-
PDF
- Split View
-
Views
-
Cite
Cite
Giulia Cisbani, Thomas B. Freeman, Denis Soulet, Martine Saint-Pierre, Dave Gagnon, Martin Parent, Robert A. Hauser, Roger A. Barker, Francesca Cicchetti, Striatal allografts in patients with Huntington’s disease: impact of diminished astrocytes and vascularization on graft viability, Brain, Volume 136, Issue 2, February 2013, Pages 433–443, https://doi.org/10.1093/brain/aws359
Close - Share Icon Share
Abstract
Neuronal transplantation has been proposed as a potential therapy to replace lost neurons in Huntington’s disease. Transplant vascularization and trophic support are important for graft survival. However, very few studies have specifically addressed graft vascularization in patients with neurological disorders. In the present study, we analysed the vasculature of the host putamen and solid grafts of foetal striatal tissue transplanted into patients with Huntington’s disease 9 and 12 years previously. Grafts were characterized by a significantly reduced number of large calibre blood vessels in comparison with the host brain. There were also significantly fewer astrocytes and gap junctions, suggesting a lack of functional blood–brain barrier components within the grafted tissue. Additionally, grafts demonstrated a nearly complete absence of pericytes (compared with the striatum) that are considered important for vascular stabilization and angiogenesis. Finally, the host striatum had a marked increase in atrophic astrocytes in comparison with controls and grafts. The extent to which the lower number of large calibre vessels and astrocytes within the transplants contributed to suboptimal graft survival is unknown. The marked increase in atrophic astrocytes in the host brain surrounding the grafts suggests that reduced host trophic support may also contribute to poor graft survival in Huntington’s disease. A better understanding of the way in which these components support allografted tissue is critical to the future development of cell-based therapies for the treatment of Huntington’s disease.
Introduction
Huntington’s disease is a devastating autosomal dominant neurodegenerative disorder caused by a CAG expansion in exon 1 of the huntingtin gene. The disease is characterized by prominent cell losses in a number of brain structures, particularly in the striatum and cortex. Clinical features include a variety of motor, cognitive and psychiatric deficits (Phillips et al., 2008). Although incurable, there are some partially effective symptomatic treatments and a range of experimental therapies that are being pursued (Qin et al., 2005; Phillips et al., 2008; Nance et al., 2012). Cell transplantation, which aims to replace the neurons of the striatum that are targeted early in the pathogenic process, has yielded some preliminary, modest and short-lived clinical benefits (Kopyov et al., 1998; Bachoud-Levi et al., 2000; Hauser et al., 2002; Rosser et al., 2002; Bachoud-Levi, 2006; Bachoud-Levi et al., 2006; Keene et al., 2007; Reuter et al., 2008; Gallina et al., 2010; Cicchetti et al., 2011). We have reported previously that graft survival in Huntington’s disease is compromised long-term (Cicchetti et al., 2009, 2011). This increases the possibility that short-term benefits are lost owing to compromised graft viability as opposed to the progression of the underlying disease.
We have undertaken post-mortem studies in five of the seven patients with Huntington’s disease who received transplants in the open label study conducted at the University of South Florida (Freeman et al., 2000a, b, c, 2011; Hauser et al., 2002; Cicchetti et al., 2009, 2011). Our observations suggest that grafted cells undergo degeneration possibly as a result of a chronic inflammatory response to the transplant and excitotoxic effects of aberrant host cortical afferents onto the grafted cells (Fan et al., 2007; Cicchetti et al., 2009, 2011; Freeman et al., 2011). In this article, we additionally examined the relationship between solid neuronal allograft vascularization and astrocytic responses to long-term transplant viability in the brains of two patients with Huntington’s disease.
Materials and methods
Patient characteristics
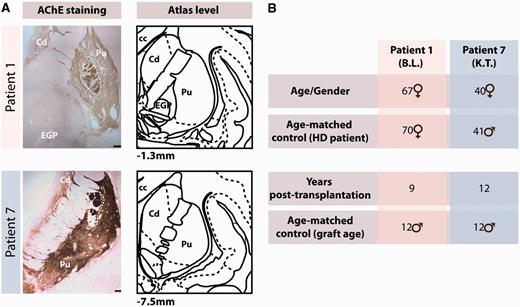
This report focuses on the histological evaluation of two brains (from Patients 1 and 7) of the initial seven patients with Huntington’s disease cohort in the open label pilot study on cell transplantation conducted at the University of South Florida (Hauser et al., 2002). These two brains were chosen because they had the best histological evidence of long-term graft survival from our cohort. Patient 1 had 42 CAG repeats (Hauser et al., 2002; Cicchetti et al., 2009), and Patient 7 had 53 CAG repeats (Hauser et al., 2002). These two female patients were transplanted at 58 and 28 years of age, and died 9 and 12 years post-transplantation, respectively, of causes unrelated to surgery. At autopsy, they were classified as having grade 3 and grade 4 disease according to the Vonsattel post-mortem rating scale (Vonsattel et al., 1985).
