-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Eimer, Angela Gosling, Bradley Duchaine, Electrophysiological markers of covert face recognition in developmental prosopagnosia, Brain, Volume 135, Issue 2, February 2012, Pages 542–554, https://doi.org/10.1093/brain/awr347
Close - Share Icon Share
Abstract
To study the existence and neural basis of covert face recognition in individuals with developmental prosopagnosia, we tested a group of 12 participants with developmental prosopagnosia in a task that required them to judge the familiarity of successively presented famous or non-famous faces. Electroencephalography was recorded during task performance, and event-related brain potentials were computed for recognized famous faces, non-recognized famous faces and non-famous faces. In six individuals with developmental prosopagnosia, non-recognized famous faces triggered an occipito-temporal N250 component, which is thought to reflect the activation of stored visual memory traces of known individual faces. In contrast to the N250, the P600f component, which is linked to late semantic stages of face identity processing, was not triggered by non-recognized famous faces. Event-related potential correlates of explicit face recognition obtained on those few trials where participants with developmental prosopagnosia classified famous faces as known or familiar, were similar to the effects previously found in participants with intact face recognition abilities, suggesting that face recognition mechanisms in individuals with developmental prosopagnosia are not qualitatively different from that of unimpaired individuals. Overall, these event-related potential results provide the first neurophysiological evidence for covert face recognition in developmental prosopagnosia, and suggest this phenomenon results from disconnected links between intact identity-specific visual memory traces and later semantic face processing stages. They also imply that the activation of stored visual representations of familiar faces is not sufficient for conscious explicit face recognition.
Introduction
People with prosopagnosia are unable to recognize and identify the faces of familiar individuals, despite normal low-level vision and intellect (Bodamer, 1947). Different types of prosopagnosia have been distinguished (De Renzi et al., 1991); apperceptive prosopagnosia is a selective deficit of face perception, while associative prosopagnosia is due to an impairment of long-term face memory, and/or disconnections between face perception and face memory. Acquired prosopagnosia can result from lesions to face-sensitive regions in occipito-temporal visual cortex, which often include the fusiform gyri (Barton, 2008). However, severe impairments in face recognition have also been found without history of neurological damage (Behrmann and Avidan, 2005; Duchaine and Nakayama, 2006a). In developmental prosopagnosia (also called ‘congenital prosopagnosia’), face recognition deficits are believed to be present from an early age, and are due to a failure to develop normally functioning face recognition mechanisms.
Although prosopagnosic individuals are severely impaired with explicit recognition of familiar faces, there is substantial physiological and behavioural evidence for ‘covert face recognition’, suggesting that some identity-sensitive face processing is present. Some patients with acquired prosopagnosia show enhanced skin conductance responses to overtly unrecognized faces of famous or personally familiar people (Bauer, 1984). Some can match printed names and famous faces with above-chance accuracy (Diamond et al., 1994), and others are faster to categorize written names when they are preceded by semantically related faces (Young et al., 1988), even though they are unable to explicitly recognize these faces. The presence or absence of covert face recognition can be task sensitive. Direct face recognition tasks (e.g. forced-choice identity judgements) require the explicit processing of facial identity, while indirect tasks (e.g. face priming) do not. The fact that some patients with acquired prosopagnosia show covert recognition effects only in direct tasks and others only in indirect tasks suggests that these tasks are sensitive to different aspects of identity-related face processing (Barton et al., 2004).
It is important to note that covert face recognition can be observed in some but by no means all patients with acquired prosopagnosia. This distinction could indicate that covert and overt face recognition are based on anatomically and functionally distinct face processing systems (Bauer, 1984); covert recognition is present when a lesion affects only the explicit system, but not when both systems are damaged. However, the observation that patients with more severe explicit face recognition deficits are less likely to show covert recognition (Schweinberger and Burton, 2003) suggests that overt and covert face recognition are both produced by a single face processing system, and reflect a continuum rather than qualitative differences in the underlying mechanisms (Burton et al., 1991; Farah et al., 1993). Interestingly, covert face recognition is found less often in patients with impaired face perception, and more frequently in patients with associative prosopagnosia, suggesting that covert recognition requires intact or relatively spared face perception (Schweinberger and Burton, 2003). Burton et al. (1991) have suggested that prosopagnosia can result from damaged links between stored visual representations of familiar individuals and semantic representations in long-term memory. The activation of visual representations of familiar faces will trigger only minimal activation of corresponding semantic memory traces, which can produce covert recognition effects, but is insufficient for overt face recognition.
The question whether covert face recognition can also be found in individuals with developmental prosopagnosia remains unresolved. Several early studies failed to find covert recognition in developmental prosopagnosia (De Haan and Campbell, 1991; Bentin et al., 1999; Barton et al., 2001), whereas Jones and Tranel (2001) reported differential skin conductance responses to unrecognized familiar versus unfamiliar faces in a child with associative developmental prosopagnosia. Recent support for covert recognition in developmental prosopagnosia comes from two group studies. Avidan and Behrmann (2008) tested six individuals with developmental prosopagnosia and found better/same/different judgements of two successively presented famous faces as compared with non-famous faces, even though the participants with developmental prosopagnosia did not recognize the famous faces explicitly. Rivolta et al. (2011) tested 11 individuals with developmental prosopagnosia, and found above-chance performance in a task where they had to decide which of two simultaneously presented faces was famous, even though these faces were not explicitly recognized in a control task. In contrast, no evidence for covert recognition was obtained in an indirect face priming task.
In summary, the nature of covert face recognition in prosopagnosia remains poorly understood. Are overt and covert face recognition produced by a single underlying face processing system or are they linked to anatomically and functionally distinct pathways? Do dissociations between overt and covert face recognition reflect deficits in perceptual stages of face processing, impairments of semantic face memory, or disconnected links between perceptual and semantic memory representations? Is covert face recognition exclusively linked to acquired prosopagnosia or can it also be demonstrated for individuals with developmental prosopagnosia who did not develop typical face recognition capabilities? Methodological problems are in part responsible for the failure to obtain clear-cut answers to these questions. With behavioural performance measures (e.g. forced-choice face recognition tests), it is difficult to distinguish covert face recognition and face recognition based on explicit knowledge, particularly when overt and covert face recognition tests are conducted at different times and in different task contexts (Avidan and Behrmann, 2008; Rivolta et al., 2011). Indirect measures (e.g. face identity priming effects) or autonomic responses such as differential skin conductance responses to familiar versus unfamiliar faces demonstrate the existence of covert face recognition, but cannot provide more specific insights into the underlying neural mechanisms.
