-
PDF
- Split View
-
Views
-
Cite
Cite
Simon Akerman, Philip R. Holland, Oliver Summ, Michele P. Lasalandra, Peter J. Goadsby, A translational in vivo model of trigeminal autonomic cephalalgias: therapeutic characterization, Brain, Volume 135, Issue 12, December 2012, Pages 3664–3675, https://doi.org/10.1093/brain/aws249
Close - Share Icon Share
Abstract
Trigeminal autonomic cephalalgias are highly disabling primary headache disorders, characterized by severe unilateral head pain and associated ipsilateral cranial autonomic features. There is limited understanding of their pathophysiology and how and where treatments act to reduce symptoms; this is significantly hindered by a lack of animal models. We have developed the first animal model to explore trigeminal autonomic cephalalgias, using stimulation within the brainstem, at the level of the superior salivatory nucleus, to activate the trigeminal autonomic reflex arc. Using electrophysiological recording of neurons of the trigeminocervical complex and laser Doppler blood flow changes around the ipsilateral lacrimal duct, superior salivatory nucleus stimulation exhibited both neuronal trigeminovascular and cranial autonomic manifestations. These responses were specifically inhibited by the autonomic ganglion blocker hexamethonium bromide. These data demonstrate that brainstem activation may be the driver of both sensory and autonomic symptoms in these disorders, and part of this activation may be via the parasympathetic outflow to the cranial vasculature. Additionally, both sensory and autonomic manifestations were significantly inhibited by highly effective treatments for trigeminal autonomic cephalalgias, such as oxygen, indomethacin and triptans, and some part of their therapeutic action appears to be specifically on the parasympathetic outflow to the cranial vasculature. Treatments more used to migraine, such as naproxen and a calcitonin gene-related peptide receptor inhibitor, olcegepant, were less effective in this model. This is the first model to represent the phenotype of trigeminal autonomic cephalalgias and their response to therapies, and indicates the parasympathetic pathway may be uniquely involved in their pathophysiology and targeted to relieve symptoms.
Introduction
The trigeminal autonomic cephalalgias (TACs) (Goadsby and Lipton, 1997), including cluster headache, are highly disabling primary headache disorders characterized by ‘severe unilateral head pain, described by females as a pain worse than childbirth’, occurring in association with ipsilateral cranial autonomic features (Headache Classification Committee of the International Headache Society, 2004). An understanding of their pathophysiology involves three major clinical features: unilateral trigeminal distribution of pain, cranial autonomic features and individually highly characteristic attack patterns (Goadsby, 2002). It is the combination of the attack pattern and phenotype that differentiates these disorders from migraine. Cluster headache attacks tend to have the longest duration with lower attack frequency (May, 2005); paroxysmal hemicrania has an intermediate duration and attack frequency (Cittadini et al., 2008); short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing have the shortest duration and many discrete attacks per day (Cohen et al., 2006); and hemicrania continua presents with unremitting pain (Cittadini and Goadsby, 2010).
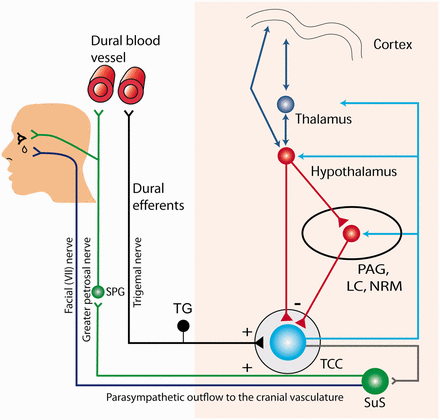
The basic anatomy and physiology of TACs are beginning to be understood, based mainly on clinical research. One of the major symptoms of TACs is the excruciating pain, which is most likely a consequence of activation of the trigeminovascular system (Goadsby et al., 2002). This includes the peripheral nerve fibres that innervate the pain-producing cranial vessels and dura mater, and the centrally projecting fibres that synapse in the trigeminocervical complex. Animal models have demonstrated activation of the trigeminocervical complex by stimulation of dural structures (Kaube et al., 1993; Hoskin et al., 1999). Stimulation of these same structures also causes neuronal activity in the superior salivatory nucleus within the pons (Knight et al., 2005), which is the origin of cells for the cranial parasympathetic autonomic vasodilator pathway (Spencer et al., 1990). This efferent projection is predominantly through the greater petrosal nerve, a branch of the facial (VIIth) cranial nerve, and its projection through the sphenopalatine ganglion (pterygopalatine ganglion in humans, Fig. 1). It is thought that this autonomic activation leads to the second important symptom in TACs: cranial autonomic features, which include lacrimation, conjunctival injection and nasal congestion and a local third-order sympathetic lesion due to carotid swelling. TACs and other primary headaches may share a common activation through the trigeminovascular system, and all primary headaches can exhibit autonomic symptoms to some degree, through reflex activation of the cranial autonomic outflow. However, in primary headaches where activation of the trigeminal autonomic reflex contributes far more significantly to their pathophysiology and as a consequence their major symptoms, they are conveniently classified together as TACs.
Overview of the major anatomical and physiological pathways believed to be involved in TACs. Pain afferents from the trigeminovascular system traverse the ophthalmic division of the trigeminal nerve, taking signals from the cranial vessels and dura mater. These inputs synapse in the trigeminocervical complex (TCC, black neuron) and project to higher brain structures such as the thalamus and cortex (blue neurons), resulting in pain perception. Activation of the trigeminovascular system by stimulation of dural structures also causes neuronal activation in the superior salivatory nucleus (SuS, via the grey neuron) within the pons, which is the origin of cells for the cranial parasympathetic autonomic vasodilator pathway. In TAC pathophysiology, there is activation of this parasympathetic reflex via the outflow from the superior salivatory nucleus, predominantly through the greater petrosal nerve (green neuron) and its relay with the sphenopalatine ganglion (SPG), but also via the facial (VIIth cranial) nerve (purple neuron). Activation of both trigeminal and autonomic nerves defines the trigeminal autonomic reflex arc and is thus integral to the pathophysiology of TACs. Additionally, it is thought that there is descending control of the trigeminocervical complex and superior salivatory nucleus through the periaqueductal grey (PAG), locus coeruleus (LC), nucleus raphe magnus (NRM) and hypothalamus (red projections). Tg = trigeminal ganglion.