The age-matched control brain for Patient 1 was a 70-year-old female previously described in Cicchetti et al. (2009). The age-matched control brain used for Patient 7 was a 41-year-old male who died of myocardial infarction. The control brain from a non-Huntington’s disease 12-year-old boy, who died from pulmonary complications of multiple trauma, was used to match the developmental stage of the grafted foetal tissue of both Huntington’s disease transplanted cases (Fig. 1B).
Macroscopic graft identification and patient demographics. (A) Macroscopic identification of the transplants based on acetylcholinesterase (AChE) staining in two patients with Huntington’s disease included in this study, and compared with corresponding atlas levels (Mai et al., 2004). Scale bars = 100 µm. (B) Summary table of all brains analysed in this study. cc = corpus callosum; Cd = caudate; EGP = external globus pallidus; HD = Huntington's disease; Pu = putamen.
Donor tissue preparation and transplantation
Methods for tissue preparation, transplantation and immunosuppression, as well as clinical and radiological evaluation have been described previously (Freeman et al., 1995, 2000a, b, c, 2011; Hauser et al., 2002; Cicchetti et al., 2009, 2011). Briefly, all patients received foetal tissue transplants from five to eight striatal primordia per site. Solid tissue transplants measured 0.5–1 mm3 and were derived from the far lateral portion of the lateral ventricular eminence to optimize the percentage of tissue of striatal origin (Freeman et al., 1995). More specifically, Patient 1 received one striatal anlage in the left and right caudate and four in the left and right putamen. Patient 7 received one far lateral portion of the lateral ventricular eminence in the left and right caudate and five and six far lateral portion of the lateral ventricular eminences in the left and right putamen, respectively. All graft sites could be identified macroscopically on post-mortem evaluation (Fig. 1A).
Tissue preparation for post-mortem histological evaluation
The brains of the transplanted patients were removed within 5 h (Patient 1) and 2 h (Patient 7) of death. Briefly, the brains were bisected, cut serially into 1-cm thick slabs and immersed in Zamboni’s fixative for 8 days at 4°C. Brain slabs were then placed in 20% sucrose in 0.1 M PBS (pH 7.4) cryoprotectant solution (Freeman et al., 2000a; Cicchetti et al., 2009). For the control brains, post-mortem delays varied between 10 and 14 h. The control brains were also bisected, sliced into 2-cm thick slabs and then fixed by immersion in 4% paraformaldehyde at 4°C for 3 days. They were then stored at 4°C in a 0.1 M PBS, pH 7.4 containing 15% sucrose and 0.1% sodium azide. Both the transplanted and control brains were sectioned using a freezing microtome (Wallman et al., 2011). These slightly different methods of tissue preservation did not appear to affect the quality of the histological stainings. The protocols for single or double immunohistochemistry as well as for acetylcholinesterase histochemical staining have been described in detail in Cicchetti et al. (2009) (also see Table 1 for antibody details).
Immunofluorescence
Post-fixation, sections were rinsed three times with KPBS and preincubated in a blocking solution containing 0.1% bovine serum albumin (Bioshop), 0.04% Triton™ X-100 (Sigma), 4% normal goat serum (Wisent Inc.) in KPBS for 30 min. After three further washes with KPBS, they were incubated in a blocking solution overnight at 4°C with primary antibodies alone or in the following combinations: von Willibrand factor (vWF), platelet-derived growth factor receptor-β (PDGFR-β)/α-smooth muscle actin (α-SMA), vWF/glial fibrillary acidic protein (GFAP) (Table 1). Type A pericytes (which emerge and proliferate from the basal lamina, releasing essential components of the extracellular matrix and contributing to the stromal tissue of the glial scar) stain positively for PDGFR-β (Goritz et al., 2011). In contrast, type B pericytes (which attach to blood vessels and fulfil the classic function of pericytes) express PDGFR-β and α-SMA (Goritz et al., 2011).