In the present study, we used event-related brain potential markers of identity-related face processing to investigate the nature of covert face recognition in prosopagnosia. Event-related potentials provide on-line measures of the neural correlates of cognitive processes on a millisecond-by-millisecond basis. Since they are acquired simultaneously with, but independent of, participants’ performance in explicit face recognition tasks, event-related potential measures are ideally suited to demonstrate dissociations between covert and overt recognition (Bobes et al., 2004). Most event-related potential investigations of face processing have measured the face-sensitive N170 component that is elicited between 150 and 200 ms after stimulus onset over lateral occipito-temporal areas (Bentin et al., 1996; Eimer et al., 2010). The N170 is linked to sensory-perceptual stages of face processing that precede the recognition of individual familiar faces (but see Jacques and Rossion, 2006), is not modulated by the difference between famous and unknown faces (Bentin and Deouell, 2000; Eimer, 2000), and is therefore not directly relevant for the study of covert face recognition. Event-related potential markers of identity-related face processing emerge at post-stimulus latencies of 200 ms and beyond. When individual faces are encountered twice in rapid succession, they trigger an enhanced negativity at inferior occipito-temporal electrodes that is maximal around 250 ms after stimulus onset, and is often larger over the right hemisphere (Begleiter et al., 1995; Schweinberger et al., 1995, 2002). This repetition-induced N250r component has been linked to the activation of a representation of a specific face in visual memory that is triggered by its match with a currently presented face (Schweinberger and Burton, 2003). The fact that the N250r component is larger for repetitions of famous faces (Herzmann et al., 2004) suggests that such memory traces are activated more strongly for faces that have pre-existing long-term representations.
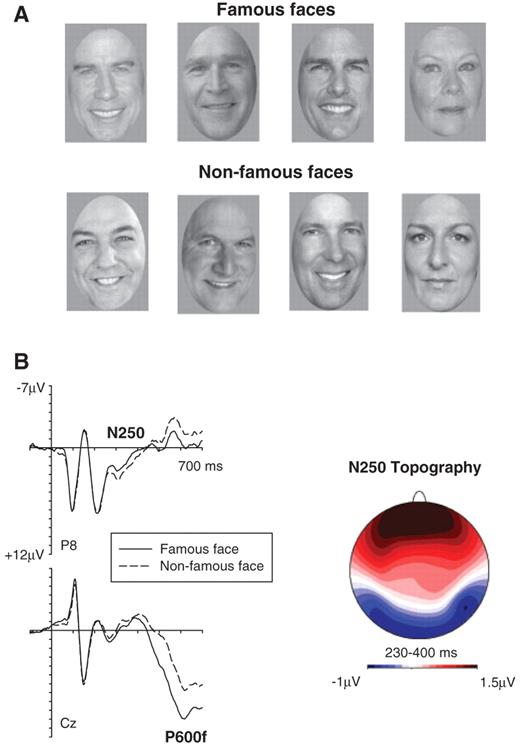
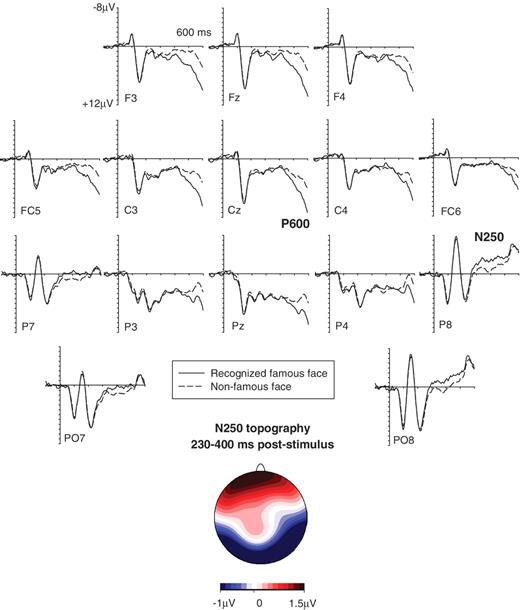
In a recent experiment (Gosling and Eimer, 2011), we obtained direct evidence for a link between the occipito-temporal N250 component and explicit face recognition. Participants made famous/non-famous judgements for sequentially presented famous and non-famous faces (Fig. 1). Relative to non-famous faces, an enhanced negativity was triggered by correctly identified famous faces between 230 and 400 ms after stimulus onset at lateral occipito-temporal electrodes (Fig. 1). Importantly, this N250 component was observed for famous faces that were judged to be definitely known, but not for famous faces that just seemed familiar, demonstrating that it is linked to explicit face recognition, rather than more unspecific face familiarity. The N250 component is triggered by images of famous faces even when they are presented for the first time, while the N250r is triggered by repeated faces, even when they are unfamiliar. In spite of this difference, the similarity of the topography of the N250 to famous faces (Fig. 1) to the N250r topography (Schweinberger et al., 2004) indicates that they may share a common neural basis. Their well-defined occipito-temporal focus suggests that these components are associated with the activation of memory representations of individual faces in ventral visual cortex. They reflect an early visual stage of face recognition where on-line perceptual representations of faces are matched with stored representations of their visual features. A successful match will trigger further explicit recognition processes, such as name retrieval and the activation of semantic or episodic information about a specific individual (Bruce and Young, 1986). Such later post-perceptual face recognition processes are associated with identity-sensitive event-related potential modulations at longer post-stimulus latencies. Relative to non-famous faces, famous faces elicit a sustained positivity (P600f) that emerges around 400 ms after stimulus onset and is broadly distributed across anterior and more posterior areas (Bentin and Deouell, 2000; Eimer, 2000). Figure 1 shows the P600f component triggered by recognized famous faces in our previous study (Gosling and Eimer, 2011).
(A) Examples of famous faces and non-famous faces used in the present experiment and in a corresponding study with non-impaired participants (Gosling and Eimer, 2011). Matching famous and non-famous faces are shown in corresponding positions. (B) Grand-averaged event-related potentials measured in the Gosling and Eimer (2011) experiment to famous faces classified as known/familiar (solid lines) and non-famous faces classified as unfamiliar/unknown (dashed lines) in the 700 ms interval after stimulus onset, showing the N250 component at right occipito-temporal electrode P8, and the P600f at midline electrode Cz. The topographic map (right) shows difference amplitudes obtained during the N250 time interval (230–400 ms post-stimulus) by subtracting event-related potential mean amplitudes in response to non-famous faces from mean amplitudes to famous faces. Enhanced negative amplitudes for famous faces are shown in blue, an enhanced positivity in red. The map was constructed by spherical spline interpolation (Perrin et al., 1989).