TACs each have unique therapeutic responses that differentiate them one from another, and from migraine (Goadsby et al., 2008). However, there is still little known about what causes TACs, and how and where treatments act to reduce symptoms, which substantially limits attempts to develop new treatments. The lack of good experimental models, which respond preferentially to the different therapies for TACs, has hindered progress in understanding their pathophysiology, reduced our ability to determine the locus of action of existing therapies and hindered the development of novel treatment strategies. Our aim was to develop an in vivo model combining trigeminovascular activation common to models of migraine, with significant activation of the parasympathetic outflow to mimic cranial autonomic symptoms, using stimulation of the trigeminal autonomic reflex. This may provide a basis to understand better the pathophysiology of TACs, and provide an opportunity to explore current strategies and develop new therapies. This in vivo model would preferentially be responsive to therapies that are effective in the various TACs, and be less responsive to specific migraine therapies. Therefore, it would need to use a different mechanism of activation to demonstrate that these therapies are acting on alternative pathways. To do this, we stimulated the superior salivatory nucleus to activate directly the trigeminal autonomic reflex arc, including the greater petrosal branch of the VIIth nerve, predicting this efferent activation would excite trigeminal afferents to the trigeminocervical complex, as well as demonstrating a parasympathetic component of symptomology.
Materials and methods
All experiments were conducted under license and approved by ethical review of the University of California, San Francisco Institutional Animal Care and Use Committee, conforming to the National Institute of Health Guide for the Care and Use of Laboratory Animals and adhering to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (Zimmermann, 1983).
Surgical preparation
Male Sprague Dawley rats (n = 136, 290–375 g) were anaesthetized with sodium pentobarbitone (60 mg/kg, intraperitoneal), and anaesthesia was maintained with propofol (15–20 mg/kg/h intravenous infusion). During the dural electrical stimulation, electrophysiological recording, animals were paralysed with pancuronium bromide (Pavulon, Organon), 0.4 mg initially and maintained with 0.2 mg every 35 min, as described previously (Akerman et al., 2007). To maintain full physiological functionality of the outflow from the superior salivatory nucleus, which is partly via the facial nerve and predominantly the greater petrosal nerve, this subgroup of animals were not paralysed. Animals were placed in a stereotaxic frame and continuously monitored for blood pressure and body temperature; the animals were artificially ventilated, and expired CO2 was measured and kept between 3.5 and 4.5%. A sufficient depth of anaesthesia was judged by the absence of paw withdrawal and corneal blink reflex, and during muscular paralysis by fluctuations of blood pressure and changes to expired CO2.
Electrophysiological recording in the trigeminocervical complex
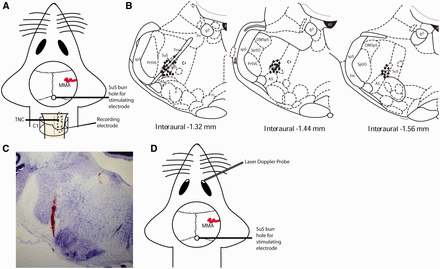
Methods for electrophysiological recording in the trigeminocervical complex have been published previously (Holland et al., 2006; Akerman et al., 2007). Briefly, a C1 hemilaminectomy was performed and the dura mater incised to expose the brainstem at the level of the caudal medulla. A tungsten recording electrode (0.5 MΩ, tip diameter 0.5 µm) was lowered into the brainstem with a piezoelectric motor controller. Extracellular recordings were made from neurons in the trigeminocervical complex and identified as having cutaneous facial receptive fields, and by superior salivatory nucleus or dura mater stimulation (see Fig. 2A for experimental set-up). The signal was amplified (total gain ×20 000–30 000) and passed through filters (300 Hz–20 KHz) and a 60-Hz noise eliminator to a second-stage amplifier. This signal was fed to a gated amplitude discriminator and analogue-to-digital converter (Cambridge Electronic Design) and to a microprocessor-based computer for analysis using Spike2 v5.
Experimental set-up and overview of stimulation sites. (A) Experimental set-up for stimulation of the superior salivatory nucleus (SuS) and recording in the trigeminocervical complex. (B) Locations of lesion sites in the area of the superior salivatory nucleus where stimulation took place. Stimulation sites in regions 1.32–1.56 mm behind the interaural point highlight that the superior salivatory nucleus was located as the stimulation site in each case, and also the proximity of the facial nerve was <250 µm from the stimulation site and therefore likely to be simultaneously activated even if motor responses were not observed. (C) Example lesion mark in an area proximate to the superior salivatory nucleus. (D) Experimental set-up for stimulation of the superior salivatory nucleus and measurement of changes in blood flow of the lacrimal duct/sac using laser Doppler flowmetry. 4V = fourth ventricle; 7 n = VIIth nerve; g7 = genu of the facial nerve; MMA = middle meningeal artery; Pr5VL = principle sensory trigeminal nucleus (ventrolateral part); sp5 = spinal trigeminal tract; Sp5O = spinal trigeminal nucleus oralis.