List of primary and secondary antibodies
| Antibody . | Company . | Catalogue number . | Dilution . |
|---|---|---|---|
| Primary antibody | |||
| Mouse Cy3 conjugated α-SMA | Sigma | C6198 | 1:200 |
| Rabbit Connexin-43 | Invitrogen | 71-0700 | 1:100 |
| Mouse GFAP | Sigma | G3893 | 1:500 |
| Rabbit GFAP | Dako | Z0334 | 1:500 |
| Mouse Neuronal nuclei | Millipore | MAB377 | 1:2500 |
| Rabbit PDGFR-β | AbCam | Ab32570 | 1:100 |
| Rabbit tyrosine hydroxylase | Pel-Freez | P40101 | 1:2500 |
| Rabbit vWF | Millipore | AB7356 | 1:500 |
| Secondary antibody | |||
| Alexa 488 goat anti-mouse | Invitrogen | A11029 | 1:500 |
| Alexa 633 goat anti-mouse | Invitrogen | A21052 | 1:500 |
| Alexa 488 goat anti-rabbit | Invitrogen | A11034 | 1:500 |
| Alexa 546 goat anti-rabbit | Invitrogen | A11035 | 1:500 |
| Alexa 633 goat anti-rabbit | Invitrogen | A21071 | 1:500 |
| Antibody . | Company . | Catalogue number . | Dilution . |
|---|---|---|---|
| Primary antibody | |||
| Mouse Cy3 conjugated α-SMA | Sigma | C6198 | 1:200 |
| Rabbit Connexin-43 | Invitrogen | 71-0700 | 1:100 |
| Mouse GFAP | Sigma | G3893 | 1:500 |
| Rabbit GFAP | Dako | Z0334 | 1:500 |
| Mouse Neuronal nuclei | Millipore | MAB377 | 1:2500 |
| Rabbit PDGFR-β | AbCam | Ab32570 | 1:100 |
| Rabbit tyrosine hydroxylase | Pel-Freez | P40101 | 1:2500 |
| Rabbit vWF | Millipore | AB7356 | 1:500 |
| Secondary antibody | |||
| Alexa 488 goat anti-mouse | Invitrogen | A11029 | 1:500 |
| Alexa 633 goat anti-mouse | Invitrogen | A21052 | 1:500 |
| Alexa 488 goat anti-rabbit | Invitrogen | A11034 | 1:500 |
| Alexa 546 goat anti-rabbit | Invitrogen | A11035 | 1:500 |
| Alexa 633 goat anti-rabbit | Invitrogen | A21071 | 1:500 |
List of primary and secondary antibodies
| Antibody . | Company . | Catalogue number . | Dilution . |
|---|---|---|---|
| Primary antibody | |||
| Mouse Cy3 conjugated α-SMA | Sigma | C6198 | 1:200 |
| Rabbit Connexin-43 | Invitrogen | 71-0700 | 1:100 |
| Mouse GFAP | Sigma | G3893 | 1:500 |
| Rabbit GFAP | Dako | Z0334 | 1:500 |
| Mouse Neuronal nuclei | Millipore | MAB377 | 1:2500 |
| Rabbit PDGFR-β | AbCam | Ab32570 | 1:100 |
| Rabbit tyrosine hydroxylase | Pel-Freez | P40101 | 1:2500 |
| Rabbit vWF | Millipore | AB7356 | 1:500 |
| Secondary antibody | |||
| Alexa 488 goat anti-mouse | Invitrogen | A11029 | 1:500 |
| Alexa 633 goat anti-mouse | Invitrogen | A21052 | 1:500 |
| Alexa 488 goat anti-rabbit | Invitrogen | A11034 | 1:500 |
| Alexa 546 goat anti-rabbit | Invitrogen | A11035 | 1:500 |
| Alexa 633 goat anti-rabbit | Invitrogen | A21071 | 1:500 |
| Antibody . | Company . | Catalogue number . | Dilution . |
|---|---|---|---|
| Primary antibody | |||
| Mouse Cy3 conjugated α-SMA | Sigma | C6198 | 1:200 |
| Rabbit Connexin-43 | Invitrogen | 71-0700 | 1:100 |
| Mouse GFAP | Sigma | G3893 | 1:500 |
| Rabbit GFAP | Dako | Z0334 | 1:500 |
| Mouse Neuronal nuclei | Millipore | MAB377 | 1:2500 |
| Rabbit PDGFR-β | AbCam | Ab32570 | 1:100 |
| Rabbit tyrosine hydroxylase | Pel-Freez | P40101 | 1:2500 |
| Rabbit vWF | Millipore | AB7356 | 1:500 |
| Secondary antibody | |||
| Alexa 488 goat anti-mouse | Invitrogen | A11029 | 1:500 |
| Alexa 633 goat anti-mouse | Invitrogen | A21052 | 1:500 |
| Alexa 488 goat anti-rabbit | Invitrogen | A11034 | 1:500 |
| Alexa 546 goat anti-rabbit | Invitrogen | A11035 | 1:500 |
| Alexa 633 goat anti-rabbit | Invitrogen | A21071 | 1:500 |
After washes in KPBS, sections were reincubated with appropriate secondary antibodies in a blocking solution for 90 min at room temperature. After rinsing, sections were incubated in KPBS containing 0.022% 4′,6-diamidino-2-phenylindole (2 mg/ml, Molecular Probes), washed and mounted on SuperFrost® slides (Fisher Scientific), coverslipped with Fluoromount-G™ (SouthernBiotech) and sealed with nail polish.
Confocal laser scanning and brightfield microscopy
Confocal laser scanning microscopy was performed using both an Olympus FV500 and LSM 5 PASCAL (Zeiss) confocal laser-scanning microscope. Images were acquired by sequential scanning and optimized by a two-frame Kalman filter and analysed using acquisition software from Olympus (Fluoview SV500 imaging software 4.3, Olympus America Inc.) and Zeiss (LSM5 Pascal Image software, Zeiss). Acquired z-series images were exported in Imaris Pro Sofware 4.5 (Bitplane AG). Reconstructions were performed using ImageJ (version 1.45s, NIH), Imaris Surpass module (Apple Corp.) and maximum intensity projections. Brightfield photomicrographs were taken by Picture Frame software (Microbrightfield) attached to an E800 Nikon microscope (Nikon Instruments). Images were prepared for illustration using Adobe Photoshop CS5 and assembled using Adobe Illustrator CS5.