In observers with intact face processing abilities, N250 and P600f components are linked to early visual and later post-perceptual stages of face recognition, respectively. The aim of the present study was to find out whether these components can also be observed in individuals with developmental prosopagnosia. We tested a group of 12 participants with developmental prosopagnosia, who all reported severe difficulties in recognizing familiar faces since childhood. Standardized tests of face processing confirmed their reports (Table 1). They then performed an explicit face recognition task in response to famous and non-famous faces while EEG was recorded. Face stimuli and task instructions were identical to our previous event-related potential study with participants with normal face recognition (Gosling and Eimer, 2011); each face had to be categorized on a four-point scale (definitely known, merely familiar, unfamiliar or definitely unknown). To simplify analyses and presentation, faces were classified as ‘recognized’ when participants judged them as known or familiar, and as ‘non-recognized’ when they were judged to be unfamiliar or unknown. As would be expected given their severe face processing impairments, the participants with developmental prosopagnosia failed to recognize most famous faces (Table 2). The main event-related potential analysis therefore focused on trials where famous faces were not recognized. Covert recognition of famous faces for at least some of the participants with developmental prosopagnosia should be reflected by systematic event-related potential differences between these faces and non-recognized non-famous faces. Such covert recognition effects might even be similar to the N250 and/or P600f components previously observed during explicit face recognition, which would link them to visual and episodic-semantic stages of identity-related face processing. The presence of N250 or P600f components in response to non-recognized famous faces was assessed with a non-parametric bootstrap procedure (Di Nocera and Ferlazzo, 2000) separately for each participant with developmental prosopagnosia. In a second analysis, event-related potentials to those few famous faces that were recognized by the participants with developmental prosopagnosia were compared with event-related potentials to non-famous faces to test whether event-related potential markers of explicit face recognition in individuals with developmental prosopagnosia are equivalent to those found in participants with intact face processing abilities.
Participants in the study
| Participants . | Age . | Gender . | CFMT . | CFPT . | ONT . |
|---|---|---|---|---|---|
| M.C.* | 41 | M | −1.38 | −1.54 | −2.46 |
| E.W.* | 32 | F | −2.64 | 0.92 | −3.43 |
| A.M.C. | 47 | F | −2.77 | −4.06 | −8.33 |
| N.E. | 31 | F | −2.77 | −1.06 | −4.17 |
| T.L.* | 51 | M | −2.26 | −0.38 | −8.38 |
| J.A. | 45 | F | −2.64 | −0.92 | −3.35 |
| A.H.* | 48 | F | −1.76 | −1.06 | −2.04 |
| A.M. | 28 | F | −2.64 | −1.74 | −2.88 |
| S.W. | 28 | F | −2.64 | −1.74 | −2.95 |
| K.S. | 29 | F | −2.9 | −0.92 | −9.03 |
| S.C.* | 22 | F | −2.64 | −0.51 | −4.15 |
| J.L.* | 66 | F | −1.76 | −2.29 | −6.27 |
| Participants . | Age . | Gender . | CFMT . | CFPT . | ONT . |
|---|---|---|---|---|---|
| M.C.* | 41 | M | −1.38 | −1.54 | −2.46 |
| E.W.* | 32 | F | −2.64 | 0.92 | −3.43 |
| A.M.C. | 47 | F | −2.77 | −4.06 | −8.33 |
| N.E. | 31 | F | −2.77 | −1.06 | −4.17 |
| T.L.* | 51 | M | −2.26 | −0.38 | −8.38 |
| J.A. | 45 | F | −2.64 | −0.92 | −3.35 |
| A.H.* | 48 | F | −1.76 | −1.06 | −2.04 |
| A.M. | 28 | F | −2.64 | −1.74 | −2.88 |
| S.W. | 28 | F | −2.64 | −1.74 | −2.95 |
| K.S. | 29 | F | −2.9 | −0.92 | −9.03 |
| S.C.* | 22 | F | −2.64 | −0.51 | −4.15 |
| J.L.* | 66 | F | −1.76 | −2.29 | −6.27 |
List of the 12 individuals with developmental prosopagnosia who participated in this study, together with z-scores of each individual's performance in the Cambridge Face Memory Test (CFMT), Cambridge Face Perception Test (CFPT) with upright faces, and Old-New Test (ONT) (see text for details). Participants marked with an asterisk showed a reliable N250 component to non-recognized famous faces. F = female; M = male.
Participants in the study
| Participants . | Age . | Gender . | CFMT . | CFPT . | ONT . |
|---|---|---|---|---|---|
| M.C.* | 41 | M | −1.38 | −1.54 | −2.46 |
| E.W.* | 32 | F | −2.64 | 0.92 | −3.43 |
| A.M.C. | 47 | F | −2.77 | −4.06 | −8.33 |
| N.E. | 31 | F | −2.77 | −1.06 | −4.17 |
| T.L.* | 51 | M | −2.26 | −0.38 | −8.38 |
| J.A. | 45 | F | −2.64 | −0.92 | −3.35 |
| A.H.* | 48 | F | −1.76 | −1.06 | −2.04 |
| A.M. | 28 | F | −2.64 | −1.74 | −2.88 |
| S.W. | 28 | F | −2.64 | −1.74 | −2.95 |
| K.S. | 29 | F | −2.9 | −0.92 | −9.03 |
| S.C.* | 22 | F | −2.64 | −0.51 | −4.15 |
| J.L.* | 66 | F | −1.76 | −2.29 | −6.27 |
| Participants . | Age . | Gender . | CFMT . | CFPT . | ONT . |
|---|---|---|---|---|---|
| M.C.* | 41 | M | −1.38 | −1.54 | −2.46 |
| E.W.* | 32 | F | −2.64 | 0.92 | −3.43 |
| A.M.C. | 47 | F | −2.77 | −4.06 | −8.33 |
| N.E. | 31 | F | −2.77 | −1.06 | −4.17 |
| T.L.* | 51 | M | −2.26 | −0.38 | −8.38 |
| J.A. | 45 | F | −2.64 | −0.92 | −3.35 |
| A.H.* | 48 | F | −1.76 | −1.06 | −2.04 |
| A.M. | 28 | F | −2.64 | −1.74 | −2.88 |
| S.W. | 28 | F | −2.64 | −1.74 | −2.95 |
| K.S. | 29 | F | −2.9 | −0.92 | −9.03 |
| S.C.* | 22 | F | −2.64 | −0.51 | −4.15 |
| J.L.* | 66 | F | −1.76 | −2.29 | −6.27 |
List of the 12 individuals with developmental prosopagnosia who participated in this study, together with z-scores of each individual's performance in the Cambridge Face Memory Test (CFMT), Cambridge Face Perception Test (CFPT) with upright faces, and Old-New Test (ONT) (see text for details). Participants marked with an asterisk showed a reliable N250 component to non-recognized famous faces. F = female; M = male.
