent and incongruent trial types, half of the time, the repeated number was the distracter (i.e. the number not matching the selection criterion) during the prime and the target (i.e. the number matching the selection criterion) on the probe. For the other half of the trials, the repeated number was the target on both the prime and the probe events. In all conditions, on 50% of trials, the selection criterion (i.e. the instruction to choose the smaller or the larger number) remained the same and 50% of the time, it switched across prime and probe events. Figure 2 presents examples of all possible control, congruent and incongruent trial configurations. Figure 2A presents examples of trials for each condition on which the selection criterion remains the same (i.e. non-switch trials), whereas Fig. 2B presents examples of trials for each condition on which the selection criterion changes (i.e. switch trials) from prime to probe.
(A) presents control (top), congruent (middle) and incongruent (bottom) trials on which the selection criterion remains the same from the prime to the probe event. (B) presents control (top), congruent (middle) and incongruent (bottom) trials on which the selection criterion changes from the prime to the probe event. A large box with thick lines indicated that the larger number in the pair was the target. A small box with thin lines indicated that the smaller number in the pair was the target. For illustrative purposes in the figure, the target is presented in bold, whereas the distracter appears in regular font. In the control condition, no numbers repeated from the prime to the probe event. In the congruent condition, a number repeated from the prime to the probe event and was consistent in terms of its relative magnitude (i.e. larger or smaller). In the incongruent condition, a number repeated from the prime to the probe event and was inconsistent in terms of its relative magnitude. To highlight the relation between the prime and the probe events in the congruent and incongruent conditions, we have circled the repeated item in the figure.
The numbers ‘two’ through to ‘seven’ appeared ∼50% of the time in the pairs across the entire experiment and during each of the four blocks of trials. By presenting items ∼50% of the time in a pair in each block of trials, we reduced the possibility that a number could be more often associated with a particular magnitude early in the experiment and then more consistently associated with the opposite magnitude later on. This constraint minimized the emergence of global number-magnitude associations, to maximize the effect of our local experimental manipulations between the prime and probe events in each condition. Trials from all conditions, each comprising a prime and probe event, were presented randomly. We could not entirely ensure that no number-magnitude biases that might oppose our experimental manipulation would be inadvertently introduced through trial randomization. Further, we could not eliminate pre-existing number-magnitude associations. To mitigate effects of biases and increase confidence that our results arose due to our experimental manipulations, as opposed to a peculiarity of trial sequencing or number pairing, the random sequence of trials generated for each patient with Parkinson’s disease was used for testing his or her matched control during both sessions. Finally, because selecting between two numbers that are closer in value (e.g. between ‘two’ and ‘three’ versus between ‘two’ and ‘five’) is more difficult, leading to higher error rates and longer response times (Holloway and Ansari, 2010), the proportion of probe events with magnitude differences of one, two and three between target and distracter numbers, was equal across conditions.
Data analysis
In Experiment 1, only response times for probe events arising on correctly performed trials—requiring accurate performance on both the prime and the probe—were included in the analyses. Facilitation scores were calculated as the mean response times for probe events in the congruent condition minus the mean response times for probe decisions in the control condition. Interference was calculated as the mean response times measured on probe events in the incongruent condition minus mean response times for probe events in the control condition. Parkinson's; disease patients facilitation and interference scores from the ON versus OFF dopamine-replacement sessions were contrasted. Analogous two-tailed t-tests were performed for control participants’ facilitation and interference scores. Their scores across sessions were treated as ON versus OFF data to parallel the order of the patient with Parkinson’s disease to whom they were matched, although controls were not treated with dopaminergic therapy in either session of the experiment.
Results
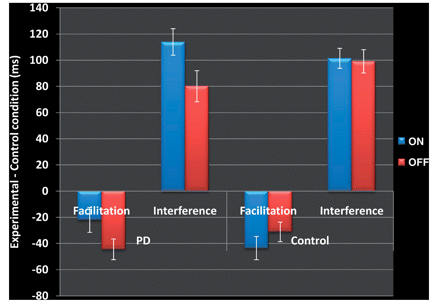
The mean response times for probe events, in each condition of both sessions, for patients with Parkinson’s disease and control participants, are presented in Table 2. The error rates for probe events in each condition of both sessions that followed correctly performed prime events also appear in this table. Significant facilitation and interference was obtained for both groups, in all sessions. Paired t-tests (two-tailed) revealed significantly less facilitation for patients with Parkinson’s disease ON relative to OFF dopamine-replacement therapy (t = −2.41, df= 21, P < 0.025). In contrast, interference was significantly greater for patients with Parkinson’s disease ON relative to OFF dopaminergic medication (t = 2.61, df = 21, P < 0.020). The between-session contrasts for control participants, analysed to parallel the ON–OFF sessions of the patients with Parkinson’s disease to whom they were matched, were not significant for facilitation (t = −1.15, df = 21, P > 0.250) or for interference (t = 0.246, df = 21, P > 0.800). Figure 3 presents the facilitation and interference scores for patients with Parkinson’s disease and control participants for each experimental session.
Experiment 1. Interference and facilitation for patients with Parkinson’s disease and control participants in both experimental sessions. Facilitation is calculated as mean response time in the congruent condition minus mean response time in the control condition, presented in milliseconds. Interference is calculated as mean response time in the incongruent condition minus mean response time in the control condition, presented in milliseconds. Facilitation and interference for patients with Parkinson’s disease measured while they were ON (in blue) versus OFF (in red) dopaminergic medication are presented. Although control participants did not receive dopaminergic therapy during either experimental session, their data are presented to correspond to the ON (in blue) versus OFF (in red) session of the patient with Parkinson’s disease to whom they were matched. Error bars represent SEM.
