-
PDF
- Split View
-
Views
-
Cite
Cite
Michael D. Spencer, Rosemary J. Holt, Lindsay R. Chura, Andrew J. Calder, John Suckling, Edward T. Bullmore, Simon Baron-Cohen, Atypical activation during the Embedded Figures Task as a functional magnetic resonance imaging endophenotype of autism, Brain, Volume 135, Issue 11, November 2012, Pages 3469–3480, https://doi.org/10.1093/brain/aws229
Close - Share Icon Share
Abstract
Atypical activation during the Embedded Figures Task has been demonstrated in autism, but has not been investigated in siblings or related to measures of clinical severity. We identified atypical activation during the Embedded Figures Task in participants with autism and unaffected siblings compared with control subjects in a number of temporal and frontal brain regions. Autism and sibling groups, however, did not differ in terms of activation during this task. This suggests that the pattern of atypical activation identified may represent a functional endophenotype of autism, related to familial risk for the condition shared between individuals with autism and their siblings. We also found that reduced activation in autism relative to control subjects in regions including associative visual and face processing areas was strongly correlated with the clinical severity of impairments in reciprocal social interaction. Behavioural performance was intact in autism and sibling groups. Results are discussed in terms of atypical information processing styles or of increased activation in temporal and frontal regions in autism and the broader phenotype. By separating the aspects of atypical activation as markers of familial risk for the condition from those that are autism-specific, our findings offer new insight into the factors that might cause the expression of autism in families, affecting some children but not others.
Introduction
Autism spectrum conditions are neurodevelopmental and cause impairments of social communication alongside unusually repetitive behaviour, narrow interests and resistance to change. Autism was first described by Kanner (1943) and is currently considered to have a prevalence of ∼1% (Weintraub, 2011). Siblings of individuals with autism have a greatly enhanced risk of developing the condition, with a prevalence estimated to be >20 times in the general population (Ritvo et al., 1989; Lauritsen et al., 2005; Constantino et al., 2010). However, it is increasingly recognized that even in relatives who do not meet diagnostic criteria for autism spectrum conditions and who are, therefore, considered ‘unaffected’, a range of subtle differences may be evident. These may take the phenotypic form of the presence of a greater number of autistic traits than would typically be exhibited in the general population (Constantino et al., 2010; Wheelwright et al., 2010) or a more aloof and rigid personality style as measured using the Broader Autism Phenotype Questionnaire (Hurley et al., 2007).
Some studies of first-degree relatives report impairments in comparable cognitive domains to those affected in autism for instance, impairments in facial emotion recognition (Baron-Cohen and Hammer, 1997; Dawson et al., 2005; Losh and Piven, 2007; Losh et al., 2009), tests of executive function, such as planning and set-shifting (Hughes et al., 1999), and even a degree of enhanced performance compared with control subjects on tasks, such as the Embedded Figures Task (EFT) (Bolte and Poustka, 2006); a task that has a number of variants, all of which require participants to visually inspect a complex pattern to search for a smaller component figure located within, and a task on which individuals with autism typically demonstrate superior performance compared with control subjects (Shah and Frith, 1983; Jolliffe and Baron-Cohen, 1997). Atypical patterns of brain activation have been reported in parents while performing the EFT (Baron-Cohen et al., 2006a).
The use of neuroimaging to investigate siblings presents a unique scientific opportunity: differences between unaffected siblings and control subjects with no family history of autism may be attributed to the effect of the genes that confer familial risk for the condition. Such differences, if also present in affected individuals, are candidate endophenotypes of autism. The term endophenotype refers to an inherited trait present in unaffected individuals to a greater extent than in the general population and which, thus, co-segregates with a condition in families, and which is present in affected individuals whether the condition is in remission or relapse (Gottesman and Gould, 2003). The particular benefit of identifying endophenotypes of a complex condition such as autism is that they are likely to be under the control of fewer genes than the condition itself—being ‘closer to the level of gene action’ (Kendler and Neale, 2010).
Despite the heritability of autism being >90%, specific genetic mechanisms have yet to be identified. Copy number variants, small regions of duplication or deletion within the genome, are more common in autism than control subjects (Marshall et al., 2008; Pinto et al., 2010). Genetic variants including those affecting SH3 and multiple ankyrin repeat domains 3 (SHANK3) (Moessner et al., 2007), cadherins (Wang et al., 2009), neurexins (Vaags et al., 2012), neuroligins (Jamain et al., 2003) and genes coding for fragile X protein (Iossifov et al., 2012) have been reported to be associated with autism, although relatively few findings have been replicated. The investigation of the genetic associations of endophenotypes would in theory benefit from greater statistical power and less phenotypic and aetiological heterogeneity than an investigation of the genetic associates of the condition itself (i.e. autism versus control subjects).
A number of theories have been proposed to explain superior performance on the EFT in autism. It is relatively well established that people with autism tend to preferentially process local as opposed to global information (Bolte et al., 2007). This local-processing bias—as described by weak central coherence theory (Happe, 1996)—may be underpinned by an impairment in the processing of global (i.e. contextual) information and/or an enhancement in the processing of local information. An alternative theory proposes a deficit in ‘hierarchization’ in autism (Mottron and Burack, 2001), whereby global and local visual processing are equally intact in autism; however, the increased importance attached by the non-autistic population to contextual information is absent.
Structural and functional MRI makes it possible to investigate unaffected relatives of individuals with autism to determine the neural basis of the broader phenotype of autism in terms of atypical neuroanatomy and brain function. Relatively few neuroimaging studies have investigated relatives of individuals with autism (principally parents, with even fewer studies investigating siblings), but such studies have tended to demonstrate a pattern of atypical structure and function of the brain comparable with that seen in autism, albeit present to a lesser degree. For instance, Baron-Cohen et al. (2006a) reported reduced activation of the middle temporal and inferior frontal gyri in parents of individuals with autism during performance of the ‘Reading the Mind in the Eyes Task’, a task that requires participants to identify different mental states from viewing stimuli comprising pictures of only the eye region of the face, and on which individuals with autism are typically impaired (Baron-Cohen et al., 1999).