Evoked neuronal responses in the trigeminocervical complex were established via stimulation of either the dura mater or the superior salivatory nucleus. To stimulate the dura mater, a bipolar stimulating electrode was placed on the dura mater adjacent to or on either side of the middle meningeal artery, and electrical stimulus was applied (0.6 Hz, 0.1–0.5 ms, 8–20 V) to activate trigeminal afferents. To stimulate the superior salivatory nucleus, a concentric bipolar tungsten-stimulating electrode (impedance 10–15 KΩ, tip diameter 3–4 µm) was stereotaxically positioned into the superior salivatory nucleus (anterior–posterior 10.20–10.80 mm from bregma or 1.2–1.8 mm from interaural, dorsal–ventral 9.2–9.6 mm and medial–lateral 2.1–2.4 mm; Paxinos and Watson, 2004) (Fig. 2B). Constant current stimulus was applied (0.5 Hz, 150-µs duration and 20–50 µA), and neuronal responses in the trigeminocervical complex were recorded. Lesion marks were made post-experiment, and the brain was removed and post-fixed to confirm stimulation location (Fig. 2C). Neurons within the trigeminocervical complex were first characterized for their cutaneous and deep receptive field, assessed through all three territories of the trigeminal nerve, and both noxious and non-noxious responses were observed. Neurons sensitive to stimulation of the ophthalmic dermatome of the trigeminal nerve were then tested for convergent input from either the dura mater or superior salivatory nucleus. Baseline responses consisted of trains of 20 stimuli delivered at 5-min intervals, stimulating either dura mater or superior salivatory nucleus. Reliable electrically evoked responses with durations in the Aδ-fibre or C-fibre range, and also cutaneous receptive field, were tested pharmacologically with therapeutics for the various TACs, as well as specific anti-migraine drugs, to characterize the reliability of the assay in predicting clinical therapeutic efficacy.
Facial flow responses
Lacrimation and tearing are common symptoms that help define the TACs (Headache Classification Committee of the International Headache Society, 2004). They are a significant component of these disorders and thought to be a consequence of cranial parasympathetic nerve activation (Goadsby, 2002; May, 2006). Within our animal model we wanted to demonstrate activation of the parasympathetic outflow to the cranium, or the autonomic reflex, after stimulation of the superior salivatory nucleus, and provide some evidence of a lacrimal response. Previously methods have used cannulation of arterial or excretory supplies to the lacrimal duct as evidence of lacrimal gland flow (Botelho et al., 1976; Botelho and Fuenmayor, 1981). However, we wanted to take a less invasive approach, as the surgery itself could affect responses, and so we measured changes in blood flow around the lacrimal gland/duct using carefully positioned laser Doppler probes as an indication of this response (Moor Instruments) (Fig. 2D). Two baseline responses were performed in response to superior salivatory nucleus stimulation (5 Hz, 500-µs duration and 40–70 µA for 10 s), and the animals were given treatment and superior salivatory nucleus stimulation repeated at 5-min intervals for 30 min.
At the end of each experiment, either electrophysiology or facial blood flow, and after euthanasia, a lesion was made in the trigeminocervical complex and superior salivatory nucleus so that the area of recording or stimulation could be identified post-experiment. The brain and spinal cord were removed and stored in 10% formalin for post-fixing for 24 h and then cryopreserved in 30% sucrose. Sections of 30-µm thickness were cut, slide mounted and contrastained with cresyl violet to highlight and identify both the recording site in the trigeminocervical complex and the stimulation site of the superior salivatory nucleus.
Data analysis
The data collected as post-stimulus histograms after electrical stimulation of the dura mater for Aδ-fibres represent the number of cells fired over a 10-ms period in the region 5–20 ms post-stimulation over the 20 collections. During superior salivatory nucleus stimulation, latency periods for Aδ-fibres were extended to 40 ms because of the greater distance for the signal to travel and activation across multiple synapses. Spontaneous activity was measured in cell firings per second (Hz). Facial flow was measured as an average increase (in arbitrary units) over the 10 s from the baseline level.
All data are expressed as mean ± SEM. Statistical analysis was performed using an ANOVA for repeated measures with Bonferroni post hoc correction for multiple comparisons used to measure the time course of significant drug intervention, using a 95% confidence interval. If Mauchly’s test of sphericity was violated, we made appropriate corrections to degrees of freedom according to Greenhouse–Geisser. Student’s paired t-test for post hoc analysis was used to test for the time points of significance, using the average of the two or three baselines for comparison, again using the criteria of Bonferroni correction. In some cases when between-groups analysis was necessary, a mixed-design repeated-measures ANOVA was used. Statistical significance was set at P < 0.05 (using SPSS 16.0 throughout).
Results
Electrophysiology
Recordings were made from 103 neurons responsive to either dural stimulation (26 neurons in 26 animals) or stimulation of the superior salivatory nucleus (77 neurons in 65 animals), and with a cutaneous receptor field that included the first (ophthalmic) division of the trigeminal nerve and occasionally the second (maxillary) division. Neurons were located in deep layers (layers IV, V and VI) of the dorsal horn of the trigeminocervical complex, which includes the cervical C1 region of the spinal cord and its transition to the trigeminal nucleus caudalis, at range of depth of 450–955 µm. Neurons responsive to dural stimulation had a latency of firing of 16 ± 2 ms and 51 ± 2 ms, respectively, for Aδ-fibre and C-fibre inputs, and the baseline spontaneous firing rate was 42.5 ± 2 Hz.