Experiment 1—mean response times (SEM) and error rates (SEM) in congruent, incongruent and control conditions for patients with Parkinson’s disease and control participants in both experimental sessions
| . | Switch congruent . | Switch incongruent . | Switch control . | Non-switch congruent . | Non-switch incongruent . | Non-switch control . |
|---|---|---|---|---|---|---|
| Parkinson’s disease | ||||||
| ON | ||||||
| Response time (SEM) | 1382.29 (43.7) | 1512.06 (49.36) | 1376.12 (44.70) | 1289.87 (44.79) | 1432.01 (43.03) | 1340.04 (43.42) |
| Error rate (SEM) | 0.051 (0.011) | 0.060 (0.015) | 0.056 (0.013) | 0.022 (0.006) | 0.031 (0.010) | 0.045 (0.009) |
| OFF | ||||||
| Response time (SEM) | 1379.56 (44.48) | 1487.14 (48.04) | 1392.67 (44.85) | 1283.83 (44.38) | 1435.47 (47.92) | 1369.66 (44.67) |
| Error rate (SEM) | 0.046 (0.012) | 0.067 (0.007) | 0.052 (0.007) | 0.017 (0.005) | 0.037 (0.008) | 0.039 (0.009) |
| Control | ||||||
| ON | ||||||
| Response time (SEM) | 1328.70 (66.87) | 1446.54 (75.24) | 1339.16 (69.86) | 1194.49 (62.65) | 1366.65 (70.34) | 1271.12 (65.86) |
| Error rate (SEM) | 0.046 (0.010) | 0.074 (0.012) | 0.050 (0.011) | 0.010 (0.003) | 0.032 (0.009) | 0.031 (0.004) |
| OFF | ||||||
| Response time (SEM) | 1331.61 (62.20) | 1455.07 (68.22) | 1339.855 (63.35) | 1222.94 (56.34) | 1373.12 (63.28) | 1290.05 (58.99) |
| Error rate (SEM) | 0.043 (0.009) | 0.071 (0.003) | 0.045 (0.007) | 0.007 (0.003) | 0.024 (0.007) | 0.033 (0.006) |
| . | Switch congruent . | Switch incongruent . | Switch control . | Non-switch congruent . | Non-switch incongruent . | Non-switch control . |
|---|---|---|---|---|---|---|
| Parkinson’s disease | ||||||
| ON | ||||||
| Response time (SEM) | 1382.29 (43.7) | 1512.06 (49.36) | 1376.12 (44.70) | 1289.87 (44.79) | 1432.01 (43.03) | 1340.04 (43.42) |
| Error rate (SEM) | 0.051 (0.011) | 0.060 (0.015) | 0.056 (0.013) | 0.022 (0.006) | 0.031 (0.010) | 0.045 (0.009) |
| OFF | ||||||
| Response time (SEM) | 1379.56 (44.48) | 1487.14 (48.04) | 1392.67 (44.85) | 1283.83 (44.38) | 1435.47 (47.92) | 1369.66 (44.67) |
| Error rate (SEM) | 0.046 (0.012) | 0.067 (0.007) | 0.052 (0.007) | 0.017 (0.005) | 0.037 (0.008) | 0.039 (0.009) |
| Control | ||||||
| ON | ||||||
| Response time (SEM) | 1328.70 (66.87) | 1446.54 (75.24) | 1339.16 (69.86) | 1194.49 (62.65) | 1366.65 (70.34) | 1271.12 (65.86) |
| Error rate (SEM) | 0.046 (0.010) | 0.074 (0.012) | 0.050 (0.011) | 0.010 (0.003) | 0.032 (0.009) | 0.031 (0.004) |
| OFF | ||||||
| Response time (SEM) | 1331.61 (62.20) | 1455.07 (68.22) | 1339.855 (63.35) | 1222.94 (56.34) | 1373.12 (63.28) | 1290.05 (58.99) |
| Error rate (SEM) | 0.043 (0.009) | 0.071 (0.003) | 0.045 (0.007) | 0.007 (0.003) | 0.024 (0.007) | 0.033 (0.006) |
Data are presented for patients with Parkinson’s disease ON versus OFF medication. Although control participants did not receive dopamine replacement during either session, their data are presented to correspond in order to ON versus OFF sessions of the patient with Parkinson’s disease to whom they were matched.
Experiment 1—mean response times (SEM) and error rates (SEM) in congruent, incongruent and control conditions for patients with Parkinson’s disease and control participants in both experimental sessions
| . | Switch congruent . | Switch incongruent . | Switch control . | Non-switch congruent . | Non-switch incongruent . | Non-switch control . |
|---|---|---|---|---|---|---|
| Parkinson’s disease | ||||||
| ON | ||||||
| Response time (SEM) | 1382.29 (43.7) | 1512.06 (49.36) | 1376.12 (44.70) | 1289.87 (44.79) | 1432.01 (43.03) | 1340.04 (43.42) |
| Error rate (SEM) | 0.051 (0.011) | 0.060 (0.015) | 0.056 (0.013) | 0.022 (0.006) | 0.031 (0.010) | 0.045 (0.009) |
| OFF | ||||||
| Response time (SEM) | 1379.56 (44.48) | 1487.14 (48.04) | 1392.67 (44.85) | 1283.83 (44.38) | 1435.47 (47.92) | 1369.66 (44.67) |
| Error rate (SEM) | 0.046 (0.012) | 0.067 (0.007) | 0.052 (0.007) | 0.017 (0.005) | 0.037 (0.008) | 0.039 (0.009) |
| Control | ||||||
| ON | ||||||
| Response time (SEM) | 1328.70 (66.87) | 1446.54 (75.24) | 1339.16 (69.86) | 1194.49 (62.65) | 1366.65 (70.34) | 1271.12 (65.86) |
| Error rate (SEM) | 0.046 (0.010) | 0.074 (0.012) | 0.050 (0.011) | 0.010 (0.003) | 0.032 (0.009) | 0.031 (0.004) |
| OFF | ||||||
| Response time (SEM) | 1331.61 (62.20) | 1455.07 (68.22) | 1339.855 (63.35) | 1222.94 (56.34) | 1373.12 (63.28) | 1290.05 (58.99) |
| Error rate (SEM) | 0.043 (0.009) | 0.071 (0.003) | 0.045 (0.007) | 0.007 (0.003) | 0.024 (0.007) | 0.033 (0.006) |
| . | Switch congruent . | Switch incongruent . | Switch control . | Non-switch congruent . | Non-switch incongruent . | Non-switch control . |
|---|---|---|---|---|---|---|
| Parkinson’s disease | ||||||
| ON | ||||||
| Response time (SEM) | 1382.29 (43.7) | 1512.06 (49.36) | 1376.12 (44.70) | 1289.87 (44.79) | 1432.01 (43.03) | 1340.04 (43.42) |
| Error rate (SEM) | 0.051 (0.011) | 0.060 (0.015) | 0.056 (0.013) | 0.022 (0.006) | 0.031 (0.010) | 0.045 (0.009) |
| OFF | ||||||
| Response time (SEM) | 1379.56 (44.48) | 1487.14 (48.04) | 1392.67 (44.85) | 1283.83 (44.38) | 1435.47 (47.92) | 1369.66 (44.67) |
| Error rate (SEM) | 0.046 (0.012) | 0.067 (0.007) | 0.052 (0.007) | 0.017 (0.005) | 0.037 (0.008) | 0.039 (0.009) |
| Control | ||||||
| ON | ||||||
| Response time (SEM) | 1328.70 (66.87) | 1446.54 (75.24) | 1339.16 (69.86) | 1194.49 (62.65) | 1366.65 (70.34) | 1271.12 (65.86) |