We recently reported that unaffected siblings of adolescents with autism demonstrated significantly reduced functional MRI activation within brain areas implicated in empathizing, theory of mind and face-processing in response to an implicit facial emotion processing task—the same regions that demonstrated reductions in autism—relative to control subjects with no family history of autism (Spencer et al., 2011). In contrast to impaired performance on tasks of facial emotion processing, performance on the EFT has generally been found to be intact or enhanced in autism. The EFT, therefore, provides an opportunity to examine functional neuroimaging correlates of familial risk for autism in the context of hypothetically intact or enhanced behavioural performance.
The aim of the present study was to investigate brain activity during the EFT in adolescents with autism spectrum conditions, their unaffected siblings and control subjects with no family history of autism. Previous functional MRI studies investigating the EFT in typically developing subjects have demonstrated that the EFT (compared with control condition) tends to activate frontal and parietal areas (Manjaly et al., 2003; Walter and Dassonville, 2011). In individuals with autism, the EFT has been reported to activate occipital and extrastriate regions to a greater extent and frontal and parietal regions to a reduced extent, compared with control subjects (Ring et al., 1999; Lee et al., 2007; Manjaly et al., 2007).
Based on our previous finding of functional neuroimaging endophenotypes of autism in siblings (Spencer et al., 2011) and the greater prevalence of broader phenotype features in first-degree relatives (Wheelwright et al., 2010), we hypothesized that siblings would show atypical activation on the EFT, comparable with that seen in autism, although perhaps to a lesser extent. We also aimed to relate atypical activation in autism to directly observed measures of autism clinical severity, defined by the Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al., 2000).
Materials and methods
Participants
Participants comprised 40 adolescents (aged 12–18 years) with autism spectrum conditions diagnosed with either classic autism or Asperger syndrome, 40 unaffected siblings and 40 typically developing control subjects (Table 1). All autism spectrum condition participants met Diagnostic and Statistical Manual of Mental Disorders, fourth edition criteria (American Psychiatric Association, 1994) for autism or Asperger syndrome and were positive on the Autism Diagnostic Interview-Revised (Lord et al., 1994) and the ADOS-G (Lord et al., 2000). Recruitment of this cohort has been previously described (Spencer et al., 2011).
Demographic characteristics of the study groups
| . | Autism group . | Sibling group . | Control group . |
|---|---|---|---|
| . | n = 38 . | n = 40 . | n = 40 . |
| Gender (male:female) | 34:4 | 12:28 | 20:20 |
| Age, years (range; SD) | 14.61 (12.01–18.53; 1.7) | 14.83 (12.01–18.95; 2.14) | 15.06 (12.08–18.17; 1.63) |
| IQ (range; SD) | 107.11 (81–146; 16.0) | 113.1 (88–133; 10.1) | 112.4 (83–136; 11.1) |
| Autism Spectrum Quotient (range; SD) | 39.55 (21–49; 6.35) | 8.65 (1–26; 5.51) | 9.10 (1–24; 5.59) |
| . | Autism group . | Sibling group . | Control group . |
|---|---|---|---|
| . | n = 38 . | n = 40 . | n = 40 . |
| Gender (male:female) | 34:4 | 12:28 | 20:20 |
| Age, years (range; SD) | 14.61 (12.01–18.53; 1.7) | 14.83 (12.01–18.95; 2.14) | 15.06 (12.08–18.17; 1.63) |
| IQ (range; SD) | 107.11 (81–146; 16.0) | 113.1 (88–133; 10.1) | 112.4 (83–136; 11.1) |
| Autism Spectrum Quotient (range; SD) | 39.55 (21–49; 6.35) | 8.65 (1–26; 5.51) | 9.10 (1–24; 5.59) |
Demographic characteristics of the study groups
| . | Autism group . | Sibling group . | Control group . |
|---|---|---|---|
| . | n = 38 . | n = 40 . | n = 40 . |
| Gender (male:female) | 34:4 | 12:28 | 20:20 |
| Age, years (range; SD) | 14.61 (12.01–18.53; 1.7) | 14.83 (12.01–18.95; 2.14) | 15.06 (12.08–18.17; 1.63) |
| IQ (range; SD) | 107.11 (81–146; 16.0) | 113.1 (88–133; 10.1) | 112.4 (83–136; 11.1) |
| Autism Spectrum Quotient (range; SD) | 39.55 (21–49; 6.35) | 8.65 (1–26; 5.51) | 9.10 (1–24; 5.59) |
| . | Autism group . | Sibling group . | Control group . |
|---|---|---|---|
| . | n = 38 . | n = 40 . | n = 40 . |
| Gender (male:female) | 34:4 | 12:28 | 20:20 |
| Age, years (range; SD) | 14.61 (12.01–18.53; 1.7) | 14.83 (12.01–18.95; 2.14) | 15.06 (12.08–18.17; 1.63) |
| IQ (range; SD) | 107.11 (81–146; 16.0) | 113.1 (88–133; 10.1) | 112.4 (83–136; 11.1) |
| Autism Spectrum Quotient (range; SD) | 39.55 (21–49; 6.35) | 8.65 (1–26; 5.51) | 9.10 (1–24; 5.59) |
Participants with autism and their siblings were recruited by approaching support groups for families with autism and schools. To minimize possible confounds relating to geography and demographics, control subjects were recruited through notices in schools and community groups in similar neighbourhoods to the participants in the autism and sibling groups. All siblings and control subjects scored below threshold (defined as a score of 15, as suggested by the authors) on a screening tool for autism spectrum conditions, the Social Communication Questionnaire (Berument et al., 1999). Autism traits were ascertained in all participants using the adolescent version of the Autism Spectrum Quotient (Baron-Cohen et al., 2006b). Siblings were full biological siblings of the participants with autism spectrum conditions, based on parental report; control subjects were defined as having no history of an autism spectrum condition within any first- or second-degree relative. It was not a requirement for sibling pairs to be matched for gender. General exclusion criteria were full-scale IQ <70 as measured using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), any psychiatric diagnosis (other than autism spectrum conditions in the autism group), any current or previous psychotropic medication, any history of seizures, any history of head injury or intracranial surgery and any history of drug abuse. The protocol was approved by the Cambridgeshire 1 Research Ethics Committee (NHS). All participants and their parents provided written informed consent.