Characterization of two distinct neuronal populations in the trigeminocervical complex after superior salivatory nucleus stimulation
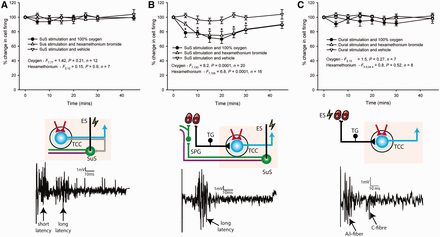
Activation of the parasympathetic outflow to the cranial vasculature was produced by stimulation of the superior salivatory nucleus, and its predominant projection through the greater petrosal branch of the VIIth (facial) cranial nerve and its projection to the sphenopalatine ganglion (see Fig. 1 for anatomical overview). Using 20 animals, two populations of neuronal responses in the trigeminocervical complex were identified with different latencies of firing. They were characterized by their response to therapeutic intervention for cluster headache, 100% O2 treatment, as well as through blockade of the parasympathetic outflow to the cranial vasculature using the specific autonomic ganglion blocker, a nicotinic cholinergic receptor antagonist, hexamethonium bromide (3 mg/kg), which does not readily cross the blood–brain barrier and blocks synaptic transmission of the autonomic ganglia (Goadsby, 1991; Toda et al., 2000; Gottselig and Messlinger, 2004). The first population of neurons fired with shorter response latency, between 3 and 20 ms, with an average response of 12.1 ± 1.0 ms. There was no significant effect of 15 min of 100% O2 treatment for 45 min, 1 l/min [F(7,77) = 1.42, P = 0.21, n = 12] or hexamethonium bromide, 10 mg/kg [F(2,12) = 0.15, P = 0.9, n = 7] on neurons firing with this short latency (Fig. 2A). The second population of neurons recorded had a much longer latency of firing, between 7 and 40 ms, with an average response of 20.4 ± 0.8 ms. These responses were significantly inhibited by treatment with 100% O2 by >30% [F(7,133) = 8.2, P = 0.0001, n = 20] and by hexamethonium bromide by >25% [F(7,105) = 6.8, P = 0.0001, n = 16] compared with controls (Fig. 2B). Baseline spontaneous firing for the aforementioned studies was 29.3 ± 2 Hz. In a separate group of animals (n = 9), vehicle-treated longer latency responses were unaltered [F(7,56) = 1.3, P = 0.29, n = 9].
As a comparison, studies were conducted using an established method of trigeminovascular nociceptive activation, with dural stimulation and recording in the trigeminocervical complex (Bergerot et al., 2006). It was found that neither oxygen [F(2,13) = 1.5, P = 0.27, n = 7], as has been demonstrated previously (Akerman et al., 2009), nor hexamethonium bromide [F(3,24) = 0.8, P = 0.52, n = 8] significantly inhibited dural-evoked responses compared with control responses (Fig. 2C).
Indomethacin and naproxen hydrochloride have differential effects on superior salivatory nucleus and dural-evoked firing
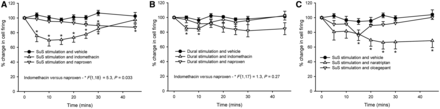
Stimulation of the superior salivatory nucleus and activation of the parasympathetic outflow to the cranial vasculature, measuring the longer latency response, appears to be the first animal model to differentiate inhibitory effects of oxygen, compared with dural-evoked stimulation. For this assay to represent a model of TACs, it should be responsive to other appropriate treatments. Indomethacin, a cyclo-oxygenase inhibitor, has such a highly characteristic effect on paroxysmal hemicranias and hemicrania continua, whereas other cyclo-oxygenase inhibitors, such as naproxen, are not consistently effective (Goadsby et al., 2008). Using the longer latency responses to superior salivatory nucleus stimulation, or dural electrical stimulation, and neuronal recording in the trigeminocervical complex, and using a single dose per animal, it was shown that indomethacin (5 mg/kg) inhibited superior salivatory nucleus-evoked firing [F(2.7,30) = 6.3, P = 0.003, n = 12] by 30% after 15 min (t11 = 3.4, P = 0.006), and dural-evoked responses [F(7,56) = 3.4, P = 0.004, n = 9] by only 15% (t8 = 3.6, P = 0.007) after 10 min. Naproxen hydrochloride (30 mg/kg) also significantly inhibited both superior salivatory nucleus-evoked firing [F(7,49) = 3.3, P = 0.006, n = 8] by 11% after 30 min (t7 = 3.5, P= 0.005) and dural-evoked responses [F(7,56)= 2.8, P= 0.015, n= 9] maximally by 17% after 25 min. When the two responses were compared, there was no significant difference between the effects of naproxen on superior salivatory nucleus versus dural-evoked firing [F(1,15)= 0.62, P= 0.45]. When the superior salivatory nucleus-evoked responses in the trigeminocervical complex for indomethacin and naproxen were compared, there was a significant difference between the groups [F(1,18)= 5.3, P= 0.033], whereas the response between the two treatments after the dural-evoked stimulus was not significantly different [F(1,17)= 1.3, P= 0.27] (Fig. 3A and B).
Response of 100% oxygen or hexamethonium bromide on dural-evoked and superior salivatory nucleus-evoked neuronal firing in the trigeminocervical complex (TCC). (A) Stimulation of the superior salivatory nucleus (SuS) evoked two populations of neuronal firing in the trigeminocervical complex, which has either a shorter or longer latency of firing (as indicated by the arrows). When 100% oxygen treatment was given for 15 min, or after treatment with the autonomic nicotinic cholinergic receptor blocker, hexamethonium bromide, there was no effect on the firing of these shorter latency responses (3–20 ms). It is believed that this evoked firing is a consequence of antidromic stimulation of the trigeminal system within the brainstem (grey neuron). (B) Neurons that fired with a longer latency (9–40 ms) were significantly inhibited by treatment with either 100% oxygen (1 l/min) or hexamethonium bromide (10 mg/kg). It is thought that this evoked response is via a different mechanism, a result of direct stimulation of the parasympathetic outflow to the cranial vasculature (green neuron), which activates trigeminal nerve endings that innervate the cranial vasculature (black neuron), resulting in neuronal activation in the trigeminocervical complex. (C) Direct activation of trigeminal afferents through stimulation of the dural vasculature (black neuron) results in Aδ-fibre neuronal responses in the trigeminocervical complex, these responses were not inhibited with treatment of either 100% oxygen or hexamethonium bromide. *P < 0.005 compared with baseline control. ES = electrical stimulation.