| Error rate (SEM) | 0.046 (0.010) | 0.074 (0.012) | 0.050 (0.011) | 0.010 (0.003) | 0.032 (0.009) | 0.031 (0.004) |
| OFF | ||||||
| Response time (SEM) | 1331.61 (62.20) | 1455.07 (68.22) | 1339.855 (63.35) | 1222.94 (56.34) | 1373.12 (63.28) | 1290.05 (58.99) |
| Error rate (SEM) | 0.043 (0.009) | 0.071 (0.003) | 0.045 (0.007) | 0.007 (0.003) | 0.024 (0.007) | 0.033 (0.006) |
Data are presented for patients with Parkinson’s disease ON versus OFF medication. Although control participants did not receive dopamine replacement during either session, their data are presented to correspond in order to ON versus OFF sessions of the patient with Parkinson’s disease to whom they were matched.
A two-sample t-test (two-tailed) revealed a statistical trend towards less interference for patients with Parkinson’s disease OFF dopamine replacement compared with controls (t = −1.73, df = 64, P = 0.090). There were no significant differences in interference scores, comparing patients with Parkinson’s disease ON dopamine replacement to controls (t = 1.15, df = 64, P > 0.235), suggesting a normalization of interference for patients with dopamine replacement. There were no significant differences comparing facilitation scores for patients with Parkinson’s disease ON (t = −1.45, df = 64, P > 0.140), or OFF (t = 0.72, df = 64, P > 0.475) dopaminergic medication relative to controls.
Response latencies on probe trials were shorter when the repeated item was consecutively the target compared with trials on which the repeated number was first the distracter and then the target from prime to probe for both patients with Parkinson’s disease and healthy controls [F(1,20)= 4.61, Mean squared error (MSe) = 1388.58, P < 0.050] and [F(1,20)= 5.83, MSe = 1729.39, P < 0.030], respectively. This selection-repetition advantage did not interact with ON–OFF medication status in patients with Parkinson’s disease [F(1,20)= 1.20, MSe = 985.64, P > 0.280], or with order of experimental sessions for control participants made to correspond with the ON–OFF sequence of the patients with Parkinson’s disease to whom they were matched [F(1,20)< 1]. Mean response times were also longer for trials in which the selection criterion changed from prime to probe (i.e. switch trials) relative to trials in which it remained the same (i.e. non-switch trials) for patients with Parkinson’s disease and controls [F(1,20)= 30.90, MSe = 2991.06, P < 0.001] and [F(1,20)= 105.76, MSe = 1657.34, P < 0.001], respectively. The cost associated with selection-criterion changes from prime to probe did not interact with medication status for patients with Parkinson’s disease or with session order for control participants’ paralleling the ON–OFF order of their Parkinson’s disease counterpart, both F(1,20)< 1.
Discussion
Consistent with our predictions, facilitation was reduced and interference was increased by dopamine replacement for patients with Parkinson’s disease in Experiment 1. There were no significant differences in facilitation or interference scores for control participants between the two experimental sessions, refuting the possibility that the ON–OFF differences observed for patients with Parkinson’s disease resulted from practice, fatigue or testing order. Further, these results cannot be attributed to a bias in the sequence of trials, inadvertently introduced through randomization, as the random order of trials generated for each patient with Parkinson’s disease was used for testing his or her matched control.
The conditions in our experiment differed from one another in only one respect: the consistency of the number-magnitude association for stimuli that repeated across a prime and a probe event. We controlled for the effects of response repetition and selection-criterion switching from prime to probe. In the congruent and incongruent conditions, there were equal numbers of trials on which the correct response was the same versus different from prime to probe. If number-magnitude relation was consistent, even if the number read aloud on the prime differed from that read aloud on the probe (i.e. if the repeated item was first the distracter and then the target), facilitation resulted. Conversely, if number-magnitude association was inconsistent, even if the number named aloud was the same on both prime and probe (i.e. if the repeated item was the target for both events), interference occurred. Overall, latencies were shorter when the response from prime to probe was repeated. This response-repetition advantage was not differentially affected by medication status in patients with Parkinson’s disease, however. Similarly, costs owing to selection-criterion switching from prime to probe (e.g. choose the larger number then the smaller number), which were equally present in our control, congruent and incongruent conditions, were not influenced by dopamine replacement in Parkinson’s disease. In our experiment, we isolated the effect of stimulus–stimulus association consistency versus inconsistency, with the overarching aim of distinguishing two processes: (i) learning associations between stimuli; and (ii) integrating competing influences on decision making. Only this variable of number-magnitude consistency across events interacted with medication status for patients with Parkinson’s disease in Experiment 1.