Embedded Figures Task protocol
Participants completed the EFT, a visual search task previously described in functional MRI studies of adolescents with autism (Manjaly et al., 2007) and typical volunteers (Manjaly et al., 2005), which requires participants to identify a component shape from within a more complex pattern. The task comprised two conditions, an EFT condition and a control task condition (for example stimuli, see Supplementary Fig. 1). In the EFT condition, the participant was presented with a stimulus comprising a small target figure and a larger pattern and was asked to decide whether the target figure was present inside the pattern. In the control task condition, the stimulus similarly comprised a small target figure and a larger pattern; however, part of the larger pattern was highlighted and the participant was asked to decide whether the target figure was the same as the highlighted part of the larger pattern. The task was designed such that the only difference between the two conditions was the additional requirement to search within the pattern for the target shape in the EFT condition, with all other aspects of the task, including responding, being identical in the two conditions.
Stimuli were presented in e-Prime version 2.0 Professional (Psychological Software Tools, Inc.). Participants were presented with eight blocks of EFT condition stimuli and eight blocks of control task stimuli. In four EFT blocks and four control task blocks, the target figure was to the right of the display (as indicated in Supplementary Fig. 1), and in the remaining four EFT blocks and four control task blocks, the target figure was to the left. Blocks were presented in one of four fixed orders (which were counterbalanced across all participants in each study group) to counterbalance target laterality within each scanning session for each individual participant and to counterbalance the condition of the initial block (i.e. ABAB versus BABA) across all participants in each study group. Each block lasted 31.2 s and comprised six stimuli presented for 4 s each, with an interstimulus interval of 1.2 s. Each block was preceded by a reminder instruction displayed for 6 s, comprising ‘Is the smaller figure present inside the larger figure?’ in the case of an EFT block and ‘Is the smaller figure the same as the highlighted part of the larger figure?’ in the case of a control task block. Participants were required to press one of two buttons to indicate their response as ‘yes’ or ‘no’ to each stimulus, using a button box held in the right hand. The task lasted for 10 min.
Behavioural data comprising accuracy and reaction time of response on the EFT and control task were analysed using analysis of variance (ANOVA) in PASW Statistics 18, Release Version 18.0.0 (SPSS, Inc.).
Imaging protocol
All participants were scanned using the same Siemens 3T Tim Trio scanner at the MRC Cognition and Brain Sciences Unit, Cambridge, UK. Functional images were acquired with a gradient echo planar imaging sequence with the following parameters: repetition time = 2000 ms, echo time = 30 ms, voxel size = 3 × 3 × 3 mm, field of view = 192 × 192 mm, 64 × 64 acquisition matrix and a 78° flip angle. Thirty-two slices were acquired descending in the transverse plane (slice thickness = 3 mm, slice gap = 25%). Each volume was acquired over 2 s, and the first three volumes were discarded to avoid equilibration effects.
Imaging data analysis
Preprocessing, first- and second-level analyses were performed in Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Cognitive Neurology, London, UK), using the automatic analysis (aa) platform (Cusack et al., 2009) (MRC Cognition and Brain Sciences Unit, Cambridge, UK) according to the standard MRC Cognition and Brain Sciences Unit pipeline comprising realignment, sinc interpolation to correct for the acquisition of different brain slices at different times, co-registration of echo planar imaging and structural scans, normalization to Montreal Neurological Institute (MNI) space (Evans et al., 1992) and smoothing using a Gaussian kernel of 10-mm full-width at half-maximum, all as previously described (Spencer et al., 2011). For each participant, functional MRI responses were modelled using a canonical haemodynamic response function, and the general linear model was used to perform a first level, within-participants analysis on the functional data from each participant individually for the primary contrast (EFT minus control task), with spatial realignment parameters entered as covariates. To characterize the patterns of activation within the brain in the three participant groups, the first-level contrast images for each study group were taken through to a second-level analysis using a random-effects model, with age and sex specified as covariates. To illustrate the general pattern of brain activity common to the three groups, a conjunction analysis determined significant activation differences (EFT minus control task) common to all three study groups, the results of which are provided in Supplementary Fig. 2 and Supplementary Table 2; the analysis used multiple comparison correction using a family-wise error (FWE) correction on a whole brain level.
To investigate possible markers of familial risk compared with autism versus control differences, we examined between-group differences in the functional MRI response in autism, sibling and control participants by undertaking second-level analyses within SPM8 using a factorial model, again taking age and sex as covariates and measuring between-group differences in activation. We used multiple comparison correction using an FWE correction on a whole brain level for our main analysis. Subsequent exploratory analyses, which were specifically declared in the relevant sections of the results, used an uncorrected whole brain level significance threshold of P = 0.001. Analyses were structured around our hypothesis that the familial risk of autism would be expressed as activation differences between siblings and control subjects, and no difference between cases and siblings. We investigated differences hypothetically associated with the shared familial risk for autism in autism and sibling groups by contrasting these groups with the control group [(autism + sibling) > control] and [control > (autism + sibling)]. The hypothesis that atypical activation on the EFT is a possible endophenotype of autism would predict that individuals with autism and their unaffected siblings would not differ significantly, whereas siblings and control subjects would differ. The hypothesis would further predict that atypical activation within autism and sibling groups relative to control subjects would occur within overlapping brain regions. We, therefore, investigated case–sibling differences by contrasting autism and sibling groups (autism > sibling; sibling > autism) and sibling–control differences by contrasting sibling and control groups (sibling > control subjects; control subjects > sibling). We also investigated case–control differences by contrasting autism and control groups (autism > control subjects; control subjects > autism). For illustration, activation maps were generated with a global threshold set at P = 0.001 uncorrected at a whole brain level, and with a cluster extent (kE) threshold set at 10 voxels. We plotted these activation maps onto an MNI152 template brain image (Evans et al., 1992) using MRIcron (Rorden and Brett, 2000).
To investigate clinical correlates of atypical activation in autism, we constructed 4-mm-radius spheres around the co-ordinates of peak activation difference between cases and control subjects (the five results for autism > control subjects and five results for control subjects > autism, listed in Table 2). We used MarsBar (Brett et al., 2002) to extract mean activation differences for the primary contrast (EFT minus control task) for each subject for each sphere. We investigated correlations between activation differences and ADOS-G scores of autism clinical severity using two-tailed partial correlations in PASW Statistics 18, controlling for age and gender.