Triptans and calcitonin gene-related peptide receptor antagonists demonstrate differential effectiveness at inhibiting superior salivatory nucleus-evoked firing
Classic anti-migraine drugs, serotonin, 5-hydroxytryptamine (5-HT)1B/1D receptor agonists, ‘triptans’ are extremely effective in the treatment of migraine (Goadsby et al., 2002), as well as being effective in animal models of durally evoked trigeminovascular nociception. Triptans are also an extremely effective abortive treatment of cluster headache (Goadsby et al., 2008). Calcitonin gene-related peptide receptor antagonists are a new and effective class of acute migraine treatments (Olesen et al., 2004), which, among other effects, inhibit neuronal firing in the trigeminocervical complex in response to dural stimulation (Storer et al., 2004); they are untried in TACs. Using a single treatment per animal, naratriptan (10 mg/kg) significantly inhibited the longer latency responses of superior salivatory nucleus-evoked firing in the trigeminocervical complex [F(7,49) = 3.5, P< 0.004, n = 8], maximally by 34% after 30 min (t7 = 4.1, P = 0.004). Olcegepant (900 µg/kg), a small molecule calcitonin gene-related peptide receptor antagonist, also significantly inhibited superior salivatory nucleus-evoked firing in the trigeminocervical complex across the time course [F(7,49) = 2.5, P = 0.031, n = 8], but not specifically at any time point (Fig. 3C).
Measuring cranial autonomic outflow using lacrimal flow
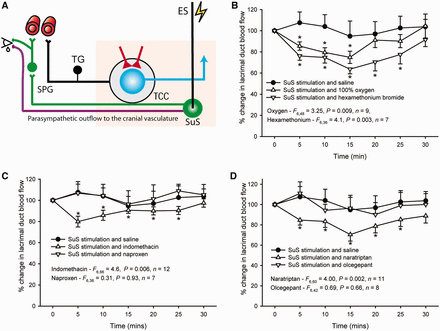
TACs are characterized by cranial autonomic features, such as lacrimation. Therefore, changes in flow in the lacrimal gland were monitored as an indication of activation of the parasympathetic outflow to the cranium, the autonomic reflex component (Fig 5A). Characteristic changes in flow in the lacrimal gland/duct were observed in response to stimulation of the superior salivatory nucleus/VIIth nerve. This effect was reproducible over 30 min (Akerman et al., 2009). Forty-seven animals were included in these studies, and hexamethonium was used in the oxygen study after 60-min washout, to determine that they both affected the same response, whereas the other treatment groups used a fresh animal/treatment. When 100% O2 was given for 15 min, there was a significant inhibition of the flow [F(6,48) = 3.25, P = 0.009, n = 9], maximally by 26% at 15 min (t8 = 4.2, P = 0.003), and when the autonomic ganglion blocker hexamethonium bromide (10 mg/kg) was used, there was a significant inhibition of flow [F(6,36) = 4.1, P = 0.003, n = 7], maximally by 36% at 15 min (t6 = 4.2, P = 0.0005; Fig. 5B). When the cyclo-oxygenase inhibitors indomethacin and naproxen were used in response to stimulation of the superior salivatory nucleus, there was a differential effect. Indomethacin significantly inhibited superior salivatory nucleus-evoked flow changes [F(6,66) = 4.6, P = 0.006, n = 12], maximally by 20% after 5 min (t11 = 3.8, P = 0.003). However, naproxen did not affect lacrimal flow responses after superior salivatory nucleus stimulation [F(6,36) = 0.31, P = 0.93, n = 7] (Fig. 5C). There was a similar differentiation of response to superior salivatory nucleus stimulation after the anti-migraine drugs naratriptan and olcegepant. Naratriptan significantly inhibited lacrimal flow changes [F(6,60) = 4.0, P = 0.002, n = 11], maximally by 29% after 15 min (t10 = 4.8, P = 0.001), whereas olcegepant had no effect on flow response [F(6,42) = 0.69, P = 0.66, n = 8] (Fig. 5D).
Response of cyclo-oxygenase inhibitors and anti-migraine drugs on dural-evoked and superior salivatory nucleus-evoked neuronal firing in the trigeminocervical complex. (A) Cyclo-oxygenase inhibitors indomethacin (5 mg/kg) and naproxen (30 mg/kg) are both able to inhibit the longer latency responses after stimulation of the superior salivatory nucleus (SuS); however, there was a significantly greater inhibition after indomethacin (30%) compared with naproxen (15%). (B) Indomethacin and naproxen were also both able to inhibit dural-evoked neuronal firing in the trigeminocervical complex, but this inhibition was not significantly different (15% and 17%, respectively) from each other. (C) Naratriptan, a 5-HT1B/1D receptor agonist, and the calcitonin gene-related peptide receptor antagonist, olcegepant, both of which are known to inhibit dural-evoked firing in the trigeminocervical complex, were also used in the superior salivatory nucleus stimulation assay. Both naratriptan (10 mg/kg) and olcegepant (900 µg/kg) were able to inhibit the long latency response of neuronal firing in the trigeminocervical complex after superior salivatory nucleus stimulation, although naratriptan did inhibit to a greater extent, 34% compared with 24% inhibition. *P < 0.05 compared with baseline control.
Responses of treatments to stimulation of the superior salivatory nucleus and measurement of changes to lacrimal flow. (A) Overview of the parasympathetic outflow to the cranial vasculature via the greater petrosal nerve (green neuron) and the facial (VIIth cranial) nerve (purple neuron). It is thought that stimulation of the superior salivatory nucleus (SuS) causes activation of the parasympathetic outflow to the face and head, via the greater petrosal nerve and, to a lesser extent, the facial nerve, and is responsible for the flow changes that occur in the lacrimal duct/gland and the autonomic symptoms seen in TACs. (B) Flow increases within the lacrimal duct/sac caused by stimulation of the superior salivatory nucleus were significantly inhibited by treatment with both 100% oxygen (1 l/min) and hexamethonium bromide (10 mg/kg), implying the response was driven solely by activation of the autonomic sphenopalatine ganglion. (C) Flow increases caused by superior salivatory nucleus stimulation were also inhibited by the cytochrome c oxidase inhibitor, indomethacin, which has characteristic effects in TACs, (5 mg/kg) by 20%, but there was no inhibition by naproxen (30 mg/kg), which is consistently ineffective as a treatment. (D) The anti-migraine treatment, 5-HT1B/1D receptor agonist, naratriptan (30 mg/kg) was also able to inhibit lacrimal duct/gland flow by 29%, whereas the calcitonin gene-related peptide receptor antagonist, olcegepant, had no effect on flow. *P < 0.05 compared with baseline control.