Mean frequencies (in percentages) of classifying famous or non-famous faces as recognized (known or familiar) or non-recognized (unfamiliar or unknown)
| Face type . | Recognized (%) . | Non-recognized (%) . |
|---|---|---|
| All participants with developmental prosopagnosia (n = 12) | ||
| Famous | 27.20 | 72.56 |
| Non-famous | 8.77 | 90.10 |
| Participants with developmental prosopagnosia with a reliable N250 to non-recognized famous faces (n = 6) | ||
| Famous | 24.29 | 75.26 |
| Non-famous | 4.13 | 95.30 |
| Face type . | Recognized (%) . | Non-recognized (%) . |
|---|---|---|
| All participants with developmental prosopagnosia (n = 12) | ||
| Famous | 27.20 | 72.56 |
| Non-famous | 8.77 | 90.10 |
| Participants with developmental prosopagnosia with a reliable N250 to non-recognized famous faces (n = 6) | ||
| Famous | 24.29 | 75.26 |
| Non-famous | 4.13 | 95.30 |
The top panel shows results across all 12 participants with developmental prosopagnosia, the bottom panel for those six participants with developmental prosopagnosia who showed event-related potential evidence for covert face recognition (reliable N250 components to non-recognized famous faces).
Mean frequencies (in percentages) of classifying famous or non-famous faces as recognized (known or familiar) or non-recognized (unfamiliar or unknown)
| Face type . | Recognized (%) . | Non-recognized (%) . |
|---|---|---|
| All participants with developmental prosopagnosia (n = 12) | ||
| Famous | 27.20 | 72.56 |
| Non-famous | 8.77 | 90.10 |
| Participants with developmental prosopagnosia with a reliable N250 to non-recognized famous faces (n = 6) | ||
| Famous | 24.29 | 75.26 |
| Non-famous | 4.13 | 95.30 |
| Face type . | Recognized (%) . | Non-recognized (%) . |
|---|---|---|
| All participants with developmental prosopagnosia (n = 12) | ||
| Famous | 27.20 | 72.56 |
| Non-famous | 8.77 | 90.10 |
| Participants with developmental prosopagnosia with a reliable N250 to non-recognized famous faces (n = 6) | ||
| Famous | 24.29 | 75.26 |
| Non-famous | 4.13 | 95.30 |
The top panel shows results across all 12 participants with developmental prosopagnosia, the bottom panel for those six participants with developmental prosopagnosia who showed event-related potential evidence for covert face recognition (reliable N250 components to non-recognized famous faces).
Participants and methods
Participants
Twelve participants with developmental prosopagnosia (10 females), aged 22–66 years, were tested. All reported severe difficulties in face recognition since childhood, and were recruited after contacting us on our research website (http://www.faceblind.org). To assess and verify their reported face recognition problems, a series of behavioural tests were conducted in two testing sessions on separate days. Table 1 shows z-scores of the performance of all 12 participants with developmental prosopagnosia in three of these tests. In the Cambridge Face Memory Test, faces of six target individuals shown in different views are memorized, and then have to be distinguished from two simultaneously presented distractor faces (see Duchaine and Nakayama, 2006b for a full description). In the Old-New Face Recognition test (Duchaine and Nakayama, 2005), 10 target faces (young females photographed under similar conditions and from the same angle) are memorized. In the test phase, target faces and 30 new faces are presented in random order, and an old/new discrimination is required for each face. In the Cambridge Face Perception Test (Duchaine et al., 2007), one target face in three-quarter view is shown above six frontal-view morphed test faces that contain a different proportion of the target face and have to be sorted according to their similarity to the target face. Faces are presented either upright or inverted. As can be seen in Table 1, all participants with developmental prosopagnosia had severe impairments in the two face recognition tests, and most of them also showed face perception deficits.
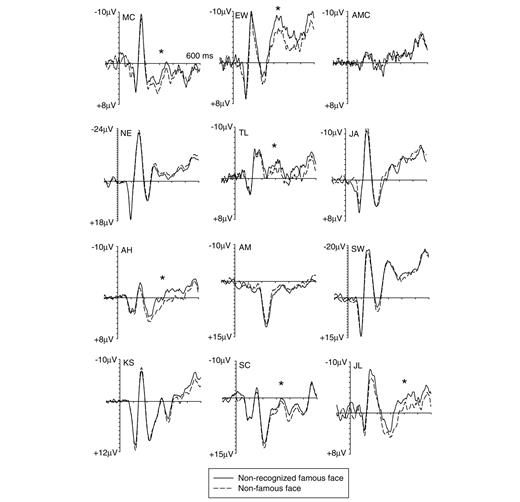
The mean age of the developmental prosopagnosia group (39 years) was not fully matched to the mean age of the participants with intact face processing tested previously (Gosling and Eimer, 2011; mean age 27.3 years), and one participant (Participant J.L.) was considerably older (66 years) than all others. However, inspection of visual event-related potentials measured for Participant J.L. revealed no age-related delays (Fig. 2), and her data were therefore retained in the sample.
Event-related potential markers of covert face recognition for individual participants with developmental prosopagnosia. Event-related potentials elicited for each of the 12 participants with developmental prosopagnosia measured at right occipito-temporal electrode P8 to famous faces (solid lines) and non-famous faces (dashed lines) on trials where these faces were classified as unfamiliar/unknown. Bootstrap analyses confirmed that six participants with developmental prosopagnosia showed a statistically reliable N250 component at P8 (indicated by asterisks) to non-recognized famous faces. Note that different voltage scales were used for individual participants.