Blood vessel quantifications and measurements
Quantifications of blood vessels labelled with vWF (for capillaries) and α-SMA (for large calibre blood vessels) were performed using a series of images collected from the host putamen and grafts at both ×10 and ×20 on a Zeiss LSM 5 PASCAL confocal laser-scanning microscope. The number of blood vessels (vWF-positive or α-SMA-positive) was calculated in each sampled field of equal area using the standardized method of ImageJ for particle analysis (Li et al., 2010; Sen et al., 2011). The results are reported as the number of positive events per field of view and expressed as a mean ± SEM. The diameter of vWF-positive capillaries or α-SMA-positive blood vessels was measured with ImageJ on a sample of 40 vessels each. The diameters are expressed as a mean ± SEM. We were unable to measure the density of blood vessels as a function of brain atrophy, as the number of brain tissue sections containing the graft was too few to allow this.
Quantifications of astrocytic cell types
The perimeters of the p-zones, non-p-zones and putamen of the Huntington’s disease and control brains were delineated using the tracing contour option in Stereo Investigator (NeuroExplorer, version 10.0; Microbrightfield). Quantifications of GFAP-positive cells (both by morphology types and total number of cells) were performed in this entire region using the Optical Fractionator probe (Stereo Investigator, Microbrightfield) installed onto an E800 Nikon microscope.
Densitometric quantification of gap junctions
Digitized brain images of the putamen were obtained with a Northern Light Desktop Illuminator (Imaging Research) using a Sony Camera Video System (XC-77) and acquired with ScionImage for Windows (Scion Corporation, version Alpha 4.0.3.2). All images were acquired at the same magnification to allow the visualization of the entire putamen in a single field. The entire putaminal structure was delineated, and the intensity of connexin-43 staining was assessed using the ImageJ software. Background intensities taken from the corpus callosum devoid of connexin-43 staining were subtracted from each measurement.
Statistical analyses
One-way ANOVA calculations were used to compare the number of GFAP-positive cells, and vWF-positive and α-SMA-positive blood vessels in each quantified region. For the GFAP-positive cell and vWF-positive vessel counts, the assumption of homogeneity was not respected. To solve this, a different variance was estimated for each group. For the number of GFAP-positive cells classified by morphology and α-SMA-positive blood vessels, normality assumption was not respected; thus, a negative binomial distribution was used. Step-down Bonferonni correction was further used to ensure that the overall significance level of the multiple comparison tests was 0.001. The analysis was performed using the MIXED and GENMOD procedures of SAS version 9.2. Diameters of vWF-positive and α-SMA-positive blood vessels were evaluated by a Student t-test (P < 0.001) using PRISM 4 (Graphpad Software). All data are expressed as mean ± SEM.
Results
The majority of transplants could be identified macroscopically (Fig. 1A). The grafts demonstrated a compartmentalized organization with the formation of striatal patchy areas referred to as p-zones that stained positively for acetylcholinesterase, as well as areas where there was no expression of striatal phenotypes (Fig. 1A), referred to as non-p-zones (Graybiel et al., 1989). These observations are similar to those described previously (Freeman et al., 2000a, 2011; Cicchetti et al., 2009).
Diminished number of large calibre blood vessels in grafted tissue
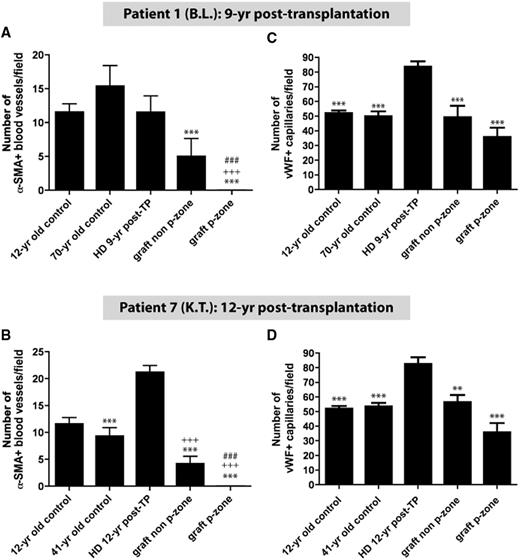
The presence of two distinct blood vessel populations was confirmed using diameter measurements on α-SMA-positive large calibre blood vessels and vWF-positive capillaries (P < 0.001; Fig. 3F). The number of large calibre (α-SMA-positive) blood vessels was lower in grafts compared with host and control brains (P < 0.001; Figs 2A and 3A). Within the grafts themselves, there was a further significant reduction in the number of large vessels in the p-zones compared with the non-p-zones of the grafts (P < 0.001; Figs 2A, B, 3A and C).