Discussion
In this study, we developed and characterized an in vivo model of the TACs, using electrical stimulation of the superior salivatory nucleus to activate the trigeminal autonomic reflex arc. The model addresses the combination of trigeminovascular neuronal activation and the cranial autonomic symptoms that are distinctive to TACs, and tests the broader question of an important role for the brainstem in these disorders. These responses are different from those generated by direct dural electrical stimulation. This novel model is also preferentially responsive to treatments for TACs over more specific migraine treatments and has allowed us, for the first time, to begin to understand their locus of action and more about the pathophysiology of TACs.
Superior salivatory nucleus stimulation produced a distinctive electrophysiological response with two populations of neuronal firing in the trigeminocervical complex, with differing latencies: those with shorter latencies of 3–20 ms, and those with more delayed latencies of 7–40 ms. These two populations were characterized by their response to the acute cluster headache treatment, 100% oxygen, followed—after 60-min washout—with medical air, and by the nicotinic autonomic ganglion blocker hexamethonium bromide. Cluster headache, the most common TAC, demonstrates a robust response to high-flow oxygen as an abortive therapy (Fogan, 1985; May et al., 2006; Cohen et al., 2009); yet, in carefully studied cohorts of patients with paroxysmal hemicrania (Cittadini et al., 2008) or short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (Cohen et al., 2006), not a single patient reported an adequate response to oxygen. Oxygen thus seems a useful treatment with which to test the new model. Oxygen specifically inhibited firing of the longer latency neuronal responses but had no effect on the shorter latency responses. Similarly, on the same neurons, hexamethonium bromide significantly inhibited the longer latency response but had no effect on the shorter latency response. Hexamethonium bromide is a specific nicotinic autonomic ganglion blocker that inhibits synaptic transmission and neuronal responses at the level of the autonomic ganglia and does not readily cross the blood–brain barrier (Goadsby, 1991; Toda et al., 2000; Gottselig and Messlinger, 2004). It is likely that hexamethonium blocks the greater petrosal nerve projection to the craniovascular/dural complex, at the level of the sphenopalatine ganglion (green nerve projection in Figs 1 and 3B). Given that oxygen followed the same pattern and level of response as hexamethonium, it is probable that the two treatments are acting on the same pathway. The data imply that the longer latency firing is a consequence of activation of the parasympathetic outflow to the cranial vasculature, which in turn activates the trigeminal projection to the trigeminal nucleus caudalis (orthodromic stimulation of the trigeminal nerve—black nerve fibre in Figs 1 and 3B), causing neuronal activation in the trigeminocervical complex. We believe that these data demonstrate that activation of this autonomic projection to the cranial vasculature, via the dura mater, can activate the trigeminovascular system and potentially contribute to the head pain experienced during TACs. It also helps us to begin to understand the locus of action of oxygen, demonstrating that it is acting on this parasympathetic outflow to the cranial vasculature, and subsequent activation of the trigeminovascular system to exert its therapeutic effects of reducing both autonomic symptoms and nociceptive traffic to the trigeminocervical complex to reduce pain symptoms.
The second population of neuronal firing in the trigeminocervical complex was unresponsive to both oxygen treatment and antagonism of the autonomic ganglia with hexamethonium. Physiologically the firing also differed because it tended to have a much shorter latency of firing, with an average of 12.1 ms, whereas the longer latency responses averaged 20.4 ms after the stimulus. It would seem likely that these cells are not a response to activation of the parasympathetic outflow to the cranium, but rather involve another pathway. We know already that as part of the trigeminal autonomic reflex arc there is likely to be a synaptic connection between the trigeminocervical complex and the superior salivatory nucleus. Indeed, it has been demonstrated that dural electrical stimulation, which causes neuronal activation in the trigeminocervical complex, also causes neuronal activation in the superior salivatory nucleus (Knight et al., 2005). It is possible that this shorter latency response is a consequence of antidromic stimulation of this synaptic connection (grey neuron in Figs 1 and 3A) causing firing in the trigeminocervical complex. This would also explain the lack of effect of the autonomic ganglion blocker hexamethonium, as this autonomic pathway is not involved. The shorter latency response is a consequence of a shorter distance and single synapse crossed in this pathway, compared with the much longer parasympathetic pathway that crosses several synapses.
This may explain why the autonomic ganglion blocker hexamethonium has no effect on this neuronal response, but it does not explain why oxygen has no effect on the trigeminal firing. it is likely then that oxygen has no effects on the trigeminovascular system per se, or specifically, the peripheral trigeminal fibre projections to the dural vasculature or the afferent projections to the trigeminal nucleus caudalis. We were able to demonstrate that oxygen has no effect on inhibiting responses to dural-evoked trigeminovascular activation, either in the periphery (Akerman et al., 2009) or at the level of the trigeminocervical complex. Similarly, hexamethonium was also ineffective at inhibiting the neuronal firing in the trigeminocervical complex. The data combined indicate that the neurons with the shorter latency of firing followed a different pathway to those with the longer latency, and it is likely to be within the brainstem. The findings suggest that it is unlikely that oxygen conveys any therapeutic benefit directly in the trigeminovascular system either at the level of the periphery or the nucleus caudalis, but rather acts at the level of the parasympathetic outflow to the cranial vasculature.