Between-group activation differences for EFT versus control task
| MNI coordinates . | P-value (uncorrected) . | Z-score . | Cluster size, kE (voxels) . | Region . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | . | . | . | . |
| Autism > control subjects | ||||||
| −58 | −6 | −18 | <0.001 | 4.12 | 288 | Left middle temporal gyrus/superior temporal sulcus |
| −56 | 0 | 8 | <0.001 | 3.76 | 271 | Left inferior frontal gyrus |
| 56 | −50 | 22 | <0.001 | 3.63 | 258 | Right superior temporal sulcus |
| 46 | 2 | −24 | <0.001 | 3.30 | 33 | Right inferior temporal gyrus |
| −44 | −54 | 22 | 0.001 | 3.22 | 14 | Left angular gyrus |
| Control subjects > autism | ||||||
| −38 | −4 | 32 | <0.001 | 3.50 | 30 | Left pre-motor cortex |
| −40 | −52 | −6 | <0.001 | 3.45 | 94 | Left fusiform gyrus |
| −44 | −84 | 18 | <0.001 | 3.43 | 17 | Left V3 (associative visual cortex) |
| −46 | −72 | −6 | 0.001 | 3.25 | 15 | Left V3 (associative visual cortex) |
| −38 | −40 | 44 | <0.001 | 3.31 | 13 | Left supramarginal gyrus |
| Sibling > control subjects | ||||||
| −56 | −6 | 30 | <0.001 | 3.88 | 114 | Left primary motor cortex |
| −56 | 8 | −18 | <0.001 | 3.80 | 93 | Left anterior superior temporal sulcus |
| −54 | 8 | 4 | <0.001 | 3.67 | 180 | Left inferior frontal gyrus |
| 38 | −12 | 6 | <0.001 | 3.56 | 60 | Right insula |
| −34 | −16 | 20 | 0.001 | 3.24 | 13 | Left insula |
| Control subjects > sibling | ||||||
| Nil | ||||||
| Autism > sibling | ||||||
| Nil | ||||||
| Sibling > autism | ||||||
| Nil | ||||||
| MNI coordinates . | P-value (uncorrected) . | Z-score . | Cluster size, kE (voxels) . | Region . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | . | . | . | . |
| Autism > control subjects | ||||||
| −58 | −6 | −18 | <0.001 | 4.12 | 288 | Left middle temporal gyrus/superior temporal sulcus |
| −56 | 0 | 8 | <0.001 | 3.76 | 271 | Left inferior frontal gyrus |
| 56 | −50 | 22 | <0.001 | 3.63 | 258 | Right superior temporal sulcus |
| 46 | 2 | −24 | <0.001 | 3.30 | 33 | Right inferior temporal gyrus |
| −44 | −54 | 22 | 0.001 | 3.22 | 14 | Left angular gyrus |
| Control subjects > autism | ||||||
| −38 | −4 | 32 | <0.001 | 3.50 | 30 | Left pre-motor cortex |
| −40 | −52 | −6 | <0.001 | 3.45 | 94 | Left fusiform gyrus |
| −44 | −84 | 18 | <0.001 | 3.43 | 17 | Left V3 (associative visual cortex) |
| −46 | −72 | −6 | 0.001 | 3.25 | 15 | Left V3 (associative visual cortex) |
| −38 | −40 | 44 | <0.001 | 3.31 | 13 | Left supramarginal gyrus |
| Sibling > control subjects | ||||||
| −56 | −6 | 30 | <0.001 | 3.88 | 114 | Left primary motor cortex |
| −56 | 8 | −18 | <0.001 | 3.80 | 93 | Left anterior superior temporal sulcus |
| −54 | 8 | 4 | <0.001 | 3.67 | 180 | Left inferior frontal gyrus |
| 38 | −12 | 6 | <0.001 | 3.56 | 60 | Right insula |
| −34 | −16 | 20 | 0.001 | 3.24 | 13 | Left insula |
| Control subjects > sibling | ||||||
| Nil | ||||||
| Autism > sibling | ||||||
| Nil | ||||||
| Sibling > autism | ||||||
| Nil | ||||||
Activated brain regions, corresponding MNI coordinates, cluster sizes, Z-scores and P-values. P-values are expressed at the uncorrected whole brain level threshold of P = 0.001; results thresholded at kE ≥ 10.
Between-group activation differences for EFT versus control task
| MNI coordinates . | P-value (uncorrected) . | Z-score . | Cluster size, kE (voxels) . | Region . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | . | . | . | . |
| Autism > control subjects | ||||||
| −58 | −6 | −18 | <0.001 | 4.12 | 288 | Left middle temporal gyrus/superior temporal sulcus |
| −56 | 0 | 8 | <0.001 | 3.76 | 271 | Left inferior frontal gyrus |
| 56 | −50 | 22 | <0.001 | 3.63 | 258 | Right superior temporal sulcus |
| 46 | 2 | −24 | <0.001 | 3.30 | 33 | Right inferior temporal gyrus |
| −44 | −54 | 22 | 0.001 | 3.22 | 14 | Left angular gyrus |
| Control subjects > autism | ||||||
| −38 | −4 | 32 | <0.001 | 3.50 | 30 | Left pre-motor cortex |
| −40 | −52 | −6 | <0.001 | 3.45 | 94 | Left fusiform gyrus |
| −44 | −84 | 18 | <0.001 | 3.43 | 17 | Left V3 (associative visual cortex) |
| −46 | −72 | −6 | 0.001 | 3.25 | 15 | Left V3 (associative visual cortex) |
| −38 | −40 | 44 | <0.001 | 3.31 | 13 | Left supramarginal gyrus |
| Sibling > control subjects | ||||||
| −56 | −6 | 30 | <0.001 | 3.88 | 114 | Left primary motor cortex |
| −56 | 8 | −18 | <0.001 | 3.80 | 93 | Left anterior superior temporal sulcus |
| −54 | 8 | 4 | <0.001 | 3.67 | 180 | Left inferior frontal gyrus |
| 38 | −12 | 6 | <0.001 | 3.56 | 60 | Right insula |
| −34 | −16 | 20 | 0.001 | 3.24 | 13 | Left insula |
| Control subjects > sibling | ||||||
| Nil | ||||||
| Autism > sibling | ||||||
| Nil | ||||||
| Sibling > autism | ||||||
| Nil | ||||||
| MNI coordinates . | P-value (uncorrected) . | Z-score . | Cluster size, kE (voxels) . | Region . | ||
|---|---|---|---|---|---|---|
| x . | y . | z . | . | . | . | . |
| Autism > control subjects | ||||||
| −58 | −6 | −18 | <0.001 | 4.12 | 288 | Left middle temporal gyrus/superior temporal sulcus |
| −56 | 0 | 8 | <0.001 | 3.76 | 271 | Left inferior frontal gyrus |
| 56 | −50 | 22 | <0.001 | 3.63 | 258 | Right superior temporal sulcus |
| 46 | 2 | −24 | <0.001 | 3.30 | 33 | Right inferior temporal gyrus |
| −44 | −54 | 22 | 0.001 | 3.22 | 14 | Left angular gyrus |