Quantifications of vWF-positive capillaries and α-SMA-positive blood vessels. Significant differences were observed between the number of α-SMA-positive (A and B) and vWF-positive (C and D) vessels in the host putamen, graft p-zones and non-p-zones. Data are expressed as a mean ± SEM. All statistical analyses were performed using the step-down Bonferroni correction method. **P < 0.01, ***P < 0.001, significant difference compared with the Huntington’s disease transplanted brain; +++P < 0.001, significant difference compared with the 12-year-old control brain; ###P < 0.001, significant difference compared with the graft non p-zone. HD = Huntington's disease; TP = transplantation; yr = year.
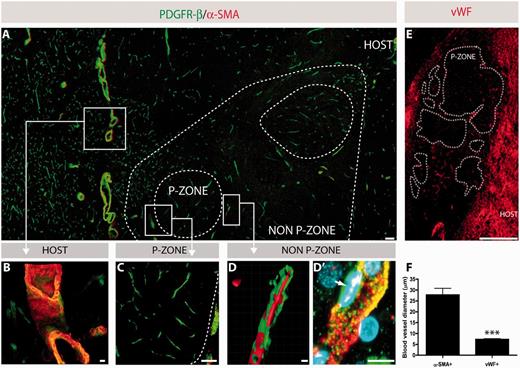
Discrepancies in vascularization between grafts and host tissue. Double immunofluorescence for type A pericytes, labelled with PDGFR-β in green, and type B pericytes, labelled with PDGFR-β and α-SMA-positive blood vessels in red (co-labelled in yellow) further revealed that large calibre blood vessels are not present within graft p-zones (dashed lines) (A and C), but are detectable in the graft non-p-zones (A, D and D’) as well as in the host (A and B). Pericytes were observed in the p-zones associated with capillaries, but not with large calibre α-SMA blood vessels (C). Various examples of blood vessels and blood-associated elements (e.g. pericytes) at higher magnification (B–D’). Larger calibre blood vessels, as observed in the host, stained positively for both PDGFR-β and α-SMA (B). Photomicrograph illustrating the exclusive presence of capillaries within a graft p-zone (C). 3D reconstruction, as performed by confocal microscopy, depicting the presence of pericytes (green) onto a blood vessel (red) (D). Higher magnification of a triple staining (PDGFR-β in green, α-SMA in red and 4′,6-diamidino-2-phenylindole in blue) illustrating pericytes and their relationship to a blood vessel, as visualized in a non-p-zone of the grafted tissue (D’). Immunofluorescence for the blood glycoprotein vWF revealed a significantly greater number of capillaries in the host parenchyma than in the grafted tissue (E, also see Fig. 2C and D). To further confirm the presence of distinct blood vessel populations, diameters were measured for 40 sampled vWF-positive and α-SMA-positive vessels, which further revealed a significant difference (F). The values are expressed as a mean ± SEM (Student’s t-test; ***P < 0.001). Scale bars: A = 100 µm; B = 10 µm; C = 100 µm, D, D’ = 10 µm; E = 1 mm.
Pericytes associated with α-SMA-positive blood vessels were found in the host (Fig. 3A and B) and in the graft non p-zones (Fig. 3A and D), but not the p-zones (Fig. 3A and C). Graft p-zones demonstrated PDGFR-β-positive pericytes associated only with capillaries (Fig. 3C). Taken together, these data suggest that there are diminished blood vessels and pericytes in grafts, and that this is more prominent in the graft p-zones than in the non-p-zones.
Immunofluorescence for the endothelial marker vWF revealed that the host putamen had a richer capillary network when compared with the grafts or control brains (P < 0.001; Figs 2C, D and 3E). These results suggest that there are no significant differences between the number of capillaries in the grafts and age-matched normal brains. However, there is a significant increase in the number of capillaries in the Huntington’s disease putamen in comparison with the normal putamen (P < 0.001; Figs 2C, D and 3E), although the extent to which this may relate to a degree of atrophy is unclear.
Astrocytic subtypes within the host putamen and grafts
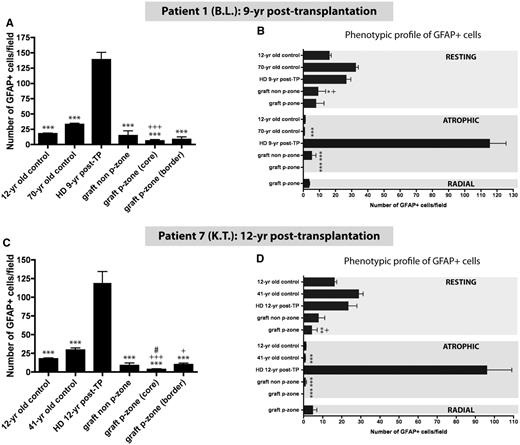
There was a significantly higher number of astrocytes in the Huntington’s disease host putamen than in any of the grafts or control putamen (P < 0.001 and P < 0.05; Figs 4A, C, 5A and C). In particular, there was a marked increase in the number of atrophic GFAP-positive cells in both the host putamen in comparison with grafts or age-matched controls. The host brain was found to have activated atrophic astrocytes typical of what is observed in Huntington’s disease (Vonsattel et al., 1985) (P < 0.001; Figs 4B, D and 5C).