Another major feature of TACs is their prominent cranial autonomic symptoms, such as lacrimation, conjunctival injection and nasal congestion. To observe an autonomic response in our animals after superior salivatory nucleus stimulation, we measured changes in blood flow around the lacrimal sac/gland as indicative of lacrimation. Changes in the flow were observed contemporaneously with superior salivatory nucleus stimulation (Akerman et al., 2009). These changes were significantly inhibited by both oxygen and the autonomic ganglion blocker to a similar extent. With the autonomic ganglion blocker also attenuating lacrimal flow in response to superior salivatory nucleus, it substantiates the hypothesis that the effects of oxygen are directly on the parasympathetic outflow to the cranial vasculature via the greater petrosal nerve and the sphenopalatine ganglion (green nerve fibre in Figs 1 and 5A) and that oxygen is probably able to relieve directly the autonomic symptoms via this mechanism.
The development of this new model and the results lend further support to the theory that the TACs are CNS neurovascular disorders (Goadsby, 2002). This states that the vascular changes seen in the cranial circulation are considered to be a response to activation of the trigeminal autonomic reflex arc. Therefore, these changes serve as a marker of brain activation rather than the driver of the headaches. This new model demonstrates that stimulation within the brainstem, at the level of the superior salivatory nucleus, is able to cause both autonomic and trigeminovascular activation, and may be a key nucleus in driving both pain and autonomic symptoms during TACs. This is supported by data showing that direct stimulation of the superior salivatory nucleus (Nakai et al., 1993), sphenopalatine ganglion (Goadsby, 1990) or activation of the VIIth cranial (facial) nerve (Goadsby, 1989) also cause increases in cerebral blood flow, dilating blood vessels and activating trigeminal nerve endings. This parasympathetic outflow to the extracranial vessels is thought to use vasoactive intestinal peptide as its primary transmitter (Goadsby and MacDonald, 1985). The release of vasoactive intestinal peptide after stimulation of the facial nerve (Goadsby and Shelley, 1990) and during TACs, including cluster headache (Goadsby and Edvinsson, 1994a) and chronic paroxysmal hemicrania (Goadsby and Edvinsson, 1996), is consistent with this pathway being activated. Indeed, recent preliminary data indicate that stimulation of the sphenopalatine ganglion in patients implanted with a neurostimulator can provide significant pain relief in patients with chronic cluster headache (Schoenen et al., 2011). This is another clear indication of the importance of the parasympathetic pathway both in the pathophysiology of these disorders and in their treatment.
Determining how the superior salivatory nucleus is activated during TACs may help us to understand further the pathophysiology of TACs. Imaging studies have demonstrated activation in the region of the posterior hypothalamus during spontaneous attacks of cluster headache (May et al., 1998a), paroxysmal hemicrania (Matharu et al., 2006), short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (May et al., 1999; Sprenger et al., 2005) and hemicrania continua (Matharu et al., 2004). PET studies during capsaicin-induced pain in the ophthalmic (first) division of the trigeminal nerve demonstrate many of the vascular changes that occur in TACs but did not demonstrate any hypothalamic activation (May et al., 1998b). It was concluded that in TACs, the vascular changes are a direct consequence of activation in the brain, or maybe specifically activation of the trigeminal autonomic reflex. It has been hypothesized further that dysfunction within the hypothalamus may be the cause of TACs. There is evidence of anatomical connections between the hypothalamus and trigeminal nucleus (Malick and Burstein, 1998; Malick et al., 2000; Benjamin et al., 2004), and the hypothalamus and the superior salivatory nucleus and its autonomic projections (Hosoya et al., 1983, 1990), and through the superior salivatory nucleus, may contribute to sustained activation of the trigeminovascular system (Burstein and Jakubowski, 2005). Also, direct hypothalamic stimulation offers relief in patients with chronic intractable cluster headache (Leone et al., 2001). Perhaps similar to theories of migraine (Goadsby et al., 2002), TACs may be considered a consequence of dysfunction of the hypothalamic descending control of trigeminal nociceptive inputs and autonomic projections to the cranial vasculature. It is possible that during cluster headache and other TACs, neurons at the level of the trigeminocervical complex are activated not only by this direct loss of descending control of trigeminal nociceptive input, but also by a loss of control of the autonomic arm of the trigeminal autonomic reflex arc.
This novel model of TACs demonstrates some of their pathophysiology and is beginning to help unravel the mechanism of action of therapies such as high-flow oxygen treatment in cluster headache. It demonstrates the importance of the superior salivatory nucleus and activation of the parasympathetic outflow to the cranial vasculature, to stimulate the trigeminovascular system. It is also able to predict the effectiveness of oxygen as an abortive therapy, over other migraine models, and is therefore unique in this respect. However, given that TACs are classified because they are thought to share a common pathophysiology, for this new assay to be considered more generically a model of TACs, it needs to demonstrate its ability to predict for TAC treatment over other primary headaches. Therefore, we used other specific TAC treatments to assess the predictability of this assay over dural electrical stimulation, which is commonly used to predict anti-migraine efficacy.
Indomethacin, a cyclo-oxygenase inhibitor, has a robust effect on paroxysmal hemicrania that is considered diagnostic (Cohen et al., 2007). Other cyclo-oxygenase inhibitors, such as naproxen, have no comparable efficacy. Using the longer-latency cell firing as our model of activation of the trigeminal autonomic reflex arc, after stimulation of the superior salivatory nucleus, it was demonstrated that indomethacin significantly inhibited evoked firing in the trigeminocervical complex by 30%. In the dural-evoked model, indomethacin also inhibited responses, but by only 15%, and there was a significant difference between the responses. Naproxen also inhibited neuronal responses in the trigeminocervical complex after superior salivatory nucleus stimulation and dural stimulation, but only by 13% and 17%, respectively. These responses were not significant from each other. However, when the responses of indomethacin and naproxen after superior salivatory nucleus stimulation were compared, indomethacin caused a significantly greater inhibition that came on more quickly. These data point to the fact that indomethacin shows greater efficacy in the superior salivatory nucleus assay, compared with other cyclo-oxygenase inhibitors and the dural electrical stimulation assay, known to have greater predictability for anti-migraine drugs. While it is likely that indomethacin is acting directly on the neurons of the trigeminovascular system, it is also acting directly on the parasympathetic outflow to the cranial vasculature. This point is supported by the fact that lacrimal duct flow changes were inhibited by indomethacin, but there was no effect of naproxen. It seems likely that both indomethacin and naproxen are acting in some way within the trigeminocervical complex to inhibit neuronal firing, but indomethacin also acts specifically on parasympathetic projections from the superior salivatory nucleus to the cranium and face, to further inhibit trigeminal responses and autonomic responses. This would again imply that superior salivatory nucleus stimulation may more accurately represent the clinical phenotype of TACs, and therefore help predict clinical therapeutic efficacy.