| Control subjects > autism | ||||||
| −38 | −4 | 32 | <0.001 | 3.50 | 30 | Left pre-motor cortex |
| −40 | −52 | −6 | <0.001 | 3.45 | 94 | Left fusiform gyrus |
| −44 | −84 | 18 | <0.001 | 3.43 | 17 | Left V3 (associative visual cortex) |
| −46 | −72 | −6 | 0.001 | 3.25 | 15 | Left V3 (associative visual cortex) |
| −38 | −40 | 44 | <0.001 | 3.31 | 13 | Left supramarginal gyrus |
| Sibling > control subjects | ||||||
| −56 | −6 | 30 | <0.001 | 3.88 | 114 | Left primary motor cortex |
| −56 | 8 | −18 | <0.001 | 3.80 | 93 | Left anterior superior temporal sulcus |
| −54 | 8 | 4 | <0.001 | 3.67 | 180 | Left inferior frontal gyrus |
| 38 | −12 | 6 | <0.001 | 3.56 | 60 | Right insula |
| −34 | −16 | 20 | 0.001 | 3.24 | 13 | Left insula |
| Control subjects > sibling | ||||||
| Nil | ||||||
| Autism > sibling | ||||||
| Nil | ||||||
| Sibling > autism | ||||||
| Nil | ||||||
Activated brain regions, corresponding MNI coordinates, cluster sizes, Z-scores and P-values. P-values are expressed at the uncorrected whole brain level threshold of P = 0.001; results thresholded at kE ≥ 10.
Results
Demographic characteristics of participant groups
A total of 118 participants completed the EFT and were included in this study. Their demographic characteristics are presented in Table 1. Groups did not differ in terms of mean age [ANOVA, F(2,115) = 0.553, P = 0.577] or IQ [ANOVA, F(2,115) = 2.622, P = 0.077]. Furthermore, whereas males were clearly over-represented in the autism group, there was no difference between siblings and control subjects in terms of sex (χ2 = 3.333, df = 1, P = 0.110). Although, as expected, the mean Autism Spectrum Quotient score was higher in the autism group than the sibling [ANOVA, F(1,76) = 528.0, P < 0.001] and control [ANOVA, F(1,76) = 506.6, P < 0.001] groups, the siblings and control subjects did not differ in terms of mean Autism Spectrum Quotient [ANOVA, F(1,78) = 0.131, P = 0.718]. This is of particular importance given our objective of identifying significant functional MRI activation differences between siblings and control subjects that are expressed despite no apparent difference between the two groups in terms of autistic behavioural phenotype.
Participants with autism had a mean score of 4.11 [range = 2–9, standard deviation (SD) = 1.61] on subdomain A (communication) of the ADOS-G, 7.89 (range = 4–17, SD = 2.86) on subdomain B (reciprocal social interaction) and 12.0 (range = 7–26, SD = 4.25) on the total score of the ADOS-G (the sum of these subdomains).
Participants with autism had a mean score of 21.4 (range = 10–37, SD = 4.98) on subdomain A (reciprocal social interaction), 17.7 (range = 10–26, SD = 4.02) on subdomain B (communication) and 6.6 (range = 3–12, SD = 2.97) on subdomain C (restricted, repetitive and stereotyped patterns of behaviour) of the Autism Diagnostic Interview-Revised.
Behavioural data analysis
Groups did not differ significantly in performance in terms of mean accuracy or reaction time on either the EFT or on the control task, or the relative difference in accuracy or reaction time between EFT and control task conditions. As indicated in Supplementary Table 1, significant effects of IQ and age on these behavioural measures were found, but no significant effects of sex or group. Only two participants (both in the autism group) scored < 50% accuracy on the control task. In case this was indicative of reduced attention during the EFT, all analyses were repeated to ensure that all statistically significant results reported were robust to the exclusion of the data from these two participants. Full details of behavioural results of the EFT are presented in Supplementary Table 1.
Atypical activation associated with autism and the broader phenotype
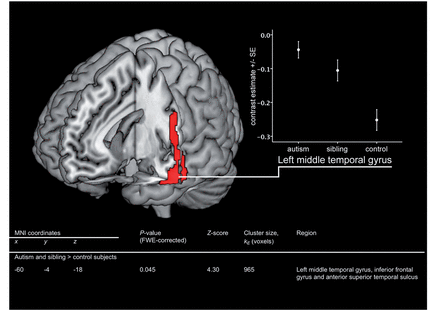
We initially investigated differences reflecting the familial risk for autism, determined as differences between the combined autism and sibling groups and the control group [(autism + sibling) > control] and [control > (autism + sibling)]. We found significantly increased activation in the combined autism and sibling groups, relative to control subjects, in a cluster involving the left anterior middle temporal gyrus, inferior frontal gyrus and anterior superior temporal sulcus. Activation in the left middle temporal gyrus was significantly increased relative to control subjects, even after correction for multiple comparisons on an FWE-corrected whole brain level (P = 0.045). Furthermore, activation within the cluster as a whole was significantly increased relative to control subjects on an FWE-corrected whole brain level (P = 0.006).
Activation in a 4-mm-radius sphere constructed around the location of the FWE-corrected significant hyperactivation in the left middle temporal gyrus was extracted and plotted for all groups (Fig. 1). The sibling group shows atypical activation, comparable with that in the autism group, but at an intermediate degree to that seen in autism. This is consistent with the hypothesis that differences in siblings represent an endophenotype of autism: a neurobiological expression of their familial risk for autism and, hence, of aspects of their genotype shared with the autism group.