Quantifications of astrocytes according to number and phenotype. Significant differences were observed between the number of GFAP-positive cells in the host putamen, graft p-zones and non-p-zones (A and C). The subdivision of the p-zones into p-zone border and p-zone core further emphasizes the different distribution of astrocytes in the p-zones as shown in Fig. 5. Additionally, different astrocytic morphologies (resting, atrophic and radial) were quantified according to their location within the host and graft (B and D). The numbers of GFAP-positive cells per sampled field of view are expressed as a mean ± SEM. All statistical analyses were performed using the Step-down Bonferroni correction method. *P < 0.05, **P < 0.01, ***P < 0.001, significant difference compared with the Huntington’s disease transplanted brain; +P < 0.05, +++P < 0.001 significant difference compared with the 12-year-old control brain; #P < 0.05, significant difference compared with the graft p-zone border. HD = Huntington’s disease; TP = transplantation; yr = year.
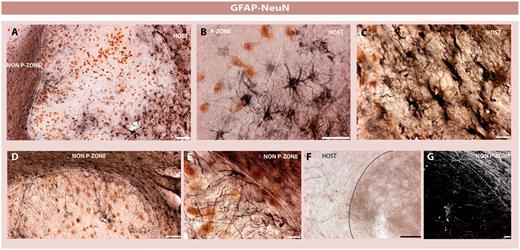
Astrocytic subtypes within the graft and surrounding brain. Double immunohistochemistry for neuronal elements (Neuronal nuclei (NeuN), revealed with the chromogen 3,3′ diaminobenzidene in brown) and astrocytes (GFAP, revealed with nickel-intensified 3,3′ diaminobenzidene in black) (A–E). Solid grafts were demarcated by an astrocytic response (A and B). Astrocytic cells, however, were rarely observed within the p-zones of the grafted tissue (A, B, D and E). When present, astrocytes projected radially within the p-zones and depicted the morphology of a resting cell (D, E and G) in comparison with the activated atrophic host astrocytes (B and C). The morphology of astrocytes differed according to tissue compartments of the graft and surrounding brain (A–E). Importantly, the circumferentially oriented astrocytes observed around the graft p-zones did not prevent the infiltration of tyrosine hydroxylase fibres arguing against the presence of a glial scar (F). Scale bars: A, B and C = 100 µm; D = 100 µm; E = 15 µm; F = 25 µm; G = 10 µm.
Within grafts, several different astrocytic subtypes were also evident. Astrocytes at the graft-host border were activated, but had a much more atrophied appearance compared with the ones found in the graft non-p-zone and its age-matched control (P < 0.001; Figs 4B, D, 5B and C). A circumferentially reactive layer of astrocytic processes was found in the transplant non p-zones surrounding the p-zones (Fig. 5D, E and G). This was not a true ‘glial scar’, as host dopaminergic and cortical striatal neurons easily penetrated this reactive layer and innervated the graft [Fig. 5F and Cicchetti et al. (2009)]. Additionally, type A pericytes, typically found in reactive scars (Goritz et al., 2011) were not found in this circumferential reactive layer.
Astrocytes were more concentrated in the periphery than in the core of the p-zones (P < 0.001 and P < 0.05; Figs 4A, C, 5A and D). Graft p-zones did not display atrophied GFAP-positive cells (P < 0.001; Figs 4B and D). A third morphological appearance of astrocytes was found at the interface of the graft p-zones and graft non-p-zones. Resting astrocytes were also observed in both compartments of the grafts, but their number was significantly lower than in the host brain or the 12-year-old control brain (P < 0.01 and P < 0.05; Figs 4B, D, 5A and D). Radially oriented astrocytes were only observed within the graft p-zones (Figs 4B, D and 5E).
Blood–brain barrier alterations within the grafts
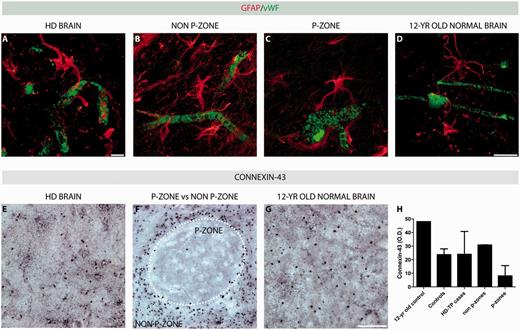
Host blood vessels were almost entirely wrapped by astrocytic end-feet (Fig. 6A). However, astrocytes seem to have an attenuated interaction with the capillaries within graft p-zones (Fig. 6C) compared with the non-p-zone (Fig. 6B) and the 12-year-old matched control (Fig. 6D). This suggests that there is a diminished blood–brain barrier within graft p-zones compared with graft non-p-zones or normal brain.