Materials and procedure
All stimuli were presented on a CRT monitor at a viewing distance of 100 cm, using E-Prime software (Psychology Software Tools). The stimulus set consisted of 80 famous and 80 non-famous faces. Except for one US resident participant with developmental prosopagnosia (see below), these faces were identical to those used in our previous study with non-impaired participants (Gosling and Eimer, 2011). Famous faces were celebrities widely known to the general public in the UK (e.g. actors/actresses, politicians, chefs, comedians, entrepreneurs, models, members of the royal family, sports personalities or musicians). For each of the 80 famous faces, one non-famous face was assigned as a direct match in terms of gender, approximate age, facial expression and low-level visual attributes such as contrast and brightness (see Fig. 1 for examples of matched famous and non-famous faces). For one of the 12 participants with developmental prosopagnosia (Participant S.C.) who was a US citizen on a short-stay in the UK, different famous faces (individuals well-known to a US audience), but the same non-famous faces were used. All face stimuli were converted to greyscale, resized and cropped into an oval shape, which removed their outer contours. They were presented at fixation, with eye gaze straight ahead, against a grey background (17.6 cd/m2), and subtended a visual angle of 7.4 × 4.9°. The average luminance of the face images was 21.9 cd/m2.
Eight experimental blocks of 80 trials were run. Famous and non-famous faces were presented with equal probability and in random order. Each individual famous and non-famous face thus appeared exactly four times throughout the experiment. A face was presented at fixation for 400 ms, followed by a blank interstimulus interval of 1300 ms. Participants were instructed to report on each trial whether they recognized a particular face by choosing one of four response alternatives (definitely known, seems familiar, seems unfamiliar or definitely unknown) that were mapped onto four horizontally arranged response keys on a purpose-built response pad. They were told to classify a face as definitely known only when they knew the person's name and profession, and to use the ‘seems familiar’ category if they perceived a face to be familiar, but were not able to name the person or state their profession. Each response key was labelled with its response category, and was mapped to the index and middle fingers of the left and right hand. The same assignment of keys and response categories was used for all participants. A training block containing 20 different famous faces and 20 different non-famous faces was delivered prior to the first experimental block. For two of the participants with developmental prosopagnosia tested (Participants A.H. and J.L.), testing had to be stopped after six or five experimental blocks, respectively, due to reported fatigue. However, the number of trials retained for both participants was sufficiently high to compute meaningful event-related potential waveforms.
After the conclusion of the experimental blocks, we tested how many of the 80 famous individuals whose faces were included in the experiment were actually known by the participants with developmental prosopagnosia. Each of them was given a list with the names of these individuals, and when appropriate, also their stage character names (e.g. Harry Potter), and were asked to indicate whether they were known or unknown to them. When a participant indicated that they knew a particular famous individual but had never seen their face prior to the experiment, this face was classified as unknown.
Electroencephalography recording and data analysis
EEG was DC recorded with a BrainAmps DC amplifier (Brain Products, Munich, Germany; upper cut-off frequency 40 Hz, 500 Hz sampling rate) and Ag–AgCl electrodes mounted on an elastic cap from 23 scalp sites (Fpz, F7, F3, Fz, F4, F8, FC5, FC6, T7, C3, Cz, C4, T8, CP5, CP6, P7, P3, Pz, P4, P8, PO7, PO8 and Oz, according to the extended international 10–20 system). Horizontal electrooculogram was recorded bipolarly from the outer canthi of both eyes. An electrode placed on the left earlobe served as reference for online recording, and EEG was re-referenced off-line to the average of the left and right earlobe. Electrode impedances were kept below 5 kΩ. No additional off-line filters were applied. EEG was epoched offline from 100 ms before to 600 ms after stimulus onset. Epochs with activity exceeding ±30 μV in the horizontal electrooculogram channel (reflecting horizontal eye movements) or ±60 μV at Fpz (indicating eye blinks or vertical eye movements) were excluded from analysis, as were epochs with voltages exceeding ±80 μV at any other electrode.
Following artefact rejection, averages were computed for trials where famous faces were correctly classified as known or familiar, trials where famous faces were judged to be unfamiliar or unknown, and trials with non-famous faces that were correctly classified as unfamiliar/unknown. All event-related potentials were computed relative to a 100 ms prestimulus baseline. Event-related potential markers of explicit face recognition were quantified by comparing event-related potentials to famous faces that were correctly categorized as known or familiar and non-famous faces classified as unfamiliar/unknown. This was done for those electrodes and time intervals where components linked to explicit face recognition (N250, P600f) were identified previously (Gosling and Eimer, 2011). N250 amplitude was measured as mean amplitudes in a 230–400 ms post-stimulus time window at right occipito-temporal electrode P8. P600f mean amplitudes were measured during the 400–600 ms time window at midline electrode Cz. These analyses included data from only 11 participants with developmental prosopagnosia, as one other (T.L.) categorized only 2.1% of all famous faces as known or familiar, resulting in an insufficient number of trials with explicitly recognized famous faces.
To identify correlates of covert face recognition, event-related potentials to non-recognized famous and non-famous faces were compared for the same time windows and electrodes. To assess the presence of statistically reliable N250 or P600f components to non-recognized famous faces for individual participants with developmental prosopagnosia, a non-parametric bootstrap procedure (Efron, 1993; Di Nocera and Ferlazzo, 2000) was used. This procedure establishes the reliability of event-related potential differences between two experimental conditions by resampling two sets of trials that are drawn randomly (with replacement) from the combined data set, and then computing the difference amplitude between the two resulting event-related potentials for a predefined time window and electrode. This procedure is repeated a large number of times (10 000 iterations in the current study). The resulting distribution of difference amplitudes has a mean value of zero, because both sample pairs are always drawn from the same data set. Based on this distribution, the reliability of an observed event-related potential difference between conditions can be assessed for individual participants. If the probability of obtaining the observed difference by chance is <5%, it can be accepted as statistically significant.
Results
Behaviour
Table 2 shows the mean frequency with which famous or non-famous faces were classified as known or familiar (recognized) or as unfamiliar/unknown (non-recognized). Because classification responses were consistent across all four presentations of the same face, values were averaged across these presentations. No response was recorded on 0.55% of all trials, which were not further analysed. Participants with developmental prosopagnosia correctly classified >90% of all non-famous faces as unfamiliar or unknown. As expected, they were very poor in recognizing famous faces. Only 12% of these faces were judged to be definitely known, and a further 15% as familiar. Recognition performance was not better for those six participants with developmental prosopagnosia who showed event-related potential evidence of covert face recognition (Table 2).