Faster selections on probe events in the congruent condition of our experiment could only result from learning the repeated number-magnitude association (Park and Kanwisher, 1994; MacDonald and Joordens, 2000; MacLeod et al., 2002; Leboe et al., 2005). In this way, facilitation in the congruent relative to the control condition provided an unambiguous measure of stimulus–stimulus association learning. In Parkinson’s disease, the ventral striatum, innervated by the relatively spared ventral tegmental area, is substantially less dopamine depleted than the dorsal striatum that receives dopamine input from the significantly degenerated substantia nigra (Braak et al., 2004). Dopamine replacement generally does not improve and has been shown occasionally to impair ventral striatum functions (Swainson et al., 2000; Cools et al., 2001; Cools, 2006). Consequently, the finding that facilitation is decreased for patients with Parkinson’s disease by administration of dopaminergic medication is consistent with our hypothesis that ventral striatum mediates learning associations between stimuli. Further, in our experiment, encoding occurred independently of the provision of explicit feedback or reward, for information that has no emotional significance, expanding the realm of learning situations that depend upon ventral striatum.
A caveat, however, is that prefrontal and limbic regions also receive dopaminergic input from ventral tegmental area and therefore associated functions might also be impaired by dopamine replacement in Parkinson’s disease (Cools, 2006). To confirm that ventral striatum underlies encoding of consistent number-magnitude associations in our task and hence mediates facilitation in the congruent condition, we conducted Experiment 2.
Slower responses for number selections on the probe events in the incongruent case could only result from (i) detecting the change in number-magnitude association from prime to probe; and (ii) integrating these divergent influences prior to enacting a selection (Park and Kanwisher, 1994; MacDonald and Joordens, 2000; MacLeod et al., 2002; Leboe et al., 2005). Patients with Parkinson’s disease, tested OFF dopamine replacement, showed less interference for incongruent compared with control trials, relative to scores obtained for healthy controls. Interference increased, becoming equivalent to that observed for control participants, with dopamine replacement. The finding of heightened facilitation in the congruent condition—our un-confounded measure of stimulus–stimulus association learning—for patients with Parkinson’s disease OFF medication, undermines a claim that lesser interference at baseline for patients with Parkinson’s disease compared with healthy controls resulted due to poorer encoding of the number-magnitude association on the prime and hence reduced detection at the time of the probe. Diminished interference in the incongruent condition for patients with Parkinson’s disease OFF medication relative to controls, that is enhanced by dopamine replacement, can either suggest (i) more efficient integration of divergent influences on decision making for patients with Parkinson’s disease compared with controls at baseline with impairment of this process upon dopamine repletion; or (ii) decreased integration of various influences on decision making for patients with Parkinson’s disease compared with controls at baseline with improvement of this process upon dopamine repletion.
We favour the latter explanation of our findings. Greater errors were noted in the incongruent condition for patients with Parkinson’s disease OFF dopaminergic medication (Table 2), inconsistent with the concept of more efficient decision making for patients with Parkinson’s disease at baseline. Our study employs a common experimental strategy of creating a context where normally advantageous cognitive processes actually hamper performance, allowing measurement of these processes. We argue that interference in the incongruent case is in fact a measure of normal dorsal striatum function. This is supported by reliable interference for healthy controls. Further, it is consistent with the previous finding of reduced interference for patients with Parkinson’s disease relative to controls if the correct selection is more salient than the distracter (Cools et al., 2010). As in our contrived incongruent condition, performance appears superior for patients with Parkinson’s disease. However, when target salience relative to distracter is reduced, greater interference manifests for patients with Parkinson’s disease relative to controls, consistent with our suggestion that the dorsal striatum’s role in promoting attention to more diverse aspects of the situation prior to selection, avoiding being guided by a single, more salient dimension, confers an overall decision advantage.
Motor and cognitive functions that rely upon the dorsal striatum are impaired at baseline and improved by dopamine replacement in Parkinson’s disease [(Poewe et al., 1991; Lange et al., 1992; Lewis et al., 2005) and see (Lange et al., 1993; Cools, 2006; MacDonald and Monchi, 2011) for reviews)]. The first interpretation of our results in the incongruent condition of Experiment 1 would therefore be contradicted by the finding that interference is mediated by dorsal striatum. Experiment 2 therefore also served to entirely disambiguate the possible interpretations of our findings in the incongruent condition of Experiment 1.
Experiment 2: contrasting facilitation and interference using functional MRI
Materials and methods
Participants
Thirteen healthy, young adults participated in Experiment 2, with seven males and six females. Participants had a mean (SEM) age of 22 (1.21) with 14.38 (0.35) mean (SEM) years of education. No participants were known for neurological or psychiatric illness. All participants gave informed consent to the protocol, which was reviewed and approved by the Joint Ethics Committee of the Regroupement Neuroimagerie Québec.
Experimental design and procedures
Participants performed four to five blocks of 72 complete prime–probe trials in the scanner, after receiving 10 practice trials to ensure that they understood the task. Trials proceeded as described in Experiment 1 except that (i) the interval between the end of the probe event and the beginning of the subsequent prime was jittered randomly between 600 and 1200 ms to maximize differences in the blood oxygen level-dependent response between events and background; and (ii) number pairs remained on the screen until the experimenter scored the accuracy of participants’ spoken responses. The experimental session was recorded and all responses were reviewed for scoring accuracy. Further, precise response times were determined using Audacity audio file processing software. As in Experiment 1, response times were calculated as the onset of a spoken response minus the time of onset of the number pair in milliseconds.