Hyperactivation in temporal and frontal regions in adolescents with autism and their siblings, relative to control subjects. Activation map indicates areas where EFT-related activation (EFT versus control task) is significantly greater in autism and sibling groups versus control subjects. Map thresholded at uncorrected P = 0.001 on whole brain level and rendered onto 3D MNI template brain image in MRIcron. The table within this figure indicates the significant result after correction for multiple comparisons on FWE-corrected whole brain level. Graph indicates means (SE) for average contrast estimate (EFT—control task) from 4-mm-radius sphere around the FWE-corrected peak result (−60, −4, −18).
No significant hypoactivation in the combined autism and sibling groups, relative to control subjects [control > (autism + sibling)], was detected at the same FWE-corrected whole brain level threshold. Furthermore, at the same corrected threshold, no significant activation differences were detected between autism and sibling groups (autism > sibling; sibling > autism).
We, therefore, conducted further exploratory analyses using an uncorrected whole brain level threshold of P = 0.001 to investigate the determinants of the significant differences observed in terms of whether they are driven by case–control or sibling–control differences.
Post hoc exploration of case–control and sibling–control differences in activation
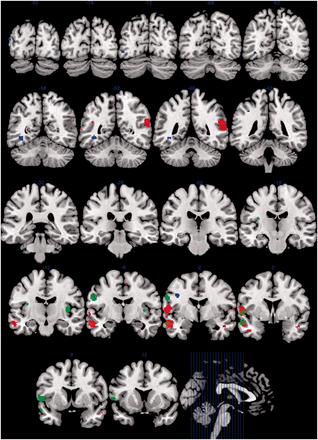
We found increased activation in autism, relative to control subjects, in similar temporal and frontal regions, namely, the left middle temporal gyrus and superior temporal sulcus (P < 0.001), left inferior frontal gyrus (P < 0.001), right superior temporal sulcus (P < 0.001), right inferior temporal gyrus (P < 0.001) and left angular gyrus (P = 0.001), all at an uncorrected whole brain level. As indicated in Fig. 2, there is overlap between the areas within which hyperactivation is evident in autism and sibling groups, relative to control subjects. Using the same threshold, we also found reduced activation in autism, relative to control subjects, in a different range of left-sided brain areas, particularly implicated in associative visual and other higher-level processing functions, namely, the left premotor cortex (P < 0.001), fusiform gyrus (P < 0.001), V3 associative visual cortex (P < 0.001) and supramarginal gyrus (P < 0.001) (Table 2 and Fig. 2).
Between-group differences in EFT-related activation in adolescents with autism, their unaffected siblings and control subjects. Red indicates greater activation in autism versus control subjects. Blue indicates greater activation in control subjects versus autism. Green indicates greater activation in siblings versus control subjects. Brown indicates overlap between greater activation in autism (red) and siblings (green) relative to control subjects. Group difference maps thresholded at uncorrected P = 0.001 on whole brain level, kE ≥ 10, and rendered as solid colours onto coronal sections of MNI template brain image in MRIcron. Number indicates y co-ordinate of coronal section. Sagittal section illustrates locations of coronal sections indicated.
We also found increased activation in unaffected siblings, relative to control subjects, in a number of temporal and frontal brain areas, namely, the left primary motor cortex (P < 0.001), left anterior superior temporal sulcus (P < 0.001), left inferior frontal gyrus (P < 0.001) and right (P < 0.001) and left (P = 0.001) insula, all at an uncorrected whole brain level. These findings are of particular importance, given that all siblings were unaffected and only differed from the control group in that the control subjects had no family history of autism.
Importantly, using the same uncorrected threshold, we did not detect any significant differences between activation in the autism and sibling groups, providing further support to the suggestion that these differences reflect the underlying familial and shared genetic risk for autism, in common between the autism and sibling groups.
Imaging correlates of autism clinical severity
We investigated the correlation between autism clinical severity, as defined by the ADOS-G score, and activation in the autism group within 4-mm-radius spheres constructed around the locations of the significant autism versus control differences, as listed in Table 2. We found that the total score on the ADOS-G (defined as the sum of subdomains A and B) was strongly negatively correlated with activation in the left supramarginal gyrus (P = 0.001), V3 associative visual cortex (P = 0.009), fusiform gyrus (P = 0.010) and premotor cortex (P = 0.022) (partial correlations, controlling for age and sex). In other words, an increasing degree of observed clinical severity on the ADOS-G was significantly associated with reduced activation on the EFT versus control task. Strikingly, although all regions of hypoactivation in autism (control subjects > autism) were significantly associated with ADOS-G scores, none of the five regions of hyperactivation in autism (autism > control) was associated with clinical severity (Table 3).
Correlation between EFT-related activation (EFT-control task) in the autism group and clinical symptom severity
| Region of hypoactivation in autism . | Correlations with autism clinical severity, P-value (correlation); df = 34 . | ||
|---|---|---|---|
| . | ADOS total score (sum of subdomains A and B) . | ADOS subdomain A (communication) . | ADOS subdomain B (reciprocal social interaction) . |
| Left pre-motor cortex | 0.022 (−0.380) | NS | 0.012 (−0.416) |
| Left fusiform gyrus | 0.010 (−0.425) | NS | 0.001 (−0.536) |
| Left V3 (associative visual cortex) | 0.016 (−0.398) | NS | 0.008 (−0.434) |
| Left supramarginal gyrus | 0.001 (−0.528) | 0.007 (−0.438) | 0.001 (−0.535) |
| Left V3 (associative visual cortex) | 0.009 (−0.428) | NS | 0.002 (−0.489) |
| Region of hypoactivation in autism . | Correlations with autism clinical severity, P-value (correlation); df = 34 . | ||
|---|---|---|---|
| . | ADOS total score (sum of subdomains A and B) . | ADOS subdomain A (communication) . | ADOS subdomain B (reciprocal social interaction) . |
| Left pre-motor cortex | 0.022 (−0.380) | NS | 0.012 (−0.416) |
| Left fusiform gyrus | 0.010 (−0.425) | NS | 0.001 (−0.536) |
| Left V3 (associative visual cortex) | 0.016 (−0.398) | NS | 0.008 (−0.434) |
| Left supramarginal gyrus | 0.001 (−0.528) | 0.007 (−0.438) | 0.001 (−0.535) |
| Left V3 (associative visual cortex) | 0.009 (−0.428) | NS | 0.002 (−0.489) |
Regions defined as 4-mm-radius spheres around the co-ordinates of significant hypoactivation in autism versus control subjects. Partial correlations, controlling for age and sex. NS = not significant.