Alteration of blood–brain barrier components within the grafts. Double immunofluorescence for endothelial cells labelled with vWF in green and astrocytes, stained with GFAP in red (A–D). Closer examination of the relationship of astrocytes with blood vessels indicates that astrocytic end-feet are rarely apposed to blood vessels found in the p-zones of the grafted tissue (C), in comparison with those found in the host (A), the non-p-zone (B) and the 12-year-old control brain (D). Immunohistochemistry for the gap junction subunit connexin-43 (revealed with nickel-intensified 3,3′ diaminobenzidene in black) demonstrates the markedly reduced expression of gap junctions in the graft p-zone (F and H) in comparison with the Huntington’s disease brain (E and H), graft non-p-zone (F and H) and putamen of the 12-year-old control brain (G and H). Scale bars: A = 10 µm; B–D = 25 µm; E–G = 100 µm. HD = Huntington’s disease; TP = transplantation; yr = year.
Gap junctions have recently been described to be essential for graft-host cell coupling (Jaderstad et al., 2011). There was high expression of gap junctions (connexin-43) in the host brain (Fig. 6E and H). Connexin-43 expression was diminished in the graft non-p-zones (Fig. 6F and H) and was even more diminished within the graft p-zones (Fig. 6F and H). The 12-year-old control brain displayed the highest intensity of connexin-43 staining (Fig. 6G and H), as has been described previously in normal CNS development (Dermietzel et al., 1989).
Discussion
Striatal grafts in patients with Huntington’s disease demonstrate significantly fewer large blood vessels in comparison with the host and control brains. This may have contributed to poor long-term graft survival (Cicchetti et al., 2009), as blood vessels are necessary for normal cell metabolism. However, while this may relate in some way to the atrophy seen in Huntington’s disease, it more likely reflects a primary abnormality as has been observed in transgenic YAC128 Huntington’s disease models (Franciosi et al., 2012). Diminished vascularization of grafts in comparison with the host has also been described in a patient with Parkinson’s disease who had good graft survival (Kordower et al., 1996).
Vascularization of grafted tissue and its integration into the host circulatory system is needed for graft survival (Lindsay et al., 1984; Broadwell et al., 1990). Several factors have been identified that participate in graft revascularization. Blood vessel supply is proportional to the dimension of the graft and method of dissection of the foetal tissue (Freeman et al., 2011). Cell suspension grafts derive vascularization from the host (Baker-Cairns et al., 1996), whereas solid grafts, which have within them an intact vessel network, anastomose with the host vasculature (Baker-Cairns et al., 1996). Therefore, solid grafts, which contain vascularization from the donor, may be more immunogenic than suspension cell transplants (Freeman et al., 2011). In our study, transplants were derived from solid tissue pieces (Freeman et al., 1995, 2000a, b, c; Hauser et al., 2002; Cicchetti et al., 2009). Additionally, the size of the cannula used for the injection could further trigger inflammation. The smaller cannula used for cell suspension grafting might minimize the brain trauma and thus, the local inflammatory reaction (Freeman et al., 2011). The immunogenicity of the blood vessels in solid grafts combined with the use of a larger needle could lead to increased inflammation, which may interfere with the vascularization and viability of grafts (Freeman et al., 2011). However, similar arguments could be made for solid grafts in Parkinson’s disease where graft viability has been far less compromised, suggesting that other factors are contributing to the loss of viability in Huntington’s disease grafts.
Host factors may have contributed to the diminished graft survival in our cases with Huntington’s disease in comparison with previously reported cases with Parkinson’s disease. Here, we noted a marked increase in atrophic astrocytes in the host putamen similar to what has been previously described in other patients with Huntington’s disease (Vonsattel et al., 1985). Therefore, it is conceivable that the poor graft survival in our patients with Huntington’s disease is due to reduced trophic support from host astrocytes.
We report several differences in the vasculature found within the host, graft non-p-zones and p-zones (Fig. 7). First, large blood vessels were more numerous in the host than graft non-p-zones and were almost non-existent in graft p-zones. In this regard, pericytes associated with large blood vessels were found in the host and graft non-p-zones, but not in the graft p-zones. Taken together, this suggests that the relative absence of large blood vessels and pericytes within the graft p-zones may be contributing to poor graft viability. The presence of large blood vessels is perhaps not expected in the transplant, given that typically, blood supply comes from locally sprouting smaller vessels (Scott and Sherman, 1984). We observed no differences between control brains and grafts with regard to capillaries. Therefore, diminished vasculature supply within graft p-zones is specifically limited to the larger blood vessels.