The mean d′ value for discriminating between famous and non-famous faces across all participants with developmental prosopagnosia was 0.90 (SD = 0.41). In our previous study (Gosling and Eimer, 2011), participants with normal face recognition categorized 72% of the same famous faces as definitely known, and an additional 10% as familiar, resulting in a d′ value for discriminating famous and non-famous faces of 1.67 (SD = 0.59). Their poor face recognition performance was not due to the fact that the participants with developmental prosopagnosia simply did not know many of the famous individuals whose faces were shown. In the interviews conducted after the experiment, on average 95.25% of these individuals were reported as known. These values ranged between 87.5% and 100% for individual participants, and did not differ between participants with developmental prosopagnosia who showed a reliable N250 component and those who did not (95.4% versus 95.1%, respectively).
Mean reaction time across both face types and all response categories was 879 ms, and did not differ reliably between famous and non-famous faces (896 versus 872 ms; t < 1). Reaction times were faster on trials where famous or non-famous faces were classified as definitely unknown (740 ms) relative to trials where they were judged as definitely known, familiar, or unfamiliar [900, 955 or 960 ms; all t(10) > 5.7, all P < 0.01].
Event-related potential markers of covert face recognition in developmental prosopagnosia
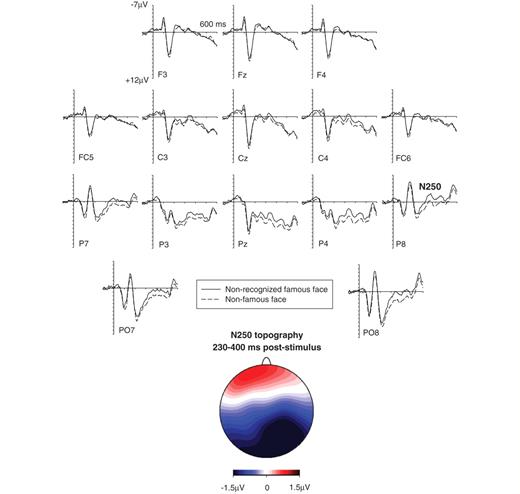
Figure 2 shows event-related potentials recorded for each of the 12 participants with developmental prosopagnosia tested at occipito-temporal electrode P8 in response to non-recognized famous faces and non-famous faces. While early components (P1, N170) did not appear to be systematically affected by the difference between objectively famous and non-famous faces, an enhanced negativity beyond 200 ms post-stimulus indicative of an N250 component was present for some but not all participants with developmental prosopagnosia. Non-parametric bootstrap analyses conducted separately for each participant on event-related potential mean amplitude differences measured at P8 during the 230–400 ms post-stimulus interval confirmed that the enhanced negativity to non-recognized famous faces was reliable in 6 out of the 12 participants with developmental prosopagnosia tested. The presence of a reliable N250 amplitude difference (i.e. an amplitude difference with a likelihood of <5% to have been produced by chance, see ‘Participants and methods’ section for details of the bootstrap analyses) for an individual participant is indicated by the asterisks in Fig. 2. The grand-averaged event-related potentials to non-recognized famous and non-famous faces for those six participants with developmental prosopagnosia, who were identified by the bootstrap analysis as having a reliable N250 component at P8 are shown in Fig. 3, together with a topographic map of event-related potential difference amplitudes for famous versus non-famous faces in the N250 time window. An enhanced occipito-temporal negativity to non-recognized famous faces relative to non-famous faces was accompanied by an enhanced anterior positivity. A further analysis (reported below) indicated that this N250 to non-recognized faces was similar to the N250 triggered during explicit face recognition.
Event-related potential correlates of covert face recognition in developmental prosopagnosia. Grand-averaged event-related potentials elicited by non-recognized famous and non-famous faces for those six participants with developmental prosopagnosia who showed a reliable N250 component in the bootstrap analyses. The topographic map shows the scalp distribution of event-related potential difference amplitudes (non-recognized famous faces minus non-famous faces) in the N250 time interval (230–400 ms post-stimulus).
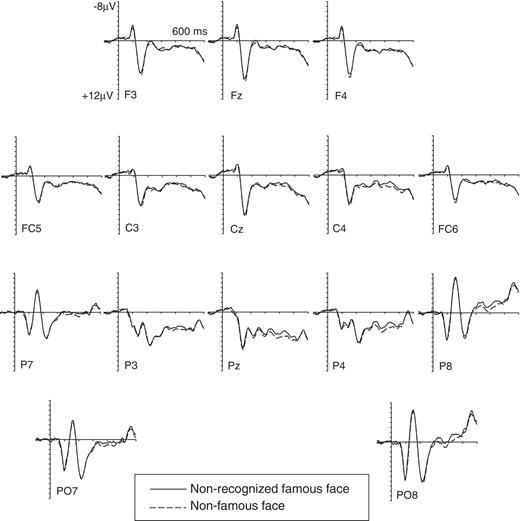
Figure 4 shows grand-averaged event-related potentials to non-recognized famous and non-famous faces, across all 12 participants with developmental prosopagnosia tested (i.e. the six participants with developmental prosopagnosia who had a reliable N250 to these faces, and the other six who did not). There was a small N250 component in response to non-recognized famous faces at lateral occipito-temporal electrodes (reflecting the fact that six participants with developmental prosopagnosia showed a reliable N250), but there was no evidence for differential event-related potential modulations to non-recognized famous versus non-famous faces in the subsequent P600f time window (400–600 ms post-stimulus). To quantitatively assess the presence of an N250 component in response to non-recognized famous faces across all 12 participants with developmental prosopagnosia tested, event-related potential mean amplitudes obtained at P8 between 230 and 400 ms post-stimulus were analysed. The main effect of face type (recognized famous versus non-famous face) was not significant, F(1,11) = 3.14; P = 0.1. The P600f component was assessed on the basis of event-related potential mean amplitudes obtained at midline electrodes Fz, Cz and Pz in the 400–600 ms post-stimulus time window. There was no main effect of face type, F(1,11) < 1, confirming that no P600f was triggered by non-recognized famous faces across all 12 participants with developmental prosopagnosia tested. To investigate whether a reliable P600f was present for any developmental prosopagnosic, event-related potential mean amplitude differences between non-recognized famous and non-famous faces in the 400–600 ms post-stimulus time window at midline electrode Cz were subjected to further bootstrap analyses. Only one of the 12 participants with developmental prosopagnosia tested (Patient M.C.) showed a reliable P600f component. Finally, an analysis of the N170 component (quantified on the basis of event-related potential mean amplitudes in the 160–200 ms post-stimulus interval at lateral occipito-parietal electrodes P7/P8) showed no effect of face type, and no interaction between face type and hemisphere, both F(1,11) < 1, confirming the absence of any link between N170 amplitudes and covert face recognition.