Correlation between EFT-related activation (EFT-control task) in the autism group and clinical symptom severity
| Region of hypoactivation in autism . | Correlations with autism clinical severity, P-value (correlation); df = 34 . | ||
|---|---|---|---|
| . | ADOS total score (sum of subdomains A and B) . | ADOS subdomain A (communication) . | ADOS subdomain B (reciprocal social interaction) . |
| Left pre-motor cortex | 0.022 (−0.380) | NS | 0.012 (−0.416) |
| Left fusiform gyrus | 0.010 (−0.425) | NS | 0.001 (−0.536) |
| Left V3 (associative visual cortex) | 0.016 (−0.398) | NS | 0.008 (−0.434) |
| Left supramarginal gyrus | 0.001 (−0.528) | 0.007 (−0.438) | 0.001 (−0.535) |
| Left V3 (associative visual cortex) | 0.009 (−0.428) | NS | 0.002 (−0.489) |
| Region of hypoactivation in autism . | Correlations with autism clinical severity, P-value (correlation); df = 34 . | ||
|---|---|---|---|
| . | ADOS total score (sum of subdomains A and B) . | ADOS subdomain A (communication) . | ADOS subdomain B (reciprocal social interaction) . |
| Left pre-motor cortex | 0.022 (−0.380) | NS | 0.012 (−0.416) |
| Left fusiform gyrus | 0.010 (−0.425) | NS | 0.001 (−0.536) |
| Left V3 (associative visual cortex) | 0.016 (−0.398) | NS | 0.008 (−0.434) |
| Left supramarginal gyrus | 0.001 (−0.528) | 0.007 (−0.438) | 0.001 (−0.535) |
| Left V3 (associative visual cortex) | 0.009 (−0.428) | NS | 0.002 (−0.489) |
Regions defined as 4-mm-radius spheres around the co-ordinates of significant hypoactivation in autism versus control subjects. Partial correlations, controlling for age and sex. NS = not significant.
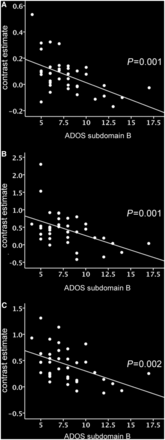
The ADOS-G total score is the sum of two subdomains: ‘subdomain A’, which measures abnormalities in communication, and ‘subdomain B’, which measures abnormalities in reciprocal social interaction. To ascertain the subdomain(s) contributing to the observed correlation, we investigated the correlation between activity in the same regions of significant hypoactivation and scores on subdomains A and B. Strikingly, activation within all regions of hypoactivation was strongly negatively correlated with impairments in reciprocal social interaction. Moreover, for all regions, the strength of the correlation was even greater than for the total ADOS-G score (Table 3 and Fig. 3). In marked contrast, however, only one region demonstrated a significant correlation between activation and impairments in communication, with this correlation being weaker than the corresponding correlation with either the total ADOS-G score or the reciprocal social communication subdomain. This provides evidence that the reduced activation in autism spectrum conditions in these brain areas during the EFT versus control task is specifically associated with clinical impairments in reciprocal social interaction.
(A–C) Correlations between impairments in reciprocal social interaction (subdomain B on the ADOS) and autism group mean contrast estimate (EFT—control task) in A, left fusiform gyrus, B, left supramarginal gyrus, and C, left V3 associative visual cortex. P-values indicate partial correlation after controlling for age and sex. CT = control task.
Discussion
The present study sheds light on an established functional imaging paradigm, the EFT. This builds on the fact that siblings of people with autism have not been previously investigated on the EFT in functional MRI studies. We found, for the first time, evidence for atypical activation in siblings versus control subjects, with no family history of autism, suggestive of a functional endophenotype of autism. The analyses also provide evidence for case–control differences in activation, but no significant difference in activation between cases and unaffected siblings.
Previous functional MRI studies have reported that atypical brain activity during performance of the EFT is different in autism compared with control subjects; however, a considerable degree of variation exists within the literature as to the location of these differences in activation. A number of studies, the first of which was from our group, suggest that people with autism tend to activate occipital and extrastriate regions to a greater extent, and frontal and parietal regions to a reduced extent, compared with control subjects (Ring et al., 1999; Lee et al., 2007; Manjaly et al., 2007). A note of caution in comparing these studies, however, is that task and control conditions vary widely across studies.
Whilst case–control findings within the present study differ from previous studies, as we found greater associative, extrastriate and occipital activation in control subjects and greater frontal and temporal activation in autism, it is notable that in the conjunction analysis, we did detect significant task-related activation, within these areas, that was common to all study groups. It may be the case that greater frontal and temporal activation in autism could reflect more effortful activation within these areas to compensate for attentional and cognitive control deficits known to occur (Solomon et al., 2009), whereas the reduced activation in visual processing areas might be due to a relative lack of requirement for effortful activation in these areas in people with autism because of underlying visual processing strengths. Such compensation could conceivably account for the lack of case–control performance differences in this and previous functional MRI studies. We found evidence for reduced activation within a range of left-sided regions in autism versus control subjects, which is concordant with a study demonstrating EFT-related activation in a network of left-sided brain regions in control subjects, but not in adolescents with autism (Manjaly et al., 2007). We demonstrated hypoactivation in left V3 associative visual cortex in autism versus control subjects; reduced activation in comparable cortical areas during the EFT has been previously reported in parents of patients with autism compared with control subjects (Baron-Cohen et al., 2006a). Furthermore, reduced functional connectivity between visuospatial and frontal regions has been reported in autism (Damarla et al., 2010).