Classic anti-migraine drugs, 5-HT1B/1D receptor agonists, ‘triptans’, in addition to being extremely effective in the treatment of migraine are also a highly effective abortive treatment of cluster headache (Cohen et al., 2007). Calcitonin gene-related peptide receptor antagonists are a new class of compounds clinically effective in the treatment of migraine (Olesen et al., 2004), but their effects on TACs are unknown. However, it is known that calcitonin gene-related peptide, alongside vasoactive intestinal peptide, is released in patients during cluster headache and paroxysmal hemicrania (Goadsby and Edvinsson, 1994b, 1996); therefore, it might be a new therapeutic option. Although both naratriptan and olcegepant significantly inhibited superior salivatory nucleus/VIIth nerve-evoked neuronal firing in the trigeminocervical complex, only naratriptan was able to inhibit lacrimal flow changes. Recent evidence indicates that serotonin, 5-HT1D receptors in rat are present on nerve endings at the level of the sphenopalatine ganglion and project to the lacrimal gland and nasal mucosa (Ivanusic et al., 2011). This might indicate why triptans can preferentially inhibit lacrimal flow after superior salivatory nucleus stimulation over calcitonin gene-related peptide receptor antagonists, as they are acting directly on receptors at the sphenopalatine ganglion to inhibit craniovascular parasympathetic projections.
To validate superior salivatory nucleus stimulation as a novel model of TACs, it was necessary to use a large number of subjects in this study. Our aim was to demonstrate several characteristics of TACs, observing both trigeminal and autonomic symptomology, using a hypothesized pathophysiology of brainstem activation, and as such, each observation had to be characterized separately. The same animals were used to first illustrate a response to oxygen during both neuronal responses and lacrimal changes, and followed after suitable washout, with the autonomic ganglion blocker, to ensure the same neuronal or lacrimal flow changes were being conducted on the same responses. To validate appropriately the response to specific TAC therapeutics in this assay, in the remaining pharmacological studies, we used one drug per animal/assay, which inevitably increases the numbers used. Additionally, when conducting repeated-measures studies, it is necessary to increase the number of subjects to try to avoid violating the sphericity assumption. Finally, to provide reliable pharmacological specificity of the model for TACs, we believed it was necessary to compare with drugs of similar class used for general primary headache that are more effective in the dural-evoked assay, as well as suitable negative (vehicle) controls. This meant up to seven different treatment interventions were necessary in some cases. To have conducted fewer studies with less pharmacological validation would not have produced the reliability of both pathophysiology and pharmacology we have demonstrated.
The overall data indicate that animal models of durally evoked trigeminovascular nociception may have limited use at predicting efficacy for treatments for TACs, with 100% oxygen treatment being the clearest example of this failure, whereas the effects of indomethacin are far more beneficial when studying the trigeminal autonomic reflex. On the other hand, using stimulation of superior salivatory nucleus/VIIth nerve not only do we see activation of the parasympathetic outflow to the cranial vasculature, as demonstrated by the facial blood flow changes, but this activation we believe is able to activate trigeminal afferents and neuronal firing within the trigeminocervical complex, as described by the trigeminal autonomic reflex arc. Indeed, two populations of neurons were characterized, not only those believed to come from the parasympathetic outflow, but also those that appear to be a result of antidromic stimulation of the trigeminal nucleus within the brainstem itself. It seems that studying the parasympathetic outflow from the superior salivatory nucleus, and perhaps inputs that modulate the superior salivatory nucleus, for example, the hypothalamus, may be integral in furthering our understanding of TACs and predicting treatment efficacy. It is noteworthy that therapeutics specifically targeting the different TACs appear to be equally effective at inhibiting both trigeminocervical complex and autonomic responses; yet, these treatments can be considered exclusive to specific TACs. It is worth bearing in mind that these disorders have been classified together because of their similar symptomology and, to some extent, pathophysiology, with hypothalamic, trigeminovascular and cranial autonomic activation demonstrated. We believe that the fact that oxygen, indomethacin and the triptans are likely to act on the parasympathetic outflow illustrates the importance of this locus in these disorders and illustrates the flexibility of the model in helping understand TACs more generally. TACs are among the most disabling of the primary headache disorders, and thus, development of a model for the disorders is of broad potential for translational studies to benefit these patients.
Funding
The work has been supported by the Sandler Family Foundation. OS received a fellowship in Neurology from Merck Sharp and Dohme, Germany.
Acknowledgements
The authors thank Anna Andreou, Annabelle Charbit and James Storer of the Headache Group at UCSF for their support and technical assistance during these experiments.
Abbreviations
References
Author notes
*Present address: Centre for Neuroregeneration, University of Edinburgh, Edinburgh, EH16 4SB, UK
†Present address: Department of Neurology, University Hospital Münster, 48149 Münster, Germany
- oxygen
- indomethacin
- migraine disorders
- vascular flow
- autonomic nervous system
- headache
- bromides
- calcitonin gene-related peptide
- autonomic ganglion
- hexamethonium
- animal model
- naproxen
- neurons
- cranium
- brain stem
- vasculature
- triptans
- trigeminal autonomic cephalalgias
- salivatory nucleus, superior
- primary headache disorders
- autonomic reflex
- lacrimal duct
- fluid flow