Grand-averaged event-related potentials elicited by non-recognized famous and non-famous faces, based on data from all 12 participants with developmental prosopagnosia tested. No P600f component was triggered by non-recognized famous faces.
Event-related potential markers of explicit face recognition in developmental prosopagnosia
Figure 5 shows grand-averaged event-related potentials to those famous faces that were classified as known or familiar, and non-famous faces classified as unfamiliar/unknown, based on the data of 11 participants with developmental prosopagnosia. Explicit face recognition was associated with N250 and P600f components, analogous to the results found previously for participants with unimpaired face processing (Gosling and Eimer, 2011). The scalp topography of the N250 component to famous faces that were explicitly recognized by the participants with developmental prosopagnosia (Fig. 5) was very similar to the N250 topography observed for normal participants (Fig. 1). The analysis of event-related potential mean amplitudes obtained in the 230–400 ms post-stimulus time window at P8 revealed a reliable effect of face type (recognized famous versus non-famous face), F(1,10) = 12.5; P < 0.01, confirming the presence of the N250 component. A significant effect of face type was also present for mean amplitudes measured in the 400–600 ms time interval at Cz, F(1,10) = 7.6; P < 0.03, confirming that the P600f component was elicited during explicit face recognition in this group of 11 participants with developmental prosopagnosia. These analyses included the between-subject factor covert recognition (participants with developmental prosopagnosia who showed a significant N250 to famous faces that were not explicitly recognized versus participants with developmental prosopagnosia who did not). No interactions between face type and covert recognition were found for the N250 or P600f analyses, both F < 1, indicating event-related potential correlates of overt face recognition were present in both groups.
Event-related potential correlates of explicit face recognition in developmental prosopagnosia. Grand-averaged event-related potentials based on data from 11 participants with developmental prosopagnosia, in response to famous faces classified as known/familiar (solid lines) and non-famous faces classified as unfamiliar/unknown (dashed lines). The topographic map shows the scalp distribution of event-related potential difference amplitudes (recognized famous faces minus non-famous faces) in the N250 time interval (230–400 ms post-stimulus).
A final analysis directly compared N250 mean amplitudes measured in the 230–400 ms interval at P8 for explicit face recognition and covert recognition for those five participants with developmental prosopagnosia who showed both a reliable N250 component to non-recognized famous faces and had a sufficient number of trials where famous faces were recognized. N250 mean amplitudes were similar in size for recognized and non-recognized famous faces (−2.14 and −1.64 µV, respectively), and did not differ significantly [t(4) = 0.9].
Discussion
A group of 12 individuals with developmental prosopagnosia performed a task where they had to classify sequentially presented famous and non-famous faces as definitely known, familiar, unfamiliar or definitely unknown. EEG was recorded during task performance, in order to obtain on-line electrophysiological markers of overt and covert face recognition. As expected, participants with developmental prosopagnosia classified only 27% of all famous faces as known or familiar (compared with an 82% recognition rate found for the same famous faces with unimpaired participants; Gosling and Eimer, 2011). To identify event-related potential correlates of covert face recognition in developmental prosopagnosia, event-related potentials measured on those trials where famous faces were judged to be unfamiliar and unknown were compared with event-related potentials in response to non-famous faces. As shown in Figs 2 and 3, 6 of the 12 participants with developmental prosopagnosia tested did show a reliable occipito-temporal N250 component to famous faces in the absence of explicit recognition. The N250 has been linked to the activation of visual memory traces of stored individual faces, and its scalp topography was very similar to the N250 topography previously observed during explicit face recognition (Gosling and Eimer, 2011). To ensure that the presence of reliable N250 components to non-recognized famous faces for these participants with developmental prosopagnosia is a meaningful result, and not just an artefact of the bootstrap analyses performed for individual participants, we used exactly the same bootstrap methods to compare event-related potentials to non-recognized famous and non-famous faces for each of the 16 individuals with intact face processing that took part in our previous study (Gosling and Eimer, 2011). None of these 16 participants showed a reliable occipito-temporal N250 component to non-recognized famous faces in the 230–400 ms post-stimulus interval, thus demonstrating that the presence of significant N250 components to non-recognized famous faces for 6 of the 12 participants with developmental prosopagnosia tested in this study is a valid finding.
The observation that the N250 component is an event-related potential marker of covert face recognition in developmental prosopagnosia confirms and extends previous observations by Avidan and Behrmann (2008) and Rivolta et al. (2011), who found behavioural evidence for covert face recognition in individuals with developmental prosopagnosia. A major problem for behavioural investigations of covert face recognition is the fact that direct and indirect measures (e.g. forced-choice face identification performance and identity-related priming effects) are obtained at different times and in different task contexts, which raises concerns about the validity of covert face recognition effects. For example, failure of a developmental prosopagnosic to recognize a photograph of Bill Clinton does not demonstrate that the developmental prosopagnosic would fail to recognize that image or another image of Clinton at another time. No such problems affect the present event-related potential study, where direct behavioural measures of explicit face recognition and indirect electrophysiological markers of identity-sensitive face processing were obtained simultaneously and independently. Therefore, the observed dissociation between these two measures provide new and methodologically solid evidence for covert face recognition in developmental prosopagnosia, and thus demonstrate that covert recognition is present for both acquired and developmental prosopagnosics. There was also no difference in response bias measure C between participants with developmental prosopagnosia who did show an N250 to non-recognized famous faces and those who did not, t(11) = 1.37; P = 0.2, confirming that the presence of an N250 in the former group was not simply due to a stronger bias towards classifying famous faces as non-familiar.
In contrast to the N250, the subsequent P600f component that is linked to the activation of semantic or episodic memory traces about a specific individual was not present on trials where a famous face was not recognized. This dissociation provides new insights into the locus of covert face recognition effects within the face processing hierarchy. The N250 is linked to early visual–perceptual stages of identity-related face processing, whereas the P600f is associated with later post-perceptual stages that involve semantic or episodic memory. The presence of an N250 component to non-recognized famous faces indicates that these faces did activate a corresponding visual memory trace, which implies that in spite of the lack of overt recognition, a successful match between on-line perceptual information and stored visual representations of famous faces did take place. The absence of a P600f component to non-recognized famous faces in five of the six participants with developmental prosopagnosia who showed a reliable N250 points to a relatively late locus of their face recognition impairments. These impairments do not seem to be linked to deficits in the visual aspects of identity-related face processing, but may instead result from the disruption of links between stored visual representations of familiar individuals and semantic or episodic representations in long-term memory.