A number of mechanisms could account for reduced task-related activation (i.e. EFT versus control task) in autism. Resting-state studies have shown a failure to deactivate a number of brain regions in autism during the resting state (Kennedy et al., 2006). It is possible, therefore, that apparently reduced EFT-related activation in autism could be underpinned by impaired deactivation during the control task. This hypothesis would hold that, despite the simpler task demands of the control condition, the participants with autism nonetheless activate associative visual areas strongly in both conditions, whereas control subjects would only demonstrate strong activation during the EFT condition. This may, perhaps, be because of a process of enhanced perceptual functioning (Mottron et al., 2006), which is associated with increased occipital and parietal activation in autism (Samson et al., 2011) or alternatively a top–down process, whereby the reduced cognitive flexibility and set-shifting that has been demonstrated in children with autism (Yerys et al., 2009) results in similar scrutiny of stimuli in both conditions.
An alternative explanation may be that information processing within the EFT occurs within an atypical location in autism versus control subjects. This explanation could account for the findings of hyperactivation in autism in temporal brain regions, in addition to hypoactivation in a number of different, primarily association, cortical regions, including fusiform and V3 visual associative regions and unimpaired performance in terms of accuracy and reaction times. Convergent evidence for atypically located visual processing, nonetheless intact in behavioural performance, comes from the face-processing literature and reports that face-processing in autism is subserved by different neural sites to control subjects, with intact reaction time and accuracy (Pierce et al., 2001).
We found evidence for strong negative correlations between autism clinical severity, as measured by the ADOS-G, and EFT-related activation. For all these measures, decreased task-related activation was associated with more severe symptoms. This is the first time that EFT-related functional MRI activation has been related to this widely used clinical diagnostic measure, and our findings provide an elucidation of the clinical correlates of atypical activation in the autism group. We found that clinical severity was associated with EFT-related hypoactivation, but not hyperactivation, in the autism group. As no hypoactivation was detected in these regions in siblings versus control subjects, it would seem that atypical activation in these regions is a clinical state-related feature of autism, rather than of the broader phenotype in relatives. Our findings suggest, however, that hyperactivation of frontal and temporal regions could be a marker of familial risk for autism, rather than of clinical state. This is supported by our finding of hyperactivation in overlapping brain regions in clinically unaffected siblings of those with autism spectrum conditions compared with control subjects.
We also found that EFT-related hypoactivation was predominantly related to clinical measures of impaired reciprocal social interaction, rather than to impaired communication in autism. Our suggestion that atypically located visual information processing in autism underlies the pattern of atypical activation found in our study could offer a tentative explanation for impairments in certain aspects of reciprocal social interaction that are dependent on visual information processing, such as impairments in eye gaze and its coordination with other modalities of social interaction, joint attention and the direction of facial expressions to others, all central to expressions of the autism phenotype (American Psychiatric Association, 1994). Furthermore, it may be that the association between an atypical EFT-related functional MRI response and reciprocal social interaction represents a speculative glimpse at features that might characterize one of a number of putative subtypes of autism, the existence of which, instead of a unitary construct of autism, has been proposed (Happe et al., 2006) as an explanation for heterogeneity within the population with autism.
Previous studies have reported conflicting findings regarding performance on the EFT in autism. A number of non-imaging studies, including a recent large study (White and Saldana, 2011), identified no difference in EFT performance in autism versus control subjects. However, superior performance on certain measures has been reported in other studies (Jolliffe and Baron-Cohen, 1997; de Jonge et al., 2006). In keeping with our findings, previous functional MRI studies using this paradigm have reported that individuals with autism perform similarly to control subjects on the EFT (Ring et al., 1999; Lee et al., 2007; Manjaly et al., 2007). This lends itself to a clearer interpretation, namely, that despite equivalent performance, different cognitive strategies are being used, reflecting different neural activation. Specifying the nature of this distinct cognitive style in cases and siblings will require further vision research.
It is notable that, within our sample, unaffected siblings and control subjects did not differ on measures of parentally reported autistic features. This may seem to be at odds with the widely accepted view that siblings of those with autism display a greater prevalence of autistic traits (Bolton et al., 1994). However, in the context of this study, it is important to note that parents of the unaffected siblings completed the Autism Spectrum Quotient for both of their children, and this introduces the possibility of reporter-bias due to a sibling contrast effect, as has been previously described (Simonoff et al., 1998; Saudino et al., 2000; Constantino et al., 2010). This does not pose a limitation on our study however, as we have neither used the Autism Spectrum Quotient in the recruitment or selection of our participants nor have we used the measure in our imaging analyses. On the contrary, the lack of any behavioural difference between siblings and control subjects highlights the remarkability of our finding of significant functional MRI differences between these two groups that do not differ behaviourally, but only in terms of the family history of autism.
Conclusion
An atypical pattern of EFT-related functional MRI activation occurs in adolescents with autism spectrum conditions and their unaffected siblings, compared with control subjects with no family history of autism spectrum conditions. This provides evidence for an atypical neural substrate of information processing during this task in autism and in the broader phenotype in siblings, reflecting the underlying genetic risk for autism as shared by the autism and sibling groups, rather than autism state-dependent factors. The pattern of atypical activation identified is a candidate functional neuroimaging endophenotype of autism and, while requiring replication, is of potential utility as a quantitative biomarker for future genetic studies. The identification of further neuroendophenotypes of autism offers an opportunity to overcome the problem of heterogeneity in autism research and could inform future developments in clinical diagnostic practice.
Funding
Clinician Scientist Fellowship from the Medical Research Council, UK [grant number G0701919] (to M.D.S.) and the Gates Cambridge Scholarship Trust (to L.R.C.).
Conflict of interest
E.T.B. is employed half-time by the University of Cambridge and half-time by GlaxoSmithKline plc. None of the other authors have any other biomedical financial interests or potential conflicts of interest.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors are grateful to all participants and their families for their participation in their study and to the autism support organizations that helped with recruitment. They are also grateful to Dr Zina Manjaly for supplying the EFT stimuli and to Dr Cinly Ooi for technical assistance. They are grateful to the anonymous reviewers of a previous version of this manuscript for their helpful feedback. They thank Dr Michael Lombardo and Dr Howard Ring for comments and discussion. This work was conducted in association with the NIHR CLAHRC for Cambridgeshire and Peterborough NHS Foundation Trust.
Abbreviations
- ADOS-G
Autism Diagnostic Observation Schedule-Generic
- EFT
Embedded Figures Task
- FWE
family-wise error
- MNI
Montreal Neurological Institute