The stimuli and conditions were as described in Experiment 1 except for the fact that for all congruent and incongruent trials, the repeated number was the distracter number on the prime and the target number on the probe. In all other respects, Experiments 1 and 2 were the same. Control trials on which the selection criterion remained the same from prime to probe are referred to as non-switch control trials. In contrast, control trials in which the selection criterion changed across prime–probe events are termed switch control trials.
Magnetic resonance imaging acquisition
Scanning was done in the 3 T Siemens Trio MRI with the Total Imaging Matrix technology scanner at the Functional Neuroimaging Unit of the CRIUGM. The session started with a scout for positioning the participant. This was followed by a T1 acquisition for anatomical localization. Four or five runs of T*2-weighted functional acquisitions followed, lasting ∼8.5 min each and consisting of 204 frames taken every 2.5 s. Each frame contained 36 slices placed along the anterior commissure/posterior commissure with a matrix of 64 × 64 pixels, an isotropic voxel size of 3.4 × 3.4 × 3.4 mm3. The flip angle was 90° and the echo time was 30 ms.
Functional magnetic resonance imaging analysis
The methods for data analysis were the same as those in Monchi et al. (2001, 2006) using the fmristat analysis software developed by Worsley et al. (2002). The first two frames in each run were discarded. Images from each run were realigned to the third frame for motion correction and were smoothed using a 6-mm full-width half-maximum isotropic Gaussian kernel. The statistical analysis of functional MRI data was based on a linear model with correlated errors. The design matrix of the linear model was first convolved with a difference of two gamma haemodynamic response functions timed to coincide with the acquisition of each slice. The correlation structure was modelled as an autoregressive process. At each voxel, the autocorrelation parameter was estimated from the least squares residuals, after a bias correction for correlation induced by the linear model. The autocorrelation parameter was first regularized by spatial smoothing and was then used to ‘whiten’ the data and the design matrix. The linear model was re-estimated using least squares on the whitened data to produce estimates of effects and their standard errors. The resulting effects and standard effect files were then spatially normalized by non-linear transformation into the standard proportional stereotaxic space of Talairach and Tournoux (1988) using the algorithm of Collins et al. (1994) and the ICBM152 atlas as an approximation. Anatomical images were also normalized to the Talairach space using the same transformation. In a second step, runs, sessions and subjects were combined using a mixed effects linear model. A random-effects analysis was performed by first estimating the ratio of the random effects variance to the fixed-effects variance, then regularizing this ratio by spatial smoothing with a Gaussian filter. The amount of smoothing was chosen to achieve 100 effective degrees of freedom (Worsley, 2005). Statistical maps were thresholded at P < 0.05 correcting for multiple comparisons using the minimum between a Bonferroni correction and random field theory in the single-group analysis. Peaks within the basal ganglia were considered predicted and are reported at a significance of P < 0.005 uncorrected (indicated by an asterisk in the tables).
Functional magnetic resonance imaging and behavioural data analysis
Only correctly performed probe events that followed correctly performed prime events were included in the response time and functional MRI analyses. Durations of probe events, calculated from the onset of the number pair to the spoken response, were explicitly included in the design matrix for functional MRI analysis. There were three contrasts of interest in Experiment 2: (i) congruent versus switch control; (ii) incongruent versus non-switch control; and (iii) switch control versus non-switch control. The congruent and switch control trials differed only in that a number was repeated and was consistent in terms of its number-magnitude association from prime to probe in the congruent condition whereas no numbers were repeated in the switch control condition (see Fig. 2 for examples). The incongruent and non-switch control trials differed only in that a number was repeated across prime and probe events that were inconsistent in terms of its relative magnitude in the incongruent case, whereas no numbers were repeated for adjacent selections in the non-switch control condition (see Fig. 2 for examples).
Results
Behavioural data
The mean response times for probe events in each condition are presented in Table 3. Error rates on probe events that followed correctly performed prime events in each condition also appear in this table. Because some participants performed four blocks of trials and others performed five, the means calculated in Experiment 2 were weighted by the number of responses on which they were based. Significant interference (t = 5.77, df = 12, P < 0.001), comparing the incongruent and non-switch control conditions and marginally significant facilitation (t = −2.14, df = 12, P = 0.053), for responses in the congruent relative to the switch control condition were observed. As in Experiment 1, latencies were significantly longer for switch- versus non-switch control trials (t = 2.94, df = 12, P < 0.025).
Experiment 2—mean response times (SEM) and error rates (SEM) in congruent, incongruent and control conditions
| . | Congruent . | Switch control . | Incongruent . | Non-switch control . |
|---|---|---|---|---|
| Response time (SEM) | 987.96 (50.09) | 1029.93 (63.17) | 998.39 (49.94) | 935.26 (46.71) |
| Error rate (SEM) | 0.088 (0.036) | 0.101 (0.020) | 0.033 (0.006) | 0.043 (0.010) |
| . | Congruent . | Switch control . | Incongruent . | Non-switch control . |
|---|---|---|---|---|
| Response time (SEM) | 987.96 (50.09) | 1029.93 (63.17) | 998.39 (49.94) | 935.26 (46.71) |
| Error rate (SEM) | 0.088 (0.036) | 0.101 (0.020) | 0.033 (0.006) | 0.043 (0.010) |
Experiment 2—mean response times (SEM) and error rates (SEM) in congruent, incongruent and control conditions
| . | Congruent . | Switch control . | Incongruent . | Non-switch control . |
|---|---|---|---|---|
| Response time (SEM) | 987.96 (50.09) | 1029.93 (63.17) | 998.39 (49.94) | 935.26 (46.71) |
| Error rate (SEM) | 0.088 (0.036) | 0.101 (0.020) | 0.033 (0.006) | 0.043 (0.010) |
| . | Congruent . | Switch control . | Incongruent . | Non-switch control . |
|---|---|---|---|---|
| Response time (SEM) | 987.96 (50.09) | 1029.93 (63.17) | 998.39 (49.94) | 935.26 (46.71) |
| Error rate (SEM) | 0.088 (0.036) | 0.101 (0.020) | 0.033 (0.006) | 0.043 (0.010) |
Functional magnetic resonance imaging data
Significant activation was observed in the left nucleus accumbens in the congruent relative to the switch control condition. Significantly greater activity also occurred in regions of right and left cerebellum for this comparison (Table 4, Fig. 4). In contrast, significantly increased activity was observed in the caudate nucleus bilaterally in the incongruent compared with the non-switch control condition (Table 4, Fig. 5). Finally, bilateral premotor, inferior parietal sulci, right inferior temporal gyrus and left superior parietal lobule revealed significantly greater activity for switch- compared with non-switch control trials. No peaks in either ventral or dorsal striatum arose at a threshold of P < 0.05 uncorrected for multiple comparisons (Table 4).