This interpretation is consistent with previous views of prosopagnosia as a disconnection phenomenon. In some individuals with developmental prosopagnosia, face recognition deficits emerge when links between stored visual representations of familiar individuals and associated semantic memory traces are damaged (Burton et al., 1991). In the face processing architecture proposed by Bruce and Young (1986), this disconnection would be located between visual face recognition units and subsequent semantic person identity nodes. A similar disconnection account has been put forward by Breen et al. (2000) to account for the patterns of dissociation between overt and covert face recognition observed in acquired prosopagnosia and in the ‘Capgras’ delusion (the belief that a close relative or friend has been replaced by an impostor). Preserved autonomic responses in the absence of explicit face recognition in acquired prosopagnosia (e.g. Bauer, 1984) result when face recognition unit–person identity node links are severed but links between face recognition units and affective responses remain intact. Conversely, the Capgras delusion is produced when face recognition unit–person identity node connections are intact, but associations between face recognition units and the affective response system are disrupted. If the N250 and P600f components observed in the present study reflect face recognition unit and person identity node activations, respectively, this hypothesis would imply that both components should be elicited in Capgras patients.
The question whether the neural processes that underlie overt or covert face recognition in individuals with developmental prosopagnosia are similar or qualitatively different from the processes that subserve normal face recognition remains controversial. Given the early onset of developmental prosopagnosia, affected individuals may never develop a typical face processing architecture, which could imply that an adult neuropsychological model that attributes face recognition deficits to damaged subcomponents within an otherwise normal system may be inappropriate (Karmiloff-Smith, 1997). The current results do not support this view, but instead demonstrate marked similarities in the processes that are activated during explicit face recognition in individuals with developmental prosopagnosia and unimpaired participants. On those trials where the participants with developmental prosopagnosia successfully recognized famous faces, an occipito-temporal N250 component was followed by a broadly distributed P600f component (Fig. 5). Because these trials were infrequent, no separate averages could be computed for faces judged to be definitely known as compared with merely familiar, in contrast to Gosling and Eimer (2011), who found that in participants with intact face recognition, N250 and P600f components were triggered by explicitly known faces only. The fact that N250 and P600f components were reliably present in response to known or familiar famous faces in the developmental prosopagnosia group suggests that there are no fundamental qualitative differences in the brain mechanisms that underlie successful face recognition between these two groups. Along similar lines, the fact that the same N250 component that is usually found during explicit face recognition was linked to covert face recognition in six participants with developmental prosopagnosia is inconsistent with the view that overt and covert recognition are associated with anatomically and functionally distinct systems (Bauer, 1984), and instead suggests that both are produced by a single face processing system (Burton et al., 1991).
The current results also suggest that the activation of stored visual face representations of specific individuals, as reflected by the N250 component, is not sufficient for overt face recognition. In order to be consciously recognized, familiar faces also need to be processed at subsequent post-perceptual semantic stages. This hypothesis is supported by the dissociation between N250 and P600f components on trials where famous faces were not recognized (Fig. 3), and by the fact that the P600f was triggered only during the explicit recognition of famous faces (Fig. 5). The strong link between the P600f component and explicit face recognition is also important for the interpretation of the N250 as an event-related potential marker of covert face recognition in developmental prosopagnosia. The presence of an N250 to famous faces that were judged to be unfamiliar could reflect very high criteria for classifying faces as famous or familiar or even response selection errors. The fact that no P600f was triggered by these faces does instead suggest that participants with developmental prosopagnosia did not have conscious access to the identity of these faces. The only exception to this general pattern was Participant M.C., who had reliable N250 and P600f components to famous faces reported to be unfamiliar or unknown. As shown in Table 1, this participant outperformed the other participants with developmental prosopagnosia in all three behavioural face processing tests, suggesting that his face recognition deficit may at least in part be located at late decision-related stages.
Evidence for covert face recognition has previously been observed for some but by no means all prosopagnosic individuals, and this was confirmed in the present study. Six of the 12 participants with developmental prosopagnosia tested showed no differential event-related potential modulations to non-recognized famous versus non-famous faces (Fig. 4). The absence of an N250 component in these individuals suggests that famous faces categorized as unfamiliar or unknown did not trigger identity-specific visual memory traces. However, the normal N250 and P600f components shown by these participants with developmental prosopagnosia to explicitly recognized famous faces suggests that even for this group, some stored visual representations of known faces remained available. A between-group comparison of z-scores in the Cambridge Face Memory Test revealed that participants with developmental prosopagnosia who showed a reliable N250 to non-recognized famous faces performed significantly better than participants with developmental prosopagnosia who did not show an N250 [t(10) = 3.0; P < 0.02]. This result supports previous suggestions that the presence or absence of covert face recognition in prosopagnosia is linked to the quality of face representations in visual memory (Schweinberger and Burton, 2003), and also suggests functional links between the degree to which visual face memory is preserved in developmental prosopagnosia and performance in face identity matching tasks. Participants with developmental prosopagnosia who showed covert face recognition also tended to perform better than the other participants with developmental prosopagnosia in the other tests shown in Table 1 (Cambridge Face Perception Test and Old-New Test), but these between-group differences were not statistically reliable. It should also be noted that the presence of residual visual face memory and covert face recognition in developmental prosopagnosia, as demonstrated by these results does not necessarily also apply to cases of acquired prosopagnosia, where face processing impairments may often involve even earlier stages of face perception (Eimer and McCarthy, 1999).
In summary, the present study has provided novel insights into the mechanisms that underlie covert and overt face recognition in individuals with developmental prosopagnosia. In half of all participants with developmental prosopagnosia tested, non-recognized famous faces triggered an N250 component. This observation not only demonstrates the existence of covert face recognition in developmental prosopagnosia, but also links this phenomenon to a disconnection between intact visual memory traces of individual faces and subsequent semantic stages of face processing. The similarity of event-related potential correlates for explicit face recognition in individuals with developmental prosopagnosia and participants with unimpaired face processing indicates that there are no fundamental qualitative differences in the basic face processing architecture between these two groups.
Funding
This research was funded by a grant from the Economic and Social Sciences Research Council (ESRC), UK, to M.E. and B.C.D.
Acknowledgements
Thanks to John Towler for helpful comments and Joanna Parketny for technical assistance.