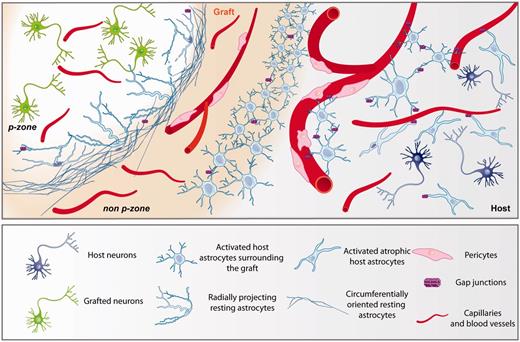
Schematic of astrocytic interaction and vascularization in grafted tissue and surrounding brain. We have observed that foetal allografts in two patients with Huntington’s disease a decade post-transplantation are characterized by the absence of critical elements of the neurovascular unit possibly contributing to poor vascularization of the transplanted cells and thus suboptimal graft survival long-term. More specifically, p-zones of the grafted tissue are not supplied by large blood vessels but strictly vascularized by capillaries. Elements associated with vasculature, such as pericytes and astrocytes, are also largely absent within the graft p-zones. In other compartments of the transplant (non-p-zones) and in the host, heterogeneous astrocytic populations were identified. For example, host astrocytes were characterized by an activated atrophied morphology. Astrocytes found at the host-graft interface demonstrated an activated morphology. The border p-zone/non-p-zone of the graft was demarcated by circumferentially oriented resting astrocytes. On the edge of the graft p-zone, additional radially projecting resting astrocytes were noted. However, this astrogliosis is permeable to the infiltration of tyrosine hydroxylase fibres originating from the host as well as to cortical connection via vGlut1 (Cicchetti et al., 2009, 2011). The absence of astrocytes within the graft is also accompanied by the lack of connexin-43, a subunit of gap junctions.
Pericytes and astrocytes are critical elements in the development and maintenance of normal vasculature (Bonkowski et al., 2011; Winkler et al., 2011). The scarcity of these elements in graft p-zones may have further contributed to the diminished graft survival in our patients with Huntington’s disease in comparison with Parkinson’s disease. It is also plausible, however, that targeted inflammatory and excitotoxicity aimed at the p-zones within the transplants (Cicchetti et al., 2009, 2011) may have caused degradation of vasculature as a secondary phenomenon. Gap junctions, and in particular connexin-43, which is abundantly expressed onto astrocytes, have recently been reported to be critical for the proper integration of the grafted cells with the host brain (Jaderstad et al., 2011). Finally, in Huntington’s disease, astrocytes show lower levels of some of the transporters that have a high affinity for glutamate, such as the glutamate transporter 1 or the glutamate aspartate transporter (Shin et al., 2005), which could lead to impairments in glutamate buffering, contributing to the excitotoxicity and degeneration of grafted cells (Cicchetti et al., 2009).
We have previously reported that microglia specifically target neurons within the graft p-zones (Cicchetti et al., 2009, 2011). This contrasts with what we have observed here, where astrocytes are distinctly absent within the graft p-zones. As astrocytes participate in the maintenance of the blood–brain barrier, it may therefore be this which is critically compromised in the p-zone of the transplanted tissue. In addition to the role of astrocytes in maintaining the blood–brain barrier around blood vessels, several types of astrocytic morphologies were observed within grafts (Fig. 7). A circumferential reactive layer of astrocytic process was found surrounding graft p-zones. Although reminiscent of a glial scar, this layer was highly permeable to the host projections to the grafts including dopaminergic and cortico-striatal glutamatergic fibres. Additionally, type A pericytes, typically found in reactive scars (Goritz et al., 2011) were not found in this circumferential reactive layer. This is therefore not a true scar.
Moreover, radially oriented resting astrocytes within the graft p-zones represented a unique morphology that has never been described in the context of transplantation. The presence of radially oriented glial fibres observed in these transplants in patients with Huntington’s disease suggests that these fibres may participate in the internal organization of embryonic graft morphology in response to developmental cues from the host brain, similar to the role radial glial fibres play in the development of the embryonic brain (Sidman et al., 1973; Gates et al., 1996; Ang et al., 2003; Anthony et al., 2004).
In conclusion, diminished graft survival in Huntington’s disease may be influenced by the presence of host atrophic astrocytes and diminished trophic support to grafts, and the relative absence of larger blood vessels and pericytes as well as an abnormal blood–brain barrier in graft p-zones. The localization of many of these observations to graft p-zones specifically sits well with our previous observations that glutamate toxicity from cortico-striatal projections targets p-zones in grafts as well. These findings suggest that there are complex interactions between inflammatory cells, blood vessels, the blood–brain barrier and grafts that all contribute to the poor long-term survival of these grafts. Similar interactions may also participate in the development of Huntington’s disease itself.
Funding
Huntington Society Canada and the International Organization of Glutaric Acidemia (IOGA) (to F.C. and D.S.). G.C. was supported by PhD recruitment scholarship from Université Laval.
Acknowledgements
The authors would like to thank Mr. Gilles Chabot for artwork and the Brain Bank of the Centre de Recherche Université Laval Robert-Giffard (CRULRG), Québec, Canada, for providing some of the control brains.
Abbreviation
- GFAP
Glial Fibrillary Acidic Protein
- PDGFR-β
platelet-derived growth factor receptor-β
- vWF
von Willibrand factor
- α-SMA
α-smooth muscle actin