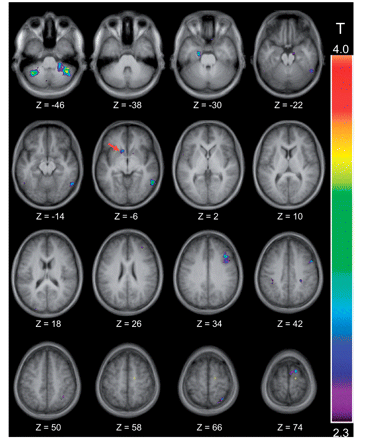
Location of the significant peaks observed in the congruent versus the switch control condition. The thresholded activation map is shown over the anatomical MRI, which is the average of the T1 acquisitions of the 13 participants transformed into stereotaxic space (ICBM152 template). Horizontal sections are shown ranging from Z = −46 to Z = 74 every 8 mm. Significant activation is observed in the left nucleus accumbens at Z = −6, which is indicated by a red arrow.
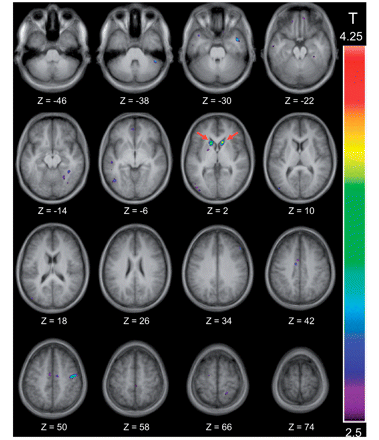
Location of the significant peaks observed in the incongruent versus the non-switch control condition. The thresholded activation map is shown over the anatomical MRI, which is the average of the T1 acquisitions of the 13 participants transformed into stereotaxic space (ICBM152 template). Horizontal sections are shown ranging from Z = −46 to Z = 74 every 8 mm. Significant activation is observed bilaterally in the caudate nucleus at Z = 2, which is indicated by two red arrows.
Experiment 2—the coordinates (x, y, z) in standard stereotaxic space and the t-values of anatomical areas of interest in all contrasts
| Anatomical area . | x, y, z . | t-stat . | Cluster size . |
|---|---|---|---|
| Congruent versus control | |||
| Right cerebellum | 38, −46, −48 | 4.29 | 2624 |
| Right cerebellum | 22, −46, −50 | 4.22 | 2624 |
| Left cerebellum | −40, −56, −48 | 3.48 | 1496 |
| Left nucleus accumbens | −12, 18, −6 | 2.94* | 176 |
| Incongruent versus control | |||
| Right caudate nucleus | 12, 22, 2 | 4.03* | 416 |
| Left caudate nucleus | −14, 18, 2 | 3.61* | 712 |
| Switch versus non-switch control trials | |||
| Right premotor | 44, 4, 36 | 4.59 | 3464 |
| Right inferior temporal gyrus | 58, −54, −8 | 4.57 | 3264 |
| Left premotor | −34, 12, 38 | 4.14 | 1840 |
| Left inferior parietal sulcus | −36, −46, 46 | 3.84 | 2960 |
| Right inferior parietal sulcus | 56, −38, 44 | 3.47 | 2720 |
| Left superior parietal lobule | −20, −72, 60 | 3.49 | 2312 |
| Anatomical area . | x, y, z . | t-stat . | Cluster size . |
|---|---|---|---|
| Congruent versus control | |||
| Right cerebellum | 38, −46, −48 | 4.29 | 2624 |
| Right cerebellum | 22, −46, −50 | 4.22 | 2624 |
| Left cerebellum | −40, −56, −48 | 3.48 | 1496 |
| Left nucleus accumbens | −12, 18, −6 | 2.94* | 176 |
| Incongruent versus control | |||
| Right caudate nucleus | 12, 22, 2 | 4.03* | 416 |
| Left caudate nucleus | −14, 18, 2 | 3.61* | 712 |
| Switch versus non-switch control trials | |||
| Right premotor | 44, 4, 36 | 4.59 | 3464 |
| Right inferior temporal gyrus | 58, −54, −8 | 4.57 | 3264 |
| Left premotor | −34, 12, 38 | 4.14 | 1840 |
| Left inferior parietal sulcus | −36, −46, 46 | 3.84 | 2960 |
| Right inferior parietal sulcus | 56, −38, 44 | 3.47 | 2720 |
| Left superior parietal lobule | −20, −72, 60 | 3.49 | 2312 |
*P < 0.005 uncorrected.
Experiment 2—the coordinates (x, y, z) in standard stereotaxic space and the t-values of anatomical areas of interest in all contrasts
| Anatomical area . | x, y, z . | t-stat . | Cluster size . |
|---|---|---|---|
| Congruent versus control | |||
| Right cerebellum | 38, −46, −48 | 4.29 | 2624 |
| Right cerebellum | 22, −46, −50 | 4.22 | 2624 |
| Left cerebellum | −40, −56, −48 | 3.48 | 1496 |
| Left nucleus accumbens | −12, 18, −6 | 2.94* | 176 |
| Incongruent versus control | |||
| Right caudate nucleus | 12, 22, 2 | 4.03* | 416 |
| Left caudate nucleus | −14, 18, 2 | 3.61* | 712 |
| Switch versus non-switch control trials | |||
| Right premotor | 44, 4, 36 | 4.59 | 3464 |
| Right inferior temporal gyrus | 58, −54, −8 | 4.57 | 3264 |
| Left premotor | −34, 12, 38 | 4.14 | 1840 |
| Left inferior parietal sulcus | −36, −46, 46 | 3.84 | 2960 |
| Right inferior parietal sulcus | 56, −38, 44 | 3.47 | 2720 |
| Left superior parietal lobule | −20, −72, 60 | 3.49 | 2312 |
| Anatomical area . | x, y, z . | t-stat . | Cluster size . |
|---|---|---|---|
| Congruent versus control | |||
| Right cerebellum | 38, −46, −48 | 4.29 | 2624 |
| Right cerebellum | 22, −46, −50 | 4.22 | 2624 |
| Left cerebellum | −40, −56, −48 | 3.48 | 1496 |
| Left nucleus accumbens | −12, 18, −6 | 2.94* | 176 |
| Incongruent versus control | |||
| Right caudate nucleus | 12, 22, 2 | 4.03* | 416 |
| Left caudate nucleus | −14, 18, 2 | 3.61* | 712 |
| Switch versus non-switch control trials | |||
| Right premotor | 44, 4, 36 | 4.59 | 3464 |
| Right inferior temporal gyrus | 58, −54, −8 | 4.57 | 3264 |
| Left premotor | −34, 12, 38 | 4.14 | 1840 |
| Left inferior parietal sulcus | −36, −46, 46 | 3.84 | 2960 |
| Right inferior parietal sulcus | 56, −38, 44 | 3.47 | 2720 |
| Left superior parietal lobule | −20, −72, 60 | 3.49 | 2312 |
*P < 0.005 uncorrected.
To be sure that no overlap existed between the ventral and dorsal striatum peaks, we lowered the threshold to a P = 0.05 uncorrected for the congruent versus the switch control and the incongruent versus the non-switch control comparisons. We performed a conjunction analysis between these contrasts. A mask created for the congruent versus the switch control contrast, at the 0.05 uncorrected threshold, revealed no activations common to the incongruent versus the non-switch control comparison; the converse was also true. The failure to uncover any significant activation in the striatum using this conjunction analysis confirms the absence of spatial overlap between the ventral striatum peak associated with facilitation and the dorsal striatum peak correlated with interference.
Discussion
Using functional MRI and our selection task, ventral striatum activation was associated with facilitation in the congruent condition, whereas more dorsal striatum was preferentially engaged in the incongruent condition. These peaks were entirely non-overlapping as confirmed by a conjunction analysis. These findings confirm the view that the ventral striatum encodes stimulus–stimulus relations in the congruent condition and that the dorsal striatum assimilates relevant influences on selection in the incongruent case. Converging with findings of Experiment 1, the dorsal striatum is not simply recruited by more attention-demanding conditions, as no regions of striatum were differentially engaged for trials on which the selection criterion changed from prime to probe, despite a significant response time cost.
General discussion
Using convergent methodologies, we achieved a within-subject, double dissociation of the ventral and dorsal striatum with different conditions of the same task in patients with Parkinson’s disease ON and OFF medication and in a functional MRI study. We proposed that the ventral striatum underlies learning stimulus–stimulus associations, yielding facilitation when number-magnitude relations are reinforced across events in our selection task. In contrast, we predicted that the dorsal striatum assimilates various influences on selection, producing interference when number-magnitude associations are incongruent across consecutive events. In Parkinson’s disease, the ventral striatum is substantially less dopamine depleted than dorsal striatum. As a result, it has been suggested that with dopamine supplementation, the ventral striatum is overdosed and its functions are impaired (Cools et al., 2001, 2006; Mehta et al., 2001, 2004; Cools, 2006), whereas the dorsal striatum becomes replete and its operations are enhanced (Poewe et al., 1991; Lange et al., 1992; Lewis et al., 2005). In line with our predictions, facilitation was reduced and interference—which at baseline was diminished relative to healthy controls’ scores—was enhanced and hence normalized by dopamine replacement for patients with Parkinson’s disease performing our selection task. Confirming our interpretation of Experiment 1 and further bolstering dissociated roles for the ventral and dorsal striatum in our task, nucleus accumbens activity correlated preferentially with facilitation and more dorsal caudate nuclei activity was related to interference in our functional MRI study.
The ventral striatum is clearly implicated in reward learning and some studies suggest that the ventral striatum even underlies selections for rewarded relative to unrewarded stimuli and responses [(Schultz, 2004; Delgado, 2007; Niv et al., 2007; Dagher and Robbins, 2009; Camara et al., 2010); see (Yin et al., 2008; Dagher and Robbins, 2009; Humphries and Prescott, 2010) for reviews]. The dorsal striatum has been implicated in response selection, response–conflict resolution and in stimulus–response and response–reward learning (Grahn et al., 2008, 2009; Yin et al., 2008; White, 2009; Balleine and O’Doherty, 2010). The current study intentionally investigated learning, independent of reward. Further, we controlled for, rather than investigated, response-repetition versus response-conflict effects. Consequently, our results neither confirm, nor refute, these previous findings. Rather, we isolated the effect of congruent versus incongruent number-magnitude relations on adjacent trials, with the aim of dissociating learning stimulus–stimulus associations from decision making where integration of divergent influences could be measured. Our results suggest that ventral and dorsal striatum underlie these different cognitive processes, consistent with a number of previous findings.
Ventral striatum in cognition
Using convergent methodologies, we show that, independent of reward, ventral striatum mediates learning-consistent stimulus–stimulus relations, on a trial-by-trial basis. We assessed ventral striatum function early in learning, before stable number-magnitude associations had been forged due to the constraint that, experiment-wide, numbers appeared equally likely as the larger and smaller items in a pair. As in our study, early in learning, the ventral striatum activity is greater for confirmation of an association relative to violation of a rule (Filoteo et al., 2005; Seger and Cincotta, 2006; Delgado, 2007; Seger et al., 2010). Treatment with dopamine supplementation also impairs sequence and probabilistic stimulus–reward learning in patients with Parkinson’s disease (Feigin et al., 2003; Shohamy et al., 2006; Torta et al., 2009; Jahanshahi et al., 2010; Seo et al., 2010). In the congruent condition of our selection task, where a consistent number-magnitude relation was repeated from prime to probe, the beginning of a pattern was signalled and even without feedback or reward, the ventral striatum was recruited, the association was encoded and responding was facilitated.
Once learning is established, the ventral striatum activity in neuroimaging studies increases over baseline tasks only for: (i) unexpected rewards, as in reward delivery for previously unrewarded stimuli or reward omission for previously rewarded items [i.e. prediction errors (Breiter et al., 2001; Ullsperger and von Cramon, 2003; Rodriguez et al., 2006; Bray and O’Doherty, 2007; Hampton and O’Doherty, 2007; Liu et al., 2007; Rolls et al., 2008)]; (ii) punishment after errors (Simoes-Franklin et al., 2010); or (iii) unexpected punishment, when reward criterion is reversed and responses to previously rewarded stimuli now elicit negative feedback (Cools et al., 2002, 2004; Seger et al., 2010). Similarly, once stimulus–reward associations have been learned, adjusting to new stimulus–reward probabilities is impaired for patients with Parkinson’s disease ON dopamine-replacement therapy (Dias et al., 1996; Swainson et al., 2000; Cools et al., 2001, 2002; Cools, 2006). Finally, the ventral striatum is differentially activated by salient (Zink et al., 2003; Cooper and Knutson, 2008; Litt et al., 2010), valued (Cooper and Knutson, 2008; Kable and Glimcher, 2010; Litt et al., 2010; Prevost et al., 2010) or novel stimuli (Seger et al., 2010; van Schouwenburg et al., 2010) and for passively received monetary or social rewards (Clithero et al., 2010). We surmise that the common theme between these situations and ours is a signal of the need for, or possibility of, new learning.
Dorsal striatum in cognition
Our results in the incongruent condition, using patients with Parkinson’s disease ON and OFF dopamine replacement in Experiment 1 and functional MRI in Experiment 2, suggest that dorsal striatum assimilates relevant information to produce more accurate selections. Our findings are coherent with results that shifting attention to more salient stimuli is accomplished more easily by patients with Parkinson’s disease than by controls (Cools et al., 2010). In contrast, switching attention to less salient or previously ignored stimuli is more impaired in patients with Parkinson’s disease and patients with dorsal striatum lesions (Benke et al., 2003; Rieger et al., 2003; Troyer et al., 2004; Cools et al., 2006, 2010; Ullsperger and von Cramon, 2006; Thoma et al., 2008; Cameron et al., 2010) and correlates with enhanced dorsal striatum activity in functional neuroimaging studies (Liu et al., 2010). Finally, the dorsal striatum is implicated in selections that require integrating information from different modalities (Thoma et al., 2008) and when various dimensions and influences (e.g. reward magnitude, subjective value and temporal discounting) are combined to enact decisions (Pine et al., 2009, 2010; Ding and Gold, 2010; Cai et al., 2011). Along with our results in the incongruent condition, these findings suggest that dorsal striatum prevents attention being directed to a single salient feature and promotes integrating more varied influences to reduce bias in selections.
No ON–OFF medication differences arose for patients with Parkinson’s disease, and the striatum was not preferentially activated using functional MRI when the selection dimension changed from prime to probe relative to when it remained constant. These trials were, nonetheless, more attention-demanding attested to by longer response latencies and greater errors. Whereas a number of investigations found that successful performance of task-switching or set-shifting depends upon (Benke et al., 2003; Rieger et al., 2003; Troyer et al., 2004; Cools et al., 2006; Ell et al., 2006; Thoma et al., 2008; Yehene et al., 2008) or preferentially activates the dorsal striatum (Rogers et al., 2000; Monchi et al., 2001; Grinband et al., 2006; van Schouwenburg et al., 2010); contradictory evidence is accumulating (Aglioti et al., 1996; Cools et al., 2006; Kehagia et al., 2009). Monchi et al. (2006) have shown specifically that uncertainty, requiring more distributed attention to the situation, rather than shifting per se engages the dorsal striatum, in line with our results and the findings of others (Daniel et al., 2010).
Implications for cognition in Parkinson’s disease
Despite consistent improvement in motor symptoms, the effect of dopamine-replacement therapy on cognition in Parkinson’s disease is less clear. Although seemingly paradoxical, our results strongly suggest that discrepancies relate to the differential reliance of various aspects of cognition on the ventral and dorsal striatum. These segments are dopamine-depleted and hence differentially affected by dopamine replacement (Cools, 2006; MacDonald and Monchi, 2011). Accordingly, defining the distinct functions of ventral and dorsal striatum will have important clinical implications. Titration of therapy in Parkinson’s disease is generally geared to optimizing dorsal striatum-mediated motor symptoms at the expense of ventral striatum operations, a consequence that is only beginning to be recognized. Enhanced awareness of this differential effect will translate to medication strategies that take into account symptoms that might be improved versus those that could deteriorate with dopamine replacement. We have shown that the ventral striatum-mediated encoding of stimulus associations is impaired, whereas the dorsal striatum-driven integration of various influences on selections is improved. Ultimately, this knowledge will lead clinicians to consider a broader range of symptoms in adjusting medication dosages to strike a better balance, based on individual patient priorities.
Funding
Canadian Institutes for Health Research (CIHR) Clinician-Scientist, Phase 1 (to P.A.MacD.); an operating grant (to O.M.; MOP-81114).
Acknowledgements
We thank Shawna Lagacé for assistance with data collection.